UV Radiation Induces Specific Changes in the Carotenoid Profile of Arabidopsis thaliana
Abstract
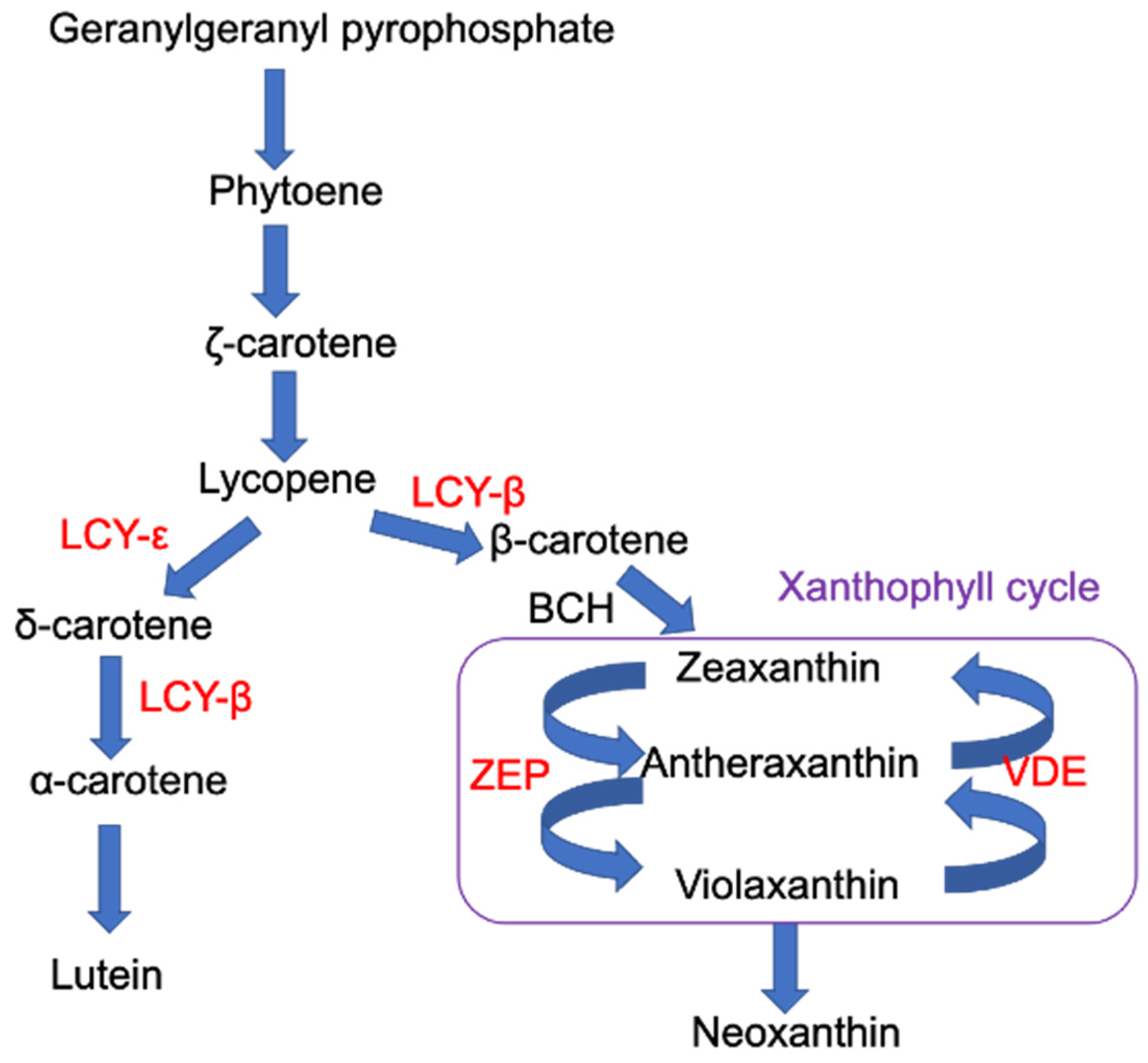
1. Introduction
2. Methodology
2.1. Plant Material
2.2. UV Treatments
2.3. Chlorophyll a Fluorescence Analysis
2.4. Carotenoid and Chlorophyll Extraction
2.5. Carotenoid and Chlorophyll Separation Using HPLC
2.6. Statistical Analysis
3. Results
3.1. UV-Induced Carotenoids in Arabidopsis Thaliana
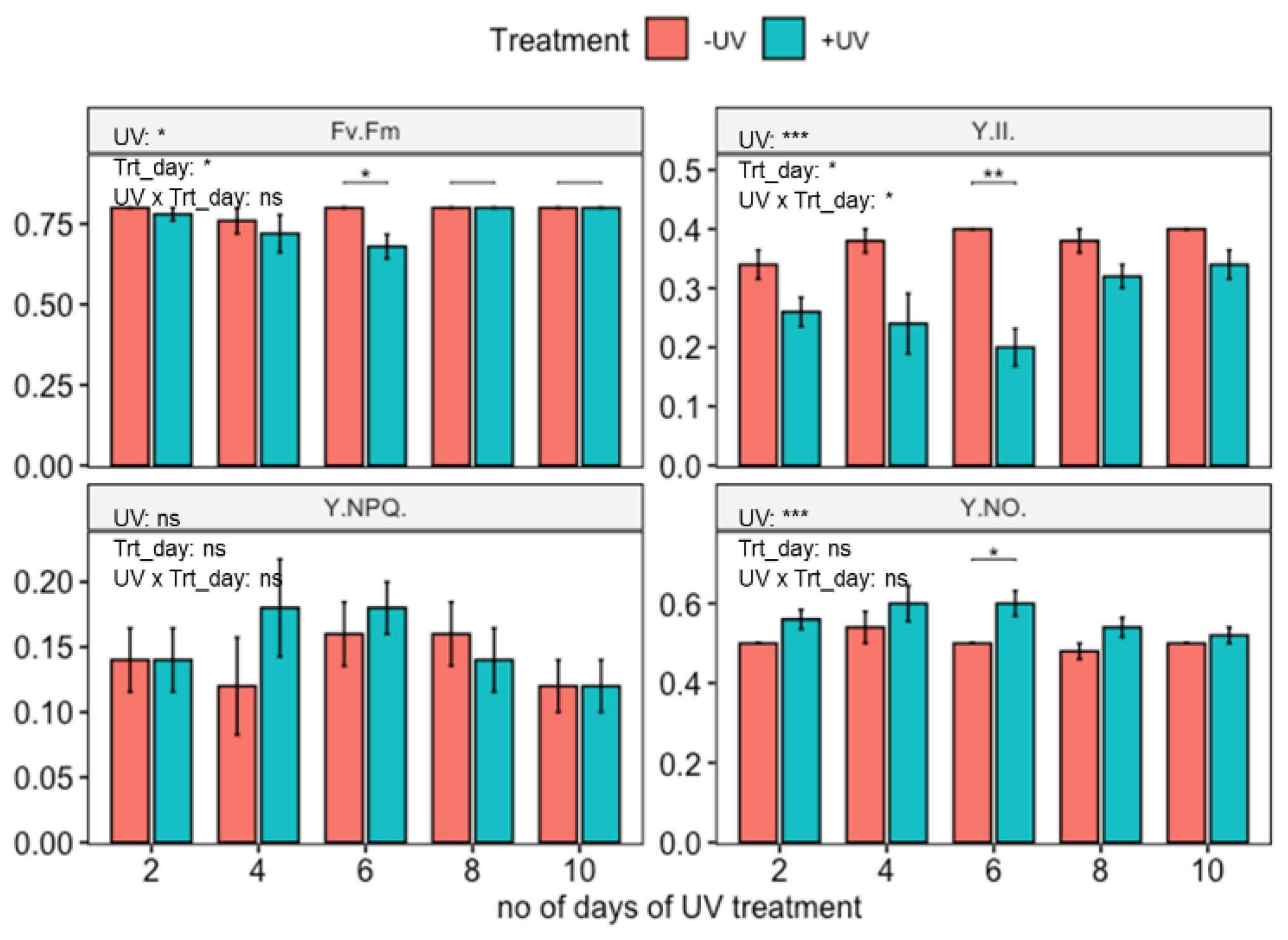
3.2. Influence of UV on Photosynthetic Parameters
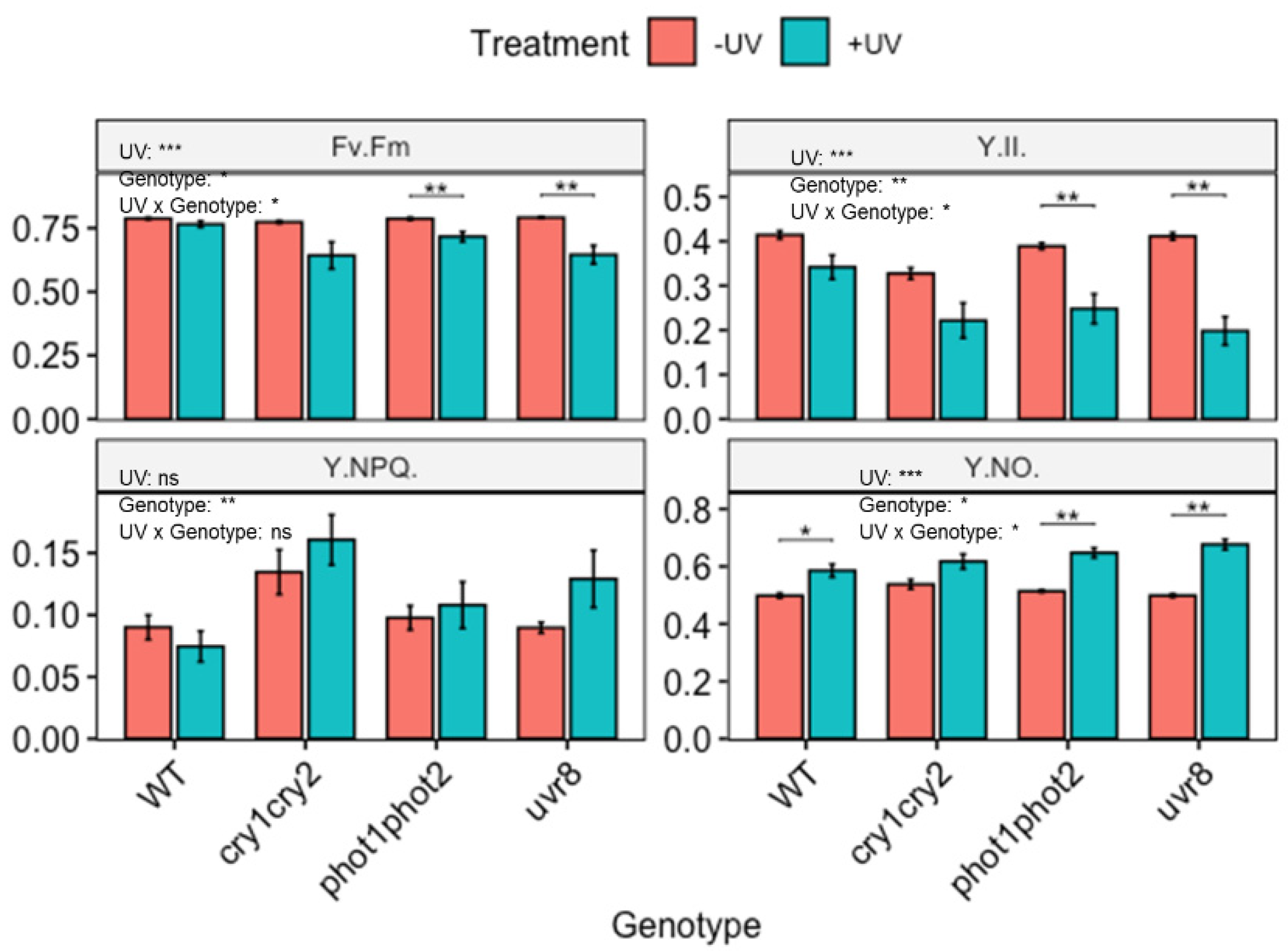
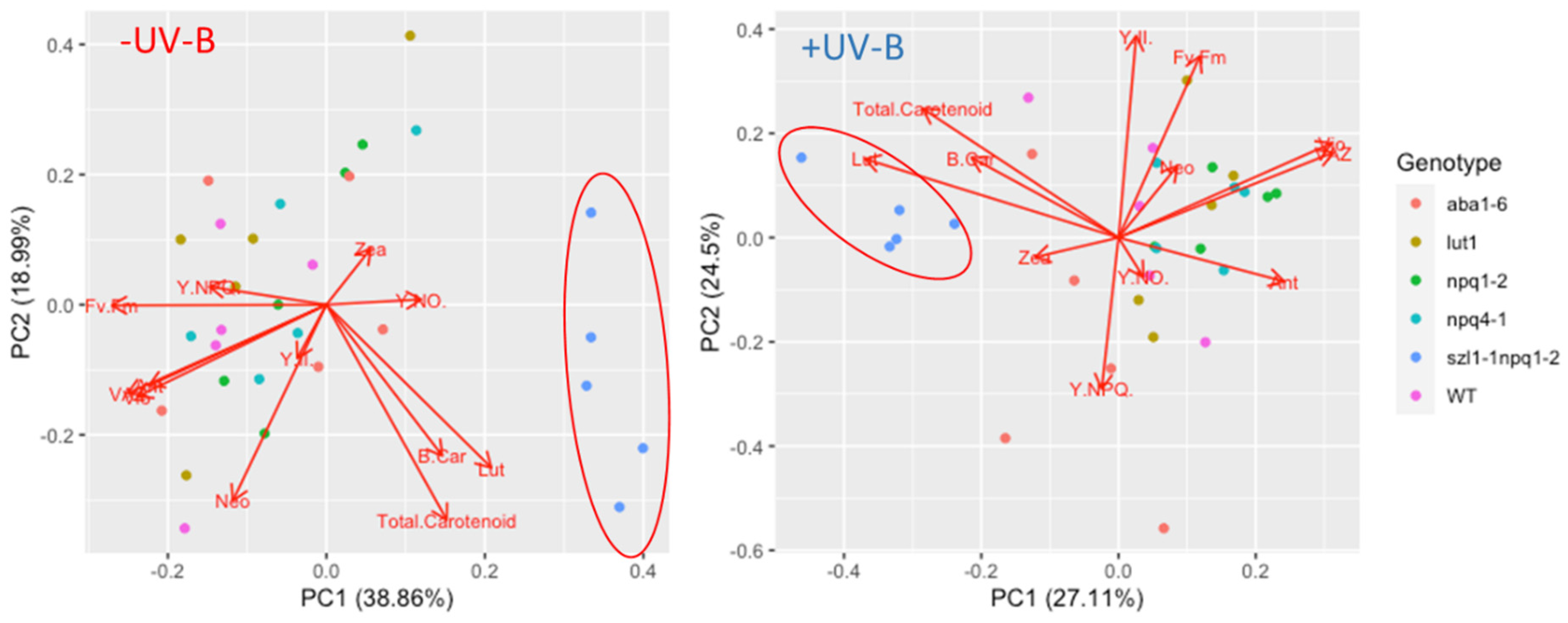
3.3. Role of Photoreceptors in the Induction of Carotenoids
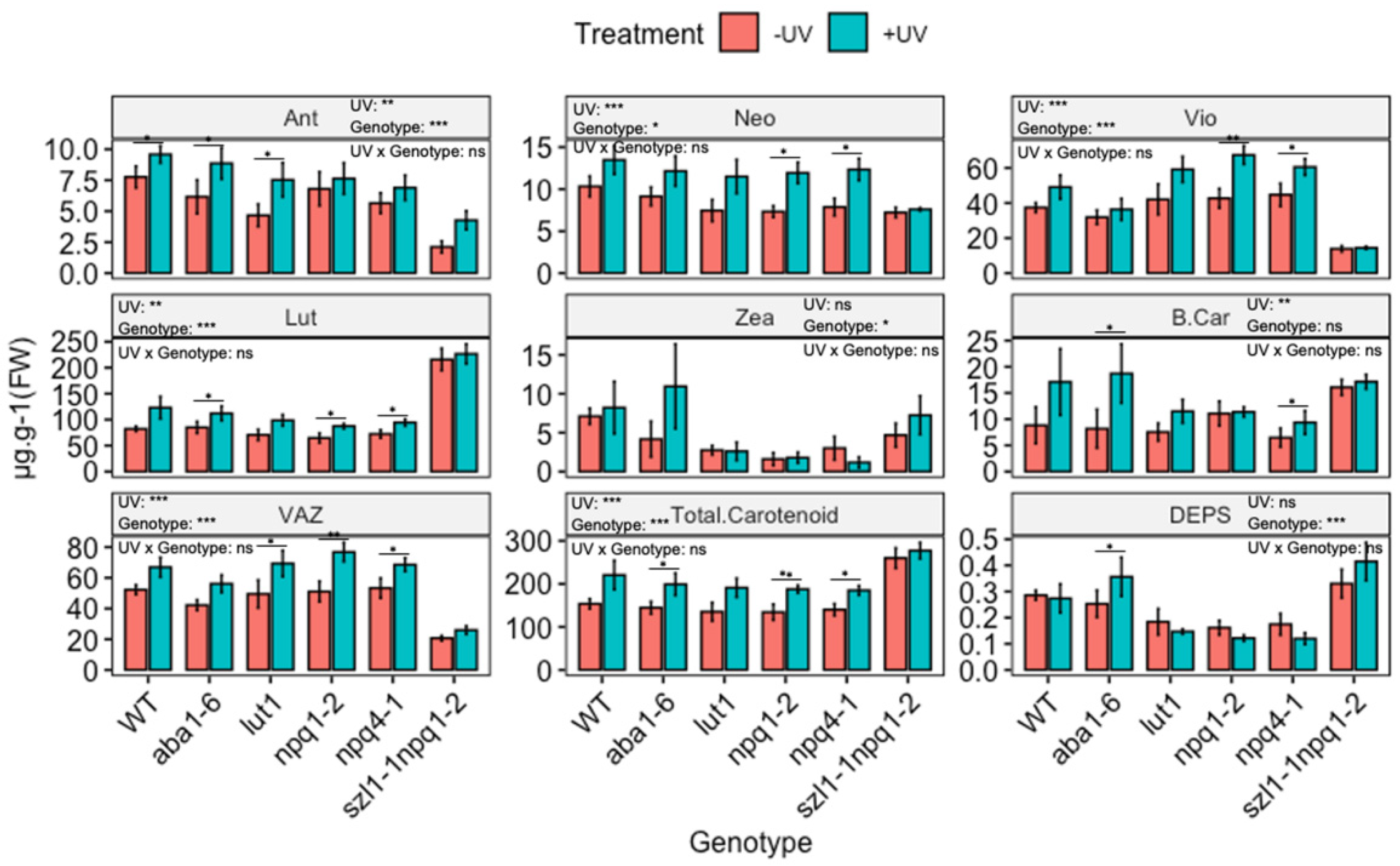
3.4. The UV-Mediated Accumulation of Carotenoids in Arabidopsis Biosynthesis Mutants
4. Discussion
4.1. UV-Induced Accumulation of Xanthophyll Cycle Pigments
4.2. UV-Induced Accumulation of Lutein
4.3. The Role of Photoreceptors in the UV-Induced Accumulation of Carotenoids
4.4. Carotenoid Profile of Arabidopsis Plants with Impaired Carotenoid Biosynthesis
4.4.1. Carotenoid Profiles under PAR
4.4.2. The Carotenoid Profile of UV-Exposed Plants
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Smith, H. Light Quality, Photoperception, and Plant Strategy. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1982, 33, 481–518. [Google Scholar]
- Huché-Thélier, L.; Crespel, L.; Le Gourrierec, J.; Morel, P.; Sakr, S.; Leduc, N. Light signaling and plant responses to blue and UV radiations-Perspectives for applications in horticulture. Environ. Exp. Bot. 2016, 121, 22–38. [Google Scholar] [CrossRef]
- Hideg, É.; Jansen, M.A.K.; Strid, Å. UV-B exposure, ROS, and stress: Inseparable companions or loosely linked associates? Trends Plant Sci. 2013, 18, 107–115. [Google Scholar] [CrossRef] [PubMed]
- Jansen, M.A.K.; Gaba, V.; Greenberg, B.M. Higher plants and UV-B radiation: Balancing damage, repair and acclimation. Trends Plant Sci. 1998, 3, 131–135. [Google Scholar] [CrossRef]
- Schreiner, M.; Martínez-Abaigar, J.; Glaab, J.; Jansen, M.A.K. UV-B Induced Secondary Plant Metabolites. Opt. Photonik 2014, 9, 34–37. [Google Scholar] [CrossRef]
- Jenkins, G.I. Photomorphogenic responses to ultraviolet-B light. Plant Cell Environ. 2017, 40, 2544–2557. [Google Scholar] [CrossRef]
- Tossi, V.E.; Regalado, J.J.; Iannicelli, J.; Laino, L.E.; Burrieza, H.P.; Escandón, A.S.; Pitta-Álvarez, S.I. Beyond Arabidopsis: Differential UV-B response mediated by UVR8 in diverse species. Front. Plant Sci. 2019, 10, 780. [Google Scholar] [CrossRef]
- Klem, K.; Holub, P.; Štroch, M.; Nezval, J.; Špunda, V.; Tříska, J.; Jansen, M.A.K.; Robson, T.M.; Urban, O. Ultraviolet and photosynthetically active radiation can both induce photoprotective capacity allowing barley to overcome high radiation stress. Plant Physiol. Biochem. 2015, 93, 74–83. [Google Scholar] [CrossRef]
- Hideg, É.; Strid, A. The effects of UV-B on the biochemistry and metabolism of plants. In UV-B Radiation and Plant Life: Molecular Biology to Ecology; Jordan, B., Ed.; CABI: London, UK, 2017; pp. 90–110. [Google Scholar] [CrossRef]
- Vandenbussche, F.; Yu, N.; Li, W.; Vanhaelewyn, L.; Hamshou, M.; Van Der Straeten, D.; Smagghe, G. An ultraviolet B condition that affects growth and defense in Arabidopsis. Plant Sci. 2018, 268, 54–63. [Google Scholar] [CrossRef]
- Verdaguer, D.; Jansen, M.A.K.; Llorens, L.; Morales, L.O.; Neugart, S. Plant Science UV-A radiation effects on higher plants: Exploring the known unknown. Plant Sci. 2017, 255, 72–81. [Google Scholar] [CrossRef]
- Brown, B.A.; Cloix, C.; Jiang, G.H.; Kaiserli, E.; Herzyk, P.; Kliebenstein, D.J.; Jenkins, G.I. A UV-B-specific signaling component orchestrates plant UV protection. Proc. Natl. Acad. Sci. USA 2005, 102, 18225–18230. [Google Scholar] [CrossRef] [PubMed]
- Morales, L.O.; Tegelberg, R.; Brosché, M.; Keinänen, M.; Lindfors, A.; Aphalo, P.J. Effects of solar UV-A and UV-B radiation on gene expression and phenolic accumulation in Betula pendula leaves. Tree Physiol. 2010, 30, 923–934. [Google Scholar] [CrossRef] [PubMed]
- Hectors, K.; Van Oevelen, S.; Geuns, J.; Guisez, Y.; Jansen, M.A.K.; Prinsen, E. Dynamic changes in plant secondary metabolites during UV acclimation in Arabidopsis thaliana. Physiol. Plant. 2014, 152, 219–230. [Google Scholar] [CrossRef] [PubMed]
- Neugart, S.; Fiol, M.; Schreiner, M.; Rohn, S.; Zrenner, R.; Kroh, L.W.; Krumbein, A. Interaction of moderate UV-B exposure and temperature on the formation of structurally different flavonol glycosides and hydroxycinnamic acid derivatives in kale (Brassica oleracea var. sabellica). J. Agric. Food Chem. 2014, 62, 4054–4062. [Google Scholar] [CrossRef] [PubMed]
- Coffey, A.; Prinsen, E.; Jansen, M.A.K.; Conway, J. The UVB photoreceptor UVR8 mediates accumulation of UV-absorbing pigments, but not changes in plant morphology, under outdoor conditions. Plant Cell Environ. 2017, 40, 2250–2260. [Google Scholar] [CrossRef]
- Celeste Dias, M.; Pinto, D.C.G.A.; Correia, C.; Moutinho-Pereira, J.; Oliveira, H.; Freitas, H.; Silva, A.M.S.; Santos, C. UV-B radiation modulates physiology and lipophilic metabolite profile in Olea europaea. J. Plant Physiol. 2018, 222, 39–50. [Google Scholar] [CrossRef]
- Contreras, R.A.; Pizarro, M.; Köhler, H.; Zamora, P.; Zúñiga, G.E. UV-B shock induces photoprotective flavonoids but not antioxidant activity in Antarctic Colobanthus quitensis (Kunth) Bartl. Environ. Exp. Bot. 2019, 159, 179–190. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, J.; Wang, H.-Z.; Wu, K.-X.; Guo, X.-R.; Mu, L.-Q.; Tang, Z.-H. Comparison of the global metabolic responses to UV-B radiation between two medicinal Astragalus species: An integrated metabolomics strategy. Environ. Exp. Bot. 2020, 176, 104094. [Google Scholar] [CrossRef]
- Badmus, U.O.; Crestani, G.; Connell, R.D.O.; Cunningham, N.; Jansen, M.A.K. UV-B induced accumulation of tocopherol in Arabidopsis thaliana is not dependent on individual UV photoreceptors. Plant Stress 2022, 5, 100105. [Google Scholar] [CrossRef]
- Vogt, T. Phenylpropanoid biosynthesis. Mol. Plant 2010, 3, 2–20. [Google Scholar] [CrossRef]
- Jansen, M.A.K.; Bilger, W.; Hideg, E.; Strid, Å.; Participants, U.W.; Urban, O. Interactive effects of UV-B radiation in a complex environment. Plant Physiol. Biochem. 2019, 134, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Badmus, U.O.; Alexander, A.; Klem, K.; Urban, O.; Jansen, M.A.K. A meta-analysis of the effects of UV radiation on the plant carotenoid pool. Plant Physiol. Biochem. 2022, 183, 36–45. [Google Scholar] [CrossRef] [PubMed]
- Britton, G. Structure and properties of carotenoids in relation to function. FASEB J. 1995, 9, 1551–1558. [Google Scholar] [CrossRef] [PubMed]
- Fraser, P.D.; Bramley, P.M. The biosynthesis and nutritional uses of carotenoids. Prog. Lipid Res. 2004, 43, 228–265. [Google Scholar] [CrossRef] [PubMed]
- Britton, G.; Liaaen-Jensen, S.; Pfander, H. Carotenoids Special Molecules, Special Properties. In Nutrition and Health; Britton, G., Liaaen-Jensen, S., Pfander, H., Eds.; Springer Science and Business Media: Berlin, Germany, 2009; pp. 1–6. Available online: http://books.google.com/books?id=c8USiLi73dUC&pgis=1 (accessed on 15 September 2022).
- Cazzaniga, S.; Bressan, M.; Carbonera, D.; Agostini, A.; Dall’osto, L. Differential Roles of Carotenes and Xanthophylls in Photosystem I Photoprotection. Biochemistry 2016, 55, 3636–3649. [Google Scholar] [CrossRef]
- Niyogi, K.K.; Björkman, O.; Grossman, A.R. The roles of specific xanthophylls in photoprotection. Proc. Natl. Acad. Sci. USA 1997, 94, 14162–14167. [Google Scholar] [CrossRef]
- Havaux, M. Carotenoids as membrane stabilizers in chloroplasts. Trends Plant Sci. 1998, 3, 147–151. [Google Scholar] [CrossRef]
- Xiao, F.G.; Shen, L.; Ji, H.F. On photoprotective mechanisms of carotenoids in light harvesting complex. Biochem. Biophys. Res. Commun. 2011, 414, 1–4. [Google Scholar] [CrossRef]
- Pogson, B.J.; Rissler, H.M. Genetic manipulation of carotenoid biosynthesis and photoprotection. Philos. Trans. R. Soc. B Biol. Sci. 2000, 355, 1395–1403. [Google Scholar] [CrossRef]
- Demmig-Adams, B.; Cohu, C.M.; Stewart, J.J.; Iii, W.W.A. Non-Photochemical Quenching and Energy Dissipation in Plants, Algae and Cyanobacteria. In Photosynthesis and Respiration; Demmig-Adams, B., Garab, G., Adams, W., III, Eds.; Springer: Dordrecht, The Netherlands, 2014; Volume 40, Available online: http://link.springer.com/10.1007/978-94-017-9032-1 (accessed on 16 September 2022).
- Bykowski, M.; Mazur, R.; Wo, J.; Suski, S. Too rigid to fold: Carotenoid-dependent decrease in thylakoid fluidity hampers the formation of chloroplast grana. Plant Physiol. 2021, 185, 210–227. [Google Scholar] [CrossRef]
- Son, M.; Pinnola, A.; Gordon, S.C.; Bassi, R.; Schlau-Cohen, G.S. Observation of dissipative chlorophyll-to-carotenoid energy transfer in light-harvesting complex II in membrane nanodiscs. Nat. Commun. 2020, 11, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Umena, Y.; Kawakami, K.; Shen, J.; Kamiya, N. Crystal structure of oxygen-evolving photosystem II at a resolution of 1.9 Å. Nature 2011, 473, 55–60. [Google Scholar] [CrossRef] [PubMed]
- Jahns, P.; Holzwarth, A.R. The role of the xanthophyll cycle and of lutein in photoprotection of photosystem II. Biochim. Biophys. Acta—Bioenerg. 2012, 1817, 182–193. [Google Scholar] [CrossRef] [PubMed]
- Xu, P.; Chukhutsina, V.U.; Nawrocki, W.J.; Schansker, G.; Bielczynski, L.W.; Lu, Y.; Karcher, D.; Bock, R.; Croce, R. Photosynthesis without b -carotene. Elife 2020, 9, e58984. [Google Scholar] [CrossRef]
- Demmig-Adams, B. Carotenoids and photoprotection in plants: A role for the xanthophyll zeaxanthin. Biochim. Biophys. Acta—Bioenerg. 1990, 1020, 1–24. [Google Scholar] [CrossRef]
- Demmig-Adams, B.; Stewart, J.J.; López-Pozo, M.; Polutchko, S.K.; Adams, W.W. Zeaxanthin, a Molecule for Photoprotection in Many Different Environments. Molecules 2020, 25, 5825. [Google Scholar] [CrossRef]
- Ablazov, A.; Mi, J.; Jamil, M.; Jia, K.P.; Wang, J.Y.; Feng, Q.; Al-Babili, S. The Apocarotenoid Zaxinone Is a Positive Regulator of Strigolactone and Abscisic Acid Biosynthesis in Arabidopsis Roots. Front. Plant Sci. 2020, 11, 1–13. [Google Scholar] [CrossRef]
- Müller, M.; Munné-Bosch, S. Hormonal impact on photosynthesis and photoprotection in plants. Plant Physiol. 2021, 185, 1500–1522. [Google Scholar] [CrossRef]
- Pang, X.; Zhang, Z.; Wen, X.; Ban, Y.; Moriguchi, T.; Yusuke, Y. Polyamines, all-purpose players in response to environment stresses in plants. Plant Stress 2007, 1, 173–188. [Google Scholar]
- Moreno, J.C.; Mi, J.; Alagoz, Y.; Al-Babili, S. Plant apocarotenoids: From retrograde signaling to interspecific communication. Plant J. 2021, 105, 351–375. [Google Scholar] [CrossRef]
- Llorente, B.; Martinez-Garcia, J.F.; Stange, C.; Rodriguez-Concepcion, M. Illuminating colors: Regulation of carotenoid biosynthesis and accumulation by light. Curr. Opin. Plant Biol. 2017, 37, 49–55. [Google Scholar] [CrossRef] [PubMed]
- Heath, J.J.; Cipollini, D.F.; Stireman, J.O., III. The role of carotenoids and their derivatives in mediating interactions between insects and their environment. Arthropod-Plant Interact. 2013, 7, 1–20. [Google Scholar] [CrossRef]
- García-Chavarría, M.; Lara-Flores, M. The use of carotenoid in aquaculture. Res. J. Fish. Hydrobiol. 2013, 8, 38–49. [Google Scholar]
- Havaux, M.; Niyogi, K.K. The violaxanthin cycle protects plants from photooxidative damage by more than one mechanism. Proc. Natl. Acad. Sci. USA 1999, 96, 8762–8767. [Google Scholar] [CrossRef] [PubMed]
- Jansen, M.A.K.; Hectors, K.; O’Brien, N.M.; Guisez, Y.; Potters, G. Plant stress and human health: Do human consumers benefit from UV-B acclimated crops? Plant Sci. 2008, 175, 449–458. [Google Scholar] [CrossRef]
- Pfündel, E.E.; Pan, R.S.; Dilley, R.A. Inhibition of violaxanthin deepoxidation by ultraviolet-B radiation in isolated chloroplasts and intact leaves. Plant Physiol. 1992, 98, 1372–1380. [Google Scholar] [CrossRef]
- Emiliani, J.; D’Andrea, L.; Falcone Ferreyra, M.L.; Maulión, E.; Rodriguez, E.; Rodriguez-Concepción, M.; Casati, P. A role for β,β-xanthophylls in Arabidopsis UV-B photoprotection. J. Exp. Bot. 2018, 69, 4921–4933. [Google Scholar] [CrossRef]
- Favory, J.J.; Stec, A.; Gruber, H.; Rizzini, L.; Oravecz, A.; Funk, M.; Albert, A.; Cloix, C.; Jenkins, G.I.; Oakeley, E.J.; et al. Interaction of COP1 and UVR8 regulates UV-B-induced photomorphogenesis and stress acclimation in Arabidopsis. EMBO J. 2009, 28, 591–601. [Google Scholar] [CrossRef]
- Mao, J.; Zhang, Y.C.; Sang, Y.; Li, Q.H.; Yang, H.Q. A role for Arabidopsis cryptochromes and COP1 in the regulation of stomatal opening. Proc. Natl. Acad. Sci. USA 2005, 102, 12270–12275. [Google Scholar] [CrossRef]
- Kinoshita, T.; Doi, M.; Suetsugu, N.; Kagawa, T.; Wada, M.; Shimazaki, K. phot1 and phot2 mediate blue light regulation of stomatal opening. Nature 2001, 414, 656–660. [Google Scholar] [CrossRef]
- Niyogi, K.K.; Grossman, A.R.; Björkman, O. Arabidopsis mutants define a central role for the xanthophyll cycle in the regulation of photosynthetic energy conversion. Plant Cell 1998, 10, 1121–1134. [Google Scholar] [CrossRef] [PubMed]
- Alonso, J.M.; Stepanova, A.N.; Leisse, T.J.; Kim, C.J.; Chen, H.; Shinn, P.; Stevenson, D.K.; Zimmerman, J.; Barajas, P.; Cheuk, R.; et al. Genome-wide insertional mutagenesis of Arabidopsis thaliana. Science 2003, 301, 653–657. [Google Scholar] [CrossRef] [PubMed]
- Li, X.P.; Björkman, O.; Shih, C.; Grossman, A.R.; Rosenquist, M.; Jansson, S.; Niyogi, K.K. A pigment-binding protein essential for regulation of photosynthetic light harvesting. Nature 2000, 403, 391–395. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Ahn, T.K.; Avenson, T.J.; Ballottari, M.; Cruz, J.A.; Kramer, D.M.; Bassi, R.; Fleming, G.R.; Keasling, J.D.; Niyogi, K.K. Lutein accumulation in the absence of zeaxanthin restores nonphotochemical quenching in the arabidopsis thaliana npq1 mutant. Plant Cell 2009, 21, 1798–1812. [Google Scholar] [CrossRef] [PubMed]
- Boyes, D.C.; Zayed, A.M.; Ascenzi, R.; McCaskill, A.J.; Hoffman, N.E.; Davis, K.R.; Görlach, J. Growth stage-based phenotypic analysis of Arabidopsis: A model for high throughput functional genomics in plants. Plant Cell 2001, 13, 1499–1510. [Google Scholar] [CrossRef]
- Aphalo, P.J.; Albert, A.; Björn, L.O.; Mcleod, A.R.; Robson, T.M.; Rosenqvist, E. Beyond the Visible: A handbook of best practice in plant UV photobiology. In COST Action FA0906 UV4growth (Issue June 2014); University of Helsinki: Helsinki, Finland, 2012. [Google Scholar] [CrossRef]
- Klughammer, C.; Schreiber, U. Complementary PS II quantum yields calculated from simple fluorescence parameters measured by PAM fluorometry and the Saturation Pulse method. PAM Appl. Notes 2008, 1, 27–35. [Google Scholar]
- Thayer, S.S.; Björkman, O. Leaf Xanthophyll content and composition in sun and shade determined by HPLC. Photosynth. Res. 1990, 23, 331–343. [Google Scholar] [CrossRef]
- Fraser, P.D.; Pinto, M.E.; Holloway, D.E.; Bramley, P.M. Application of high-performance liquid chromatography with photodiode array detection to the metabolic profiling of plant isoprenoids. Plant J. 2000, 24, 551–558. [Google Scholar] [CrossRef]
- Venables, W.N.; Ripley, B.D. Modern Applied Statistics with S-Plus; Springer Science & Business Media: Berlin, Germany, 2013; Available online: https://www.rdocumentation.org/packages/stats/versions/3.6.2/topics/prcomp (accessed on 12 August 2002).
- Esteban, R.; Barrutia, O.; Artetxe, U.; Fernández-Marín, B.; Hernández, A.; García-Plazaola, J.I. Internal and external factors affecting photosynthetic pigment composition in plants: A meta-analytical approach. New Phytol. 2015, 206, 268–280. [Google Scholar] [CrossRef]
- Biswas, D.K.; Ma, B.L.; Xu, H.; Li, Y.; Jiang, G. Lutein-mediated photoprotection of photosynthetic machinery in Arabidopsis thaliana exposed to chronic low ultraviolet-B radiation. J. Plant Physiol. 2020, 248, 153160. [Google Scholar] [CrossRef]
- Lee, J.-H.; Shibata, S.; Goto, E. Time-Course of Changes in Photosynthesis and Secondary Metabolites in Canola (Brassica napus) Under Different UV-B Irradiation Levels in a Plant Factory With Artificial Light. Front. Plant Sci. 2021, 12, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Moreira-Rodríguez, M.; Nair, V.; Benavides, J.; Cisneros-Zevallos, L.; Jacobo-Velázquez, D.A. UVA, UVB light, and methyl jasmonate, alone or combined, redirect the biosynthesis of glucosinolates, phenolics, carotenoids, and chlorophylls in broccoli sprouts. Int. J. Mol. Sci. 2017, 18, 2330. [Google Scholar] [CrossRef]
- García-Plazaola, J.I.; Hernández, A.; Olano, J.M.; Becerril, J.M. The operation of the lutein epoxide cycle correlates with energy dissipation. Funct. Plant Biol. 2003, 30, 319–324. [Google Scholar] [CrossRef] [PubMed]
- Rai, N.; Morales, L.O.; Aphalo, P.J. Perception of solar UV radiation by plants: Photoreceptors and mechanisms. Plant Physiol. 2021, 186, 1382–1396. [Google Scholar] [CrossRef] [PubMed]
- Ulm, R.; Jenkins, G.I. Q&A: How do plants sense and respond to UV-B radiation? BMC Biol. 2015, 13, 4–9. [Google Scholar] [CrossRef]
- Clayton, W.A.; Albert, N.W.; Thrimawithana, A.H.; McGhie, T.K.; Deroles, S.C.; Schwinn, K.E.; Warren, B.A.; McLachlan, A.R.G.; Bowman, J.L.; Jordan, B.R.; et al. UVR8-mediated induction of flavonoid biosynthesis for UVB tolerance is conserved between the liverwort Marchantia polymorpha and flowering plants. Plant J. 2018, 96, 503–517. [Google Scholar] [CrossRef]
- Brelsford, C.C.; Morales, L.O.; Nezval, J.; Kotilainen, T.K.; Hartikainen, S.M.; Aphalo, P.J.; Robson, T.M. Do UV-A radiation and blue light during growth prime leaves to cope with acute high light in photoreceptor mutants of Arabidopsis thaliana? Physiol. Plant. 2019, 165, 537–554. [Google Scholar] [CrossRef]
- Pogson, B.; McDonald, K.A.; Truong, M.; Britton, G.; DellaPenna, D. Arabidopsis carotenoid mutants demonstrate that lutein is not essential for photosynthesis in higher plants. Plant Cell 1996, 8, 1627–1639. [Google Scholar] [CrossRef]
- Cazzaniga, S.; Li, Z.; Niyogi, K.K.; Bassi, R.; Dall’Osto, L. The Arabidopsis szl1 mutant reveals a Critical role of β-carotene in photosystem I photoprotection. Plant Physiol. 2012, 159, 1745–1758. [Google Scholar] [CrossRef]
- Jansen, M.A.K.; Ač, A.; Klem, K.; Urban, O. A meta-analysis of the interactive effects of UV and drought on plants. Plant Cell Environ. 2022, 45, 41–54. [Google Scholar] [CrossRef]
- Castagna, A.; Csepregi, K.; Neugart, S.; Zipoli, G.; Večeřová, K.; Jakab, G.; Jug, T.; Llorens, L.; Martínez-Abaigar, J.; Martínez-Lüscher, J.; et al. Environmental plasticity of Pinot noir grapevine leaves: A trans-European study of morphological and biochemical changes along a 1,500-km latitudinal climatic gradient. Plant Cell Environ. 2017, 40, 2790–2805. [Google Scholar] [CrossRef] [PubMed]


| Mutants and (NASC Code) | Mutant Name | Target Gene/Enzyme | Deficiency/Function | References Detailing Mutants |
|---|---|---|---|---|
| aba1 (npq2) N3772 | ABA-DEFICIENT-1 | Zeaxanthin epoxidase | ABA deficiency | [54] |
| lut1/salk_097275c N655128 | LUTEIN-1 | Lycopene ε cyclase | Lutein deficiency | [55] |
| npq1-2 N69600 | NON-PHOTOCHEMICAL QUENCHING 1 | Violaxanthin de-epoxidase | Reduced nonphotochemical quenching due to the absence of zeaxanthin formation | [54] |
| npq4 N66021 | NON-PHOTOCHEMICAL QUENCHING 4 | Chlorophyll a-b binding family protein | Defective in pH-dependent npq of chlorophyll fluorescence | [56] |
| szl1-1npq1-2 N66023 | SUPRESOR OF ZEAXANTHIN-LESS 1-NON-PHOTOCHEMICAL QUENCHING 1 | Lycopene β-cyclase/violaxanthin de-epoxidase | Restores npq defects of npq1-2 mutant. | [57] |
| Treatment | Filter Used | PAR | UV-B | UV-A | Biologically Effective Dose | |
|---|---|---|---|---|---|---|
| UV-B | UV-A | |||||
| −UV | Mylar | 188 µmol m−2 s−1 | 0.00 W m−2 | 0.25 W m−2 | 0.14 kJ m−2 | 0.18 kJ m−2 |
| +UV | Cellulose acetate | 188 µmol m−2 s−1 | 0.22 W m−2 | 0.37 W m−2 | 2.53 kJ m−2 | 0.28 kJ m−2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Badmus, U.O.; Crestani, G.; Cunningham, N.; Havaux, M.; Urban, O.; Jansen, M.A.K. UV Radiation Induces Specific Changes in the Carotenoid Profile of Arabidopsis thaliana. Biomolecules 2022, 12, 1879. https://doi.org/10.3390/biom12121879
Badmus UO, Crestani G, Cunningham N, Havaux M, Urban O, Jansen MAK. UV Radiation Induces Specific Changes in the Carotenoid Profile of Arabidopsis thaliana. Biomolecules. 2022; 12(12):1879. https://doi.org/10.3390/biom12121879
Chicago/Turabian StyleBadmus, Uthman O., Gaia Crestani, Natalie Cunningham, Michel Havaux, Otmar Urban, and Marcel A. K. Jansen. 2022. "UV Radiation Induces Specific Changes in the Carotenoid Profile of Arabidopsis thaliana" Biomolecules 12, no. 12: 1879. https://doi.org/10.3390/biom12121879
APA StyleBadmus, U. O., Crestani, G., Cunningham, N., Havaux, M., Urban, O., & Jansen, M. A. K. (2022). UV Radiation Induces Specific Changes in the Carotenoid Profile of Arabidopsis thaliana. Biomolecules, 12(12), 1879. https://doi.org/10.3390/biom12121879










