Spinal Astrocyte-Neuron Lactate Shuttle Contributes to the Pituitary Adenylate Cyclase-Activating Polypeptide/PAC1 Receptor-Induced Nociceptive Behaviors in Mice
Abstract
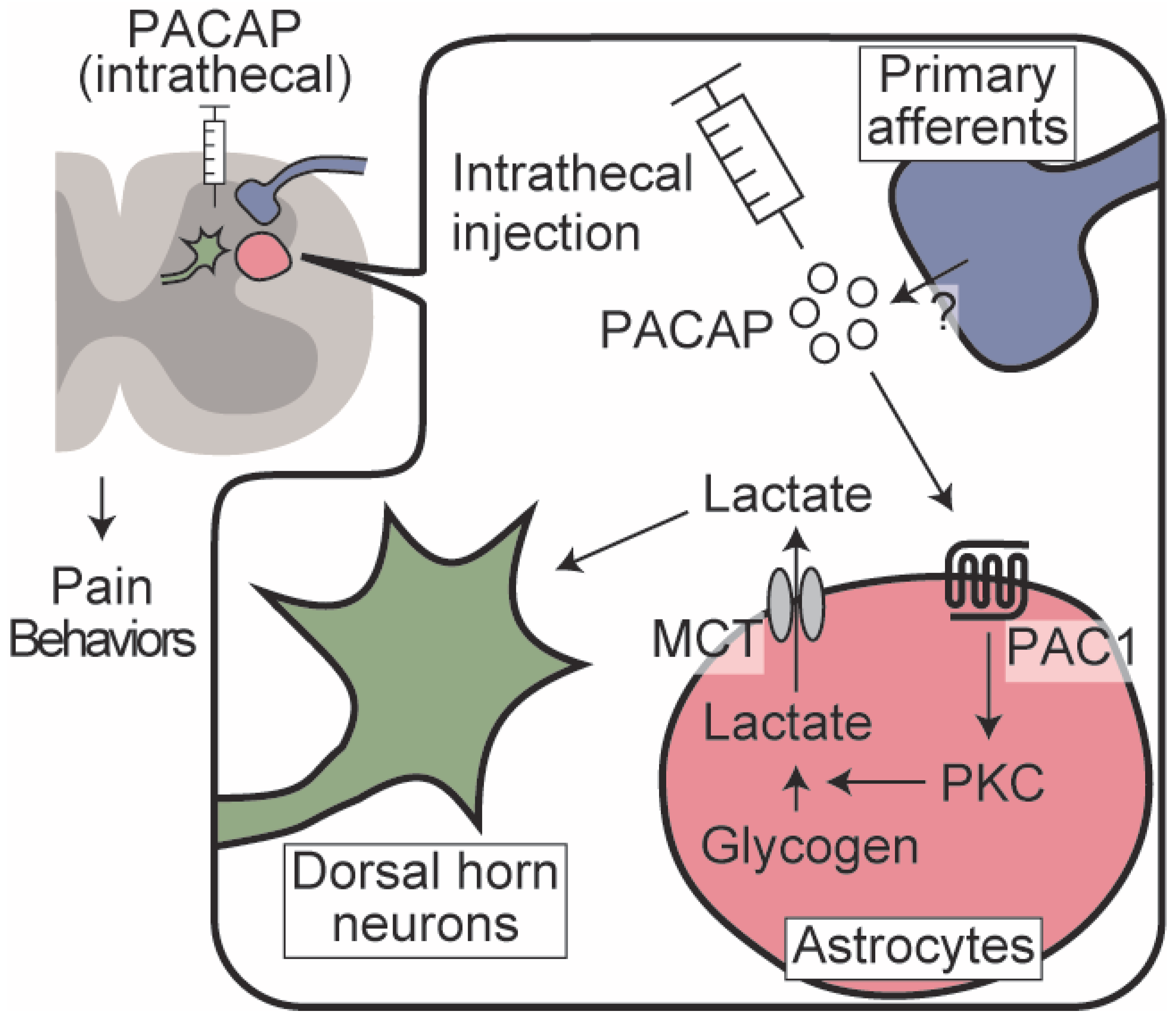
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Drugs
2.3. Intrathecal Injection and Behavioral Observation
2.4. Cultured Spinal Cord Astrocytes
2.5. Immunocytochemistry
2.6. Glycogen Assay
2.7. L-Lactate Assay
2.8. Spinal Nerve Ligation (SNL) Surgery
2.9. Ethics
2.10. Statistical Analysis
3. Results
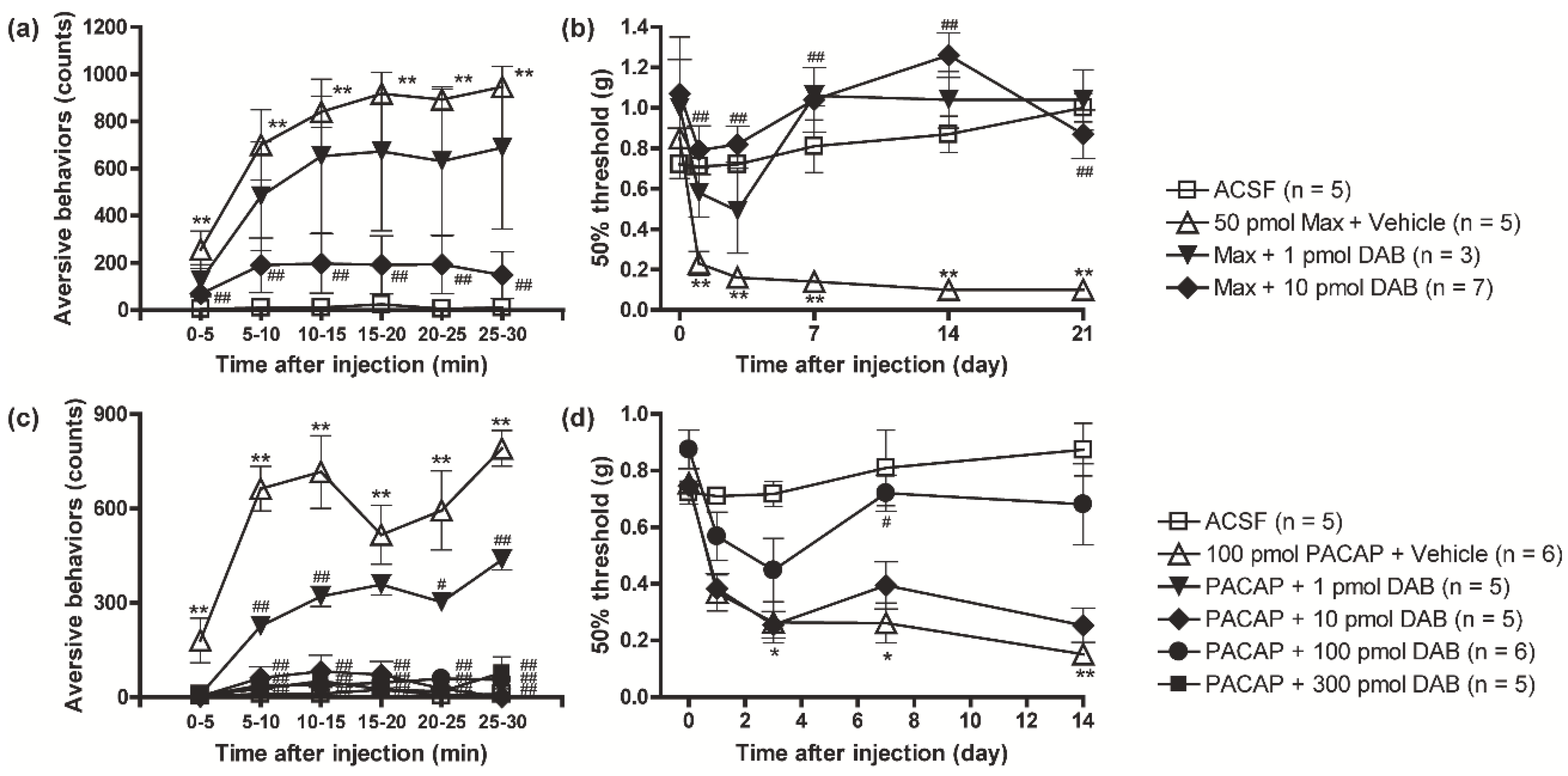
3.1. PACAP/PAC1 Receptor-Induced Nociceptive Behaviors Were Attenuated by the Inhibition of Glycogen Phosphorylase with DAB
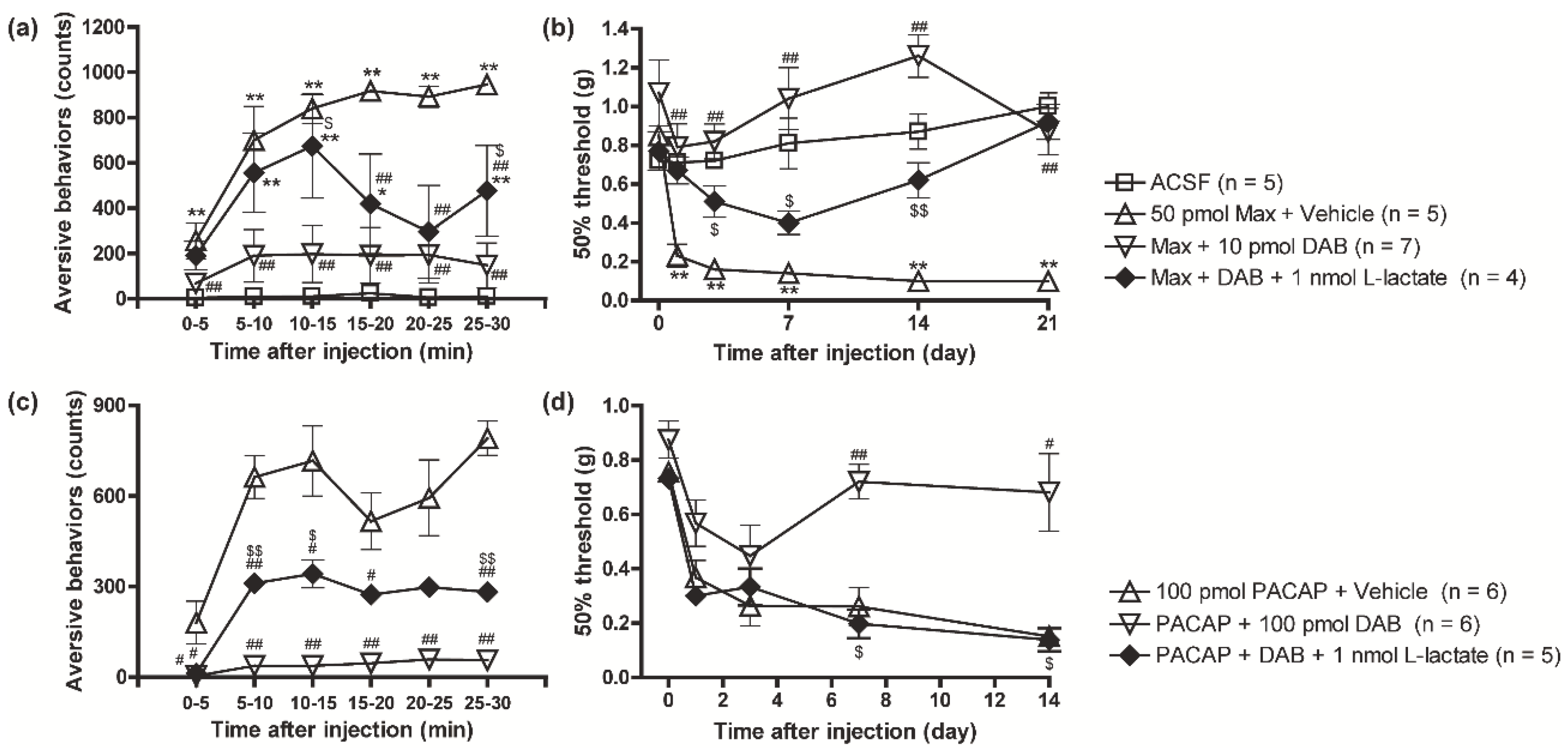
3.2. Suppression of the PAC1 Receptor-Evoked Nociceptive Behaviors by DAB Was Reversed by Intrathecal Co-Injection of L-Lactate
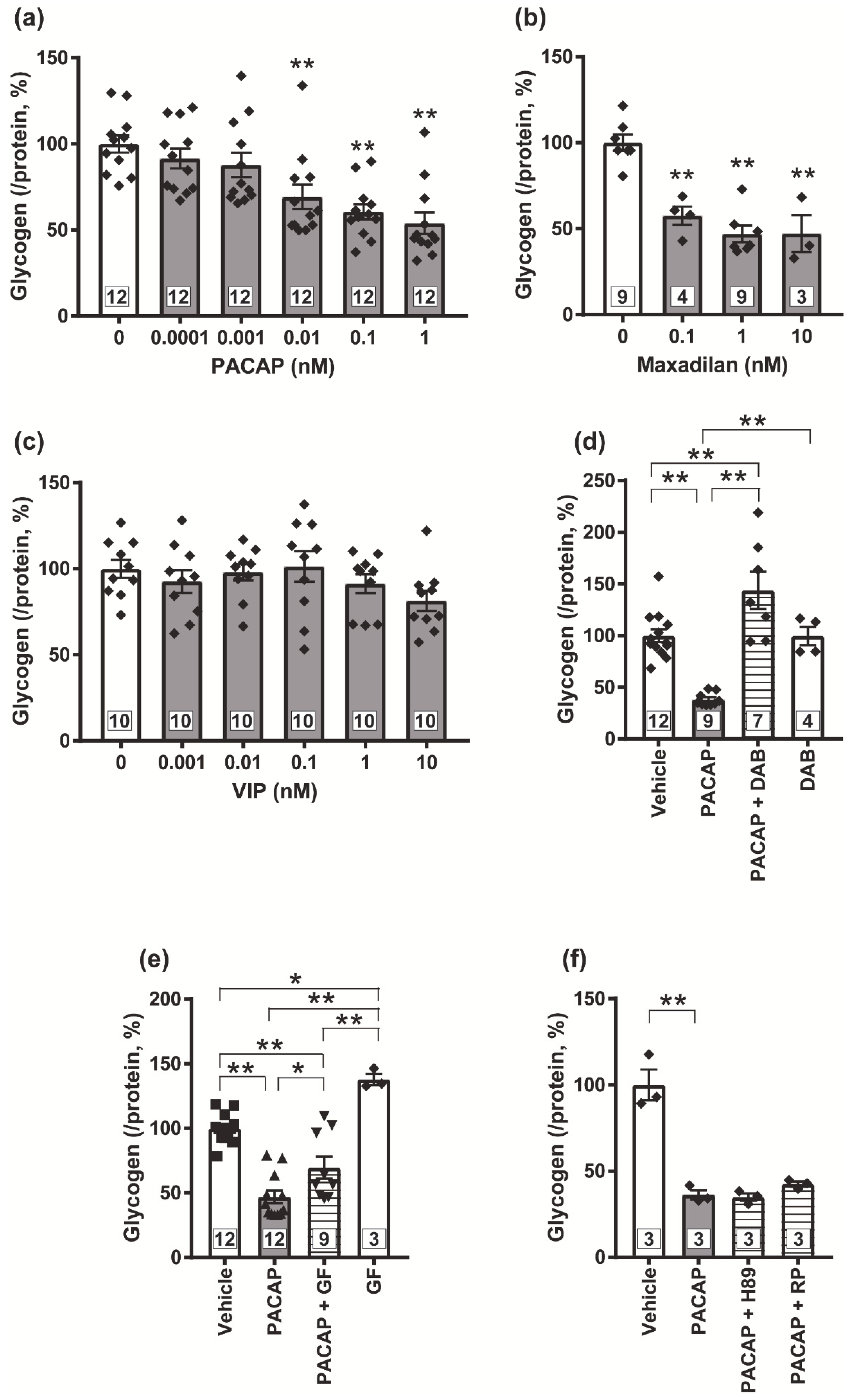
3.3. Possible Involvement of PKC in the PACAP/PAC1 Receptor-Evoked Glycogenolysis in Cultured Spinal Cord Astrocytes
3.4. PKC Is Crucial for the PACAP/PAC1 Receptor-Evoked L-Lactate Secretion in the Cultured Spinal Astrocytes
3.5. Blockade of the PACAP/PAC1 Receptor-Induced Nociceptive Behaviors by the PKC Inhibitor, GF109203X
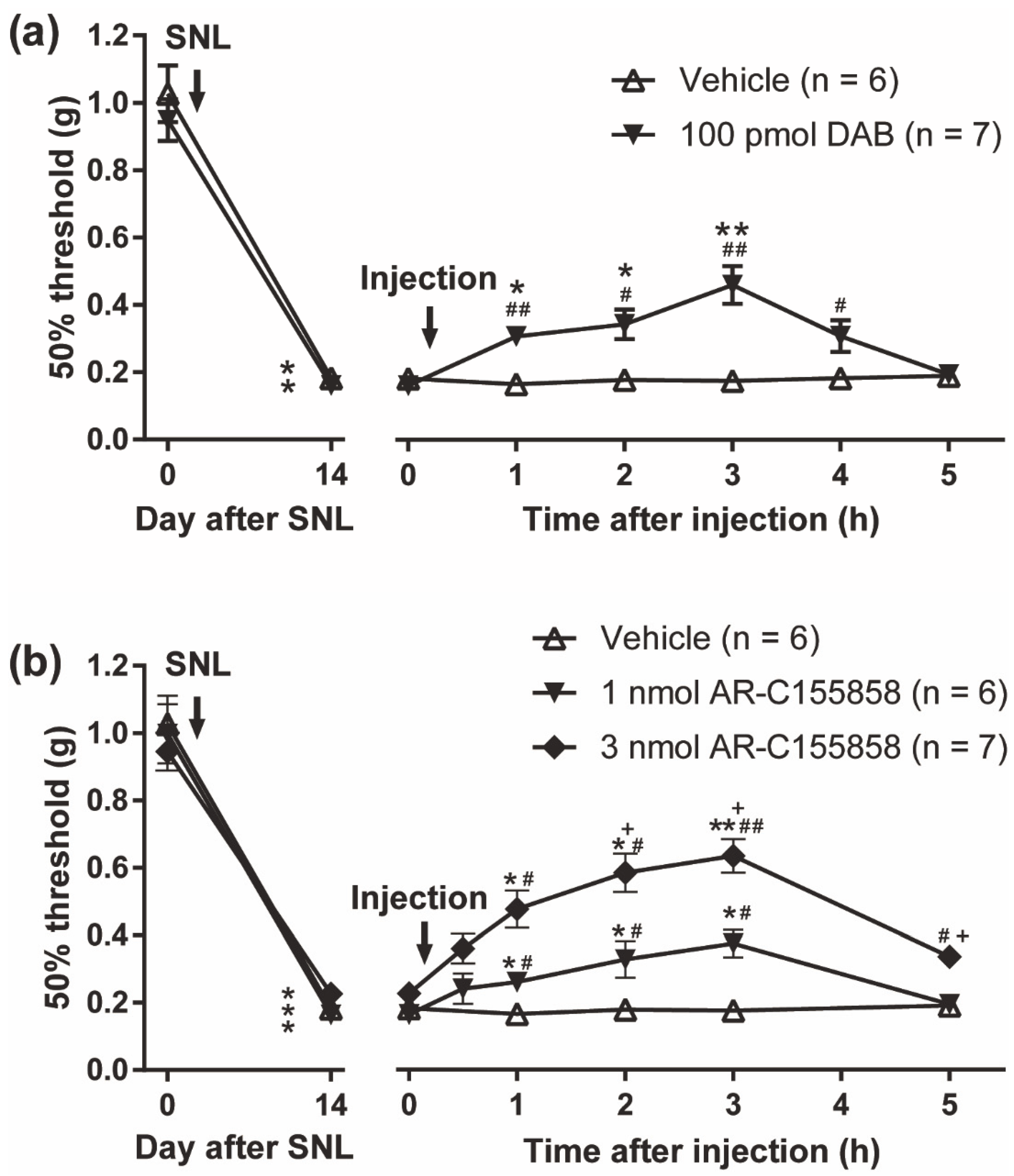
3.6. Pharmacological Inhibition of Monocarboxylate Transporters Attenuated the PACAP/PAC1 Receptor-Induced Nociceptive Behaviors
3.7. Reversal of Spinal Nerve Injury (SNL)-Induced Mechanical Allodynia by the PYGB Inhibitor, DAB and MCT Inhibitor, AR-C155858
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| 4-CIN | α-cyano-4-hydroxycinnamate |
| AC | adenylate cyclase |
| ACSF | artificial cerebrospinal fluid |
| AMPA | a-3-hydroxy-5-methyl-4-isoxazole propionic acid |
| ANLS | astrocyte-neuron lactate shuttle |
| CNPase | 2′,3′-cyclic-nucleotide 3′-phosphodiesterase |
| CNS | central nervous system |
| DAB | 1,4-dideoxy-1,4-imino-d-arabinitol |
| DREADDs | designer receptors exclusively activated by designer drugs |
| GF109203X | bisindolylmaleimide I |
| GFAP | glial fibrillary acidic protein |
| Iba1 | ionized calcium binding adaptor molecule-1 |
| MAP2 | microtubule associated protein-2 |
| Max | maxadilan |
| MCTs | monocarboxylate transporters |
| NMDA | N-methyl-D-aspartate |
| PAC1 | PACAP type 1 |
| PACAP | pituitary adenylate cyclase-activating polypeptide |
| PBS | phosphate buffered saline |
| PKA | protein kinase A |
| PKC | protein kinase C |
| PMA | 12-myristate 13-acetate |
| PYGB | glycogen phosphorylase |
| Rp-8-Br-cAMPS | 8-Bromoadenosine-3′,5′-cyclic monophosphorothioate, Rp-isomer |
| Sq22.536 | 9-(tetrahydrofuran-2-yl)-9h-purin-6-amine |
| VIP | vasoactive intestinal polypeptide |
| VPAC | VIP/PACAP |
| SNL | spinal nerve ligation |
References
- Magistretti, P.J.; Allaman, I. A Cellular Perspective on Brain Energy Metabolism and Functional Imaging. Neuron 2015, 86, 883–901. [Google Scholar] [CrossRef] [PubMed]
- Magistretti, P.J.; Allaman, I. Lactate in the brain: From metabolic end-product to signalling molecule. Nat. Rev. Neurosci. 2018, 19, 235–249. [Google Scholar] [CrossRef] [PubMed]
- Newman, L.A.; Korol, D.L.; Gold, P.E. Lactate Produced by Glycogenolysis in Astrocytes Regulates Memory Processing. PLoS ONE 2011, 6, e28427. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, A.; Stern, S.A.; Bozdagi, O.; Huntley, G.W.; Walker, R.H.; Magistretti, P.J.; Alberini, C.M. Astrocyte-Neuron Lactate Transport Is Required for Long-Term Memory Formation. Cell 2011, 144, 810–823. [Google Scholar] [CrossRef] [PubMed]
- Kambe, Y.; Thi, T.N.; Hashiguchi, K.; Sameshima, Y.; Yamashita, A.; Kurihara, T.; Miyata, A. The dorsal hippocampal protein targeting to glycogen maintains ionotropic glutamate receptor subunits expression and contributes to working and short-term memories in mice. J. Pharmacol. Sci. 2022, 148, 108–115. [Google Scholar] [CrossRef]
- Duran, J.; Saez, I.; Gruart, A.; Guinovart, J.J.; Delgado-García, J.M. Impairment in Long-Term Memory Formation and Learning-Dependent Synaptic Plasticity in Mice Lacking Glycogen Synthase in the Brain. J. Cereb. Blood Flow Metab. 2013, 33, 550–556. [Google Scholar] [CrossRef]
- Boury-Jamot, B.; Carrard, A.; Martin, J.-L.; Halfon, O.; Magistretti, P.J.; Boutrel, B. Disrupting astrocyte–neuron lactate transfer persistently reduces conditioned responses to cocaine. Mol. Psychiatry 2015, 21, 1070–1076. [Google Scholar] [CrossRef]
- Zhang, Y.; Xue, Y.; Meng, S.; Luo, Y.; Liang, J.; Li, J.; Ai, S.; Sun, C.; Shen, H.; Zhu, W.; et al. Inhibition of Lactate Transport Erases Drug Memory and Prevents Drug Relapse. Biol. Psychiatry 2016, 79, 928–939. [Google Scholar] [CrossRef]
- Nagase, M.; Takahashi, Y.; Watabe, A.M.; Kubo, Y.; Kato, F. On-Site Energy Supply at Synapses through Monocarboxylate Transporters Maintains Excitatory Synaptic Transmission. J. Neurosci. 2014, 34, 2605–2617. [Google Scholar] [CrossRef]
- Yang, J.; Ruchti, E.; Petit, J.-M.; Jourdain, P.; Grenningloh, G.; Allaman, I.; Magistretti, P.J. Lactate promotes plasticity gene expression by potentiating NMDA signaling in neurons. Proc. Natl. Acad. Sci. USA 2014, 111, 12228–12233. [Google Scholar] [CrossRef]
- Kambe, Y. Recent behavioral findings of pathophysiological involvement of lactate in the central nervous system. Biochim. Biophys. Acta Gen. Subj. 2022, 1866, 130137. [Google Scholar] [CrossRef]
- Miyamoto, K.; Ishikura, K.-I.; Kume, K.; Ohsawa, M. Astrocyte-neuron lactate shuttle sensitizes nociceptive transmission in the spinal cord. Glia 2019, 67, 27–36. [Google Scholar] [CrossRef]
- Miyata, A.; Arimura, A.; Dahl, R.R.; Minamino, N.; Uehara, A.; Jiang, L.; Culler, M.D.; Coy, D.H. Isolation of a novel 38 residue-hypothalamic polypeptide which stimulates adenylate cyclase in pituitary cells. Biochem. Biophys. Res. Commun. 1989, 164, 567–574. [Google Scholar] [CrossRef]
- Miyata, A.; Jiang, L.; Dahl, R.D.; Kitada, C.; Kubo, K.; Fujino, M.; Minamino, N.; Arimura, A. Isolation of a neuropeptide corresponding to the N-terminal 27 residues of the pituitary adenylate cyclase activating polypeptide with 38 residues (PACAP38). Biochem. Biophys. Res. Commun. 1990, 170, 643–648. [Google Scholar] [CrossRef]
- Deutsch, P.J.; Sun, Y. The 38-amino acid form of pituitary adenylate cyclase-activating polypeptide stimulates dual signaling cascades in PC12 cells and promotes neurite outgrowth. J. Biol. Chem. 1992, 267, 5108–5113. [Google Scholar] [CrossRef]
- Dickinson, T.; Mitchell, R.; Robberecht, P.; Fleetwood-Walker, S.M. The role of VIP/PACAP receptor subtypes in spinal somatosensory processing in rats with an experimental peripheral mononeuropathy. Neuropharmacology 1999, 38, 167–180. [Google Scholar] [CrossRef][Green Version]
- Jongsma, H.; Danielsen, N.; Sundler, F.; Kanje, M. Alteration of PACAP distribution and PACAP receptor binding in the rat sensory nervous system following sciatic nerve transection. Brain Res. 2000, 853, 186–196. [Google Scholar] [CrossRef]
- Sakashita, Y.; Kurihara, T.; Uchida, D.; Tatsuno, I.; Yamamoto, T. Involvement of PACAP receptor in primary afferent fibre-evoked responses of ventral roots in the neonatal rat spinal cord. Br. J. Pharmacol. 2001, 132, 1769–1776. [Google Scholar] [CrossRef]
- Vaudry, D.; Falluel-Morel, A.; Bourgault, S.; Basille, M.; Burel, D.; Wurtz, O.; Fournier, A.; Chow, B.K.C.; Hashimoto, H.; Galas, L.; et al. Pituitary Adenylate Cyclase-Activating Polypeptide and Its Receptors: 20 Years after the Discovery. Pharmacol. Rev. 2009, 61, 283–357. [Google Scholar] [CrossRef]
- Yokai, M.; Kurihara, T.; Miyata, A. Spinal astrocytic activation contributes to both induction and maintenance of pituitary adenylate cyclase-activating polypeptide type 1 receptor-induced long-lasting mechanical allodynia in mice. Mol. Pain 2016, 12, 1744806916646383. [Google Scholar] [CrossRef]
- Moller, K.; Zhang, Y.-Z.; Håkanson, R.; Luts, A.; Sjölund, B.; Uddman, R.; Sundler, F. Pituitary adenylate cyclase activating peptide is a sensory neuropeptide: Immunocytochemical and immunochemical evidence. Neuroscience 1993, 57, 725–732. [Google Scholar] [CrossRef] [PubMed]
- Dun, N.; Miyazaki, T.; Tang, H.; Dun, E. Pituitary adenylate cyclase activating polypeptide immunoreactivity in the rat spinal cord and medulla: Implication of sensory and autonomic functions. Neuroscience 1996, 73, 677–686. [Google Scholar] [CrossRef] [PubMed]
- Dun, E.; Huang, R.; Dun, S.; Dun, N. Pituitary adenylate cyclase activating polypeptide-immunoreactivity in human spinal cord and dorsal root ganglia. Brain Res. 1996, 721, 233–237. [Google Scholar] [CrossRef]
- Narita, M.; Dun, S.L.; Dun, N.J.; Tseng, L.F. Hyperalgesia induced by pituitary adenylate cyclase-activating polypeptide in the mouse spinal cord. Eur. J. Pharmacol. 1996, 311, 121–126. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Shi, T.-J.; Ji, R.-R.; Zhang, Y.-T.; Sundler, F.; Hannibal, J.; Fahrenkrug, J.; Hökfelt, T. Expression of pituitary adenylate cyclase-activating polypeptide in dorsal root ganglia following axotomy: Time course and coexistence. Brain Res. 1995, 705, 149–158. [Google Scholar] [CrossRef]
- Zhang, Y.; Danielsen, N.; Sundler, F.; Mulder, H. Pituitary adenylate cyclase-activating peptide is upregulated in sensory neurons by inflammation. NeuroReport 1998, 9, 2833–2836. [Google Scholar] [CrossRef]
- Wallin, H.; Pettersson, L.; Verge, V.; Danielsen, N. Effect of anti-nerve growth factor treatment on pituitary adenylate cyclase activating polypeptide expression in adult sensory neurons exposed to adjuvant induced inflammation. Neuroscience 2003, 120, 325–331. [Google Scholar] [CrossRef]
- Mabuchi, T.; Shintani, N.; Matsumura, S.; Okuda-Ashitaka, E.; Hashimoto, H.; Muratani, T.; Minami, T.; Baba, A.; Ito, S. Pituitary Adenylate Cyclase-Activating Polypeptide Is Required for the Development of Spinal Sensitization and Induction of Neuropathic Pain. J. Neurosci. 2004, 24, 7283–7291. [Google Scholar] [CrossRef]
- Moro, O.; Lerner, E.A. Maxadilan, the Vasodilator from Sand Flies, Is a Specific Pituitary Adenylate Cyclase Activating Peptide Type I Receptor Agonist. J. Biol. Chem. 1997, 272, 966–970. [Google Scholar] [CrossRef]
- Shimizu, T.; Katahira, M.; Sugawara, H.; Inoue, K.; Miyata, A. Diverse effects of intrathecal pituitary adenylate cyclase-activating polypeptide on nociceptive transmission in mice spinal cord. Regul. Pept. 2004, 123, 117–122. [Google Scholar] [CrossRef]
- Ohnou, T.; Yokai, M.; Kurihara, T.; Hasegawa-Moriyama, M.; Shimizu, T.; Inoue, K.; Kambe, Y.; Kanmura, Y.; Miyata, A. Pituitary adenylate cyclase-activating polypeptide type 1 receptor signaling evokes long-lasting nociceptive behaviors through the activation of spinal astrocytes in mice. J. Pharmacol. Sci. 2016, 130, 194–203. [Google Scholar] [CrossRef]
- Pierre, K.; Pellerin, L. Monocarboxylate transporters in the central nervous system: Distribution, regulation and function. J. Neurochem. 2005, 94, 1–14. [Google Scholar] [CrossRef]
- Ovens, M.J.; Davies, A.J.; Wilson, M.C.; Murray, C.M.; Halestrap, A.P. AR-C155858 is a potent inhibitor of monocarboxylate transporters MCT1 and MCT2 that binds to an intracellular site involving transmembrane helices 7–10. Biochem. J. 2010, 425, 523–530. [Google Scholar] [CrossRef]
- Cataldo, A.M.; Broadwell, R.D. Cytochemical identification of cerebral glycogen and glucose-6-phosphatase activity under normal and experimental conditions. II. Choroid plexus and ependymal epithelia, endothelia and pericytes. J. Neurocytol. 1986, 15, 511–524. [Google Scholar] [CrossRef]
- Kambe, Y.; Yamauchi, Y.; Nguyen, T.T.; Nguyen, T.T.; Ago, Y.; Shintani, N.; Hashimoto, H.; Yoshitake, S.; Yoshitake, T.; Kehr, J.; et al. The pivotal role of pituitary adenylate cyclase-activating polypeptide for lactate production and secretion in astrocytes during fear memory. Pharmacol. Rep. 2021, 73, 1109–1121. [Google Scholar] [CrossRef]
- Chaplan, S.R.; Bach, F.W.; Pogrel, J.W.; Chung, J.M.; Yaksh, T.L. Quantitative assessment of tactile allodynia in the rat paw. J. Neurosci. Methods 1994, 53, 55–63. [Google Scholar] [CrossRef]
- Kambe, Y.; Miyata, A. Role of Mitochondrial Activation in PACAP Dependent Neurite Outgrowth. J. Mol. Neurosci. 2012, 48, 550–557. [Google Scholar] [CrossRef]
- Ogura, M.; Nakamichi, N.; Takano, K.; Oikawa, H.; Kambe, Y.; Ohno, Y.; Taniura, H.; Yoneda, Y. Functional expression of A glutamine transporter responsive to down-regulation by lipopolysaccharide through reduced promoter activity in cultured rat neocortical astrocytes. J. Neurosci. Res. 2006, 83, 1447–1460. [Google Scholar] [CrossRef]
- Kambe, Y.; Nakamichi, N.; Georgiev, D.D.; Nakamura, N.; Taniura, H.; Yoneda, Y. Insensitivity to glutamate neurotoxicity mediated by NMDA receptors in association with delayed mitochondrial membrane potential disruption in cultured rat cortical neurons. J. Neurochem. 2008, 105, 1886–1900. [Google Scholar] [CrossRef]
- Hanada, T.; Kurihara, T.; Tokudome, M.; Tokimura, H.; Arita, K.; Miyata, A. Development and pharmacological verification of a new mouse model of central post-stroke pain. Neurosci. Res. 2014, 78, 72–80. [Google Scholar] [CrossRef]
- Zhang, W.; Xiao, D.; Li, X.; Zhang, Y.; Rasouli, J.; Casella, G.; Boehm, A.; Hwang, D.; Ishikawa, L.L.; Thome, R.; et al. SIRT1 inactivation switches reactive astrocytes to an anti-inflammatory phenotype in CNS autoimmunity. J. Clin. Investig. 2022, 132, e151803. [Google Scholar] [CrossRef] [PubMed]
- A Tarczyluk, M.; A Nagel, D.; O’Neil, J.D.; Parri, R.; Tse, E.; Coleman, M.D.; Hill, E. Functional Astrocyte-Neuron Lactate Shuttle in a Human Stem Cell-Derived Neuronal Network. J. Cereb. Blood Flow Metab. 2013, 33, 1386–1393. [Google Scholar] [CrossRef] [PubMed]
- Saegusa, H.; Kurihara, T.; Zong, S.; Kazuno, A.; Matsuda, Y.; Nonaka, T.; Han, W.; Toriyama, H.; Tanabe, T. Suppression of inflammatory and neuropathic pain symptoms in mice lacking the N-type Ca2+ channel. EMBO J. 2001, 20, 2349–2356. [Google Scholar] [CrossRef] [PubMed]
- Sakurai, E.; Kurihara, T.; Kouchi, K.; Saegusa, H.; Zong, S.; Tanabe, T. Upregulation of Casein Kinase 1∊ in Dorsal Root Ganglia and Spinal Cord after Mouse Spinal Nerve Injury Contributes to Neuropathic Pain. Mol. Pain 2009, 5, 74. [Google Scholar] [CrossRef] [PubMed]
- Davis-Taber, R.; Baker, S.; Lehto, S.G.; Zhong, C.; Surowy, C.S.; Faltynek, C.R.; Scott, V.E.; Honore, P. Central Pituitary Adenylate Cyclase 1 Receptors Modulate Nociceptive Behaviors in Both Inflammatory and Neuropathic Pain States. J. Pain 2008, 9, 449–456. [Google Scholar] [CrossRef]
- Takasaki, I.; Watanabe, A.; Yokai, M.; Watanabe, Y.; Hayakawa, D.; Nagashima, R.; Fukuchi, M.; Okada, T.; Toyooka, N.; Miyata, A.; et al. In Silico Screening Identified Novel Small-molecule Antagonists of PAC1 Receptor. J. Pharmacol. Exp. Ther. 2018, 365, 1–8. [Google Scholar] [CrossRef]
- Takasaki, I.; Ogashi, H.; Okada, T.; Shimodaira, A.; Hayakawa, D.; Watanabe, A.; Miyata, A.; Kurihara, T.; Gouda, H.; Toyooka, N. Synthesis of a novel and potent small-molecule antagonist of PAC1 receptor for the treatment of neuropathic pain. Eur. J. Med. Chem. 2020, 186, 111902. [Google Scholar] [CrossRef]
- Takasaki, I.; Watanabe, A.; Okada, T.; Kanayama, D.; Nagashima, R.; Shudo, M.; Shimodaira, A.; Nunomura, K.; Lin, B.; Watanabe, Y.; et al. Design and synthesis of pyrido[2,3-d]pyrimidine derivatives for a novel PAC1 receptor antagonist. Eur. J. Med. Chem. 2022, 231, 114160. [Google Scholar] [CrossRef]
- Sandkühler, J. Understanding LTP in Pain Pathways. Mol. Pain 2007, 3, 9. [Google Scholar] [CrossRef]
- Kuner, R.; Flor, H. Structural plasticity and reorganisation in chronic pain. Nat. Rev. Neurosci. 2016, 18, 20–30. [Google Scholar] [CrossRef]
- Grace, P.M.; Hutchinson, M.R.; Maier, S.F.; Watkins, L.R. Pathological pain and the neuroimmune interface. Nat. Rev. Immunol. 2014, 14, 217–231. [Google Scholar] [CrossRef]
- Ji, R.-R.; Xu, Z.-Z.; Gao, Y.-J. Emerging targets in neuroinflammation-driven chronic pain. Nat. Rev. Drug Discov. 2014, 13, 533–548. [Google Scholar] [CrossRef]
- Brunet, J.F.; Allaman, I.; Magistretti, P.J.; Pellerin, L. Glycogen Metabolism as a Marker of Astrocyte Differentiation. J. Cereb. Blood Flow Metab. 2009, 30, 51–55. [Google Scholar] [CrossRef]
- Baron, A.; Monnier, D.; Roatti, A.; Baertschi, A.J. Pituitary adenylate cyclase-activating polypeptide activates K(ATP) current in rat atrial myocytes. Am. J. Physiol. Heart Circ. Physiol. 2001, 280, H1058–H1065. [Google Scholar] [CrossRef] [PubMed]
- Gibbs, M.E. Role of Glycogenolysis in Memory and Learning: Regulation by Noradrenaline, Serotonin and ATP. Front. Integr. Neurosci. 2016, 9, 70. [Google Scholar] [CrossRef]
- Sorg, O.; Magistretti, P.J. Characterization of the glycogenolysis elicited by vasoactive intestinal peptide, noradrenaline and adenosine in primary cultures of mouse cerebral cortical astrocytes. Brain Res. 1991, 563, 227–233. [Google Scholar] [CrossRef]
- Velázquez, K.T.; Mohammad, H.; Sweitzer, S.M. Protein kinase C in pain: Involvement of multiple isoforms. Pharmacol. Res. 2007, 55, 578–589. [Google Scholar] [CrossRef]
- Gosselin, R.-D.; Meylan, P.; Decosterd, I. Extracellular microvesicles from astrocytes contain functional glutamate transporters: Regulation by protein kinase C and cell activation. Front. Cell. Neurosci. 2013, 7, 251. [Google Scholar] [CrossRef]
- Narumi, K.; Furugen, A.; Kobayashi, M.; Otake, S.; Itagaki, S.; Iseki, K. Regulation of Monocarboxylate Transporter 1 in Skeletal Muscle Cells by Intracellular Signaling Pathways. Biol. Pharm. Bull. 2010, 33, 1568–1573. [Google Scholar] [CrossRef]
- Narumi, K.; Kobayashi, M.; Otake, S.; Furugen, A.; Takahashi, N.; Ogura, J.; Itagaki, S.; Hirano, T.; Yamaguchi, H.; Iseki, K. Regulation of human monocarboxylate transporter 4 in skeletal muscle cells: The role of protein kinase C (PKC). Int. J. Pharm. 2012, 428, 25–32. [Google Scholar] [CrossRef]
- Smith, J.P.; Uhernik, A.L.; Li, L.; Liu, Z.; Drewes, L.R. Regulation of Mct1 by cAMP-dependent internalization in rat brain endothelial cells. Brain Res. 2012, 1480, 1–11. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kambe, Y.; Youkai, M.; Hashiguchi, K.; Sameshima, Y.; Takasaki, I.; Miyata, A.; Kurihara, T. Spinal Astrocyte-Neuron Lactate Shuttle Contributes to the Pituitary Adenylate Cyclase-Activating Polypeptide/PAC1 Receptor-Induced Nociceptive Behaviors in Mice. Biomolecules 2022, 12, 1859. https://doi.org/10.3390/biom12121859
Kambe Y, Youkai M, Hashiguchi K, Sameshima Y, Takasaki I, Miyata A, Kurihara T. Spinal Astrocyte-Neuron Lactate Shuttle Contributes to the Pituitary Adenylate Cyclase-Activating Polypeptide/PAC1 Receptor-Induced Nociceptive Behaviors in Mice. Biomolecules. 2022; 12(12):1859. https://doi.org/10.3390/biom12121859
Chicago/Turabian StyleKambe, Yuki, Masafumi Youkai, Kohei Hashiguchi, Yoshimune Sameshima, Ichiro Takasaki, Atsuro Miyata, and Takashi Kurihara. 2022. "Spinal Astrocyte-Neuron Lactate Shuttle Contributes to the Pituitary Adenylate Cyclase-Activating Polypeptide/PAC1 Receptor-Induced Nociceptive Behaviors in Mice" Biomolecules 12, no. 12: 1859. https://doi.org/10.3390/biom12121859
APA StyleKambe, Y., Youkai, M., Hashiguchi, K., Sameshima, Y., Takasaki, I., Miyata, A., & Kurihara, T. (2022). Spinal Astrocyte-Neuron Lactate Shuttle Contributes to the Pituitary Adenylate Cyclase-Activating Polypeptide/PAC1 Receptor-Induced Nociceptive Behaviors in Mice. Biomolecules, 12(12), 1859. https://doi.org/10.3390/biom12121859





