Elevated TAF12 Expression Predicts Poor Prognosis in Glioma Patients: Evidence from Bioinformatic and Immunohistochemical Analyses
Abstract
1. Introduction
2. Materials and Methods
2.1. Dataset Selection
2.2. Data Downloading and Preprocessing/Data Acquisition
2.3. Immunohistochemistry
2.4. Bioinformatics Analysis
2.5. Analysis of Immune Response
2.6. Meta-Analysis
2.7. Statistical Analysis
3. Results
3.1. Characteristics of Patients
3.2. Transcriptional Levels of TAF12 Were Upregulated in Glioma
3.3. TAF12 Expression Is Associated with Glioma Grade, Subtype and Molecular Features
3.4. High TAF12 Expression Predicts Poor Prognosis in Glioma Patients
3.5. TAF12 Is an Independent Prognostic Factor for Glioma Patients
3.6. TAF12 May Be Involved in the Malignant Progression of Glioma
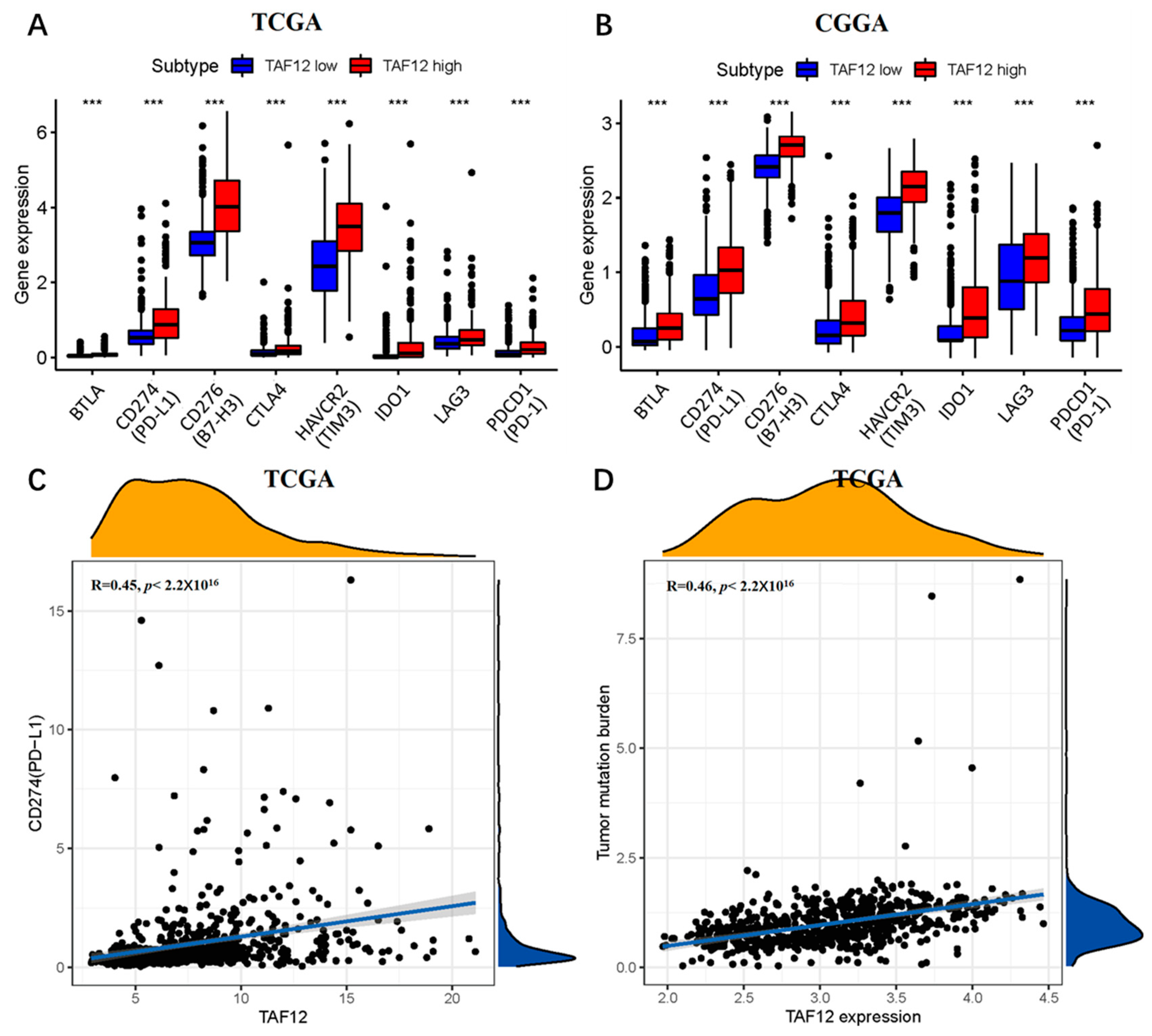
3.7. TAF12 Is Associated with Immune and Inflammatory Responses in Glioma
3.8. High TAF12 Expression Is Associated with Greater Sensitivity of Glioma to Immunotherapy
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ostrom, Q.T.; Patil, N.; Cioffi, G.; Waite, K.; Kruchko, C.; Barnholtz-Sloan, J.S. CBTRUS Statistical Report: Primary Brain and Other Central Nervous System Tumors Diagnosed in the United States in 2013–2017. Neuro Oncol. 2020, 22 (Suppl. 2), iv1–iv96. [Google Scholar] [CrossRef] [PubMed]
- Jiang, T.; Nam, D.H.; Ram, Z.; Poon, W.S.; Wang, J.; Boldbaatar, D.; Mao, Y.; Ma, W.; Mao, Q.; You, Y.; et al. Clinical practice guidelines for the management of adult diffuse gliomas. Cancer Lett. 2021, 499, 60–72. [Google Scholar] [CrossRef] [PubMed]
- Joo, Y.J.; Ficarro, S.B.; Soares, L.M.; Chun, Y.; Marto, J.A.; Buratowski, S. Downstream promoter interactions of TFIID TAFs facilitate transcription reinitiation. Genes Dev. 2017, 31, 2162–2174. [Google Scholar] [CrossRef] [PubMed]
- Patel, A.B.; Louder, R.K.; Greber, B.J.; Grünberg, S.; Luo, J.; Fang, J.; Liu, Y.; Ranish, J.; Hahn, S.; Nogales, E. Structure of human TFIID and mechanism of TBP loading onto promoter DNA. Science 2018, 362, eaau8872. [Google Scholar] [CrossRef]
- Voulgari, A.; Voskou, S.; Tora, L.; Davidson, I.; Sasazuki, T.; Shirasawa, S.; Pintzas, A. TATA box-binding protein-associated factor 12 is important for RAS-induced transformation properties of colorectal cancer cells. Mol. Cancer Res. 2008, 6, 1071–1083. [Google Scholar] [CrossRef]
- Tong, Y.; Merino, D.; Nimmervoll, B.; Gupta, K.; Wang, Y.D.; Finkelstein, D.; Dalton, J.; Ellison, D.W.; Ma, X.; Zhang, J.; et al. Cross-Species Genomics Identifies TAF12, NFYC, and RAD54L as Choroid Plexus Carcinoma Oncogenes. Cancer Cell 2015, 27, 712–727. [Google Scholar] [CrossRef]
- Ren, J.; Lou, M.; Shi, J.; Xue, Y.; Cui, D. Identifying the genes regulated by IDH1 via gene-chip in glioma cell U87. Int. J. Clin. Exp. Med. 2015, 8, 18090–18098. [Google Scholar]
- Wijethilake, N.; Meedeniya, D.; Chitraranjan, C.; Perera, I. Survival Prediction and Risk Estimation of Glioma Patients Using mRNA Expressions; IEEE: Piscataway, NJ, USA, 2020. [Google Scholar]
- Zhao, Z.; Zhang, K.-N.; Wang, Q.; Li, G.; Zeng, F.; Zhang, Y.; Wu, F.; Chai, R.; Wang, Z.; Zhang, C.; et al. Chinese Glioma Genome Atlas (CGGA): A Comprehensive Resource with Functional Genomic Data from Chinese Glioma Patients. Genom. Proteom. Bioinform. 2021, 19, 1–12. [Google Scholar] [CrossRef]
- Gravendeel, L.A.M.; Kouwenhoven, M.C.M.; Gevaert, O.; de Rooi, J.J.; Stubbs, A.P.; Duijm, J.E.; Daemen, A.; Bleeker, F.E.; Bralten, L.B.C.; Kloosterhof, N.K.; et al. Intrinsic gene expression profiles of gliomas are a better predictor of survival than histology. Cancer Res. 2009, 69, 9065–9072. [Google Scholar] [CrossRef]
- Leek, J.T.; Johnson, W.E.; Parker, H.S.; Jaffe, A.E.; Storey, J.D. The sva package for removing batch effects and other unwanted variation in high-throughput experiments. Bioinformatics 2012, 28, 882–883. [Google Scholar] [CrossRef]
- Ritchie, M.E.; Phipson, B.; Wu, D.; Hu, Y.; Law, C.W.; Shi, W.; Smyth, G.K. limma powers differential expression analyses for RNA-sequencing and microarray studies. Nucleic Acids Res. 2015, 43, e47. [Google Scholar] [CrossRef] [PubMed]
- Feng, E.; Liang, T.; Wang, X.; Du, J.; Tang, K.; Wang, X.; Wang, F.; You, G. Correlation of alteration of HLA-F expression and clinical characterization in 593 brain glioma samples. J. Neuroinflammation 2019, 16, 33. [Google Scholar] [CrossRef] [PubMed]
- Tang, Z.; Kang, B.; Li, C.; Chen, T.; Zhang, Z. GEPIA2: An enhanced web server for large-scale expression profiling and interactive analysis. Nucleic Acids Res. 2019, 47, W556–W560. [Google Scholar] [CrossRef] [PubMed]
- Yu, G.; Wang, L.-G.; Han, Y.; He, Q.-Y. clusterProfiler: An R package for comparing biological themes among gene clusters. OMICS 2012, 16, 284–287. [Google Scholar] [CrossRef] [PubMed]
- Subramanian, A.; Tamayo, P.; Mootha, V.K.; Mukherjee, S.; Ebert, B.L.; Gillette, M.A.; Paulovich, A.; Pomeroy, S.L.; Golub, T.R.; Lander, E.S.; et al. Gene set enrichment analysis: A knowledge-based approach for interpreting genome-wide expression profiles. Proc. Natl. Acad. Sci. USA 2005, 102, 15545–15550. [Google Scholar] [CrossRef]
- Liberzon, A.; Birger, C.; Thorvaldsdóttir, H.; Ghandi, M.; Mesirov, J.P.; Tamayo, P. The Molecular Signatures Database (MSigDB) hallmark gene set collection. Cell Syst. 2015, 1, 417–425. [Google Scholar] [CrossRef]
- He, Y.; Jiang, Z.; Chen, C.; Wang, X. Classification of triple-negative breast cancers based on Immunogenomic profiling. J. Exp. Clin. Cancer Res. 2018, 37, 327. [Google Scholar] [CrossRef]
- Hänzelmann, S.; Castelo, R.; Guinney, J. GSVA: Gene set variation analysis for microarray and RNA-seq data. BMC Bioinform. 2013, 14, 7. [Google Scholar] [CrossRef]
- Yoshihara, K.; Shahmoradgoli, M.; Martínez, E.; Vegesna, R.; Kim, H.; Torres-Garcia, W.; Treviño, V.; Shen, H.; Laird, P.W.; Levine, D.A.; et al. Inferring tumour purity and stromal and immune cell admixture from expression data. Nat. Commun. 2013, 4, 2612. [Google Scholar] [CrossRef]
- Newman, A.M.; Liu, C.L.; Green, M.R.; Gentles, A.J.; Feng, W.; Xu, Y.; Hoang, C.D.; Diehn, M.; Alizadeh, A.A. Robust enumeration of cell subsets from tissue expression profiles. Nat. Methods 2015, 12, 453–457. [Google Scholar] [CrossRef]
- Higgins, J.P.T.; Thompson, S.G.; Deeks, J.J.; Altman, D.G. Measuring inconsistency in meta-analyses. BMJ 2003, 327, 557–560. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Zhang, J.; Xuan, S.; Liu, Z.; Wang, Y.; Zhao, P. Molecular and Clinicopathological Characterization of a Prognostic Immune Gene Signature Associated With MGMT Methylation in Glioblastoma. Front. Cell Dev. Biol. 2021, 9, 600506. [Google Scholar] [CrossRef] [PubMed]
- Bagchi, S.; Yuan, R.; Engleman, E.G. Immune Checkpoint Inhibitors for the Treatment of Cancer: Clinical Impact and 2 Mechanisms of Response and Resistance. Annu. Rev. Pathol. 2021, 16, 223–249. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.; Tang, L.; Li, X.; Fan, F.; Liu, Z. Immunotherapy for glioma: Current management and future application. Cancer Lett. 2020, 476, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Denissov, S.; van Driel, M.; Voit, R.; Hekkelman, M.; Hulsen, T.; Hernandez, N.; Grummt, I.; Wehrens, R.; Stunnenberg, H. Identification of novel functional TBP-binding sites and general factor repertoires. EMBO J. 2007, 26, 944–954. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Milazzo, J.P.; Somerville, T.D.D.; Tarumoto, Y.; Huang, Y.H.; Ostrander, E.L.; Wilkinson, J.E.; Challen, G.A.; Vakoc, C.R. A TFIID-SAGA Perturbation that Targets MYB and Suppresses Acute Myeloid Leukemia. Cancer Cell 2018, 33, 13–28.e8. [Google Scholar] [CrossRef]
- Bach, D.-H.; Zhang, W.; Sood, A.K. Chromosomal Instability in Tumor Initiation and Development. Cancer Res. 2019, 79, 3995–4002. [Google Scholar] [CrossRef]
- Hanahan, D. Hallmarks of Cancer: New Dimensions. Cancer Discov. 2022, 12, 31–46. [Google Scholar] [CrossRef]
- Duffy, S.; Fam, H.K.; Wang, Y.K.; Styles, E.B.; Kim, J.H.; Ang, J.S.; Singh, T.; Larionov, V.; Shah, S.P.; Andrews, B.; et al. Overexpression screens identify conserved dosage chromosome instability genes in yeast and human cancer. Proc. Natl. Acad. Sci. USA 2016, 113, 9967–9976. [Google Scholar] [CrossRef]
- Quail, D.F.; Joyce, J.A. Microenvironmental regulation of tumor progression and metastasis. Nat. Med. 2013, 19, 1423–1437. [Google Scholar] [CrossRef]
- Mantovani, A.; Sica, A. Macrophages, innate immunity and cancer: Balance, tolerance, and diversity. Curr. Opin. Immunol. 2010, 22, 231–237. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Zhao, Y.; Yan, H.; Yang, Y.; Shen, S.; Dai, X.; Ji, X.; Ji, F.; Gong, X.G.; Li, L.; et al. Single tumor-initiating cells evade immune clearance by recruiting type II macrophages. Genes Dev. 2017, 31, 247–259. [Google Scholar] [CrossRef] [PubMed]
- Ruffell, B.; Affara, N.I.; Coussens, L.M. Differential macrophage programming in the tumor microenvironment. Trends Immunol. 2012, 33, 119–126. [Google Scholar] [CrossRef] [PubMed]
- Ohue, Y.; Nishikawa, H. Regulatory T (Treg) cells in cancer: Can Treg cells be a new therapeutic target? Cancer Sci. 2019, 110, 2080–2089. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Galat, V.; Galat, Y.; Lee, Y.K.A.; Wainwright, D.; Wu, J. NK cell-based cancer immunotherapy: From basic biology to clinical development. J. Hematol. Oncol. 2021, 14, 7. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Chan, H.L.; Chen, P. Immune Checkpoint Inhibitors: Basics and Challenges. Curr. Med. Chem. 2019, 26, 3009–3025. [Google Scholar] [CrossRef]
- Wainwright, D.A.; Chang, A.L.; Dey, M.; Balyasnikova, I.V.; Kim, C.K.; Tobias, A.; Cheng, Y.; Kim, J.W.; Qiao, J.; Zhang, L.; et al. Durable therapeutic efficacy utilizing combinatorial blockade against IDO, CTLA-4, and PD-L1 in mice with brain tumors. Clin. Cancer Res. 2014, 20, 5290–5301. [Google Scholar] [CrossRef]
- Hanihara, M.; Kawataki, T.; Oh-Oka, K.; Mitsuka, K.; Nakao, A.; Kinouchi, H. Synergistic antitumor effect with indoleamine 2,3-dioxygenase inhibition and temozolomide in a murine glioma model. J. Neurosurg. 2016, 124, 1594–1601. [Google Scholar] [CrossRef]
- Castro, M.G.; Baker, G.J.; Lowenstein, P.R. Blocking immunosuppressive checkpoints for glioma therapy: The more the Merrier! Clin. Cancer Res. 2014, 20, 5147–5149. [Google Scholar] [CrossRef]
- Reardon, D.A.; Brandes, A.A.; Omuro, A.; Mulholland, P.; Lim, M.; Wick, A.; Baehring, J.; Ahluwalia, M.S.; Roth, P.; Bahr, O.; et al. Effect of Nivolumab vs Bevacizumab in Patients With Recurrent Glioblastoma: The CheckMate 143 Phase 3 Randomized Clinical Trial. JAMA Oncol. 2020, 6, 1003–1010. [Google Scholar] [CrossRef]
- Omuro, A.; Brandes, A.A.; Carpentier, A.F.; Idbaih, A.; Reardon, D.A.; Cloughesy, T.; Sumrall, A.; Baehring, J.; van den Bent, M.; Bahr, O.; et al. Radiotherapy Combined With Nivolumab or Temozolomide for Newly Diagnosed Glioblastoma With Unmethylated MGMT Promoter: An International Randomized Phase 3 Trial. Neuro Oncol. 2022, noac099. [Google Scholar] [CrossRef] [PubMed]
- Lim, M.; Weller, M.; Idbaih, A.; Steinbach, J.; Finocchiaro, G.; Raval, R.R.; Ansstas, G.; Baehring, J.; Taylor, J.W.; Honnorat, J.; et al. Phase 3 Trial of Chemoradiotherapy With Temozolomide Plus Nivolumab or Placebo for Newly Diagnosed Glioblastoma With Methylated MGMT Promoter. Neuro Oncol. 2022, 24, 1935–1949. [Google Scholar] [CrossRef] [PubMed]
- Maleki Vareki, S. High and low mutational burden tumors versus immunologically hot and cold tumors and response to immune checkpoint inhibitors. J. Immunother. Cancer 2018, 6, 157. [Google Scholar] [CrossRef] [PubMed]






| Characteristic | TCGA | CGGA | GSE16011 | Ditan |
|---|---|---|---|---|
| Samples | 703 | 1018 | 284 | 28 |
| Age (years) | ||||
| ≤40 | 236 | 434 | 82 | 8 |
| >40 | 375 | 583 | 194 | 20 |
| Gender | ||||
| Male | 357 | 601 | 184 | 15 |
| Female | 254 | 417 | 92 | 13 |
| IDH1 status | ||||
| mutation | 376 | 531 | 81 | 11 |
| wild type | 227 | 435 | 140 | 17 |
| 1p/19q codeletion status | ||||
| codeleted | 150 | 212 | 45 | 6 |
| non-codeleted | 455 | 728 | 73 | 22 |
| Grade ※ | ||||
| Ⅱ | 214 | 291 | 24 | 5 |
| Ⅲ | 237 | 334 | 99 | 10 |
| Ⅳ | 160 | 388 | 145 | 13 |
| Histology ※ | ||||
| non-tumor tissue | 5 | 8 | ||
| astrocytoma | 167 | 389 | 29 | 9 |
| oligodendroglioma | 171 | 206 | 52 | 5 |
| oligoastrocytoma | 113 | 29 | 28 | 1 |
| glioblastoma | 160 | 388 | 159 | 13 |
| 5th WHO classification | ||||
| Astrocytoma, IDH-mutant, 1p/19q non-codeleted | 219 | 175 | 44 | 5 |
| Oligodendroglioma, IDH-mutant, 1p/19q codeleted | 149 | 121 | 27 | 6 |
| Glioblastoma, IDH-wildtype | 130 | 158 | 78 | 15 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guo, X.; Chen, J.; Fang, A.; Ji, Q.; Chen, F.; Zhou, X.; Li, X.; Li, W. Elevated TAF12 Expression Predicts Poor Prognosis in Glioma Patients: Evidence from Bioinformatic and Immunohistochemical Analyses. Biomolecules 2022, 12, 1847. https://doi.org/10.3390/biom12121847
Guo X, Chen J, Fang A, Ji Q, Chen F, Zhou X, Li X, Li W. Elevated TAF12 Expression Predicts Poor Prognosis in Glioma Patients: Evidence from Bioinformatic and Immunohistochemical Analyses. Biomolecules. 2022; 12(12):1847. https://doi.org/10.3390/biom12121847
Chicago/Turabian StyleGuo, Xiaodi, Jiamin Chen, Aizhong Fang, Qiang Ji, Feng Chen, Xingang Zhou, Xinyi Li, and Wenbin Li. 2022. "Elevated TAF12 Expression Predicts Poor Prognosis in Glioma Patients: Evidence from Bioinformatic and Immunohistochemical Analyses" Biomolecules 12, no. 12: 1847. https://doi.org/10.3390/biom12121847
APA StyleGuo, X., Chen, J., Fang, A., Ji, Q., Chen, F., Zhou, X., Li, X., & Li, W. (2022). Elevated TAF12 Expression Predicts Poor Prognosis in Glioma Patients: Evidence from Bioinformatic and Immunohistochemical Analyses. Biomolecules, 12(12), 1847. https://doi.org/10.3390/biom12121847






