A Novel and Validated 8-Pyroptosis-Related Genes Based Risk Prediction Model for Diffuse Large B Cell Lymphoma
Abstract
:1. Introduction
2. Materials and Methods
2.1. Data Acquisition
2.2. Pathway Enrichment Analysis
2.3. Establishment and Validation of the Risk Prediction Model
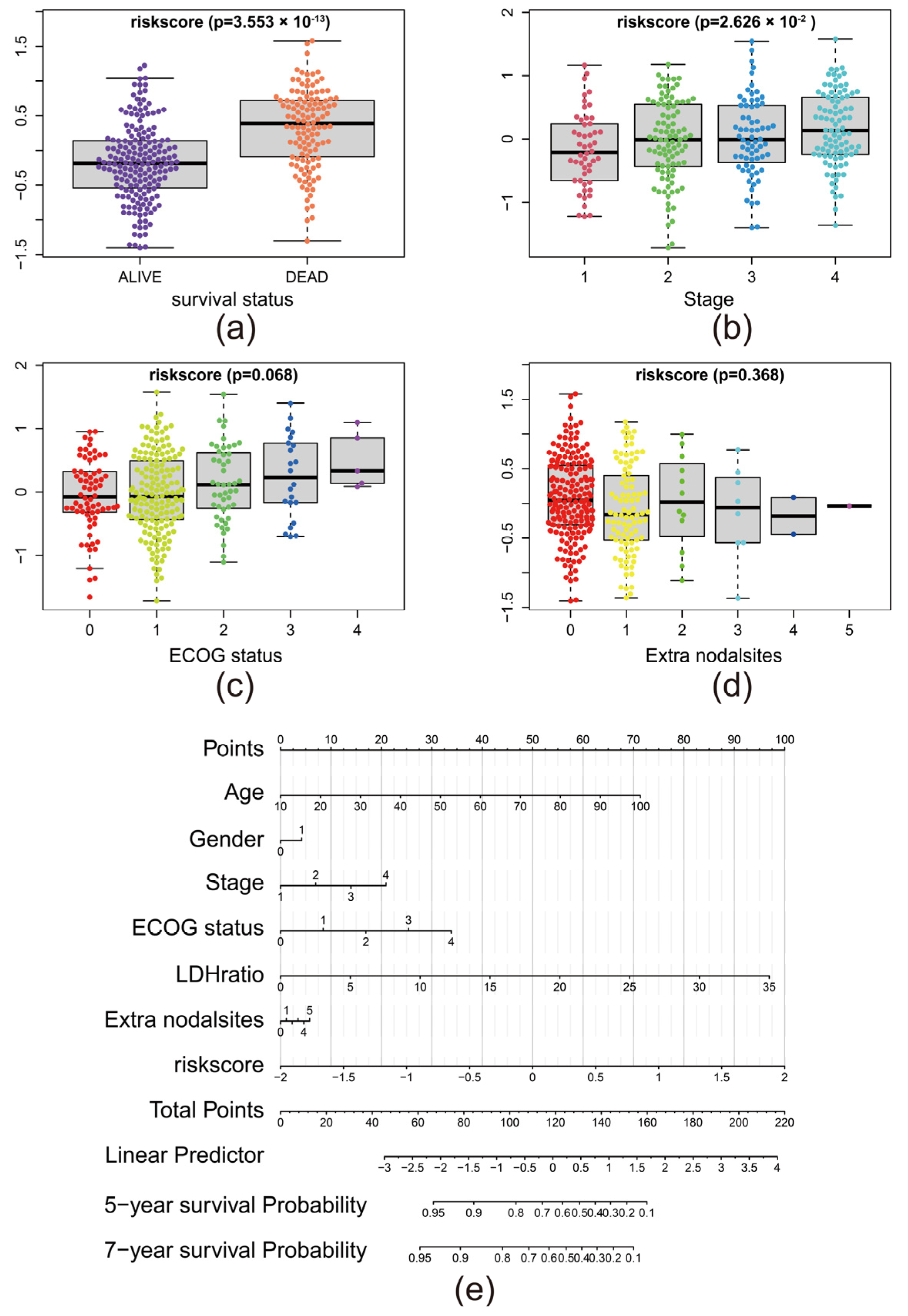
2.4. Construction of Nomogram
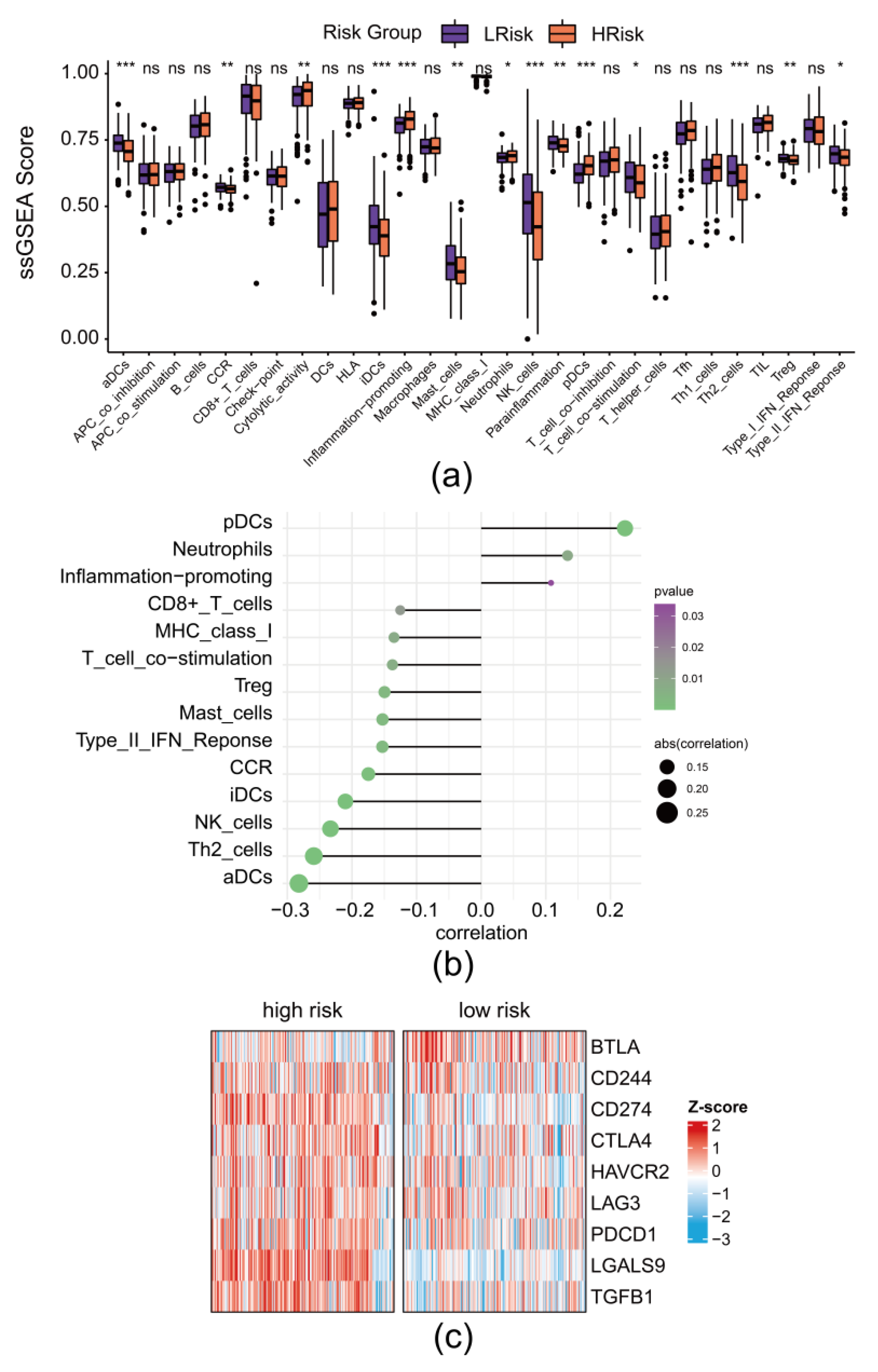
2.5. Immune Components Prediction
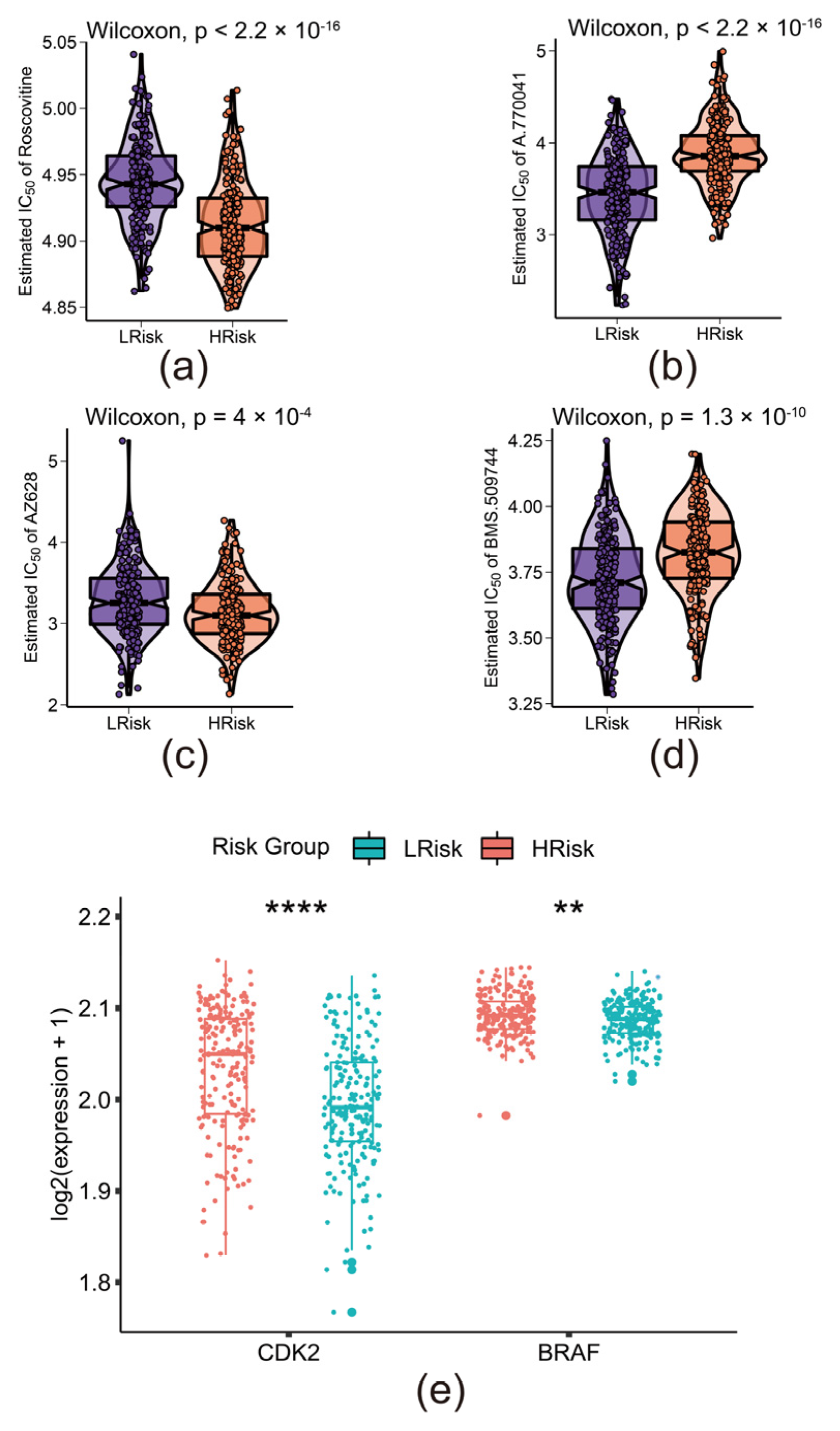
2.6. Prediction of Chemosensitivity
2.7. Cell Culture
2.8. RT-qPCR
2.9. Statistical Analysis
3. Results
3.1. Identification of 24 Prognostic Pyroptosis Related Genes (PRGs) in DLBCL
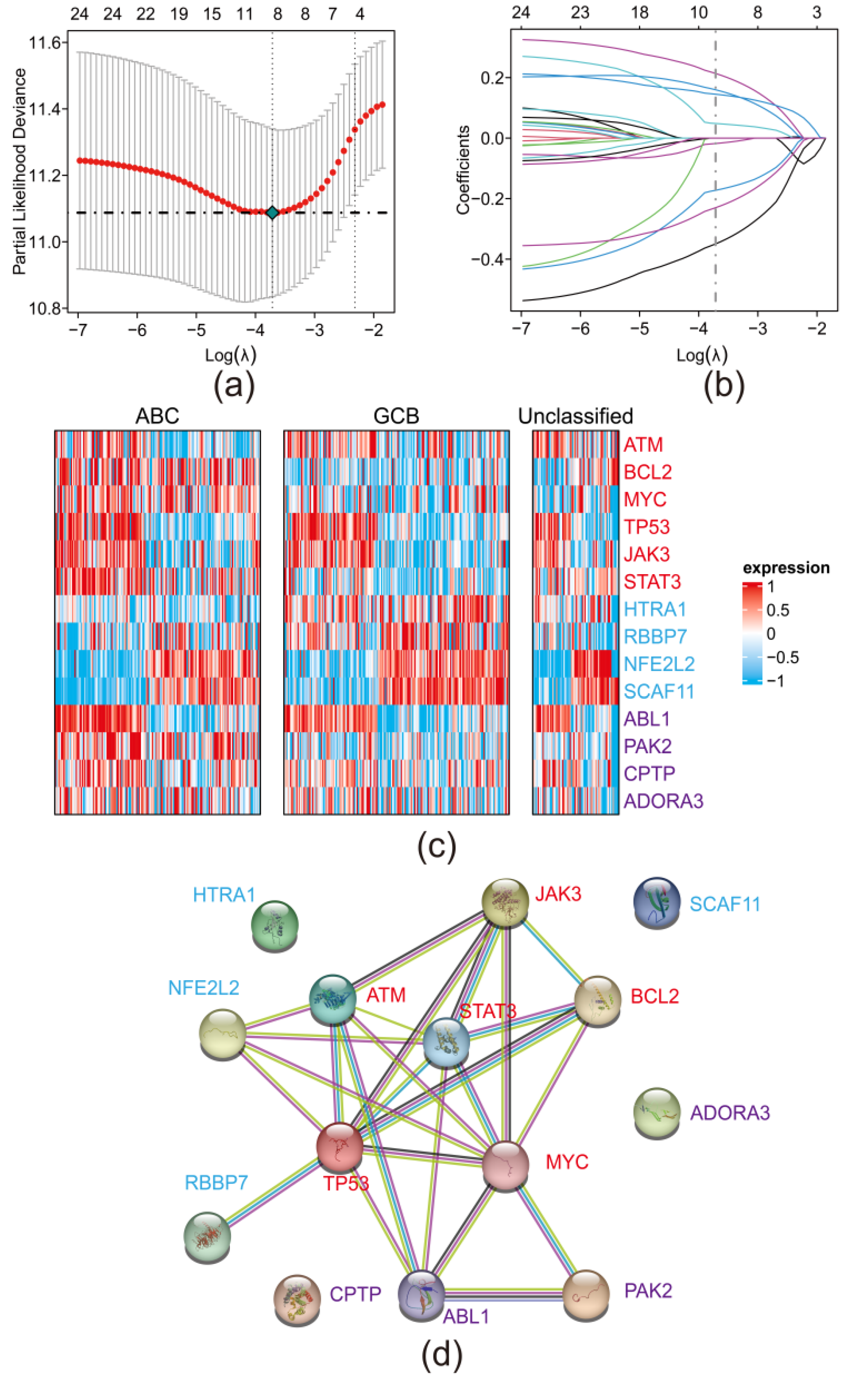
3.2. Establishment of a PRGs Oriented Risk Model via LASSO Regression
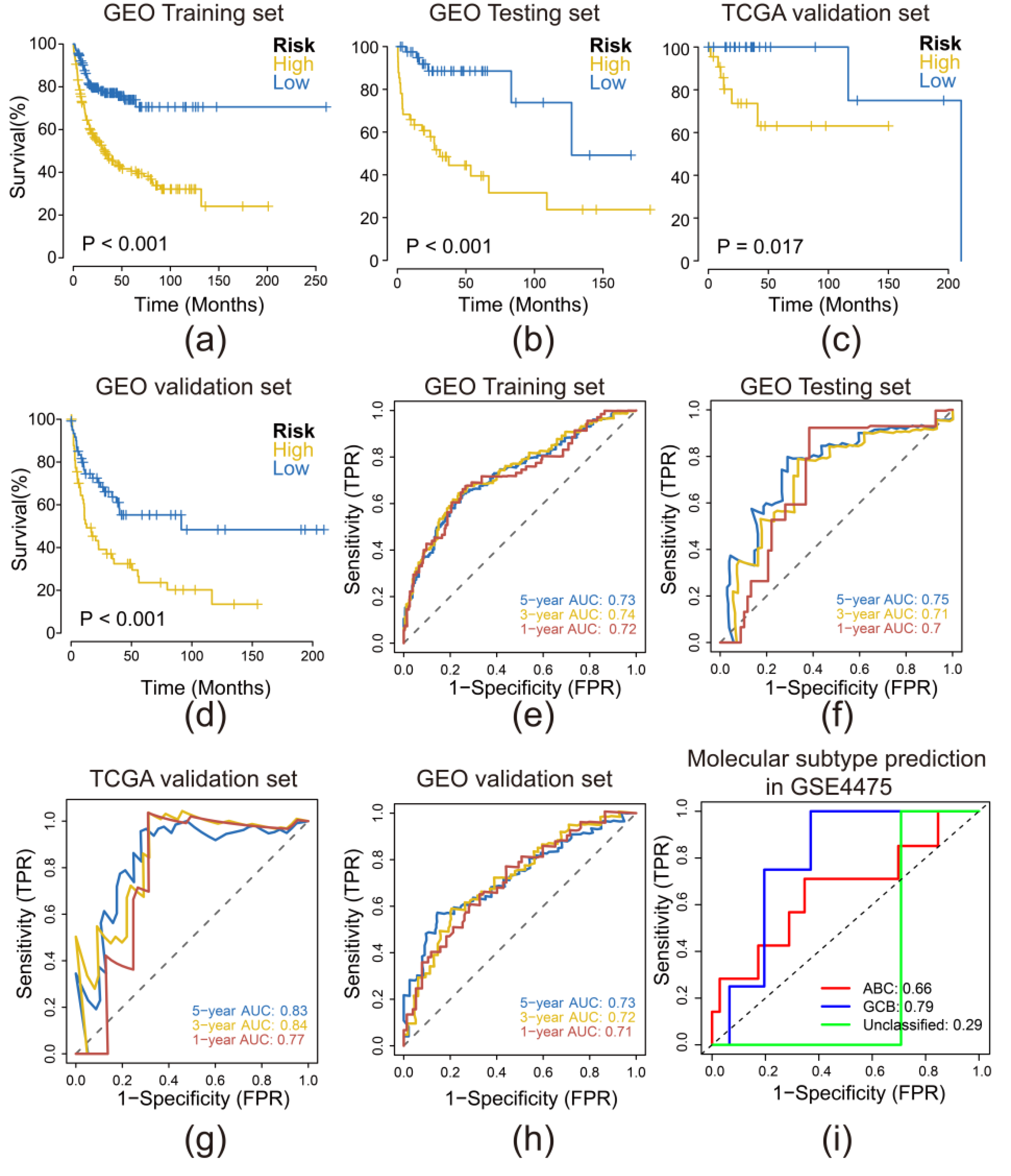
3.3. Risk Model Performed Sensitively and Robust in Internal and External Validation Datasets
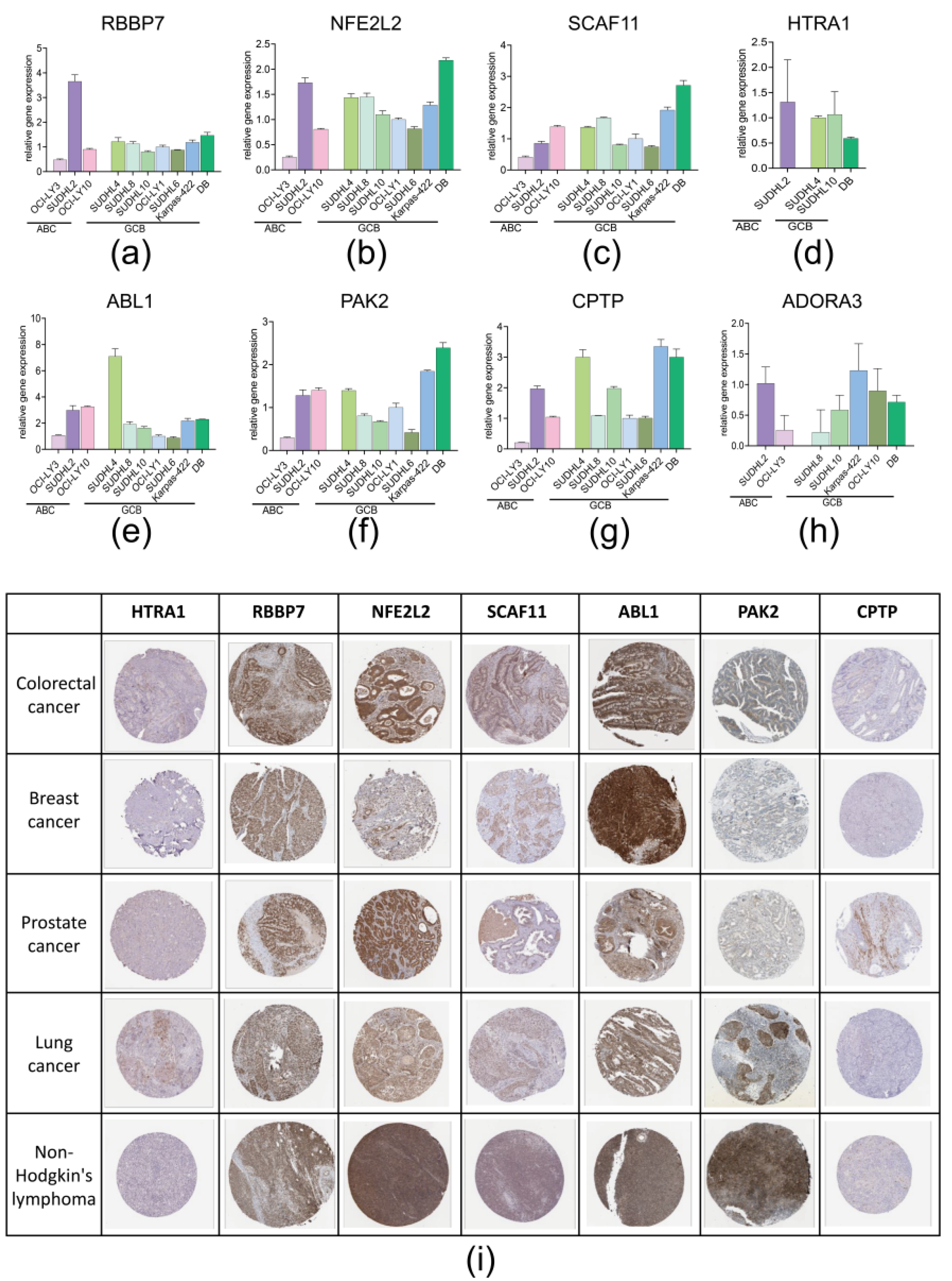
3.4. RT-qPCR and IHC Slice Image Confirmed the Expression of 8 PRGs in Real World
3.5. The Risk Score Predicted the Advance of DLBCL
3.6. The Risk Model Predicted the Sensitivity of Novel Chemotherapy
3.7. The Risk Model Revealed the Remodeling of Immune Landscape in DLBCL
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Swerdlow, S.H. (Ed.) WHO Classification of Tumours of Haematopoietic and Lymphoid Tissues; IARC Press: Lyon, France, 2017. [Google Scholar]
- Schmitz, R.; Wright, G.W.; Huang, D.W.; Johnson, C.A.; Phelan, J.D.; Wang, J.Q.; Roulland, S.; Kasbekar, M.; Young, R.M.; Shaffer, A.L.; et al. Genetics and Pathogenesis of Diffuse Large B-Cell Lymphoma. N. Engl. J. Med. 2018, 378, 1396–1407. [Google Scholar] [CrossRef]
- Alizadeh, A.A.; Eisen, M.B.; Davis, R.E.; Ma, C.; Lossos, I.S.; Rosenwald, A.; Boldrick, J.C.; Sabet, H.; Tran, T.; Yu, X.; et al. Distinct types of diffuse large B-cell lymphoma identified by gene expression profiling. Nature 2000, 403, 503–511. [Google Scholar] [CrossRef] [PubMed]
- Sehn, L.H.; Salles, G. Diffuse Large B-Cell Lymphoma. N. Engl. J. Med. 2021, 384, 842–858. [Google Scholar] [CrossRef] [PubMed]
- Wilson, W.H.; Young, R.M.; Schmitz, R.; Yang, Y.; Pittaluga, S.; Wright, G.; Lih, C.-J.; Williams, P.M.; Shaffer, A.L.; Gerecitano, J.; et al. Targeting B cell receptor signaling with ibrutinib in diffuse large B cell lymphoma. Nat. Med. 2015, 21, 922–926. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Barta, S.K. Diffuse large B-cell lymphoma: 2019 update on diagnosis, risk stratification, and treatment. Am. J.Hematol. 2019, 94, 604–616. [Google Scholar] [CrossRef] [Green Version]
- Chen, X.; He, W.T.; Hu, L.; Li, J.; Fang, Y.; Wang, X.; Xu, X.; Wang, Z.; Huang, K.; Han, J. Pyroptosis is driven by non-selective gasdermin-D pore and its morphology is different from MLKL channel-mediated necroptosis. Cell Res. 2016, 26, 1007–1020. [Google Scholar] [CrossRef]
- Frank, D.; Vince, J.E. Pyroptosis versus necroptosis: Similarities, differences, and crosstalk. Cell Death Differ. 2019, 26, 99–114. [Google Scholar] [CrossRef] [Green Version]
- Wei, X.; Xie, F.; Zhou, X.; Wu, Y.; Yan, H.; Liu, T.; Huang, J.; Wang, F.; Zhou, F.; Zhang, L. Role of pyroptosis in inflammation and cancer. Cell Mol. Immunol. 2022, 19, 971–992. [Google Scholar] [CrossRef]
- Yu, P.; Zhang, X.; Liu, N.; Tang, L.; Peng, C.; Chen, X. Pyroptosis: Mechanisms and diseases. Signal Transduct. Target Ther. 2021, 6, 128. [Google Scholar] [CrossRef]
- Fang, Y.; Tian, S.; Pan, Y.; Li, W.; Wang, Q.; Tang, Y.; Yu, T.; Wu, X.; Shi, Y.; Ma, P.; et al. Pyroptosis: A new frontier in cancer. Biomed. Pharmacother. 2020, 121, 109595. [Google Scholar] [CrossRef]
- Wu, T.; Dai, Y. Tumor microenvironment and therapeutic response. Cancer Lett. 2017, 387, 61–68. [Google Scholar] [CrossRef]
- Wang, L.; Yuan, W.; Li, L.; Shen, Z.; Geng, Q.; Zheng, Y.; Zhao, J. Immunogenomic-Based Analysis of Hierarchical Clustering of Diffuse Large Cell Lymphoma. J. Immunol. Res. 2022, 2022, 9544827. [Google Scholar] [CrossRef] [PubMed]
- Tan, Y.; Sun, R.; Liu, L.; Yang, D.; Xiang, Q.; Li, L.; Tang, J.; Qiu, Z.; Peng, W.; Wang, Y.; et al. Tumor Suppressor DRD2 Facilitates M1 Macrophages and Restricts NF-κB Signaling to Trigger Pyroptosis in Breast Cancer. Theranostics 2021, 11, 5214–5231. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Xu, S.-W.; Teng, Y.; Zhu, M.; Guo, Q.-Y.; Wang, Y.-W.; Mao, X.-L.; Li, S.-W.; Luo, W.-D. The Dark Side of Pyroptosis of Diffuse Large B-Cell Lymphoma in B-Cell Non-Hodgkin Lymphoma: Mediating the Specific Inflammatory Microenvironment. Front. Cell Dev. Biol. 2021, 9, 779123. [Google Scholar] [CrossRef]
- Xiao, B.; Liu, L.; Li, A.; Xiang, C.; Wang, P.; Li, H.; Xiao, T. Identification and Verification of Immune-Related Gene Prognostic Signature Based on ssGSEA for Osteosarcoma. Front. Oncol. 2020, 10, 607622. [Google Scholar] [CrossRef]
- Hänzelmann, S.; Castelo, R.; Guinney, A. GSVA: Gene set variation analysis for microarray and RNA-seq data. BMC Bioinform. 2013, 14, 7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miao, Y.; Medeiros, L.J.; Li, Y.; Li, J.; Young, K.H. Genetic alterations and their clinical implications in DLBCL. Nat. Rev. Clin. Oncol. 2019, 16, 634–652. [Google Scholar] [CrossRef]
- Deng, M.; Sun, S.; Zhao, R.; Guan, R.; Zhang, Z.; Li, S.; Wei, W.; Guo, R. The pyroptosis-related gene signature predicts prognosis and indicates immune activity in hepatocellular carcinoma. Mol. Med. 2022, 28, 16. [Google Scholar] [CrossRef]
- Xu, D.; Ji, Z.; Qiang, L. Molecular characteristics, clinical implication, and cancer immunity interactions of pyroptosis-related genes in breast cancer. Front. Med. 2021, 8, 702638. [Google Scholar] [CrossRef]
- Chen, P.; Li, Y.; Li, N.; Shen, L.; Li, Z. Comprehensive analysis of pyroptosis-associated in molecular classification, immunity and prognostic of glioma. Front. Genet. 2021, 12, 781538. [Google Scholar] [CrossRef]
- Pan, X.Q.; Huang, W.; Jin, L.W.; Lin, H.Z.; Xu, X.Y. A novel pyroptosis-related prognostic signature for risk stratification and clinical prognosis in clear cell renal cell carcinoma. Dis. Mark. 2022, 2022, 8093837. [Google Scholar] [CrossRef] [PubMed]
- Ruan, J.; Wang, S.; Wang, J. Mechanism and regulation of pyroptosis-mediated in cancer cell death. Chem. Biol. Interact. 2020, 323, 109052. [Google Scholar] [CrossRef] [PubMed]
- Greuber, E.K.; Smith-Pearson, P.; Wang, J.; Pendergast, A.M. Role of ABL family kinases in cancer: From leukaemia to solid tumours. Nat. Rev. Cancer 2013, 13, 559–571. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, C.; Navab, R.; Iakovlev, V.; Leng, Y.; Zhang, J.; Tsao, M.S.; Siminovitch, K.; McCready, D.R.; Done, S.J. Abelson interactor protein-1 positively regulates breast cancer cell proliferation, migration, and invasion. Mol. Cancer Res. 2007, 5, 1031–1039. [Google Scholar] [CrossRef] [Green Version]
- Cui, M.; Yu, W.; Dong, J.; Chen, J.; Zhang, X.; Liu, Y. Downregulation of ABI1 expression affects the progression and prognosis of human gastric carcinoma. Med. Oncol. 2010, 27, 632–639. [Google Scholar] [CrossRef]
- Chitsike, L.; Ding, X.; Breslin, P.; Qiu, W. ABL1 is Overexpressed and Activated in Hepatocellular Carcinoma. J. Cancer Tumor Int. 2017, 6, 1–8. [Google Scholar] [CrossRef]
- Kumar, R.; Sanawar, R.; Li, X.; Li, F. Structure, biochemistry, and biology of PAK kinases. Gene 2017, 605, 20–31. [Google Scholar] [CrossRef] [Green Version]
- Flate, E.; Stalvey, J.R. Motility of select ovarian cancer cell lines: Effect of extra-cellular matrix proteins and the involvement of PAK2. Int. J. Oncol. 2014, 45, 1401–1411. [Google Scholar] [CrossRef] [Green Version]
- Hao, S.; Luo, C.; Abukiwan, A.; Wang, G.; He, J.; Huang, L.; Weber, C.E.; Lv, N.; Xiao, X.; Eichmüller, S.B.; et al. miR-137 inhibits proliferation of melanoma cells by targeting PAK2. Exp. Dermatol. 2015, 24, 947–952. [Google Scholar] [CrossRef]
- Deng, W.W.; Wu, L.; Bu, L.L.; Liu, J.F.; Li, Y.C.; Ma, S.R.; Yu, G.T.; Mao, L.; Zhang, W.F.; Sun, Z.J. PAK2 promotes migration and proliferation of salivary gland adenoid cystic carcinoma. Am. J. Transl. Res. 2016, 8, 3387–3397. [Google Scholar]
- Zhou, Z.; Gao, Z.; Yan, W.; Zhang, Y.; Huang, J.; Xiong, K. Adenosine A3 receptor activated in H2O2 oxidative stress of primary open-angle glaucoma. Ann. Transl. Med. 2021, 9, 526. [Google Scholar] [CrossRef] [PubMed]
- Mishra, S.K.; Gao, Y.-G.; Deng, Y.; Chalfant, C.E.; Hinchcliffe, E.H.; Brown, R.E. CPTP: A sphingolipid transfer protein that regulates autophagy and inflammasome activation. Autophagy 2018, 14, 862–879. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Federico, M.; Symonds, C.E.; Bagella, L.; Rizzolio, F.; Fanale, D.; Russo, A.; Giordano, A. R-Roscovitine (Seliciclib) prevents DNA damage-induced cyclin A1 upregulation and hinders non-homologous end-joining (NHEJ) DNA repair. Mol. Cancer 2010, 9, 208. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Whittaker, S.R.; Theurillat, J.P.; Van Allen, E.; Wagle, N.; Hsiao, J.; Cowley, G.S.; Schadendorf, D.; Root, D.E.; Garraway, L.A. A genome-scale RNA interference screen implicates NF1 loss in resistance to RAF inhibition. Cancer Discov. 2013, 3, 350–362. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Montagut, C.; Sharma, S.V.; Shioda, T.; McDermott, U.; Ulman, M.; Ulkus, L.E.; Dias-Santagata, D.; Stubbs, H.; Lee, D.Y.; Singh, A.; et al. Elevated CRAF as a potential mechanism of acquired resistance to BRAF inhibition in melanoma. Cancer Res. 2008, 68, 4853–4861. [Google Scholar] [CrossRef]

| Genes | Forward Primer (5′-3′) | Reverse Primer (5′-3′) |
|---|---|---|
| HTRA1 | TCCCAACAGTTTGCGCCATAA | CCGGCACCTCTCGTTTAGAAA |
| RBBP7 | GAGGAGCGTGTCATCAATGAA | GCATGGGTCATAACCAGGTCATA |
| NFE2L2 | TTCCCGGTCACATCGAGAG | TCCTGTTGCATACCGTCTAAATC |
| SCAF11 | TGAAAGCAAAGTGTACCAACCT | GGCTCTCTATAAGCTCCTCTGT |
| ABL1 | TGAAAAGCTCCGGGTCTTAGG | TTGACTGGCGTGATGTAGTTG |
| PAK2 | CACCCGCAGTAGTGACAGAG | GGGTCAATTACAGACCGTGTG |
| CPTP | TCTGCGCCGACTCCTACAA | CTCGCCTAGCATCTGCACG |
| ADORA3 | GGCCAATGTTACCTACATCACC | CCAGGGCTAGAGAGACAATGAA |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, J.; Wang, W.; Ma, J.; Xu, Z. A Novel and Validated 8-Pyroptosis-Related Genes Based Risk Prediction Model for Diffuse Large B Cell Lymphoma. Biomolecules 2022, 12, 1835. https://doi.org/10.3390/biom12121835
Ma J, Wang W, Ma J, Xu Z. A Novel and Validated 8-Pyroptosis-Related Genes Based Risk Prediction Model for Diffuse Large B Cell Lymphoma. Biomolecules. 2022; 12(12):1835. https://doi.org/10.3390/biom12121835
Chicago/Turabian StyleMa, Junrui, Wenhan Wang, Jiao Ma, and Zizhen Xu. 2022. "A Novel and Validated 8-Pyroptosis-Related Genes Based Risk Prediction Model for Diffuse Large B Cell Lymphoma" Biomolecules 12, no. 12: 1835. https://doi.org/10.3390/biom12121835
APA StyleMa, J., Wang, W., Ma, J., & Xu, Z. (2022). A Novel and Validated 8-Pyroptosis-Related Genes Based Risk Prediction Model for Diffuse Large B Cell Lymphoma. Biomolecules, 12(12), 1835. https://doi.org/10.3390/biom12121835






