Considerations for the Use of Photobiomodulation in the Treatment of Retinal Diseases
Abstract
1. Introduction
2. Methods
3. Wavelength of Light for Exerting PBM
4. Energy Density of Light Irradiation
5. Parameters of Light Irradiation
6. Potential Molecular Mechanisms of PBM for Treating Retinal Diseases
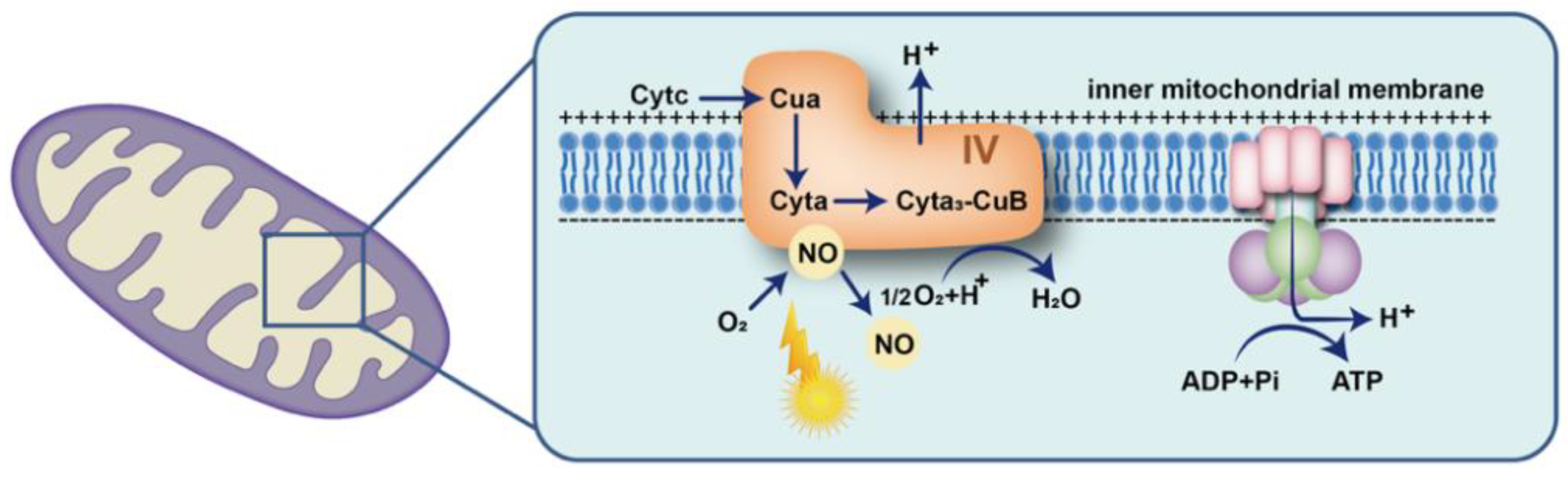
6.1. Acting on Cytochrome C Oxidase to Increase Energy Supply
6.2. Influencing Intracellular Redox Levels
6.3. Regulating Cellular Inflammatory Factor Release
7. Biological Effect of PBM on Retinal Cells
8. Therapeutic Effect of PBM on Retinal Diseases
9. Summary
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Hamblin, M.R. Mechanisms and Mitochondrial Redox Signaling in Photobiomodulation. Photochem. Photobiol. 2018, 94, 199–212. [Google Scholar] [CrossRef] [PubMed]
- Merry, G.F.; Munk, M.R.; Dotson, R.S.; Walker, M.G.; Devenyi, R.G. Photobiomodulation reduces drusen volume and improves visual acuity and contrast sensitivity in dry age-related macular degeneration. Acta Ophthalmol. 2017, 95, e270–e277. [Google Scholar] [CrossRef] [PubMed]
- Shen, W.; Teo, K.Y.C.; Wood, J.P.M.; Vaze, A.; Chidlow, G.; Ao, J.; Lee, S.R.; Yam, M.X.; Cornish, E.E.; Fraser-Bell, S.; et al. Preclinical and clinical studies of photobiomodulation therapy for macular oedema. Diabetologia 2020, 63, 1900–1915. [Google Scholar] [CrossRef] [PubMed]
- Natoli, R.; Valter, K.; Barbosa, M.; Dahlstrom, J.; Rutar, M.; Kent, A.; Provis, J. 670 nm photobiomodulation as a novel protection against retinopathy of prematurity: Evidence from oxygen induced retinopathy models. PLoS ONE 2013, 8, e72135. [Google Scholar] [CrossRef]
- Eells, J.T.; Henry, M.M.; Summerfelt, P.; Wong-Riley, M.T.; Buchmann, E.V.; Kane, M.; Whelan, N.T.; Whelan, H.T. Therapeutic photobiomodulation for methanol-induced retinal toxicity. Proc. Natl. Acad. Sci. USA 2003, 100, 3439–3444. [Google Scholar] [CrossRef]
- Zhu, Q.; Xiao, S.; Hua, Z.; Yang, D.; Hu, M.; Zhu, Y.T.; Zhong, H. Near Infrared (NIR) Light Therapy of Eye Diseases: A Review. Int. J. Med. Sci. 2021, 18, 109–119. [Google Scholar] [CrossRef]
- Geneva, II, Photobiomodulation for the treatment of retinal diseases: A review. Int. J. Ophthalmol. 2016, 9, 145–152.
- Beirne, K.; Rozanowska, M.; Votruba, M. Photostimulation of mitochondria as a treatment for retinal neurodegeneration. Mitochondrion 2017, 36, 85–95. [Google Scholar] [CrossRef]
- Henein, C.; Steel, D.H. Photobiomodulation for non-exudative age-related macular degeneration. Cochrane Database Syst. Rev. 2021, 5, Cd013029. [Google Scholar] [CrossRef][Green Version]
- Boulton, M.; Rozanowska, M.; Rozanowski, B. Retinal photodamage. J. Photochem. Photobiol. B 2001, 64, 144–161. [Google Scholar] [CrossRef]
- Fan, B.; Zhang, C.; Chi, J.; Liang, Y.; Bao, X.; Cong, Y.; Yu, B.; Li, X.; Li, G.Y. The Molecular Mechanism of Retina Light Injury Focusing on Damage from Short Wavelength Light. Oxid. Med. Cell Longev. 2022, 2022, 8482149. [Google Scholar] [CrossRef] [PubMed]
- Youssef, P.N.; Sheibani, N.; Albert, D.M. Retinal light toxicity. Eye 2011, 25, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Heinig, N.; Schumann, U.; Calzia, D.; Panfoli, I.; Ader, M.; Schmidt, M.H.H.; Funk, R.H.W.; Roehlecke, C. Photobiomodulation Mediates Neuroprotection against Blue Light Induced Retinal Photoreceptor Degeneration. Int. J. Mol. Sci. 2020, 21, 2370. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.; Du, Y.; Liu, H.; Tang, J.; Veenstra, A.; Kern, T.S. Photobiomodulation Inhibits Long-term Structural and Functional Lesions of Diabetic Retinopathy. Diabetes 2018, 67, 291–298. [Google Scholar] [CrossRef] [PubMed]
- Karu, T.I.; Pyatibrat, L.V.; Kolyakov, S.F.; Afanasyeva, N.I. Absorption measurements of a cell monolayer relevant to phototherapy: Reduction of cytochrome c oxidase under near IR radiation. J. Photochem. Photobiol. B 2005, 81, 98–106. [Google Scholar] [CrossRef]
- Karu, T. Primary and secondary mechanisms of action of visible to near-IR radiation on cells. J. Photochem. Photobiol. B 1999, 49, 1–17. [Google Scholar] [CrossRef]
- Karu, T.I. Multiple roles of cytochrome C oxidase in mammalian cells under action of red and IR-A radiation. IUBMB Life 2010, 62, 607–610. [Google Scholar] [CrossRef] [PubMed]
- Wong-Riley, M.T.; Liang, H.L.; Eells, J.T.; Chance, B.; Henry, M.M.; Buchmann, E.; Kane, M.; Whelan, H.T. Photobiomodulation directly benefits primary neurons functionally inactivated by toxins: Role of cytochrome c oxidase. J. Biol. Chem. 2005, 280, 4761–4771. [Google Scholar] [CrossRef]
- Gupta, A.; Dai, T.; Hamblin, M.R. Effect of red and near-infrared wavelengths on low-level laser (light) therapy-induced healing of partial-thickness dermal abrasion in mice. Lasers Med. Sci. 2014, 29, 257–265. [Google Scholar] [CrossRef]
- Tang, J.; Du, Y.; Lee, C.A.; Talahalli, R.; Eells, J.T.; Kern, T.S. Low-intensity far-red light inhibits early lesions that contribute to diabetic retinopathy: In vivo and in vitro. Invest. Ophthalmol. Vis. Sci. 2013, 54, 3681–3690. [Google Scholar] [CrossRef]
- Zein, R.; Selting, W.; Hamblin, M.R. Review of light parameters and photobiomodulation efficacy: Dive into complexity. J. Biomed. Opt. 2018, 23, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Albarracin, R.; Valter, K. 670 nm red light preconditioning supports Müller cell function: Evidence from the white light-induced damage model in the rat retina. Photochem. Photobiol. 2012, 88, 1418–1427. [Google Scholar] [CrossRef] [PubMed]
- Marco, F.D.; Romeo, S.; Nandasena, C.; Purushothuman, S.; Adams, C.; Bisti, S.; Stone, J. The time course of action of two neuroprotectants, dietary saffron and photobiomodulation, assessed in the rat retina. Am. J. Neurodegener. Dis. 2013, 2, 208–220. [Google Scholar] [PubMed]
- Lu, Y.Z.; Fernando, N.; Natoli, R.; Madigan, M.; Valter, K. 670nm light treatment following retinal injury modulates Müller cell gliosis: Evidence from in vivo and in vitro stress models. Exp. Eye Res. 2018, 169, 1–12. [Google Scholar] [CrossRef]
- Di Paolo, M. Sequential PBM-Saffron Treatment in an Animal Model of Retinal Degeneration. Medicina 2021, 57, 1059. [Google Scholar] [CrossRef] [PubMed]
- Saliba, A.; Du, Y.; Liu, H.; Patel, S.; Roberts, R.; Berkowitz, B.A.; Kern, T.S. Photobiomodulation Mitigates Diabetes-Induced Retinopathy by Direct and Indirect Mechanisms: Evidence from Intervention Studies in Pigmented Mice. PLoS ONE 2015, 10, e0139003. [Google Scholar] [CrossRef] [PubMed]
- Begum, R.; Powner, M.B.; Hudson, N.; Hogg, C.; Jeffery, G. Treatment with 670 nm light up regulates cytochrome C oxidase expression and reduces inflammation in an age-related macular degeneration model. PLoS ONE 2013, 8, e57828. [Google Scholar] [CrossRef] [PubMed]
- Gopalakrishnan, S.; Mehrvar, S.; Maleki, S.; Schmitt, H.; Summerfelt, P.; Dubis, A.M.; Abroe, B.; Connor, T.B., Jr.; Carroll, J.; Huddleston, W.; et al. Photobiomodulation preserves mitochondrial redox state and is retinoprotective in a rodent model of retinitis pigmentosa. Sci. Rep. 2020, 10, 20382. [Google Scholar] [CrossRef]
- Siqueira, R.C.; Belíssimo, L.M.; Pinho, T.S.; Dourado, L.F.N.; Alves, A.P.; de Paiva, M.R.B.; Ajero, U.; Cunha, A.D.S. Short-Term Results of Photobiomodulation Using Light-Emitting Diode Light of 670 nm in Eyes with Age-Related Macular Degeneration. Photobiomodul. Photomed. Laser Surg. 2021, 39, 581–586. [Google Scholar] [CrossRef]
- Markowitz, S.N.; Devenyi, R.G.; Munk, M.R.; Croissant, C.L.; Tedford, S.E.; Rückert, R.; Walker, M.G.; Patino, B.E.; Chen, L.; Nido, M.; et al. A double-masked, randomized, sham-controlled, single-center study with photobiomodulation for the treatment of dry age-related macular degeneration. Retina 2020, 40, 1471–1482. [Google Scholar] [CrossRef]
- Tang, J.; Herda, A.A.; Kern, T.S. Photobiomodulation in the treatment of patients with non-center-involving diabetic macular oedema. Br. J. Ophthalmol. 2014, 98, 1013–1015. [Google Scholar] [CrossRef] [PubMed]
- Grewal, M.K.; Sivapathasuntharam, C.; Chandra, S.; Gurudas, S.; Chong, V.; Bird, A.; Jeffery, G.; Sivaprasad, S. A Pilot Study Evaluating the Effects of 670 nm Photobiomodulation in Healthy Ageing and Age-Related Macular Degeneration. J. Clin. Med. 2020, 9, 1001. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.E.; Glassman, A.R.; Josic, K.; Melia, M.; Aiello, L.P.; Baker, C.; Eells, J.T.; Jampol, L.M.; Kern, T.S.; Marcus, D.; et al. A Randomized Trial of Photobiomodulation Therapy for Center-Involved Diabetic Macular Edema with Good Visual Acuity (Protocol AE). Ophthalmol. Retin. 2022, 6, 298–307. [Google Scholar] [CrossRef] [PubMed]
- Kent, A.L.; Abdel-Latif, M.E.; Cochrane, T.; Broom, M.; Dahlstrom, J.E.; Essex, R.W.; Shadbolt, B.; Natoli, R. A pilot randomised clinical trial of 670 nm red light for reducing retinopathy of prematurity. Pediatr. Res. 2020, 87, 131–136. [Google Scholar] [CrossRef] [PubMed]
- Passarella, S.; Ostuni, A.; Atlante, A.; Quagliariello, E. Increase in the ADP/ATP exchange in rat liver mitochondria irradiated in vitro by helium-neon laser. Biochem. Biophys. Res. Commun. 1988, 156, 978–986. [Google Scholar] [CrossRef]
- Karu, T. Photobiology of low-power laser effects. Health Phys. 1989, 56, 691–704. [Google Scholar] [CrossRef]
- Hamblin, M.R. Mechanisms and applications of the anti-inflammatory effects of photobiomodulation. AIMS Biophys. 2017, 4, 337–361. [Google Scholar] [CrossRef]
- de Freitas, L.F.; Hamblin, M.R. Proposed Mechanisms of Photobiomodulation or Low-Level Light Therapy. IEEE J. Sel. Top. Quantum Electron. 2016, 22, 348–364. [Google Scholar] [CrossRef]
- Moncada, S.; Erusalimsky, J.D. Does nitric oxide modulate mitochondrial energy generation and apoptosis? Nat. Rev. Mol. Cell Biol. 2002, 3, 214–220. [Google Scholar] [CrossRef]
- Baik, J.S.; Lee, T.Y.; Kim, N.G.; Pak, K.; Ko, S.H.; Min, J.H.; Shin, Y.I. Effects of Photobiomodulation on Changes in Cognitive Function and Regional Cerebral Blood Flow in Patients with Mild Cognitive Impairment: A Pilot Uncontrolled Trial. J. Alzheimers Dis. 2021, 83, 1513–1519. [Google Scholar] [CrossRef]
- Pope, N.J.; Powell, S.M.; Wigle, J.C.; Denton, M.L. Wavelength- and irradiance-dependent changes in intracellular nitric oxide level. J. Biomed. Opt. 2020, 25, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Farivar, S.; Malekshahabi, T.; Shiari, R. Biological effects of low level laser therapy. J. Lasers Med. Sci. 2014, 5, 58–62. [Google Scholar] [PubMed]
- Kim, J.; Won, J.Y. Effect of Photobiomodulation in Suppression of Oxidative Stress on Retinal Pigment Epithelium. Int. J. Mol. Sci. 2022, 23, 6413. [Google Scholar] [CrossRef]
- Huang, Y.Y.; Nagata, K.; Tedford, C.E.; McCarthy, T.; Hamblin, M.R. Low-level laser therapy (LLLT) reduces oxidative stress in primary cortical neurons in vitro. J. Biophotonics 2013, 6, 829–838. [Google Scholar] [CrossRef] [PubMed]
- Kokkinopoulos, I.; Colman, A.; Hogg, C.; Heckenlively, J.; Jeffery, G. Age-related retinal inflammation is reduced by 670 nm light via increased mitochondrial membrane potential. Neurobiol. Aging 2013, 34, 602–609. [Google Scholar] [CrossRef]
- Nayak, K.; Misra, M. A review on recent drug delivery systems for posterior segment of eye. Biomed. Pharmacother. 2018, 107, 1564–1582. [Google Scholar] [CrossRef]
- Pfeiffer, R.L.; Marc, R.E.; Jones, B.W. Müller Cell Metabolic Signatures: Evolutionary Conservation and Disruption in Disease. Trends Endocrinol. Metab. 2020, 31, 320–329. [Google Scholar] [CrossRef]
- Quinn, N.; Csincsik, L.; Flynn, E.; Curcio, C.A.; Kiss, S.; Sadda, S.R.; Hogg, R.; Peto, T.; Lengyel, I. The clinical relevance of visualising the peripheral retina. Prog. Retin. Eye Res. 2019, 68, 83–109. [Google Scholar] [CrossRef]
- Strauss, O. The retinal pigment epithelium in visual function. Physiol. Rev. 2005, 85, 845–881. [Google Scholar] [CrossRef]
- Laha, B.; Stafford, B.K.; Huberman, A.D. Regenerating optic pathways from the eye to the brain. Science 2017, 356, 1031–1034. [Google Scholar] [CrossRef]
- Kang, E.Y.; Liu, P.K.; Wen, Y.T.; Quinn, P.M.J.; Levi, S.R.; Wang, N.K.; Tsai, R.K. Role of Oxidative Stress in Ocular Diseases Associated with Retinal Ganglion Cells Degeneration. Antioxidants 2021, 10, 1948. [Google Scholar] [CrossRef] [PubMed]
- Beirne, K.; Rozanowska, M.; Votruba, M. Red Light Treatment in an Axotomy Model of Neurodegeneration. Photochem. Photobiol. 2016, 92, 624–631. [Google Scholar] [CrossRef] [PubMed]
- Beirne, K.; Freeman, T.J.; Rozanowska, M.; Votruba, M. Red Light Irradiation In Vivo Upregulates DJ-1 in the Retinal Ganglion Cell Layer and Protects against Axotomy-Related Dendritic Pruning. Int. J. Mol. Sci. 2021, 22, 8380. [Google Scholar] [CrossRef]
- Naifeh, J.; Kaufman, E.J. Color Vision. In StatPearls; StatPearls Publishing LLC.: Treasure Island, FL, USA, 2022. [Google Scholar]
- Eells, J.T. Mitochondrial Dysfunction in the Aging Retina. Biology 2019, 8, 31. [Google Scholar] [CrossRef] [PubMed]
- Korhonen, E.; Hytti, M.; Piippo, N.; Kaarniranta, K.; Kauppinen, A. Antimycin A-induced mitochondrial dysfunction regulates inflammasome signaling in human retinal pigment epithelial cells. Exp. Eye Res. 2021, 209, 108687. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Zhang, J.; Zheng, Y.; Zhang, Y.; Zhang, X.J.; Wang, H.; Du, Y.; Guan, J.; Wang, X.; Fu, J. NAD(+) improves cognitive function and reduces neuroinflammation by ameliorating mitochondrial damage and decreasing ROS production in chronic cerebral hypoperfusion models through Sirt1/PGC-1α pathway. J. Neuroinflammation 2021, 18, 207. [Google Scholar] [CrossRef]
- Shinhmar, H.; Grewal, M.; Sivaprasad, S.; Hogg, C.; Chong, V.; Neveu, M.; Jeffery, G. Optically Improved Mitochondrial Function Redeems Aged Human Visual Decline. J. Gerontol. A Biol. Sci. Med. Sci. 2020, 75, e49–e52. [Google Scholar] [CrossRef]
- Fuma, S.; Murase, H.; Kuse, Y.; Tsuruma, K.; Shimazawa, M.; Hara, H. Photobiomodulation with 670 nm light increased phagocytosis in human retinal pigment epithelial cells. Mol. Vis. 2015, 21, 883–892. [Google Scholar]
- Casaroli-Marano, R.P.; Alforja, S.; Giralt, J.; Farah, M.E. Epimacular brachytherapy for wet AMD: Current perspectives. Clin. Ophthalmol. 2014, 8, 1661–1670. [Google Scholar] [CrossRef][Green Version]
- Al-Zamil, W.M.; Yassin, S.A. Recent developments in age-related macular degeneration: A review. Clin. Interv. Aging 2017, 12, 1313–1330. [Google Scholar] [CrossRef]
- Deng, Y.; Qiao, L.; Du, M.; Qu, C.; Wan, L.; Li, J.; Huang, L. Age-related macular degeneration: Epidemiology, genetics, pathophysiology, diagnosis, and targeted therapy. Genes Dis. 2022, 9, 62–79. [Google Scholar] [CrossRef] [PubMed]
- Romero-Aroca, P. Targeting the pathophysiology of diabetic macular edema. Diabetes Care 2010, 33, 2484–2485. [Google Scholar] [CrossRef] [PubMed]
- Albarracin, R.; Eells, J.; Valter, K. Photobiomodulation protects the retina from light-induced photoreceptor degeneration. Invest. Ophthalmol. Vis. Sci. 2011, 52, 3582–3592. [Google Scholar] [CrossRef] [PubMed]
- Silva, R. Myopic maculopathy: A review. Ophthalmologica 2012, 228, 197–213. [Google Scholar] [CrossRef]
- Haarman, A.E.G.; Enthoven, C.A.; Tideman, J.W.L.; Tedja, M.S.; Verhoeven, V.J.M.; Klaver, C.C.W. The Complications of Myopia: A Review and Meta-Analysis. Invest. Ophthalmol. Vis. Sci. 2020, 61, 49. [Google Scholar] [CrossRef]
- Alvarez-Peregrina, C.; Sánchez-Tena, M.; Martinez-Perez, C.; Villa-Collar, C. The Relationship Between Screen and Outdoor Time with Rates of Myopia in Spanish Children. Front. Public Health 2020, 8, 560378. [Google Scholar] [CrossRef]
- Landis, E.G.; Park, H.N.; Chrenek, M.; He, L.; Sidhu, C.; Chakraborty, R.; Strickland, R.; Iuvone, P.M.; Pardue, M.T. Ambient Light Regulates Retinal Dopamine Signaling and Myopia Susceptibility. Invest. Ophthalmol. Vis. Sci. 2021, 62, 28. [Google Scholar] [CrossRef]
- Jones-Jordan, L.A.; Sinnott, L.T.; Chu, R.H.; Cotter, S.A.; Kleinstein, R.N.; Manny, R.E.; Mutti, D.O.; Twelker, J.D.; Zadnik, K. Myopia Progression as a Function of Sex, Age, and Ethnicity. Investig. Ophthalmol. Vis. Sci. 2021, 62, 36. [Google Scholar] [CrossRef]
- Gawne, T.J.; Ward, A.H.; Norton, T.T. Long-wavelength (red) light produces hyperopia in juvenile and adolescent tree shrews. Vis. Res. 2017, 140, 55–65. [Google Scholar] [CrossRef]
- Ward, A.H.; Norton, T.T.; Huisingh, C.E.; Gawne, T.J. The hyperopic effect of narrow-band long-wavelength light in tree shrews increases non-linearly with duration. Vis. Res. 2018, 146–147, 9–17. [Google Scholar] [CrossRef]
- Hung, L.F.; Arumugam, B.; She, Z.; Ostrin, L.; Smith, E.L., 3rd. Narrow-band, long-wavelength lighting promotes hyperopia and retards vision-induced myopia in infant rhesus monkeys. Exp. Eye Res. 2018, 176, 147–160. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Zhu, Z.; Tan, X.; Kong, X.; Zhong, H.; Zhang, J.; Xiong, R.; Yuan, Y.; Zeng, J.; Morgan, I.G.; et al. Effect of Repeated Low-Level Red-Light Therapy for Myopia Control in Children: A Multicenter Randomized Controlled Trial. Ophthalmology 2021, 129, 509–519. [Google Scholar] [CrossRef] [PubMed]
| Type of Retinal Disease | First Author (Year) | Subject (No.) | λ (nm) | Dose | Irradiance (Duration) | Illumination Method | Result |
|---|---|---|---|---|---|---|---|
| Retinal Degeneration | Albarracin 2012 [22] | rats (36) | 670 nm LED | 9 J/cm2 | 60 mW/cm2 3 min 5 days | Eye level was approximately 2.5 cm away from the light source | Ameliorates the light-induced alterations in the expression of Müller-cell specific markers for structure, stress, metabolism, and inflammation |
| Retinal Degeneration | Marco 2013 [23] | rats | 670 nm LED | 5 J/cm2 | 3 min 2–10 days | The WARP 75’s emission plate was placed on the “ceiling” 1–2 cm from the animal’s eye | Reduces photoreceptor death, preserves the population of surviving photoreceptors, and reduces the upregulation of glial fibrillary acidic protein in Müller cells |
| Retinal Degeneration | Lu 2018 [24] | rats (39) | 670 nm LED | 9 J/cm2 | 60 mW/cm2, 3 min 5 days | Animals were positioned so that both eyes were approximately 2.5 cm away from the light source | Mitigates the production of Müller cell-related pro-inflammatory cytokines, reduces microglia/macrophage (MG/MΦ) recruitment into the outer retina |
| Retinal Degeneration | Di Paolo 2021 [25] | rats (25) | 670 nm LED | 4.0–4.5 J/cm2 | 3 min 7 days | Placed 2.5 cm away from the animal | Preserves retinal thickness and gliosis and microglia invasion |
| DR | Tang 2013 [20] | rats (80) | 670 nm LED | 6 J/cm2 | 25 mW/cm2 240 s 10 weeks | Approximately 1 inch above the animal, and exposed to whole-body irradiation | Ameliorates lesions of DR in vivo |
| DR | Saliba 2015 [26] | C57BL/6J mice (60) | 670 nm LED | 4.8 J/cm2 | 20 mW/cm2 240 s 10 weeks | 2–3 cm distance used between the device and the animal | Inhibits early changes of DR |
| DR | Cheng 2018 [14] | C57BL/6J mice | 670 nm LED | 5 J/cm2 | 25 mW/cm2 240 s 8 months | The light provided illumination evenly across the entire back of the animals. | Inhibits the functional and histopathologic features of early DR |
| ROP | Natoli 2013 [4] | C57BL/6J mice or rats | 670 nm LED | 9 J/cm2 | 50 mw/cm2 3 min (P7–P17, mice) (P0–P18, rats) | Each animal was held approximately 2.5 cm from the light source | Neovascularization, vaso-obliteration, and abnormal peripheral branching patterns of retinal vessels in oxygen-induced retinopathy |
| AMD | Begum 2013 [27] | mice (29) | 670 nm LED | 7.2 J/cm2 | 20 mW/cm2 6 min twice a day 14 days | In the form of supplemented environmental light | Reduces inflammation probably via cytochrome c oxidase activation in mice even |
| RP | Gopalakrishnan 2020 [28] | rats | 830 nm LED | 4.5 J/cm2 | 25 mW/cm2 180 s, 5 days per week (p10–p25) | LED array was positioned directly over to the animal’s head at a distance of 2 cm exposing both eyes. | Preserves mitochondrial metabolic state and attenuates photoreceptor loss |
| Type of Retinal Disease | First Author (Year) | Subject (No.) | λ (nm) | Dose | Irradiance Treatment Duration | Illumination Method | Result |
|---|---|---|---|---|---|---|---|
| AMD | Siqueira 2021 [29] | Human (10) | 670 nm LED | 5 J/cm2 | 50 mW/cm2 88 s 3 times/week for 3 weeks | Kept closed during PBM therapy, and the device was positioned 2 cm away from the eye | Improves VA and macular perimetry |
| AMD | Markowitz 2020 [30] | Human (46) | 590 nm 660 nm 850 nm LED | None | 590 nm = 5 mW/cm2 660 nm = 65 mW/cm2 850 nm = 8 mW/cm2 (250 s) (3× per week for 3–4 weeks) over 1 year | 590 nm and 850 nm eyes open, 660 nm eyes closed | Improves clinical and anatomical outcomes with more robust benefits observed in subjects |
| AMD | Merry 2017 [2] | Human (42) | 590 nm 670 nm 790 nm LED | 670 nm (4–7.7 J/cm2), 590 nm, 790 nm (0.1 J/cm2) | 670 nm,50–80 mW/cm2 88 s, 590 nm and 790 nm 0.6 mW for 35 s 3× per week for 3 weeks | None | Improves function and anatomical outcomes in dry AMD |
| DME | Shen 2020 [3] | Human (21) | 670 nm Laser | 2. 25 J/cm2 9 J/cm2 18 J/cm2 | 25, 100, or 200 mW/cm2 90 s for 5 weeks | None | Reduction in CMT at all three settings at 6 months |
| DME | Tang 2014 [31] | Human (4) | 670 nm LED | 25 J/cm2 | Twice daily for 2–9 month | Devices were held an inch away from the closed treatment eye. | Significantly reduces focal retinal thickening |
| Type of Retinal Disease | First Author (Year) | Subject (No.) | λ (nm) | Dose | Irradiance Treatment Duration | Illumination Method | Result |
|---|---|---|---|---|---|---|---|
| AMD | Grewal 2020 [32] | Human (31) | 670 nm LED | 4.8 J/cm2 | 40 mW/cm2 120 s, 12 months | looking at the red light to the front of the study eye | No effect in patients who have already progressed to intermediate AMD |
| DME | Kim 2022 [33] | Human (135) | 670 nm LED | 4.5 J/cm2 | <50 mW/cm2, 90 s, twice daily for 4 months | The device is worn as a single eye patch to direct the treatment effect to the study eye | Although safe and well-tolerated, it was not found to be effective for the treatment of CI-DME in eyes with good vision |
| ROP | Kent 2020 [34] | Neonate < 30 weeks gestation or <1150 g (86) | 670 nm LED | 9 J/cm2 | 15 min daily until 34 weeks corrected age | LED was placed on the isolette 20–25 cm above the baby | This small pilot study did not show a difference in severity of ROP but may indicate an improvement in survival |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, C.-X.; Lou, Y.; Chi, J.; Bao, X.-L.; Fan, B.; Li, G.-Y. Considerations for the Use of Photobiomodulation in the Treatment of Retinal Diseases. Biomolecules 2022, 12, 1811. https://doi.org/10.3390/biom12121811
Zhang C-X, Lou Y, Chi J, Bao X-L, Fan B, Li G-Y. Considerations for the Use of Photobiomodulation in the Treatment of Retinal Diseases. Biomolecules. 2022; 12(12):1811. https://doi.org/10.3390/biom12121811
Chicago/Turabian StyleZhang, Chun-Xia, Yan Lou, Jing Chi, Xiao-Li Bao, Bin Fan, and Guang-Yu Li. 2022. "Considerations for the Use of Photobiomodulation in the Treatment of Retinal Diseases" Biomolecules 12, no. 12: 1811. https://doi.org/10.3390/biom12121811
APA StyleZhang, C.-X., Lou, Y., Chi, J., Bao, X.-L., Fan, B., & Li, G.-Y. (2022). Considerations for the Use of Photobiomodulation in the Treatment of Retinal Diseases. Biomolecules, 12(12), 1811. https://doi.org/10.3390/biom12121811







