Deetect: A Deep Learning-Based Image Analysis Tool for Quantification of Adherent Cell Populations on Oxygenator Membranes after Extracorporeal Membrane Oxygenation Therapy
Abstract
:1. Introduction
2. Materials and Methods
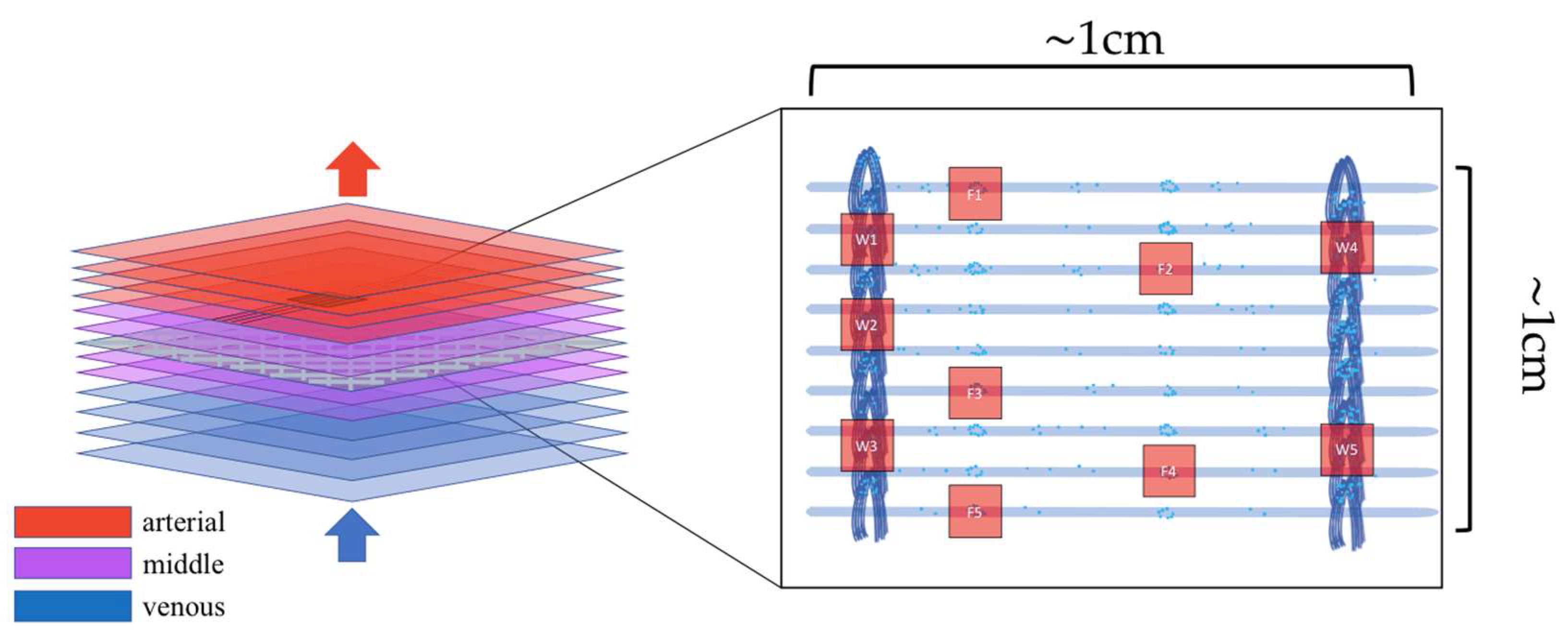
2.1. Experimental Dataset
Preparation of the Biological Samples
2.2. Confocal Laser Scanning Microscopy
3. Results
3.1. Cell Quantification Methods
3.1.1. Manual Counting
3.1.2. Conventional Segmentation and Counting via Fiji
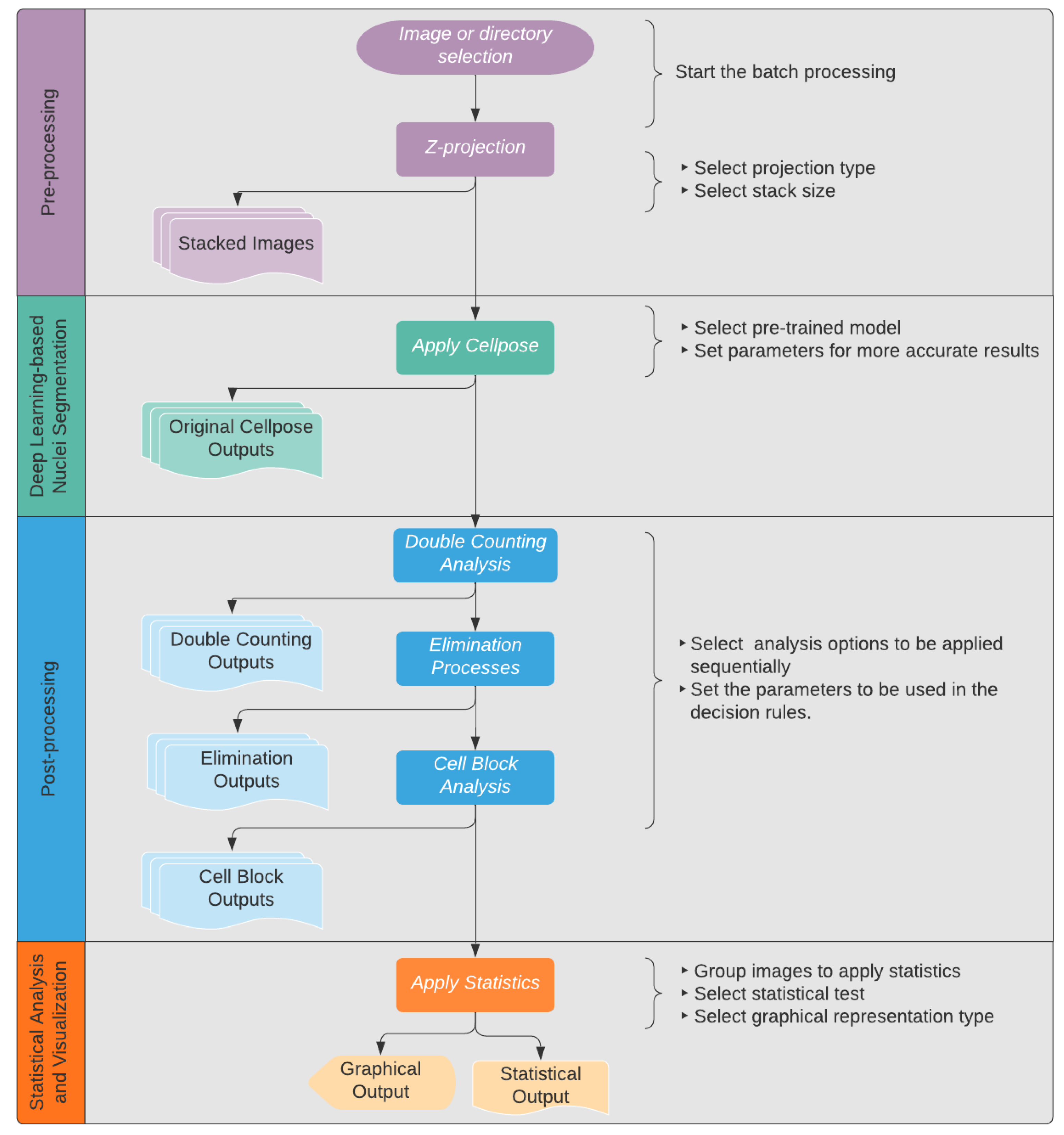
3.1.3. AI-Based Cell Quantification Tool–Deetect
- Batch processing: This feature allows the processing of multiple image sequences in a short amount of time. After opening Deetect, the users can decide whether they want to analyze one image to test the parameters or to do a batch processing of multiple image sequences to generate results for a bigger dataset. In the latter case, they can simply select a directory, and all contained sequences will be processed in the same way.
- Z-projection: Merging images into a maximum projection can be convenient [16]. However, for optimal cell counting, the examiner must avoid crowded projections with blurred cell contours where individual cells can no longer be distinguished. To avoid crowded projections, partial stacking is a good solution. In Deetect, there are six options for z-projection algorithms: maximum, minimum, mean, median, standard deviation, and sum. For stacking, the user can choose between the following options:5-Z-stacking, where the first and the last 5 Z-planes are summed up into two substacks; 2-Z-stacking, where the whole stack of 10 Z-planes is divided into five substacks, each containing two consecutive Z-planes summed up; and the complete projection showing all 10 Z-planes summed up.
- Segmentation: To segment the nuclei, a deep learning-based segmentation method called Cellpose [11] was used. It is a state-of-the-art method for cellular segmentation that has been shown to outperform other, well-known methods [10,11,17]. Cellpose was chosen because of its strong ability to generalize, which means that it is able to segment biological pictures out of the box without being trained on the specific dataset. This is attributed to the different convolutional neural networks that are provided in the program that were pretrained on a highly variable dataset of biological images with over 70,000 segmented objects [11]. In order to produce reliable results, some of the parameters and models Cellpose uses must be adapted to the dataset being analyzed. Deetect’s GUI allows the user to choose different Cellpose models and parameters to apply the best settings for their analysis.For the dataset generated in this study, the cytoplasm model from Cellpose was used because it achieved the best segmentation (data not shown). The performance parameters of Cellpose were set to flow threshold = 0.6 and mask threshold = 0.0.
- Double-counting analysis: One of the artifacts of partial stacking is the double-counting problem. Some nuclei are represented in two substacks after partial stacking, and these nuclei will be detected twice during the segmentation and counting process. This situation is shown in Figure 6. Because of this problem, partial stacking projections may result in unwanted double-counting of nuclei. To address this issue, a method based on colocalization analysis for the detection and elimination of such double-counting artifacts was developed. The algorithm compares all nuclei of two sequential projections (Figure 6). If a nucleus from one projection has an intersection with a nucleus from an adjacent projection that is greater than a user-defined threshold, the algorithm marks the smallest of the two nuclei as a double-counted nucleus. This user-defined threshold is called the intersection percentage (default value is 60%). The nucleus that would have been double counted is outlined in red in the resulting output image (Figure 7).
- Elimination processes: Artifacts can occur in very sensitive deep-learning algorithms. In this study, Deetect was able to detect cell nuclei whose structure could not be recognized by the human eye. These counts cannot be confirmed by the investigator and must therefore be removed from the data in a subsequent exclusion procedure. Additionally, very small structures appeared that could not be identified as a cell nucleus. In this study, it was assumed that these were artifacts caused by hemolysis during long-term ECMO support [18,19]. To avoid result distortion by these unwanted small and invisible structures, elimination rules were defined. Small, artifactual structures that should not be counted can be eliminated by defining a small nucleus area limit in the GUI. Detected nuclei that cannot be verified visually were eliminated using a visibility limit. The default setting is 16 because it represents the first distinguishable color from pure black [20].The rules of the elimination processes are applied on all detected nuclei. As a result, all problematic nuclei are outlined with blue (invisible nucleus) and yellow (small nucleus) in the output image (Figure 7).
- The rule for the elimination of small nuclei: the area of a nucleus is less than the user-defined small nucleus area limit.
- ⚬
- nucleus_area < small_nucleus_area_limit (default value: 100 px).
- The rule for the elimination of invisible nuclei: more than 50% of the pixels of the nucleus have an intensity value less than the visible intensity.
- ⚬
- invisible_pixel: if intensity < 16 (visibility_limit).
- ⚬
- percent_of_invisible_pixel > 50.
- Cell block analysis: Detecting nuclear boundaries in areas of high cell accumulation is a common problem for quantification algorithms. When strong light reflections occur in places with increased cell density, nuclei borders are hard to distinguish. This can often lead to undercounting, e.g., if the cluster is counted as one nucleus instead of the actual quantity within the cell cluster. To define this type of cell cluster, a rule-based analysis was developed. After identifying those cell clusters as “big nuclei,” the algorithm approximately calculates the number of nuclei present using the average nucleus area, which was determined to be 400 px in this project. In the resulting image (Figure 7), the big nuclei are labeled with gray outlines.
- The rule for big nuclei: the area of a nucleus is bigger than the user-defined big nucleus area limit.
- ⚬
- nucleus_area > big_nucleus_area_limit.
- ⚬
- >50% of the pixels have an intensity > 127.
- ⚬
- approximate_cell_count = big_nucleus_area/average_nucleus_area.
- Statistics and visualization: Deetect allows statistical analysis and data visualization. After analyzing the image sequences, the user can group the generated data as desired in Deetect’s statistics module and compare different groups using statistical tests. In this study, the Mann–Whitney U test was performed after the cell quantification process to compare different predilection sites for increased cell deposition with each other. It is a non-parametric test that does not require a normal distribution and is therefore integrated as the default setting. For group analysis of the predilection sites from different parts of the oxygenator, the Kruskal–Wallis test and post hoc Dunn’s test were applied.
3.2. Evaluation of Deetect
3.3. Applicability of Deetect for the Comparison of Predilection Sites (Warps and Hollow Fibers)
4. Discussion
4.1. Cell Quantification Methods
4.2. Comparison of the Methods
4.3. Imaging and Applicability of Deetect for the Comparison of Predilection Sites
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Millar, J.E.; Fanning, J.P.; McDonald, C.I.; McAuley, D.F.; Fraser, J.F. The inflammatory response to extracorporeal membrane oxygenation (ECMO): A review of the pathophysiology. Crit. Care 2016, 20, 1–10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ki, K.K.; Millar, J.E.; Langguth, D.; Passmore, M.R.; McDonald, C.I.; Shekar, K.; Shankar-Hari, M.; Cho, H.J.; Suen, J.Y.; Fraser, J.F. Current Understanding of Leukocyte Phenotypic and Functional Modulation During Extracorporeal Membrane Oxygenation: A Narrative Review. Front. Immunol. 2021, 11, 600684. [Google Scholar] [CrossRef] [PubMed]
- Ontaneda, A.; Annich, G.M. Novel Surfaces in Extracorporeal Membrane Oxygenation Circuits. Front. Med. 2018, 5, 321. [Google Scholar] [CrossRef] [PubMed]
- Brix-Christensen, V.; Tønnesen, E.; Hjortdal, V.E.; Chew, M.; Flø, C.; Marqversen, J.; Hansen, J.F.; Andersen, N.T.; Ravn, H.B. Neutrophils and platelets accumulate in the heart, lungs, and kidneys after cardiopulmonary bypass in neonatal pigs. Crit. Care Med. 2002, 30, 670–676. [Google Scholar] [CrossRef] [PubMed]
- Plotz, F.B.; Van Oeveren, W.; Bartlett, R.H.; Wildevuur, C.R.H. Blood activation during neonatal extracorporeal life support. J. Thorac. Cardiovasc. Surg. 1993, 105, 823–832. [Google Scholar] [CrossRef]
- Burrell, A.J.C.; Lubnow, M.; Enger, T.B.; Nanjayya, V.B.; Philipp, A.; Malfertheiner, M.V.; Lunz, D.; Bein, T.; Pellegrino, V.A.; Müller, T. The impact of venovenous extracorporeal membrane oxygenation on cytokine levels in patients with severe acute respiratory distress syndrome: A prospective, observational study. Crit. care Resusc. J. Australas. Acad. Crit. Care Med. 2017, 19, 37–44. [Google Scholar]
- Lehle, K.; Friedl, L.; Wilm, J.; Philipp, A.; Müller, T.; Lubnow, M.; Schmid, C. Accumulation of Multipotent Progenitor Cells on Polymethylpentene Membranes During Extracorporeal Membrane Oxygenation. Artif. Organs 2016, 40, 577–585. [Google Scholar] [CrossRef]
- Wilm, J.; Philipp, A.; Müller, T.; Bredthauer, A.; Gleich, O.; Schmid, C.; Lehle, K. Leukocyte Adhesion as an Indicator of Oxygenator Thrombosis During Extracorporeal Membrane Oxygenation Therapy? ASAIO J. 2018, 64, 24–30. [Google Scholar] [CrossRef]
- Lehle, K.; Philipp, A.; Gleich, O.; Holzamer, A.; Müller, T.; Bein, T.; Schmid, C. Efficiency in extracorporeal membrane oxygenation-cellular deposits on polymethypentene membranes increase resistance to blood flow and reduce gas exchange capacity. ASAIO J. 2008, 54, 612–617. [Google Scholar] [CrossRef]
- Rasse, T.M.; Hollandi, R.; Horvath, P. OpSeF: Open Source Python Framework for Collaborative Instance Segmentation of Bioimages. Front. Bioeng. Biotechnol. 2020, 8, 1–15. [Google Scholar] [CrossRef]
- Stringer, C.; Wang, T.; Michaelos, M.; Pachitariu, M. Cellpose: A generalist algorithm for cellular segmentation. Nat. Methods 2021, 18, 100–106. [Google Scholar] [CrossRef] [PubMed]
- Bredthauer, A.; Wilm, J.; Philipp, A.; Foltan, M.; Mueller, T.; Lehle, K. Oxygenator Design Might Influence the Adhesion of Leukocytes and Deposits of von Willebrand Fibres on the Surface of Gas Exchange Membranes during ECMO Introduction; EuroELSO: London, UK, 2017. [Google Scholar]
- Schindelin, J.; Arganda-Carreras, I.; Frise, E.; Kaynig, V.; Longair, M.; Pietzsch, T.; Preibisch, S.; Rueden, C.; Saalfeld, S.; Schmid, B.; et al. Fiji: An open-source platform for biological-image analysis. Nat. Methods 2012, 9, 676–682. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, C.H.; Tam, P.K.S. An iterative algorithm for minimum cross entropy thresholding. Pattern Recognit. Lett. 1998, 19, 771–776. [Google Scholar] [CrossRef]
- Ronneberger, O.; Fischer, P.; Brox, T. U-Net: Convolutional Networks for Biomedical Image Segmentation. In Proceedings of the Medical Image Computing and Computer-Assisted Intervention—MICCAI 2015; Navab, N., Hornegger, J., Wells, W.M., Frangi, A.F., Eds.; Springer International Publishing: Cham, Switzerland, 2015; pp. 234–241. [Google Scholar]
- Shihavuddin, A.; Basu, S.; Rexhepaj, E.; Delestro, F.; Menezes, N.; Sigoillot, S.M.; Del Nery, E.; Selimi, F.; Spassky, N.; Genovesio, A. Smooth 2D manifold extraction from 3D image stack. Nat. Commun. 2017, 8, 15554. [Google Scholar] [CrossRef] [Green Version]
- Kromp, F.; Fischer, L.; Bozsaky, E.; Ambros, I.M.; Dorr, W.; Beiske, K.; Ambros, P.F.; Hanbury, A.; Taschner-Mandl, S. Evaluation of Deep Learning Architectures for Complex Immunofluorescence Nuclear Image Segmentation. IEEE Trans. Med. Imaging 2021, 40, 1934–1949. [Google Scholar] [CrossRef]
- Chan, C.H.H.; Ki, K.K.; Zhang, M.; Asnicar, C.; Cho, H.J.; Ainola, C.; Bouquet, M.; Heinsar, S.; Pauls, J.P.; Li Bassi, G.; et al. Extracorporeal Membrane Oxygenation-Induced Hemolysis: An In Vitro Study to Appraise Causative Factors. Membranes 2021, 11, 313. [Google Scholar] [CrossRef]
- Kawahito, S.; Maeda, T.; Yoshikawa, M.; Takano, T.; Nonaka, K.; Linneweber, J.; Mikami, M.; Motomura, T.; Ichikawa, S.; Glueck, J.; et al. Blood trauma induced by clinically accepted oxygenators. ASAIO J. 2001, 47, 492–495. [Google Scholar] [CrossRef]
- Adjusting Your Monitor. Available online: https://daystarvisions.com/Adjust.html (accessed on 10 June 2022).
- Dunn, K.W.; Kamocka, M.M.; McDonald, J.H. A practical guide to evaluating colocalization in biological microscopy. Am. J. Physiol. Cell Physiol. 2011, 300, C723–C742. [Google Scholar] [CrossRef] [Green Version]
- Stirling, D.R.; Swain-Bowden, M.J.; Lucas, A.M.; Carpenter, A.E.; Cimini, B.A.; Goodman, A. CellProfiler 4: Improvements in speed, utility and usability. BMC Bioinformatics 2021, 22, 1–11. [Google Scholar] [CrossRef]
- von Chamier, L.; Laine, R.F.; Jukkala, J.; Spahn, C.; Krentzel, D.; Nehme, E.; Lerche, M.; Hernández-Pérez, S.; Mattila, P.K.; Karinou, E.; et al. Democratising deep learning for microscopy with ZeroCostDL4Mic. Nat. Commun. 2021, 12, 1–18. [Google Scholar] [CrossRef]
- Gerst, R.; Cseresnyés, Z.; Figge, M.T. JIPipe: Visual batch processing for ImageJ. Res. Sq. 2022. [Google Scholar] [CrossRef]
- Ershov, D.; Phan, M.-S.; Pylvänäinen, J.W.; Rigaud, S.U.; Le Blanc, L.; Charles-Orszag, A.; Conway, J.R.W.; Laine, R.F.; Roy, N.H.; Bonazzi, D.; et al. Bringing TrackMate into the era of machine-learning and deep-learning. bioRxiv 2021. [Google Scholar] [CrossRef]
- Steiger, T.; Foltan, M.; Philipp, A.; Mueller, T.; Gruber, M.; Bredthauer, A.; Krenkel, L.; Birkenmaier, C.; Lehle, K. Accumulations of von Willebrand factor within ECMO oxygenators: Potential indicator of coagulation abnormalities in critically ill patients? Artif. Organs 2019, 43, 1065–1076. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dhahri, M.; Rodriguez-Ruiz, V.; Aid-Launais, R.; Ollivier, V.; Pavon-Djavid, G.; Journé, C.; Louedec, L.; Chaubet, F.; Letourneur, D.; Maaroufi, R.M.; et al. In vitro and in vivo hemocompatibility evaluation of a new dermatan sulfate-modified PET patch for vascular repair surgery. J. Biomed. Mater. Res. B. Appl. Biomater. 2017, 105, 2001–2009. [Google Scholar] [CrossRef]
- Gawlikowski, M.; El Fray, M.; Janiczak, K.; Zawidlak-Węgrzyńska, B.; Kustosz, R. In-vitro biocompatibility and hemocompatibility study of new pet copolyesters intended for heart assist devices. Polymers 2020, 12, 2857. [Google Scholar] [CrossRef]
- Giol, E.D.; Van Vlierberghe, S.; Unger, R.E.; Schaubroeck, D.; Ottevaere, H.; Thienpont, H.; Kirkpatrick, C.J.; Dubruel, P. Endothelialization and Anticoagulation Potential of Surface-Modified PET Intended for Vascular Applications. Macromol. Biosci. 2018, 18, e1800125. [Google Scholar] [CrossRef]
- Wang, Y.B.; Shi, K.H.; Jiang, H.L.; Gong, Y.K. Significantly reduced adsorption and activation of blood components in a membrane oxygenator system coated with crosslinkable zwitterionic copolymer. Acta Biomater. 2016, 40, 153–161. [Google Scholar] [CrossRef]









| Predilection Site | Z-Scan Range (m ± SD) |
|---|---|
| Warps | 52.27 ± 25.51 µm (n = 60) |
| Hollow fibers | 50.74 ± 26.45 µm (n = 55) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hoeren, F.; Görmez, Z.; Richter, M.; Troidl, K. Deetect: A Deep Learning-Based Image Analysis Tool for Quantification of Adherent Cell Populations on Oxygenator Membranes after Extracorporeal Membrane Oxygenation Therapy. Biomolecules 2022, 12, 1810. https://doi.org/10.3390/biom12121810
Hoeren F, Görmez Z, Richter M, Troidl K. Deetect: A Deep Learning-Based Image Analysis Tool for Quantification of Adherent Cell Populations on Oxygenator Membranes after Extracorporeal Membrane Oxygenation Therapy. Biomolecules. 2022; 12(12):1810. https://doi.org/10.3390/biom12121810
Chicago/Turabian StyleHoeren, Felix, Zeliha Görmez, Manfred Richter, and Kerstin Troidl. 2022. "Deetect: A Deep Learning-Based Image Analysis Tool for Quantification of Adherent Cell Populations on Oxygenator Membranes after Extracorporeal Membrane Oxygenation Therapy" Biomolecules 12, no. 12: 1810. https://doi.org/10.3390/biom12121810
APA StyleHoeren, F., Görmez, Z., Richter, M., & Troidl, K. (2022). Deetect: A Deep Learning-Based Image Analysis Tool for Quantification of Adherent Cell Populations on Oxygenator Membranes after Extracorporeal Membrane Oxygenation Therapy. Biomolecules, 12(12), 1810. https://doi.org/10.3390/biom12121810






