Fabrication of Collagen–Hyaluronic Acid Cryogels by Directional Freezing Mimicking Cartilage Arcade-like Structure
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methacrylate Hyaluronic Acid (MeHA) Synthesis
2.3. Pin Plate Set-Up
2.4. CollGTA First Network Directional Freezing and Cryogelation
2.5. CollGTA-MeHA Double Network Formation
2.6. Swelling Testing
2.7. Scaffold Morphology
2.7.1. Light Microscopy and OrientationJ Analysis
2.7.2. Scanning Electron Microscopy (SEM)
2.8. Compression Testing
2.8.1. Single Compression
2.8.2. Fatigue Resistance
2.9. Cell Culture Experiments
2.9.1. Cell Source and Expansion
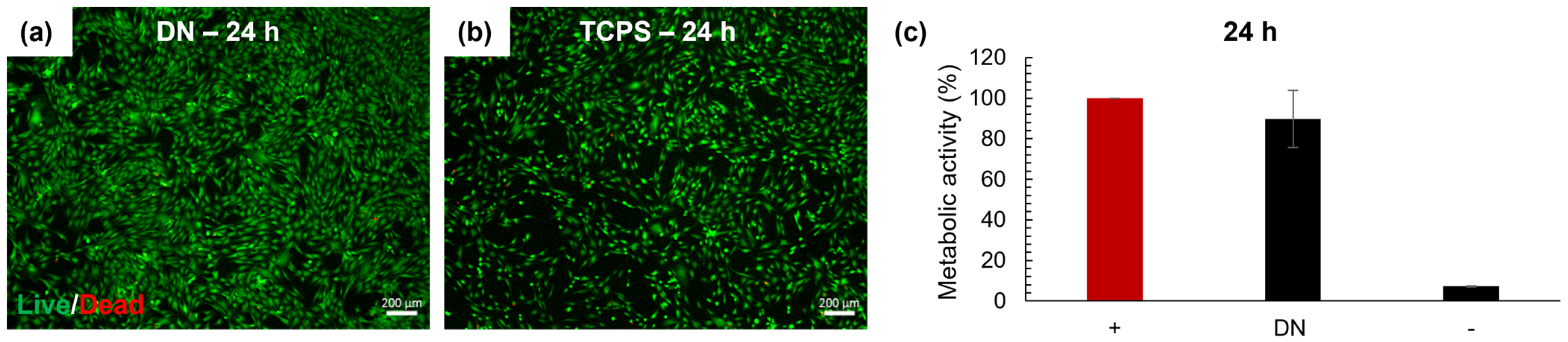
2.9.2. Evaluation of Cytotoxicity
3. Results and Discussion
3.1. Preparation of the CollGTA-MeHA Cryogels
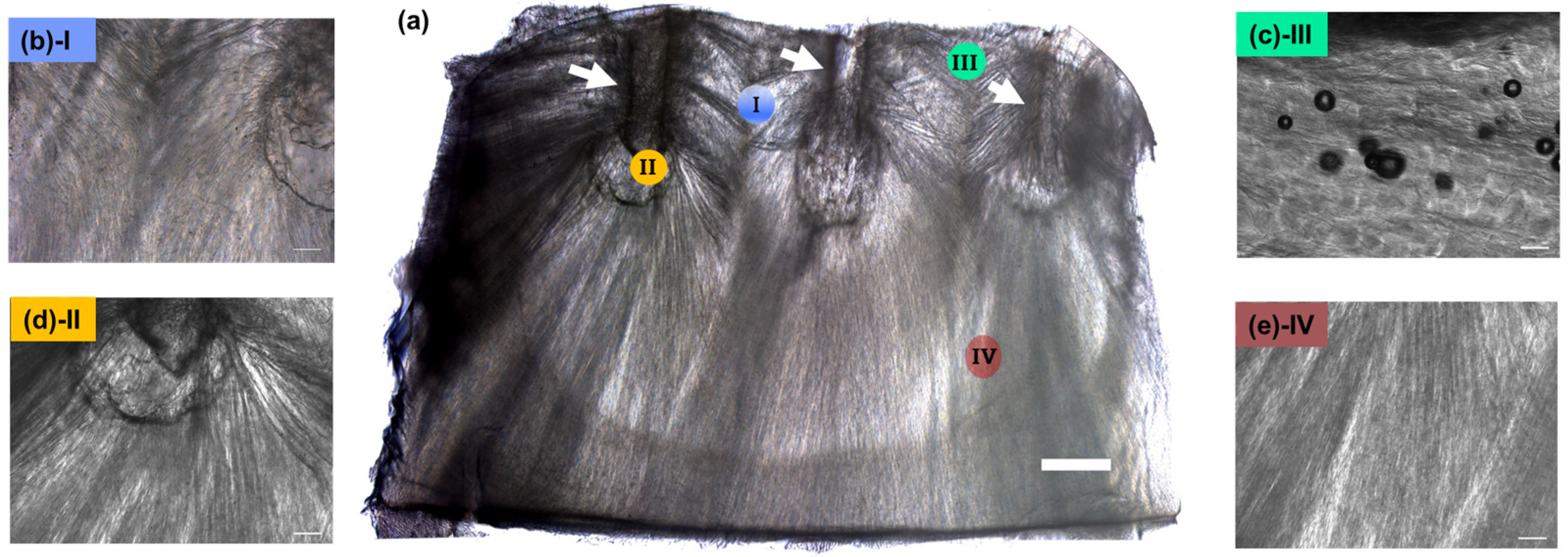
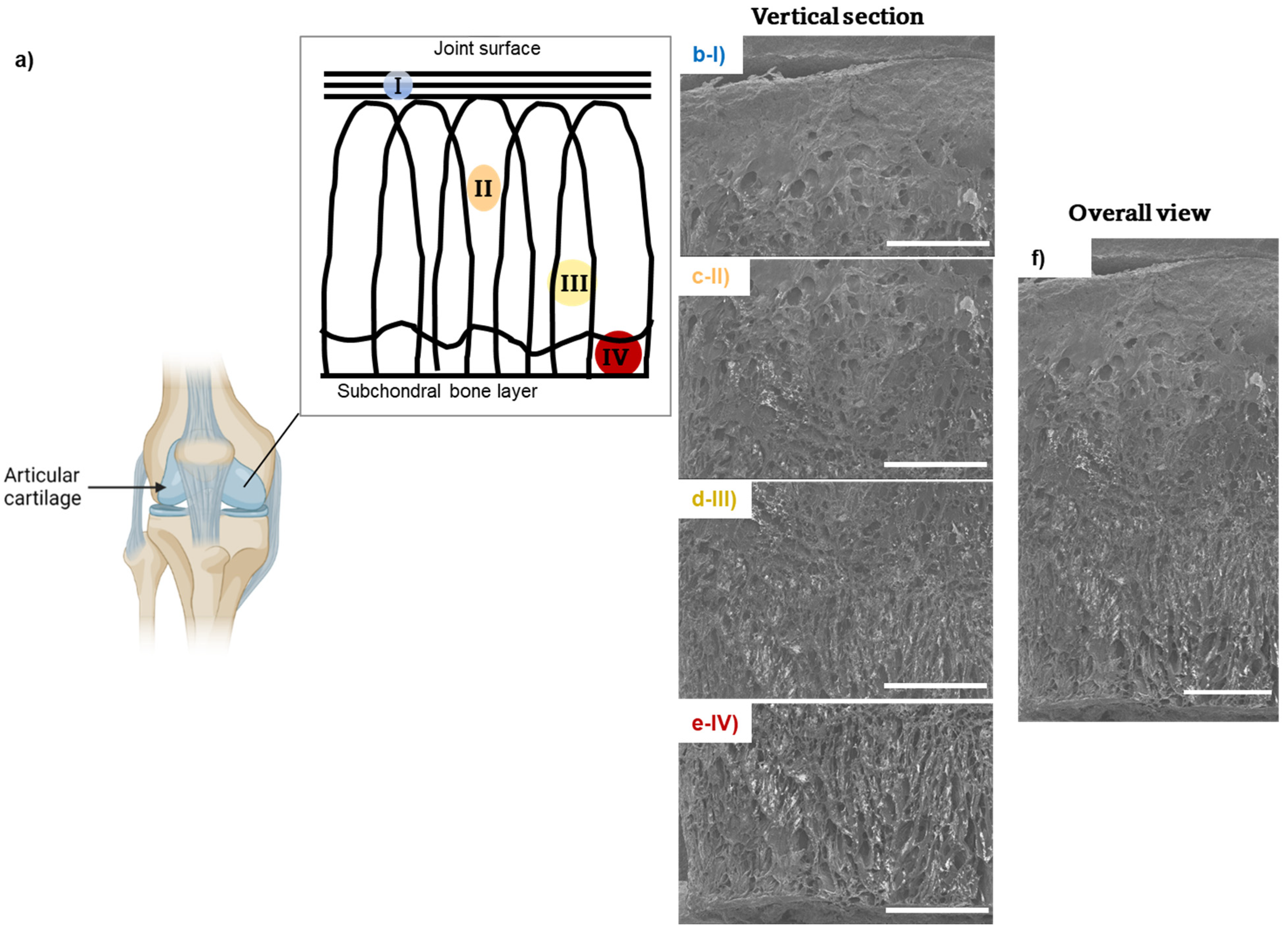
3.2. Arcade-like Structures of CollGTA-MeHA Cryogels
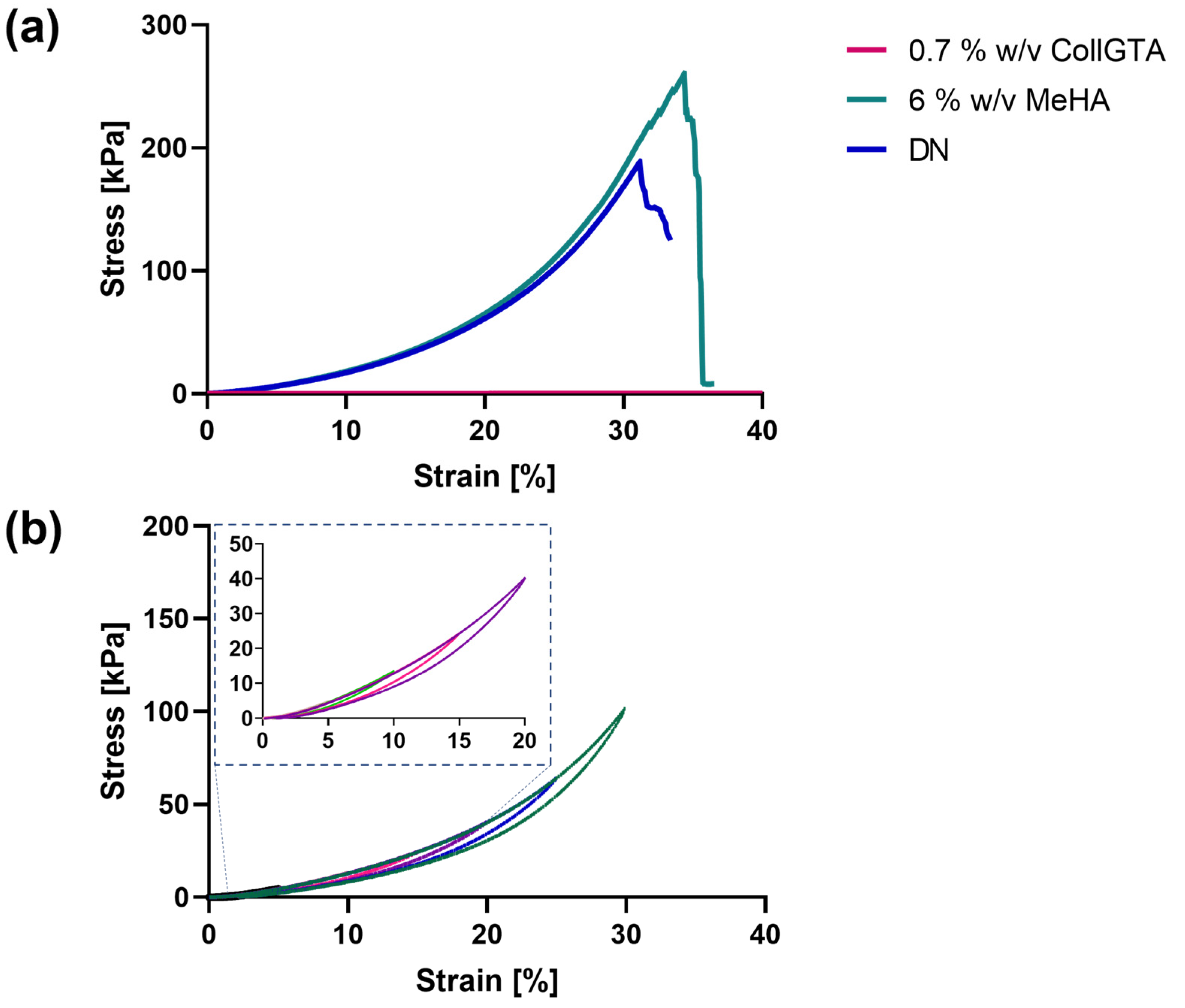
3.3. Mechanical Properties of CollGTA-MeHA Cryogel Double Networks
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Benninghoff, A. Form und Bau der Gelenkknorpel in ihren Beziehungen zur Funktion. Z. Zellforsch. Mikrosk. Anat. 1925, 2, 783–862. [Google Scholar] [CrossRef]
- Eschweiler, J.; Horn, N.; Rath, B.; Betsch, M.; Baroncini, A.; Tingart, M.; Migliorini, F. The Biomechanics of Cartilage—An Overview. Life 2021, 11, 302. [Google Scholar] [CrossRef] [PubMed]
- Poole, A.R.; Kojima, T.; Yasuda, T.; Mwale, F.; Kobayashi, M.; Laverty, S. Composition and Structure of Articular Cartilage: A Template for Tissue Repair. Clin. Orthop. Relat. Res. 2001, 391, S26–S33. [Google Scholar]
- Datta, P.; Vyas, V.; Dhara, S.; Chowdhury, A.R.; Barui, A. Anisotropy Properties of Tissues: A Basis for Fabrication of Biomimetic Anisotropic Scaffolds for Tissue Engineering. J. Bionic Eng. 2019, 16, 842–868. [Google Scholar]
- Armiento, A.R.; Stoddart, M.J.; Alini, M.; Eglin, D. Biomaterials for articular cartilage tissue engineering: Learning from biology. Acta Biomater. 2018, 65, 1–20. [Google Scholar] [PubMed]
- Chondrogenic Differentiation of Human Mesenchymal Stem Cells on Oriented Nanofibrous Scaffolds: Engineering the Superficial Zone of Articular Cartilage. Tissue Eng. Part A 2009, 15, 913–921.
- Schwab, A.; Hélary, C.; Richards, R.G.; Alini, M.; Eglin, D.; D’Este, M. Tissue mimetic hyaluronan bioink containing collagen fibers with controlled orientation modulating cell migration and alignment. Mater. Today Bio 2020, 7, 100058. [Google Scholar]
- Daly, A.C.; Kelly, D.J. Biofabrication of spatially organised tissues by directing the growth of cellular spheroids within 3D printed polymeric microchambers. Biomaterials 2019, 197, 194–206. [Google Scholar] [CrossRef]
- Pot, M.W.; Faraj, K.A.; Adawy, A.; van Enckevort, W.J.P.; van Moerkerk, H.T.B.; Vlieg, E.; Daamen, W.F.; van Kuppevelt, T.H. Versatile Wedge-Based System for the Construction of Unidirectional Collagen Scaffolds by Directional Freezing: Practical and Theoretical Considerations. ACS Appl. Mater. Interfaces 2015, 7, 8495–8505. [Google Scholar] [CrossRef]
- Hixon, K.R.; Lu, T.; Sell, S.A. A comprehensive review of cryogels and their roles in tissue engineering applications. Acta Biomater. 2017, 62, 29–41. [Google Scholar]
- Rogers, Z.J.; Bencherif, S.A. Cryogelation and Cryogels. Gels 2019, 5, 46. [Google Scholar] [CrossRef] [PubMed]
- Loebel, C.; Rodell, C.B.; Chen, M.H.; Burdick, J.A. Shear-thinning and self-healing hydrogels as injectable therapeutics and for 3D-printing. Nat. Protoc. 2017, 12, 1521–1541. [Google Scholar] [CrossRef] [PubMed]
- Assael, M.J.; Botsios, S.; Gialou, K.; Metaxa, I.N. Thermal Conductivity of Polymethyl Methacrylate (PMMA) and Borosilicate Crown Glass BK7. Int. J. Thermophys. 2005, 26, 1595–1605. [Google Scholar] [CrossRef]
- Carvill, J. 3-Thermodynamics and heat transfer. In Mechanical Engineer’s Data Handbook; Carvill, J., Ed.; Butterworth-Heinemann: Oxford, UK, 1993; pp. 102–145. [Google Scholar]
- Tang, Y.; Qiu, S.; Miao, Q.; Zhao, K. Fabrication of lamellar porous alumina with regular structure via directional solidification with multiple cold sources. J. Porous Mater. 2018, 25, 443–450. [Google Scholar] [CrossRef]
- Bencherif, S.; Braschler, T.; Renaud, P. Advances in the design of macroporous polymer scaffolds for potential applications in dentistry. J. Periodontal Implant. Sci. 2013, 43, 251–261. [Google Scholar] [CrossRef]
- Kumar, A.; Mishra, R.; Reinwald, Y.; Bhat, S. Cryogels: Freezing unveiled by thawing. Mater. Today 2010, 13, 42–44. [Google Scholar] [CrossRef]
- Davis, S.; Roldo, M.; Blunn, G.; Tozzi, G.; Roncada, T. Influence of the Mechanical Environment on the Regeneration of Osteochondral Defects. Front. Bioeng. Biotechnol. 2021, 9, 603408. [Google Scholar] [CrossRef]
- LaPrade, R.F. Practical Orthopaedic Sports Medicine and Arthroscopy. JBJS 2007, 89, 2579. [Google Scholar] [CrossRef]
- Malda, J.; Groll, J.; van Weeren, P.R. Rethinking articular cartilage regeneration based on a 250-year-old statement. Nat. Rev. Rheumatol. 2019, 15, 571–572. [Google Scholar] [CrossRef]
- Murphy, W.L.; McDevitt, T.C.; Engler, A.J. Materials as stem cell regulators. Nat. Mater. 2014, 13, 547–557. [Google Scholar] [CrossRef]
- Nuernberger, S.; Cyran, N.; Albrecht, C.; Redl, H.; Vécsei, V.; Marlovits, S. The influence of scaffold architecture on chondrocyte distribution and behavior in matrix-associated chondrocyte transplantation grafts. Biomaterials 2011, 32, 1032–1040. [Google Scholar] [CrossRef] [PubMed]
- Sophia Fox, A.J.; Bedi, A.; Rodeo, S.A. The basic science of articular cartilage: Structure, composition, and function. Sports Health 2009, 1, 461–468. [Google Scholar] [CrossRef] [PubMed]
- Deville, S. Freeze-Casting of Porous Ceramics: A Review of Current Achievements and Issues. Adv. Eng. Mater. 2008, 10, 155–169. [Google Scholar] [CrossRef]
- Tong, L.; Hao, Z.; Wan, C.; Wen, S. Detection of depth-depend changes in porcine cartilage after wear test using Raman spectroscopy. J. Biophotonics 2018, 11, e201700217. [Google Scholar] [CrossRef] [PubMed]
- Rezakhaniha, R.; Agianniotis, A.; Schrauwen, J.T.C.; Griffa, A.; Sage, D.; Bouten, C.V.C.; van de Vosse, F.N.; Unser, M.; Stergiopulos, N. Experimental investigation of collagen waviness and orientation in the arterial adventitia using confocal laser scanning microscopy. Biomech. Model. Mechanobiol. 2012, 11, 461–473. [Google Scholar] [CrossRef]
- Beck, E.C.; Barragan, M.; Tadros, M.H.; Gehrke, S.H.; Detamore, M.S. Approaching the compressive modulus of articular cartilage with a decellularized cartilage-based hydrogel. Acta Biomater. 2016, 38, 94–105. [Google Scholar] [CrossRef]
- Jaumard, N.V.; Welch, W.C.; Winkelstein, B.A. Spinal facet joint biomechanics and mechanotransduction in normal, injury and degenerative conditions. J. Biomech. Eng. 2011, 133, 071010. [Google Scholar] [CrossRef]
- Little, C.J.; Bawolin, N.K.; Chen, X. Mechanical properties of natural cartilage and tissue-engineered constructs. Tissue Eng. Part B Rev. 2011, 17, 213–227. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yamamoto, T.; Randriantsilefisoa, R.; Sprecher, C.M.; D’Este, M. Fabrication of Collagen–Hyaluronic Acid Cryogels by Directional Freezing Mimicking Cartilage Arcade-like Structure. Biomolecules 2022, 12, 1809. https://doi.org/10.3390/biom12121809
Yamamoto T, Randriantsilefisoa R, Sprecher CM, D’Este M. Fabrication of Collagen–Hyaluronic Acid Cryogels by Directional Freezing Mimicking Cartilage Arcade-like Structure. Biomolecules. 2022; 12(12):1809. https://doi.org/10.3390/biom12121809
Chicago/Turabian StyleYamamoto, Taiyo, Rotsiniaina Randriantsilefisoa, Christoph Martin Sprecher, and Matteo D’Este. 2022. "Fabrication of Collagen–Hyaluronic Acid Cryogels by Directional Freezing Mimicking Cartilage Arcade-like Structure" Biomolecules 12, no. 12: 1809. https://doi.org/10.3390/biom12121809
APA StyleYamamoto, T., Randriantsilefisoa, R., Sprecher, C. M., & D’Este, M. (2022). Fabrication of Collagen–Hyaluronic Acid Cryogels by Directional Freezing Mimicking Cartilage Arcade-like Structure. Biomolecules, 12(12), 1809. https://doi.org/10.3390/biom12121809




