Metabolic Profiling of Chestnut Shell (Castanea crenata) Cultivars Using UPLC-QTOF-MS and Their Antioxidant Capacity
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Plant Materials
2.3. Sample Preparation
2.4. Mass Spectrometry Analysis and Data Processing
2.5. In Vitro Activities of the Chestnut Shell Extracts
2.5.1. Preparation of the Extracts
2.5.2. Total Phenolic Content (TPC)
2.5.3. 2,2-Diphenylpicrylhydrazyl (DPPH) Radical Scavenging Assay
2.5.4. Ferric Reducing Antioxidant Power (FRAP) Assay
2.5.5. Determination of Intracellular ROS Scavenging Activity
2.6. Statistical Methods
3. Results and Discussion
3.1. The Metabolic Composition of Whole C. crenata Shells
3.2. Differences in Metabolite Levels Associated with Whole C. crenata Shells
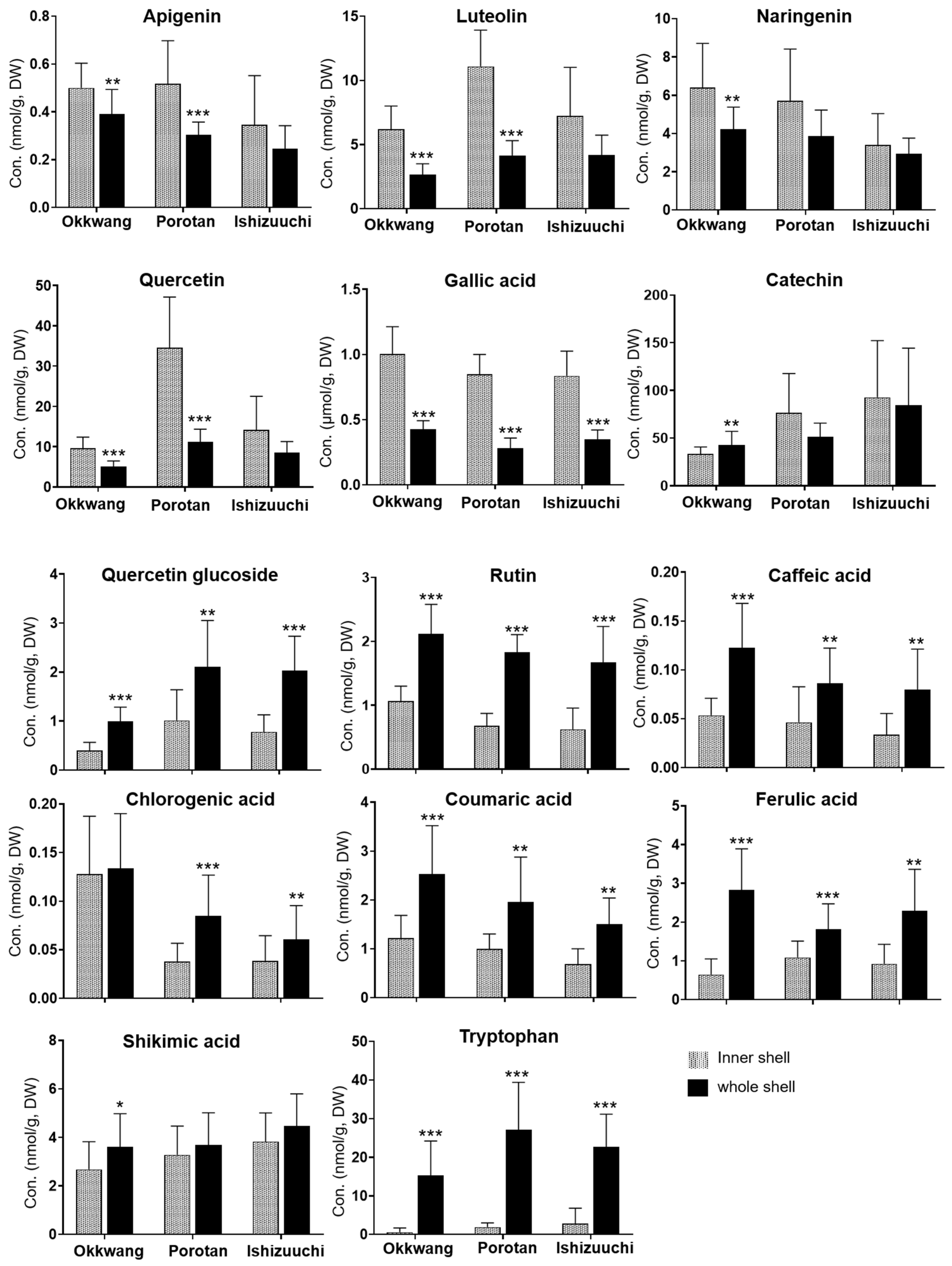
3.3. Metabolite Quantification in Inner and Whole Shells of C. crenata
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cravotto, C.; Grillo, G.; Binello, A.; Gallina, L.; Olivares-Vicente, M.; Herranz-Lopez, M.; Micol, V.; Barrajon-Catalan, E.; Cravotto, G. Bioactive Antioxidant Compounds from Chestnut Peels through Semi-Industrial Subcritical Water Extraction. Antioxidants 2022, 11, 988. [Google Scholar] [CrossRef] [PubMed]
- Korea Statistical Information Service. Available online: http://kosis.kr (accessed on 30 September 2022).
- Jeong, H.; Jo, Y.; Jeong, J.; Jin, D.; Song, B.; Jin, Y.; Kim, M.; Lee, U.; Heo, H. Change in the chemical composition of chest-nuts (Castanea crenata) from different Periods. Korean J. Food Sci. Technol. 2012, 44, 393–400. [Google Scholar] [CrossRef] [Green Version]
- Vella, F.M.; Laratta, B.; La Cara, F.; Morana, A. Recovery of bioactive molecules from chestnut (Castanea sativa Mill.) by-products through extraction by different solvents. Nat. Prod. Res. 2018, 32, 1022–1032. [Google Scholar] [CrossRef] [PubMed]
- Cerulli, A.; Napolitano, A.; Masullo, M.; Hosek, J.; Pizza, C.; Piacente, S. Chestnut shells (Italian cultivar “Marrone di Roccadaspide” PGI): Antioxidant activity and chemical investigation with in depth LC-HRMS/MS(n) rationalization of tannins. Food Res. Int. 2020, 129, 108787. [Google Scholar] [CrossRef]
- Jeong, H.R.; Jo, Y.N.; Jeong, J.H.; Jin, D.E.; Song, B.G.; Choi, S.J.; Shin, D.H.; Heo, H.J. Antiamnesic effects of ethyl acetate fraction from chestnut (Castanea crenata var. dulcis) inner skin on Abeta(25-35)-induced cognitive deficits in mice. J. Med. Food 2012, 15, 1051–1056. [Google Scholar] [CrossRef] [Green Version]
- Kang, H. Inhibition of lipopolysaccharide-induced neuroinflammatory events in Bv-2 microglia by chestnut peel extract. Trop. J. Pharm. Res. 2014, 13, 1615. [Google Scholar] [CrossRef] [Green Version]
- Mizutani, T.; Shizuka, F.; Matsuzawa, T.; Amano, Y.; Arikawa, Y. Anti-glycation activity of Japanese Chestnut (Castanea crenata) inner skin extract is beneficial for Type 2 Diabetes in a rat model. J. Anti-Aging Med. 2014, 10, 112–119. [Google Scholar]
- Noh, J.R.; Gang, G.T.; Kim, Y.H.; Yang, K.J.; Hwang, J.H.; Lee, H.S.; Oh, W.K.; Song, K.S.; Lee, C.H. Antioxidant effects of the chestnut (Castanea crenata) inner shell extract in t-BHP-treated HepG2 cells, and CCl4- and high-fat diet-treated mice. Food Chem. Toxicol. 2010, 48, 3177–3183. [Google Scholar] [CrossRef]
- Jung, B.S.; Lee, N.K.; Na, D.S.; Yu, H.H.; Paik, H.D. Comparative analysis of the antioxidant and anticancer activities of chestnut inner shell extracts prepared with various solvents. J. Sci. Food Agric. 2016, 96, 2097–2102. [Google Scholar] [CrossRef]
- Sorice, A.; Siano, F.; Capone, F.; Guerriero, E.; Picariello, G.; Budillon, A.; Ciliberto, G.; Paolucci, M.; Costantini, S.; Volpe, M.G. Potential Anticancer Effects of Polyphenols from Chestnut Shell Extracts: Modulation of Cell Growth, and Cytokinomic and Metabolomic Profiles. Molecules 2016, 21, 1411. [Google Scholar] [CrossRef] [Green Version]
- Zhang, H.; Luo, X.; Ke, J.; Duan, Y.; He, Y.; Zhang, D.; Cai, M.; Sun, G.; Sun, X. Procyanidins, from Castanea mollissima Bl. shell, induces autophagy following apoptosis associated with PI3K/AKT/mTOR inhibition in HepG2 cells. Biomed. Pharmacother. 2016, 81, 15–24. [Google Scholar] [CrossRef] [PubMed]
- Fei, W.; Xuan, Y.; Jian, X.; Yue, W.; Yuejun, Y.; Yu, J.; Huifang, X.; Yuancai, L.; Yifu, Y.; Xiangwei, Z. One New Phenolic Compound from Castanea mollissima Shells and its Suppression of HepatomaCell Proliferation and Inflammation by Inhibiting NF-kappaB Pathway. Int. J. Mol. Sci. 2019, 20, 466. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rodrigues, F.; Santos, J.; Pimentel, F.B.; Braga, N.; Palmeira-de-Oliveira, A.; Oliveira, M.B.P. Promising new applications of Castanea sativa shell: Nutritional composition, antioxidant activity, amino acids and vitamin E profile. Food Funct. 2015, 6, 2854–2860. [Google Scholar] [CrossRef] [PubMed]
- Squillaci, G.; Apone, F.; Sena, L.M.; Carola, A.; Tito, A.; Bimonte, M.; Lucia, A.D.; Colucci, G.; Cara, F.L.; Morana, A. Chestnut (Castanea sativa Mill.) industrial wastes as a valued bioresource for the production of active ingredients. Process Biochem. 2018, 64, 228–236. [Google Scholar] [CrossRef]
- Dettmer, K.; Aronov, P.A.; Hammock, B.D. Mass spectrometry-based metabolomics. Mass Spectrom. Rev. 2007, 26, 51–78. [Google Scholar] [CrossRef]
- Sheth, B.P.; Thaker, V.S. Plant systems biology: Insights, advances and challenges. Planta 2014, 240, 33–54. [Google Scholar] [CrossRef]
- Kiefer, P.; Portais, J.C.; Vorholt, J.A. Quantitative metabolome analysis using liquid chromatography-high-resolution mass spectrometry. Anal. Biochem. 2008, 382, 94–100. [Google Scholar] [CrossRef]
- Kim, M.S.; Jin, J.S.; Kwak, Y.S.; Hwang, G.S. Metabolic Response of Strawberry (Fragaria × ananassa) Leaves Exposed to the Angular Leaf Spot Bacterium (Xanthomonas fragariae). J. Agric. Food Chem. 2016, 64, 1889–1898. [Google Scholar] [CrossRef]
- Kim, M.; Lee, U.; Kim, S.; Hwang, M.; Lee, M. Comparison of Nut Characteristics between Korean Native Chestnut Accessions and Prevailing Cultivars Cultivated in Korea. In III International Chestnut Congress; International Society for Horticultural Science: Chaves, Portugal, 2004; Volume 69, pp. 299–304. [Google Scholar]
- Seo, D.-J.; Chung, M.-J.; Kim, D.-J.; You, J.-K.; Choe, M. Nutritional Constituent Analysis of Korean Chestnuts. J. Korean Soc. Food Sci. Nutr. 2009, 38, 166–176. [Google Scholar] [CrossRef]
- Woldegiorgis, A.Z.; Abate, D.; Haki, G.D.; Ziegler, G.R. Antioxidant property of edible mushrooms collected from Ethiopia. Food Chem. 2014, 157, 30–36. [Google Scholar] [CrossRef]
- Bak, M.J.; Jeong, W.S.; Kim, K.B. Detoxifying effect of fermented black ginseng on H2O2-induced oxidative stress in HepG2 cells. Int. J. Mol. Med. 2014, 34, 1516–1522. [Google Scholar] [CrossRef] [PubMed]
- METLIN. Available online: https://metlin.scripps.edu/ (accessed on 30 September 2022).
- Salminen, J.P.; Karonen, M.; Sinkkonen, J. Chemical ecology of tannins: Recent developments in tannin chemistry reveal new structures and structure-activity patterns. Chemistry 2011, 17, 2806–2816. [Google Scholar] [CrossRef] [PubMed]
- Sanz, M.; Cadahia, E.; Esteruelas, E.; Muñoz, A.N.M.; Fernandez de Simon, B.; Hernandez, T.; Estrella, I. Phenolic compounds in chestnut (Castanea sativa Mill.) heartwood. Effect of toasting at cooperage. J. Agric. Food Chem. 2010, 58, 9631–9640. [Google Scholar] [CrossRef] [PubMed]
- Moilanen, J.; Sinkkonen, J.; Salminen, J.-P. Characterization of bioactive plant ellagitannins by chromatographic, spectroscopic and mass spectrometric methods. Chemoecology 2013, 23, 165–179. [Google Scholar] [CrossRef]
- Campo, M.; Pinelli, P.; Romani, A. Hydrolyzable Tannins from Sweet Chestnut Fractions Obtained by a Sustainable and Eco-friendly Industrial Process. Nat. Prod. Commun. 2015, 11, 409–415. [Google Scholar] [CrossRef] [Green Version]
- Sangiovanni, E.; Piazza, S.; Vrhovsek, U.; Fumagalli, M.; Khalilpour, S.; Masuero, D.; Di Lorenzo, C.; Colombo, L.; Mattivi, F.; De Fabiani, E.; et al. A bio-guided approach for the development of a chestnut-based proanthocyanidin-enriched nutraceutical with potential anti-gastritis properties. Pharmacol. Res. 2018, 134, 145–155. [Google Scholar] [CrossRef]
- Rauf, A.; Imran, M.; Abu-Izneid, T.; Iahtisham Ul, H.; Patel, S.; Pan, X.; Naz, S.; Sanches Silva, A.; Saeed, F.; Rasul Suleria, H.A. Proanthocyanidins: A comprehensive review. Biomed. Pharmacother. 2019, 116, 108999. [Google Scholar] [CrossRef]
- Ferreira, D.; Slade, D. Oligomeric proanthocyanidins: Naturally occurring O-heterocycles. Nat. Prod. Rep. 2002, 19, 517–541. [Google Scholar] [CrossRef]
- Jaiswal, R.; Jayasinghe, L.; Kuhnert, N. Identification and characterization of proanthocyanidins of 16 members of the Rhododendron genus (Ericaceae) by tandem LC-MS. J. Mass Spectrom. 2012, 47, 502–515. [Google Scholar] [CrossRef]
- Seleem, D.; Pardi, V.; Murata, R.M. Review of flavonoids: A diverse group of natural compounds with anti-Candida albicans activity in vitro. Arch. Oral Biol. 2017, 76, 76–83. [Google Scholar] [CrossRef]
- Sánchez-Rabaneda, F.; Jáuregui, O.; Lamuela-Raventós, R.M.; Viladomat, F.; Bastida, J.; Codina, C. Qualitative analysis of phenolic compounds in apple pomace using liquid chromatography coupled to mass spectrometry in tandem mode. Rapid Commun. Mass Spectrom. 2004, 18, 553–563. [Google Scholar] [CrossRef]
- Abu El-Soud, W.; Hegab, M.M.; AbdElgawad, H.; Zinta, G.; Asard, H. Ability of ellagic acid to alleviate osmotic stress on chickpea seedlings. Plant Physiol. Biochem. 2013, 71, 173–183. [Google Scholar] [CrossRef] [PubMed]
- Landete, J.M. Ellagitannins, ellagic acid and their derived metabolites: A review about source, metabolism, functions and health. Food Res. Int. 2011, 44, 1150–1160. [Google Scholar] [CrossRef]
- Al Zahrani, N.A.; El-Shishtawy, R.M.; Asiri, A.M. Recent developments of gallic acid derivatives and their hybrids in medicinal chemistry: A review. Eur. J. Med. Chem. 2020, 204, 112609. [Google Scholar] [CrossRef] [PubMed]
- Shetty, K.; Paliyath, G.; Pometto, A.; Levin, R.E. Food Biotechnology, 2nd ed.; Taylor & Francis: London, UK, 2006. [Google Scholar]
- Ciucure, C.T.; Geana, E.-I.; Sandru, C.; Tita, O.; Botu, M. Phytochemical and Nutritional Profile Composition in Fruits of Different Sweet Chestnut (Castanea sativa Mill.) Cultivars Grown in Romania. Separations 2022, 9, 66. [Google Scholar] [CrossRef]
- Xu, Z.; Meenu, M.; Chen, P.; Xu, B. Comparative Study on Phytochemical Profiles and Antioxidant Capacities of Chestnuts Produced in Different Geographic Area in China. Antioxidants 2020, 9, 190. [Google Scholar] [CrossRef] [Green Version]
- Yang, F.; Liu, Q.; Pan, S.; Xu, C.; Xiong, Y.L. Chemical composition and quality traits of Chinese chestnuts (Castanea mollissima) produced in different ecological regions. Food Biosci. 2015, 11, 33–42. [Google Scholar] [CrossRef]
- Nie, H.; Chen, H.; Li, G.; Su, K.; Song, M.; Duan, Z.; Li, X.; Cao, X.; Huang, J.; Huang, S.; et al. Comparison of flavonoids and phenylpropanoids compounds in Chinese water chestnut processed with different methods. Food Chem. 2021, 335, 127662. [Google Scholar] [CrossRef]



| No | Compound | Rt (min) | Ionization Mode | Molecular Formula | Observed Precursor Ions (m/z) | Difference (ppm) | Product Ions (m/z) |
|---|---|---|---|---|---|---|---|
| Ellagitannins | |||||||
| 1 | HHDP-glucose | 2.02 | [M−H]− | C20H18O14 | 481.0627 | 1.87 | 300, 275 |
| 2 | Galloyl-HHDP-glucose | 2.75 | [M−H]− | C27H22O18 | 633.0721 | −0.95 | 481, 463, 300, 275 |
| 3 | NHTP-HHDP-glucose | 2.76 | [M−H]− | C41H26O26 | 933.0625 | −0.96 | 915, 631, 613, 425, 301 |
| 4 | Bis-HHDP-glucose | 2.91 | [M−H]− | C34H24O22 | 783.0662 | −2.43 | 765, 481, 301, 275 |
| 5 | Bis-HHDP-glucose | 2.93 | [M−H]− | C34H24O22 | 783.0674 | −0.89 | 765, 481, 301, 275 |
| 6 | HHDP-valoneoyl-glucose | 3.17 | [M−H]− | C41H28O27 | 951.0723 | −1.68 | 907, 783, 465, 301 |
| 7 | Galloyl-HHDP-glucose | 3.20 | [M−H]− | C27H22O18 | 633.0718 | −1.42 | 481, 463, 300, 275 |
| 8 | Digalloyl-HHDP-glucose | 3.62 | [M−H]− | C34H26O22 | 785.0829 | −1.02 | 633, 615, 463, 301, 275 |
| 9 | Trigalloyl-HHDP-glucose | 3.95 | [M−H]− | C41H30O26 | 937.0946 | −0.11 | 767, 633, 617, 465, 301 |
| Proanthocyanidinsa | |||||||
| 10 | GC-GC-C B-type trimer | 2.47 | [M−H]− | C45H38O20 | 897.1866 | −1.34 | 729, 711, 425, 407, 303, 289 |
| 11 | GC-GC-C B-type trimer | 2.61 | [M−H]− | C45H38O20 | 897.1861 | −1.89 | 729, 711, 425, 407, 303, 289 |
| 12 | GC-GC-GC B-type trimer | 2.62 | [M−H]− | C45H38O21 | 913.1799 | −3.07 | 727, 559, 423, 305, 303 |
| 13 | GC-GC-GC B-type trimer | 2.67 | [M−H]− | C45H38O21 | 913.1822 | −0.55 | 727, 559, 423, 305, 303 |
| 14 | GC-GC B-type dimer | 2.76 | [M−H]− | C30H26O14 | 609.1232 | −1.97 | 591, 483, 441, 423, 305 |
| 15 | C-C-C B-type trimer | 2.82 | [M−H]− | C45H38O18 | 865.1974 | −0.58 | 847, 713, 695, 577, 425, 407, 289, 287 |
| 16 | GC-GC-GC B-type trimer | 2.89 | [M−H]− | C45H38O21 | 913.1827 | 0.00 | 727, 559, 423, 305, 303 |
| 17 | GC-GC B-type dimer | 3.16 | [M−H]− | C30H26O14 | 609.1238 | −0.99 | 591, 483, 441, 423, 305 |
| 18 | C-C B-type dimer | 3.24 | [M−H]− | C30H26O12 | 577.1345 | −0.17 | 425, 407, 289, 245, 125 |
| 19 | C-C-C B-type trimer | 3.46 | [M−H]− | C45H38O18 | 865.1967 | −1.39 | 847, 713, 695, 577, 425, 407, 289, 287 |
| 20 | C(G)-C B-type dimer | 3.77 | [M−H]− | C37H30O16 | 729.1446 | −1.23 | 577, 559, 451, 425, 407, 289, 287 |
| 21 | C-C B-type dimer | 3.78 | [M−H]− | C30H26O12 | 577.1349 | 0.52 | 425, 407, 289, 245, 125 |
| 22 | C-C B-type dimer | 3.95 | [M−H]− | C30H26O12 | 577.1341 | −0.87 | 425, 407, 289, 245, 125 |
| 23 | C(G)-C B-type dimer | 4.36 | [M−H]− | C37H30O16 | 729.1487 | 4.39 | 577, 559, 451, 425, 407, 289, 287 |
| Flavonoids | |||||||
| 24 | Epigallocatechin | 2.93 | [M−H]− | C15H14O7 | 305.0652 | −2.95 | 261, 179, 167 |
| 25 | Catechin * | 3.50 | [M−H]− | C15H14O6 | 289.0717 | 1.73 | 245, 221, 203 |
| 26 | Myricetin hexose | 4.03 | [M−H]− | C21H20O13 | 479.0833 | 1.67 | 317 |
| 27 | Rutin * | 4.22 | [M−H]− | C27H30O16 | 609.1472 | 2.79 | 301, 273 |
| 28 | Quercetin glucoside * | 4.35 | [M−H]− | C21H20O12 | 463.0882 | 1.30 | 301, 271 |
| 29 | Myricetin | 5.01 | [M−H]− | C15H10O8 | 317.031 | 4.10 | 151 |
| 30 | Naringenin glucoside | 5.14 | [M−H]− | C21H22O10 | 433.1138 | 0.92 | 271, 151, 119 |
| 31 | Narigenin * | 5.31 | [M+H]+ | C15H12O5 | 273.0749 | −5.13 | 153, 119 |
| 32 | Kaempferol rutinoside | 5.71 | [M−H]− | C27H30O15 | 593.1507 | 0.17 | 285 |
| 33 | Luteolin * | 5.84 | [M+H]+ | C15H10O6 | 287.0546 | −3.14 | 153 |
| 34 | Quercetin * | 5.86 | [M−H]− | C15H10O7 | 301.0354 | 1.99 | 178, 151 |
| 35 | Kaempferol coumaroyl hexose | 5.86 | [M+H]+ | C30H26O13 | 595.1457 | 1.01 | 309, 287 |
| 36 | Eriodictyol | 5.89 | [M+H]+ | C15H12O6 | 289.0695 | −5.88 | 153, 135 |
| 37 | Kaempferol | 6.01 | [M+H]+ | C15H10O6 | 287.0552 | −1.05 | 165, 153 |
| 38 | Naringin | 6.62 | [M−H]− | C27H32O14 | 579.1710 | −0.52 | 459, 271 |
| 39 | Apigenin * | 6.69 | [M−H]− | C15H10O5 | 269.0468 | 6.69 | 151, 117 |
| 40 | Isorhamnetin | 7.17 | [M−H]− | C16H12O7 | 315.0532 | 8.89 | 300, 151 |
| Ellagic acid derivatives | |||||||
| 41 | Ellagic acid hexose | 3.76 | [M−H]− | C20H16O13 | 463.0513 | 0.22 | 301 |
| 42 | Ellagic acid pentose | 4.07 | [M−H]− | C19H14O12 | 433.0427 | 4.62 | 301 |
| 43 | Ellagic acid deoxyhexose | 4.19 | [M−H]− | C20H16O12 | 447.058 | 3.80 | 301 |
| 44 | Ellagic acid | 4.34 | [M−H]− | C14H6O8 | 300.9994 | 3.32 | 257, 229 |
| 45 | Methylellagic acid | 4.95 | [M−H]− | C15H8O8 | 315.0151 | 3.49 | 300 |
| 46 | Dimethylellagic acid | 6.18 | [M−H]− | C16H10O8 | 329.0295 | −0.61 | 314, 299 |
| 47 | Dimethylellagic acid | 6.31 | [M−H]− | C16H10O8 | 329.0297 | 0.00 | 314, 299 |
| 48 | Trimethylellagic acid | 7.88 | [M−H]− | C17H12O8 | 343.0456 | 0.87 | 328, 299, 284 |
| Gallic acid derivatives | |||||||
| 49 | Galloylglucose | 0.72 | [M−H]− | C13H16O10 | 331.0655 | −3.02 | 169, 125 |
| 50 | Gallic acid | 2.39 | [M−H]− | C7H6O5 | 169.0134 | −1.78 | 125 |
| 51 | Digalloyl glucose | 3.11 | [M−H]− | C20H20O14 | 483.0766 | −1.66 | 465, 331, 313, 169 |
| 52 | Digalloyl glucose | 3.33 | [M−H]− | C20H20O14 | 483.0781 | 1.45 | 465, 331, 313, 169 |
| 53 | Ttrigalloyl glucose | 3.69 | [M−H]− | C27H24O18 | 635.087 | −2.20 | 483, 465, 331, 313 |
| 54 | Trigalloyl glucose | 3.70 | [M−H]− | C27H24O18 | 635.0908 | 3.78 | 483, 465, 331, 313 |
| 55 | Tetragalloyl glucose | 4.15 | [M−H]− | C34H28O22 | 787.1012 | 2.29 | 635, 617, 483, 465, 447, 331 |
| 56 | Tetragalloyl glucose | 4.16 | [M−H]− | C34H28O22 | 787.0999 | 0.64 | 635, 617, 483, 465, 447, 331 |
| Amino acids | |||||||
| 57 | Asparagin * | 0.50 | [M−H]− | C4H8N2O3 | 131.0455 | −0.76 | 114, 95, 70 |
| 58 | Arginine * | 0.54 | [M+H]+ | C6H14N4O2 | 175.1186 | −5.14 | 158, 130, 116 |
| 59 | Proline * | 0.54 | [M+H]+ | C5H9NO2 | 116.0714 | 2.24 | 70 |
| 60 | Glutamate * | 0.54 | [M−H]− | C5H9NO4 | 146.0448 | −3.42 | 128, 102 |
| 61 | Betaine * | 0.57 | [M+H]+ | C5H11NO2 | 118.0858 | −8.47 | 58, 59 |
| 62 | Glutamine * | 0.55 | [M+H]+ | C5H10N2O3 | 147.0758 | −7.48 | 102, 84 |
| 63 | Phenylalanine * | 2.81 | [M+H]+ | C9H11NO2 | 166.0860 | −4.82 | 120, 103 |
| 64 | Tryptophan * | 3.32 | [M+H]+ | C11H12N2O2 | 205.097 | −3.41 | 188, 170, 118 |
| Organic acids | |||||||
| 65 | Fumaric acid * | 0.42 | [M−H]− | C4H4O4 | 115.0032 | 0.87 | 71 |
| 66 | Citric acid * | 0.7 | [M−H]− | C6H8O7 | 191.0198 | 3.66 | 111, 87, 85 |
| 67 | Malic acid * | 0.7 | [M−H]− | C4H6O5 | 133.0133 | −3.01 | 115, 89, 71 |
| Phenolic acid | |||||||
| 68 | Coumaric acid * | 0.39 | [M−H]− | C9H8O3 | 163.0385 | −6.13 | 119, 117, 93 |
| 69 | Caffeic acid * | 0.4 | [M−H]− | C9H8O4 | 179.0348 | 2.23 | 135, 134, 107 |
| 70 | Quinic acid * | 0.61 | [M−H]− | C7H12O6 | 191.055 | −2.62 | 173, 127, 93, 85 |
| 71 | Salicylic acid * | 3.59 | [M+H]+ | C7H6O3 | 139.0387 | −5.75 | 121, 93 |
| 72 | Ferulic acid | 3.84 | [M+H]+ | C10H10O4 | 195.0646 | −5.64 | 177 |
| 73 | Phloretin | 6.6 | [M−H]− | C15H14O5 | 273.0777 | 5.13 | 179, 167 |
| Samples | Total Phenol Content (mg GAE/g) a | DPPH Free Radical Scavenging Activity IC50 (mg/L) | FRAP Value (mmol Fe/g Dry Weight) (%) |
|---|---|---|---|
| Okkwang | 32.57 ± 6.06 | 42.23 ± 9.61 | 6.70 ± 1.28 |
| Daebo | 25.32 ± 5.77 | 53.76 ± 16.10 | 5.16 ± 1.19 |
| Riheiguri | 22.27 ± 7.38 | 57.52 ± 13.11 | 4.65 ± 1.42 |
| Porotan | 35.55 ± 3.91 | 40.29 ± 5.69 | 7.35 ± 1.13 |
| Ishizuuchi | 44.80 ± 8.59 | 29.64 ± 9.83 | 9.23 ± 1.78 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nam, M.; Yu, J.M.; Park, Y.R.; Kim, Y.-S.; Kim, J.-H.; Kim, M.-S. Metabolic Profiling of Chestnut Shell (Castanea crenata) Cultivars Using UPLC-QTOF-MS and Their Antioxidant Capacity. Biomolecules 2022, 12, 1797. https://doi.org/10.3390/biom12121797
Nam M, Yu JM, Park YR, Kim Y-S, Kim J-H, Kim M-S. Metabolic Profiling of Chestnut Shell (Castanea crenata) Cultivars Using UPLC-QTOF-MS and Their Antioxidant Capacity. Biomolecules. 2022; 12(12):1797. https://doi.org/10.3390/biom12121797
Chicago/Turabian StyleNam, Miso, Ja Myung Yu, Young Ran Park, Young-Sik Kim, Jae-Ho Kim, and Min-Sun Kim. 2022. "Metabolic Profiling of Chestnut Shell (Castanea crenata) Cultivars Using UPLC-QTOF-MS and Their Antioxidant Capacity" Biomolecules 12, no. 12: 1797. https://doi.org/10.3390/biom12121797
APA StyleNam, M., Yu, J. M., Park, Y. R., Kim, Y.-S., Kim, J.-H., & Kim, M.-S. (2022). Metabolic Profiling of Chestnut Shell (Castanea crenata) Cultivars Using UPLC-QTOF-MS and Their Antioxidant Capacity. Biomolecules, 12(12), 1797. https://doi.org/10.3390/biom12121797




