IL-18BP Improves Early Graft Function and Survival in Lewis–Brown Norway Rat Orthotopic Liver Transplantation Model
Abstract
:1. Introduction
2. Materials and Methods
2.1. Adeno-Associated Virus-8 (AAV-8) Construction
2.2. Animals
2.3. Tail Vein Injection of AAV-8
2.4. Surgery for Organ Transplantation
2.5. Acquisition of Blood and Tissue
2.6. Serum Analysis
2.7. Histologic and Immunohistochemical Analyses
2.8. Quantitative Real-Time PCR (qRT-PCR) Analysis
2.9. Western Blotting Analysis
2.10. Statistical Analysis
3. Results
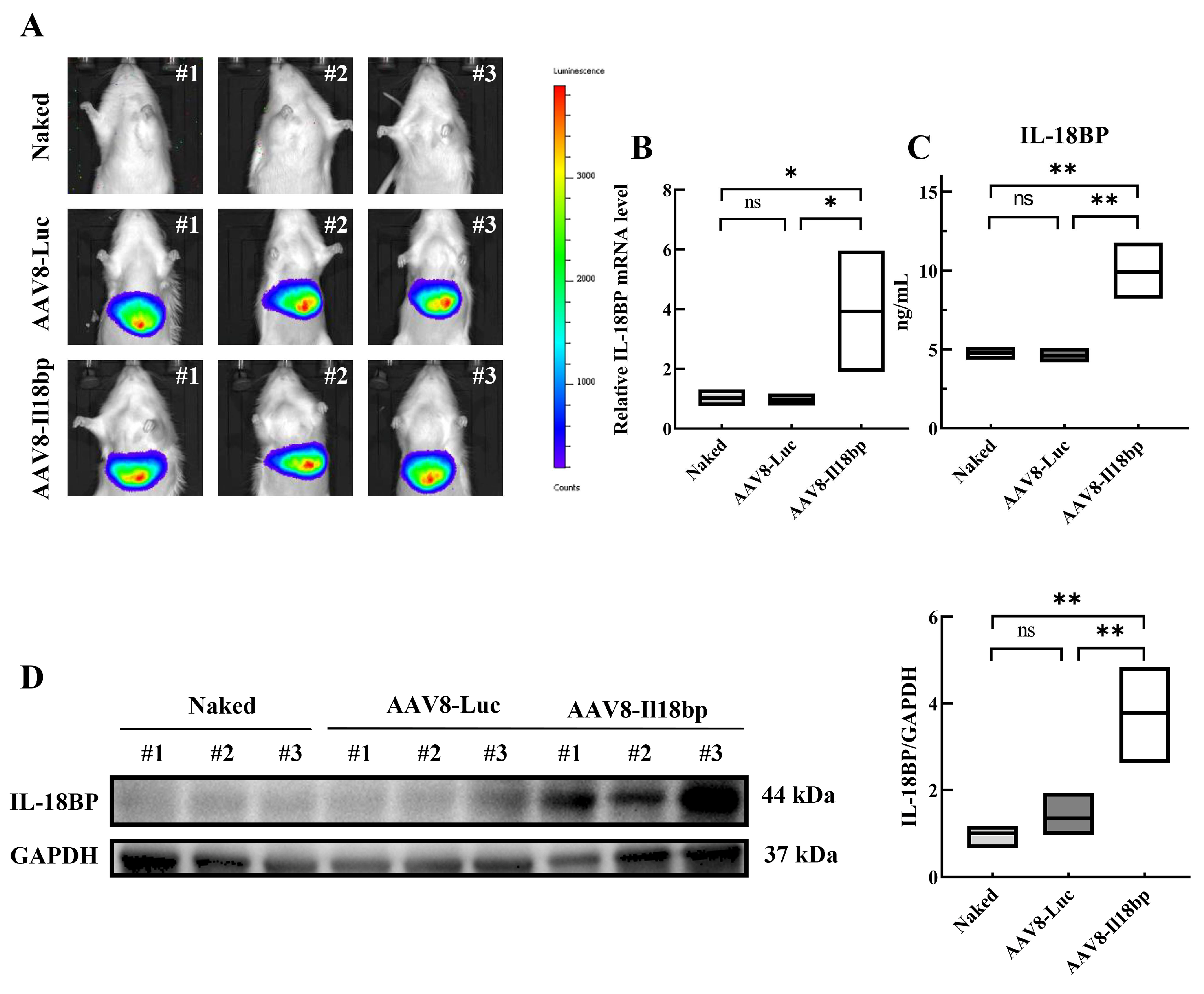
3.1. AAV8-Il18bp Treatment Caused Specific IL-18BP Overexpression in the Liver of LEW Rats
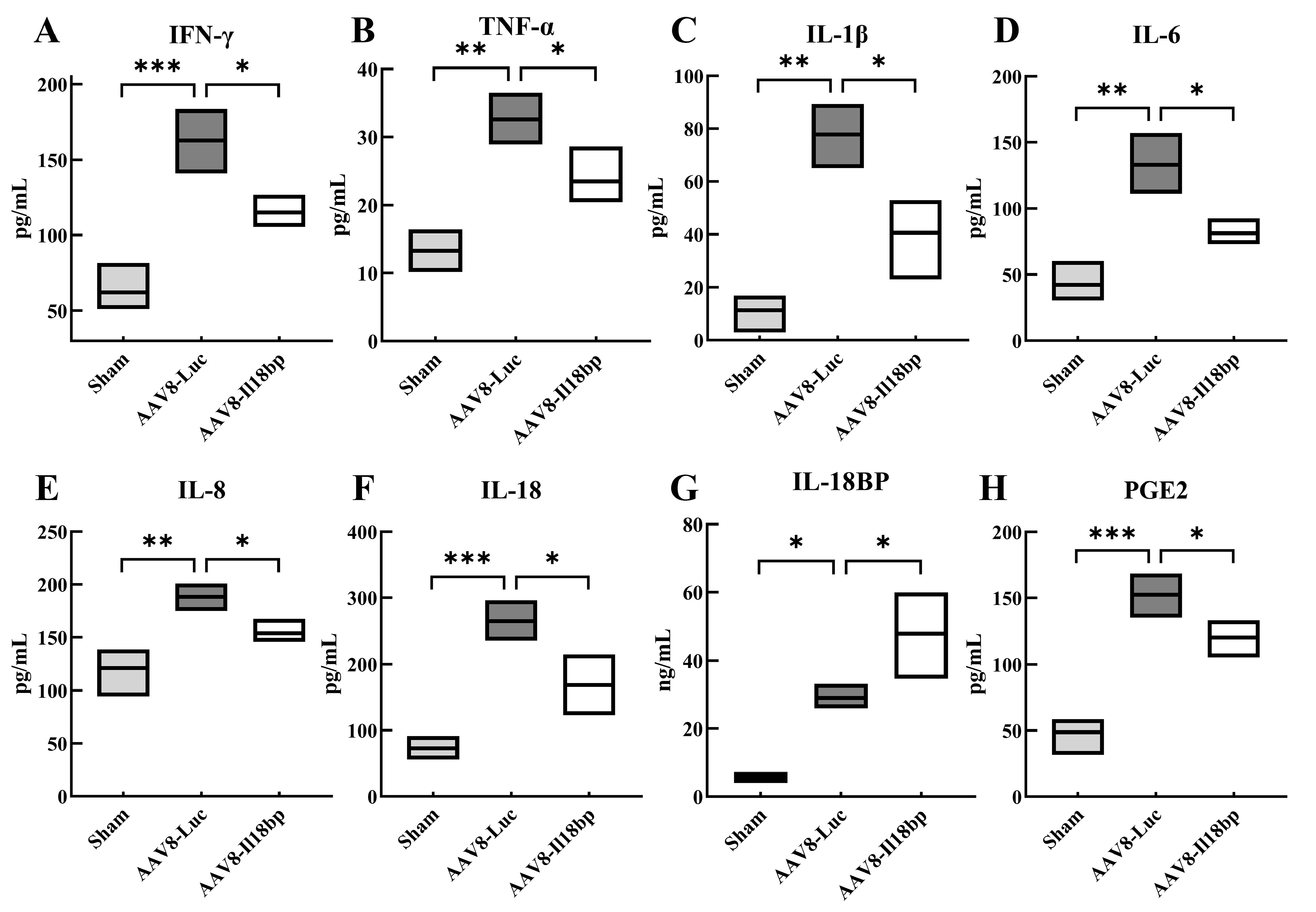
3.2. Levels of Inflammatory Factors in Groups with Different Treatment
3.3. Increased IL-18BP Levels Protected Liver Function from Immune Rejection after LEW-BN OLTx
3.4. Elevated IL-18BP Levels Protected the Liver from Apoptosis after LEW-BN OLTx
3.5. IL-18BP Overexpression Reduced CD56 Expression after LEW-BN OLTx
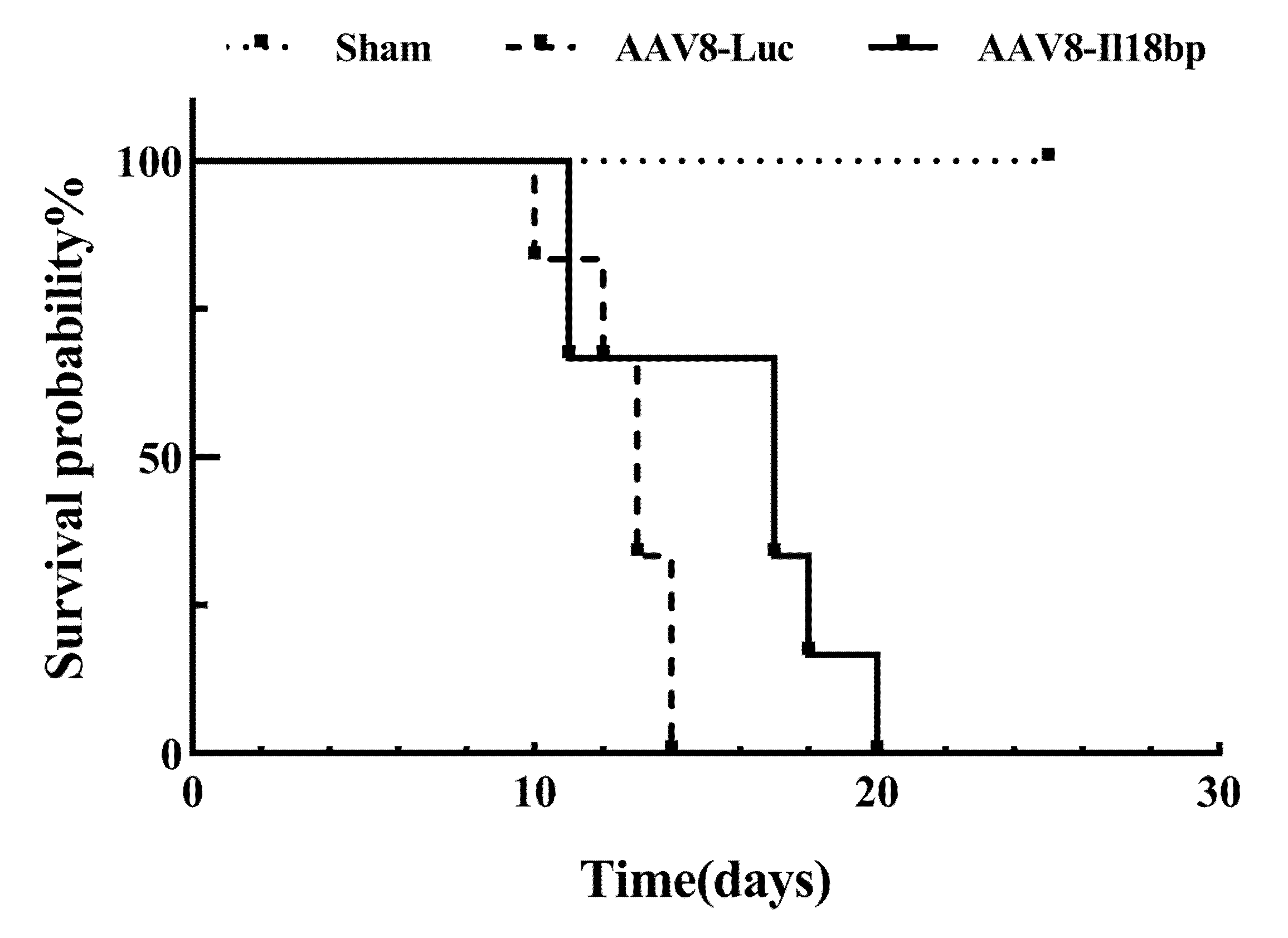
3.6. High Level of IL-18BP Prolonged Survival Time of the Recipients after OLTx
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Samuel, D.; Coilly, A. Management of patients with liver diseases on the waiting list for transplantation: A major impact to the success of liver transplantation. BMC Med. 2018, 16, 113. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lu, T.; Yang, B.; Wang, R.; Qin, C. Xenotransplantation: Current Status in Preclinical Research. Front. Immunol. 2019, 10, 3060. [Google Scholar] [CrossRef]
- Zhang, X.; Li, X.; Yang, Z.; Tao, K.; Wang, Q.; Dai, B.; Qu, S.; Peng, W.; Zhang, H.; Cooper, D.K.C.; et al. A review of pig liver xenotransplantation: Current problems and recent progress. Xenotransplantation 2019, 26, e12497. [Google Scholar] [CrossRef] [PubMed]
- Hein, R.; Sake, H.J.; Pokoyski, C.; Hundrieser, J.; Brinkmann, A.; Baars, W.; Nowak-Imialek, M.; Lucas-Hahn, A.; Figueiredo, C.; Schuberth, H.J.; et al. Triple (GGTA1, CMAH, B2M) modified pigs expressing an SLA class I(low) phenotype-Effects on immune status and susceptibility to human immune responses. Am. J. Transpl. 2020, 20, 988–998. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bongoni, A.K.; Kiermeir, D.; Schnider, J.; Jenni, H.; Garimella, P.; Bähr, A.; Klymiuk, N.; Wolf, E.; Ayares, D.; Voegelin, E.; et al. Transgenic Expression of Human CD46 on Porcine Endothelium: Effect on Coagulation and Fibrinolytic Cascades During Ex Vivo Human-to-Pig Limb Xenoperfusions. Transplantation 2015, 99, 2061–2069. [Google Scholar] [CrossRef]
- Matsunami, K.; Miyagawa, S.; Nakai, R.; Murase, A.; Shirakura, R. The possible use of HLA-G1 and G3 in the inhibition of NK cell-mediated swine endothelial cell lysis. Clin. Exp. Immunol. 2001, 126, 165–172. [Google Scholar] [CrossRef]
- Cooper, D.K.C.; Hara, H.; Iwase, H.; Yamamoto, T.; Jagdale, A.; Kumar, V.; Mannon, R.B.; Hanaway, M.J.; Anderson, D.J.; Eckhoff, D.E. Clinical Pig Kidney Xenotransplantation: How Close Are We? J. Am. Soc. Nephrol. JASN 2020, 31, 12–21. [Google Scholar] [CrossRef]
- Xu, H.; Gundry, S.R.; Hancock, W.W.; Matsumiya, G.; Zuppan, C.W.; Morimoto, T.; Slater, J.; Bailey, L.L. Prolonged discordant xenograft survival and delayed xenograft rejection in a pig-to-baboon orthotopic cardiac xenograft model. J. Thorac. Cardiovasc. Surg. 1998, 115, 1342–1349. [Google Scholar] [CrossRef] [Green Version]
- Quan, D.; Bravery, C.; Chavez, G.; Richards, A.; Cruz, G.; Copeman, L.; Atkinson, C.; Holmes, B.; Davies, H.; Cozzi, E.; et al. Identification, detection, and in vitro characterization of cynomolgus monkey natural killer cells in delayed xenograft rejection of hDAF transgenic porcine renal xenografts. Transpl. Proc. 2000, 32, 936–937. [Google Scholar] [CrossRef]
- Lin, Y.; Vandeputte, M.; Waer, M. Natural killer cell- and macrophage-mediated rejection of concordant xenografts in the absence of T and B cell responses. J. Immunol. 1997, 158, 5658–5667. [Google Scholar]
- Horvath-Arcidiacono, J.A.; Porter, C.M.; Bloom, E.T. Human NK cells can lyse porcine endothelial cells independent of their expression of Galalpha(1,3)-Gal and killing is enhanced by activation of either effector or target cells. Xenotransplantation 2006, 13, 318–327. [Google Scholar] [CrossRef]
- Harmon, C.; Sanchez-Fueyo, A.; O’Farrelly, C.; Houlihan, D.D. Natural Killer Cells and Liver Transplantation: Orchestrators of Rejection or Tolerance? Am. J. Transpl. 2016, 16, 751–757. [Google Scholar] [CrossRef]
- Okamura, H.; Tsutsi, H.; Komatsu, T.; Yutsudo, M.; Hakura, A.; Tanimoto, T.; Torigoe, K.; Okura, T.; Nukada, Y.; Hattori, K.; et al. Cloning of a new cytokine that induces IFN-gamma production by T cells. Nature 1995, 378, 88–91. [Google Scholar] [CrossRef]
- Huang, C.; Wang, Y.; Li, X.; Ren, L.; Zhao, J.; Hu, Y.; Zhang, L.; Fan, G.; Xu, J.; Gu, X.; et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 2020, 395, 497–506. [Google Scholar] [CrossRef] [Green Version]
- Novick, D.; Elbirt, D.; Miller, G.; Dinarello, C.A.; Rubinstein, M.; Sthoeger, Z.M. High circulating levels of free interleukin-18 in patients with active SLE in the presence of elevated levels of interleukin-18 binding protein. J. Autoimmun. 2010, 34, 121–126. [Google Scholar] [CrossRef]
- Novick, D.; Kim, S.H.; Fantuzzi, G.; Reznikov, L.L.; Dinarello, C.A.; Rubinstein, M. Interleukin-18 binding protein: A novel modulator of the Th1 cytokine response. Immunity 1999, 10, 127–136. [Google Scholar] [CrossRef] [Green Version]
- Kim, S.H.; Eisenstein, M.; Reznikov, L.; Fantuzzi, G.; Novick, D.; Rubinstein, M.; Dinarello, C.A. Structural requirements of six naturally occurring isoforms of the IL-18 binding protein to inhibit IL-18. Proc. Natl. Acad. Sci. USA 2000, 97, 1190–1195. [Google Scholar] [CrossRef] [Green Version]
- Nold-Petry, C.A.; Lehrnbecher, T.; Jarisch, A.; Schwabe, D.; Pfeilschifter, J.M.; Muhl, H.; Nold, M.F. Failure of interferon gamma to induce the anti-inflammatory interleukin 18 binding protein in familial hemophagocytosis. PLoS ONE 2010, 5, e8663. [Google Scholar] [CrossRef] [Green Version]
- Weiss, E.S.; Girard-Guyonvarc’h, C.; Holzinger, D.; de Jesus, A.A.; Tariq, Z.; Picarsic, J.; Schiffrin, E.J.; Foell, D.; Grom, A.A.; Ammann, S.; et al. Interleukin-18 diagnostically distinguishes and pathogenically promotes human and murine macrophage activation syndrome. Blood 2018, 131, 1442–1455. [Google Scholar] [CrossRef]
- Yasin, S.; Fall, N.; Brown, R.A.; Henderlight, M.; Canna, S.W.; Girard-Guyonvarc’h, C.; Gabay, C.; Grom, A.A.; Schulert, G.S. IL-18 as a biomarker linking systemic juvenile idiopathic arthritis and macrophage activation syndrome. Rheumatology 2020, 59, 361–366. [Google Scholar] [CrossRef]
- Chen, O.; Shan, N.; Zhu, X.; Wang, Y.; Ren, P.; Wei, D.; Sun, R. The imbalance of IL-18/IL-18BP in patients with systemic juvenile idiopathic arthritis. Acta Biochim. Biophys. Sin. 2013, 45, 339–341. [Google Scholar] [CrossRef] [PubMed]
- Niu, X.L.; Huang, Y.; Gao, Y.L.; Sun, Y.Z.; Han, Y.; Chen, H.D.; Gao, X.H.; Qi, R.Q. Interleukin-18 exacerbates skin inflammation and affects microabscesses and scale formation in a mouse model of imiquimod-induced psoriasis. Chin. Med. J. 2019, 132, 690–698. [Google Scholar] [CrossRef] [PubMed]
- Girard-Guyonvarc’h, C.; Harel, M.; Gabay, C. The Role of Interleukin 18/Interleukin 18-Binding Protein in Adult-Onset Still’s Disease and Systemic Juvenile Idiopathic Arthritis. J. Clin. Med. 2022, 11, 430. [Google Scholar] [CrossRef] [PubMed]
- Kaplanski, G. Interleukin-18: Biological properties and role in disease pathogenesis. Immunol. Rev. 2018, 281, 138–153. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gabay, C.; Fautrel, B.; Rech, J.; Spertini, F.; Feist, E.; Kötter, I.; Hachulla, E.; Morel, J.; Schaeverbeke, T.; Hamidou, M.A.; et al. Open-label, multicentre, dose-escalating phase II clinical trial on the safety and efficacy of tadekinig alfa (IL-18BP) in adult-onset Still’s disease. Ann. Rheum. Dis. 2018, 77, 840–847. [Google Scholar] [CrossRef]
- Faggioni, R.; Cattley, R.C.; Guo, J.; Flores, S.; Brown, H.; Qi, M.; Yin, S.; Hill, D.; Scully, S.; Chen, C.; et al. IL-18-binding protein protects against lipopolysaccharide- induced lethality and prevents the development of Fas/Fas ligand-mediated models of liver disease in mice. J. Immunol. 2001, 167, 5913–5920. [Google Scholar] [CrossRef] [Green Version]
- Siegmund, B.; Sennello, J.A.; Lehr, H.A.; Senaldi, G.; Dinarello, C.A.; Fantuzzi, G. Frontline: Interferon regulatory factor-1 as a protective gene in intestinal inflammation: Role of TCR gamma delta T cells and interleukin-18-binding protein. Eur. J. Immunol. 2004, 34, 2356–2364. [Google Scholar] [CrossRef]
- Liu, Y.M.; Chen, Y.; Li, J.Z.; Gong, J.P. Up-regulation of Galectin-9 in vivo results in immunosuppressive effects and prolongs survival of liver allograft in rats. Immunol. Lett. 2014, 162, 217–222. [Google Scholar] [CrossRef]
- Wang, D.; Tai, P.W.L.; Gao, G. Adeno-associated virus vector as a platform for gene therapy delivery. Nat. Rev. Drug Discov. 2019, 18, 358–378. [Google Scholar] [CrossRef]
- Newberry, E.P.; Hall, Z.; Xie, Y.; Molitor, E.A.; Bayguinov, P.O.; Strout, G.W.; Fitzpatrick, J.A.J.; Brunt, E.M.; Griffin, J.L.; Davidson, N.O. Liver-Specific Deletion of Mouse Tm6sf2 Promotes Steatosis, Fibrosis, and Hepatocellular Cancer. Hepatology 2021, 74, 1203–1219. [Google Scholar] [CrossRef]
- Vozenilek, A.E.; Blackburn, C.M.R.; Schilke, R.M.; Chandran, S.; Castore, R.; Klein, R.L.; Woolard, M.D. AAV8-mediated overexpression of mPCSK9 in liver differs between male and female mice. Atherosclerosis 2018, 278, 66–72. [Google Scholar] [CrossRef]
- Kamada, N.; Calne, R.Y. Orthotopic liver transplantation in the rat. Technique using cuff for portal vein anastomosis and biliary drainage. Transplantation 1979, 28, 47–50. [Google Scholar] [CrossRef]
- Jiang, J.W.; Ren, Z.G.; Lu, H.F.; Zhang, H.; Li, A.; Cui, G.Y.; Jia, J.J.; Xie, H.Y.; Chen, X.H.; He, Y.; et al. Optimal immunosuppressor induces stable gut microbiota after liver transplantation. World J. Gastroenterol. 2018, 24, 3871–3883. [Google Scholar] [CrossRef]
- Liu, S.F.; Newton, R.; Evans, T.W.; Barnes, P.J. Differential regulation of cyclo-oxygenase-1 and cyclo-oxygenase-2 gene expression by lipopolysaccharide treatment in vivo in the rat. Clin. Sci. 1996, 90, 301–306. [Google Scholar] [CrossRef]
- Anderson, G.D.; Hauser, S.D.; McGarity, K.L.; Bremer, M.E.; Isakson, P.C.; Gregory, S.A. Selective inhibition of cyclooxygenase (COX)-2 reverses inflammation and expression of COX-2 and interleukin 6 in rat adjuvant arthritis. J. Clin. Investig. 1996, 97, 2672–2679. [Google Scholar] [CrossRef] [Green Version]
- Portanova, J.P.; Zhang, Y.; Anderson, G.D.; Hauser, S.D.; Masferrer, J.L.; Seibert, K.; Gregory, S.A.; Isakson, P.C. Selective neutralization of prostaglandin E2 blocks inflammation, hyperalgesia, and interleukin 6 production in vivo. J. Exp. Med. 1996, 184, 883–891. [Google Scholar] [CrossRef] [Green Version]
- Montalvo-Javé, E.E.; Ortega-Salgado, J.A.; Castell, A.; Carrasco-Daza, D.; Jay, D.; Gleason, R.; Muñoz, E.; Montalvo-Arenas, C.; Hernández-Muñoz, R.; Piña, E. Piroxicam and meloxicam ameliorate hepatic oxidative stress and protein carbonylation in Kupffer and sinusoidal endothelial cells promoted by ischemia-reperfusion injury. Transpl. Int. Off. J. Eur. Soc. Organ Transplant. 2011, 24, 489–500. [Google Scholar] [CrossRef]
- Ozturk, H.; Gezici, A.; Ozturk, H. The effect of celecoxib, a selective COX-2 inhibitor, on liver ischemia/reperfusion-induced oxidative stress in rats. Hepatol. Res. Off. J. Jpn. Soc. Hepatol. 2006, 34, 76–83. [Google Scholar] [CrossRef]
- Roskams, T.; De Vos, R.; Van Eyken, P.; Myazaki, H.; Van Damme, B.; Desmet, V. Hepatic OV-6 expression in human liver disease and rat experiments: Evidence for hepatic progenitor cells in man. J. Hepatol. 1998, 29, 455–463. [Google Scholar] [CrossRef]
- Roskams, T.; De Vos, R.; Desmet, V. ‘Undifferentiated progenitor cells’ in focal nodular hyperplasia of the liver. Histopathology 1996, 28, 291–299. [Google Scholar] [CrossRef]
- Van Den Heuvel, M.C.; Slooff, M.J.; Visser, L.; Muller, M.; De Jong, K.P.; Poppema, S.; Gouw, A.S. Expression of anti-OV6 antibody and anti-N-CAM antibody along the biliary line of normal and diseased human livers. Hepatology 2001, 33, 1387–1393. [Google Scholar] [CrossRef] [PubMed]
- Ghayur, T.; Banerjee, S.; Hugunin, M.; Butler, D.; Herzog, L.; Carter, A.; Quintal, L.; Sekut, L.; Talanian, R.; Paskind, M.; et al. Caspase-1 processes IFN-gamma-inducing factor and regulates LPS-induced IFN-gamma production. Nature 1997, 386, 619–623. [Google Scholar] [CrossRef] [PubMed]
- Nakanishi, K.; Yoshimoto, T.; Tsutsui, H.; Okamura, H. Interleukin-18 regulates both Th1 and Th2 responses. Annu. Rev. Immunol. 2001, 19, 423–474. [Google Scholar] [CrossRef] [PubMed]
- Robinson, D.; Shibuya, K.; Mui, A.; Zonin, F.; Murphy, E.; Sana, T.; Hartley, S.B.; Menon, S.; Kastelein, R.; Bazan, F.; et al. IGIF does not drive Th1 development but synergizes with IL-12 for interferon-gamma production and activates IRAK and NFkappaB. Immunity 1997, 7, 571–581. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Suzuki, N.; Chen, N.J.; Millar, D.G.; Suzuki, S.; Horacek, T.; Hara, H.; Bouchard, D.; Nakanishi, K.; Penninger, J.M.; Ohashi, P.S.; et al. IL-1 receptor-associated kinase 4 is essential for IL-18-mediated NK and Th1 cell responses. J. Immunol. 2003, 170, 4031–4035. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ono, S.; Obara, H.; Takayanagi, A.; Tanabe, M.; Kawachi, S.; Itano, O.; Shinoda, M.; Kitago, M.; Hibi, T.; Chiba, T.; et al. Suppressive effects of interleukin-18 on liver function in rat liver allografts. J. Surg. Res. 2012, 176, 293–300. [Google Scholar] [CrossRef]
- Chen, Z.; Chu, D.; Castro-Perez, J.M.; Ni, W.; Zhang, A.; Krsmanovic, M.L.; Xie, D.; Shah, V.; Stout, S.J.; McLaren, D.G.; et al. AAV8-mediated long-term expression of human LCAT significantly improves lipid profiles in hCETP;Ldlr(+/−) mice. J. Cardiovasc. Transl. Res. 2011, 4, 801–810. [Google Scholar] [CrossRef]
- Nathwani, A.C.; Reiss, U.M.; Tuddenham, E.G.; Rosales, C.; Chowdary, P.; McIntosh, J.; Della Peruta, M.; Lheriteau, E.; Patel, N.; Raj, D.; et al. Long-term safety and efficacy of factor IX gene therapy in hemophilia B. N. Engl. J. Med. 2014, 371, 1994–2004. [Google Scholar] [CrossRef] [Green Version]
- Obara, H.; Nagasaki, K.; Hsieh, C.L.; Ogura, Y.; Esquivel, C.O.; Martinez, O.M.; Krams, S.M. IFN-gamma, produced by NK cells that infiltrate liver allografts early after transplantation, links the innate and adaptive immune responses. Am. J. Transpl. 2005, 5, 2094–2103. [Google Scholar] [CrossRef] [Green Version]
- Striz, I.; Krasna, E.; Honsova, E.; Lacha, J.; Petrickova, K.; Jaresova, M.; Lodererova, A.; Bohmova, R.; Valhova, S.; Slavcev, A.; et al. Interleukin 18 (IL-18) upregulation in acute rejection of kidney allograft. Immunol. Lett. 2005, 99, 30–35. [Google Scholar] [CrossRef]
- Vecchié, A.; Bonaventura, A.; Toldo, S.; Dagna, L.; Dinarello, C.A.; Abbate, A. IL-18 and infections: Is there a role for targeted therapies? J. Cell. Physiol. 2021, 236, 1638–1657. [Google Scholar] [CrossRef]
- Lee, J.H.; Cho, D.H.; Park, H.J. IL-18 and Cutaneous Inflammatory Diseases. Int. J. Mol. Sci. 2015, 16, 29357–29369. [Google Scholar] [CrossRef] [Green Version]
- Hu, B.; Ren, J.; Luo, Y.; Keith, B.; Young, R.M.; Scholler, J.; Zhao, Y.; June, C.H. Augmentation of Antitumor Immunity by Human and Mouse CAR T Cells Secreting IL-18. Cell Rep. 2017, 20, 3025–3033. [Google Scholar] [CrossRef] [Green Version]
- Felderhoff-Mueser, U.; Schmidt, O.I.; Oberholzer, A.; Bührer, C.; Stahel, P.F. IL-18: A key player in neuroinflammation and neurodegeneration? Trends Neurosci. 2005, 28, 487–493. [Google Scholar] [CrossRef]




| Gene (Rat) | Forward Primer | Reverse Primer |
|---|---|---|
| GAPDH | GCTGAGTATGTCGTGGAGTCTAC | TTCCACGATGCCAAAGTTGTCAT |
| IL-18BP | CAGAGAAAGAAGTGCCACTGAATG | GGATCCACAAACAGACAGGAGAA |
| IL-18 | TCGTAAACCAGGCTTTCACTTCT | CAGGCAGCTAGGATTACGGAAAG |
| IFN | GCACACTCATTGAAAGCCTAGAA | AGGTGCGATTCGATGACACTTAT |
| TNF | GCACACTCATTGAAAGCCTAGAA | CCTTGAAGAGAACCTGGGAGTAG |
| IL-1β | TGCTAGTGTGTGATGTTCCCATTA | GTCGTTGCTTGTCTCTCCTTGTA |
| IL-6 | TTCCGTTTCTACCTGGAGTTTGT | TTAGGAGAGCATTGGAAGTTGGG |
| IL-10 | GTGACAATAACTGCACCCACTTC | GGGCATCACTTCTACCAGGTAAA |
| COX-2 | CTTCCTCCTGTGGCTGATGACTG | GGTCCTCGCTTCTGATCTGTCTTG |
| Western Blotting Antibody | Company | Dilution |
|---|---|---|
| IL-18BP | Abcam (ab52914) | 1:5000 |
| Bax | Proteintech Group (60267-1-Ig) | 1:5000 |
| Bcl-2 | Proteintech Group (60178-1-Ig) | 1:1000 |
| Caspase-3 | Proteintech Group (66470-2-Ig) | 1:1000 |
| Cleaved caspase-3 | Proteintech Group (66470-2-Ig) | 1:1000 |
| COX-2 | Proteintech Group (66351-1-Ig) | 1:1000 |
| PARP1 | Proteintech Group (66520-1-Ig) | 1:5000 |
| Cleaved PARP1 | Proteintech Group (66520-1-Ig) | 1:5000 |
| GAPDH | Proteintech Group (10494-1-AP) | 1:10,000 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Meng, Q.; Wu, W.; Zhang, W.; Yuan, J.; Yang, L.; Zhang, X.; Tao, K. IL-18BP Improves Early Graft Function and Survival in Lewis–Brown Norway Rat Orthotopic Liver Transplantation Model. Biomolecules 2022, 12, 1801. https://doi.org/10.3390/biom12121801
Meng Q, Wu W, Zhang W, Yuan J, Yang L, Zhang X, Tao K. IL-18BP Improves Early Graft Function and Survival in Lewis–Brown Norway Rat Orthotopic Liver Transplantation Model. Biomolecules. 2022; 12(12):1801. https://doi.org/10.3390/biom12121801
Chicago/Turabian StyleMeng, Qiang, Weikang Wu, Wenjie Zhang, Juzheng Yuan, Long Yang, Xuan Zhang, and Kaishan Tao. 2022. "IL-18BP Improves Early Graft Function and Survival in Lewis–Brown Norway Rat Orthotopic Liver Transplantation Model" Biomolecules 12, no. 12: 1801. https://doi.org/10.3390/biom12121801
APA StyleMeng, Q., Wu, W., Zhang, W., Yuan, J., Yang, L., Zhang, X., & Tao, K. (2022). IL-18BP Improves Early Graft Function and Survival in Lewis–Brown Norway Rat Orthotopic Liver Transplantation Model. Biomolecules, 12(12), 1801. https://doi.org/10.3390/biom12121801






