A Large-Scale High-Throughput Screen for Modulators of SERCA Activity
Abstract
1. Introduction
2. Materials and Methods
2.1. Compound Handling
2.2. SR Preparations
2.3. HTS NADH-Coupled Enzyme Assay Preparation and Measurements
2.4. Ca2+-Uptake Assays
3. Results
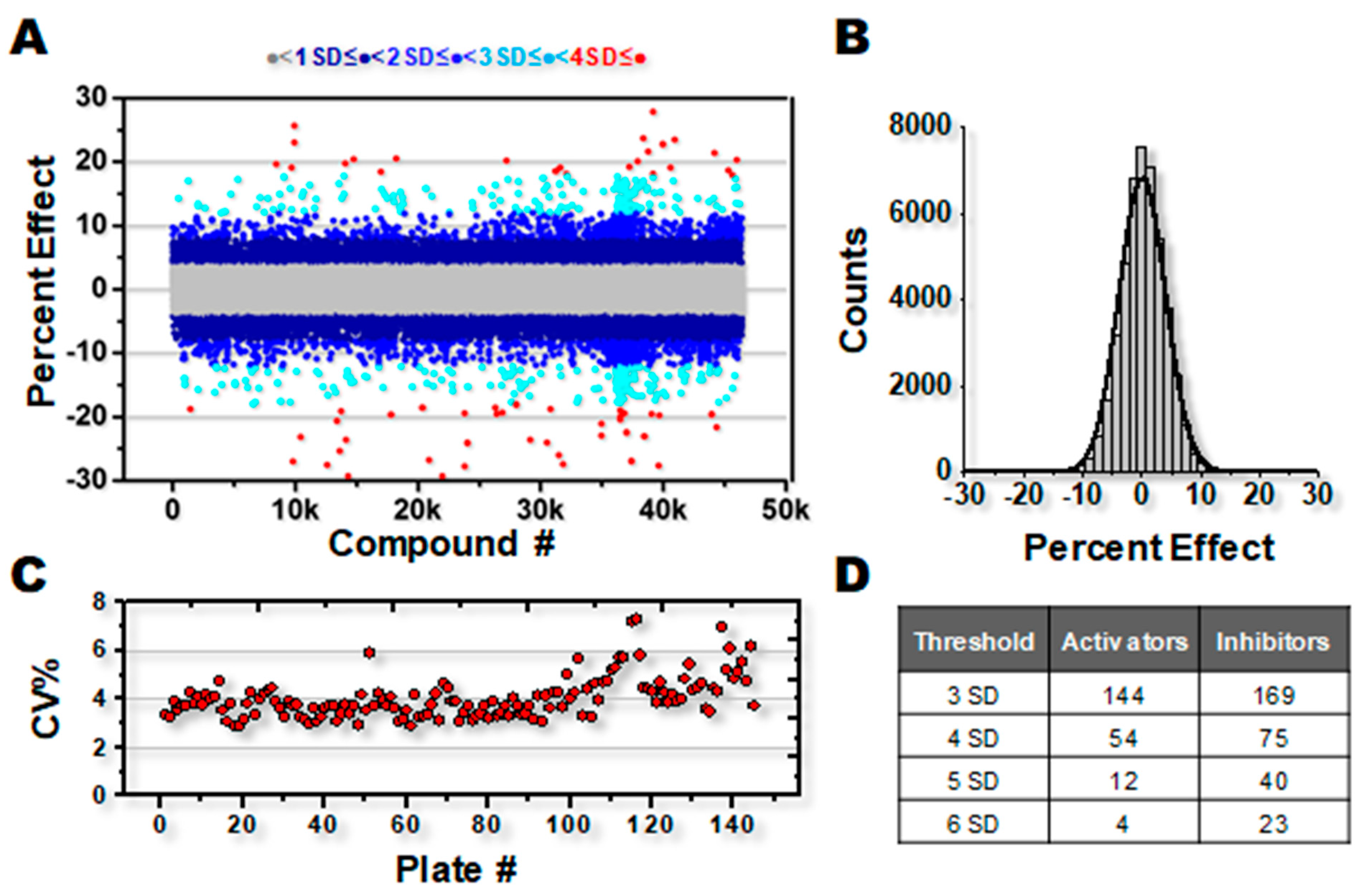
3.1. High-Throughput Screening for SERCA Modulators
3.2. Characterization of Hit Compounds in Skeletal SR ATPase Assay
3.3. Characterization of Hit Compounds in Skeletal SR Ca2+ Uptake Assays
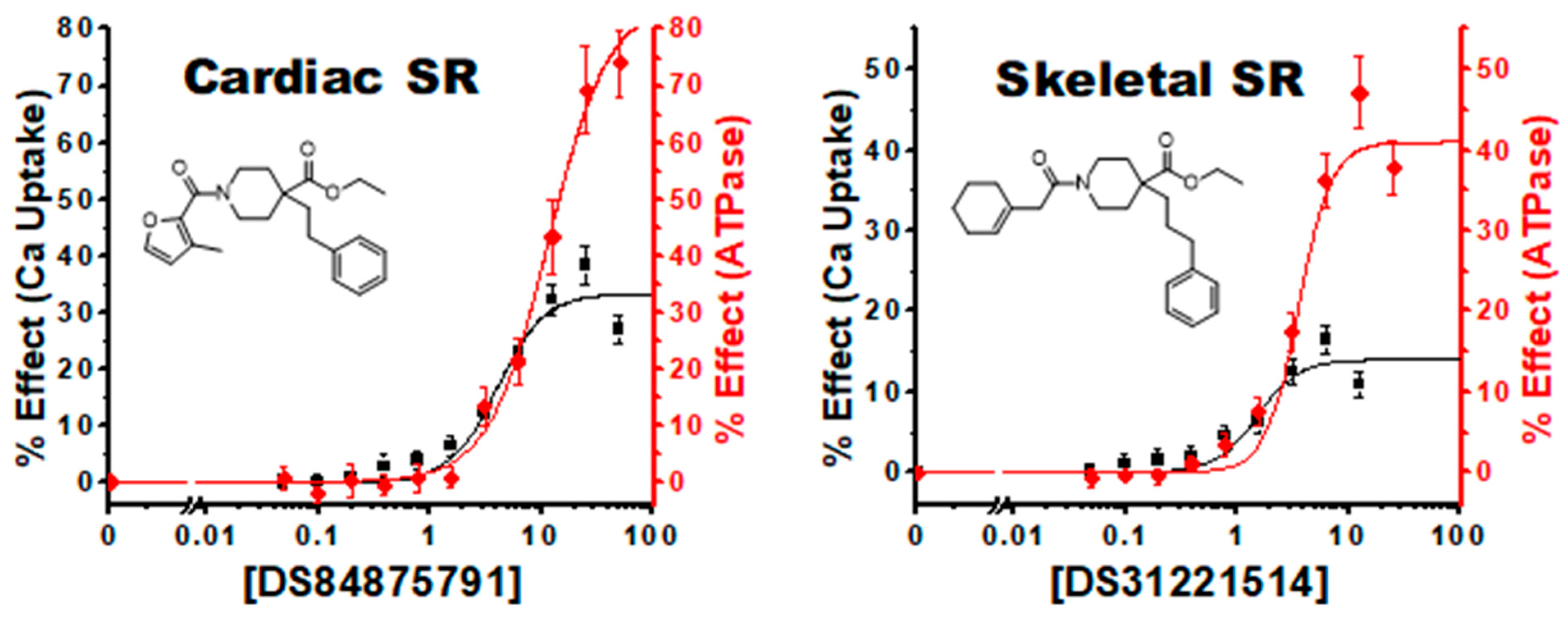
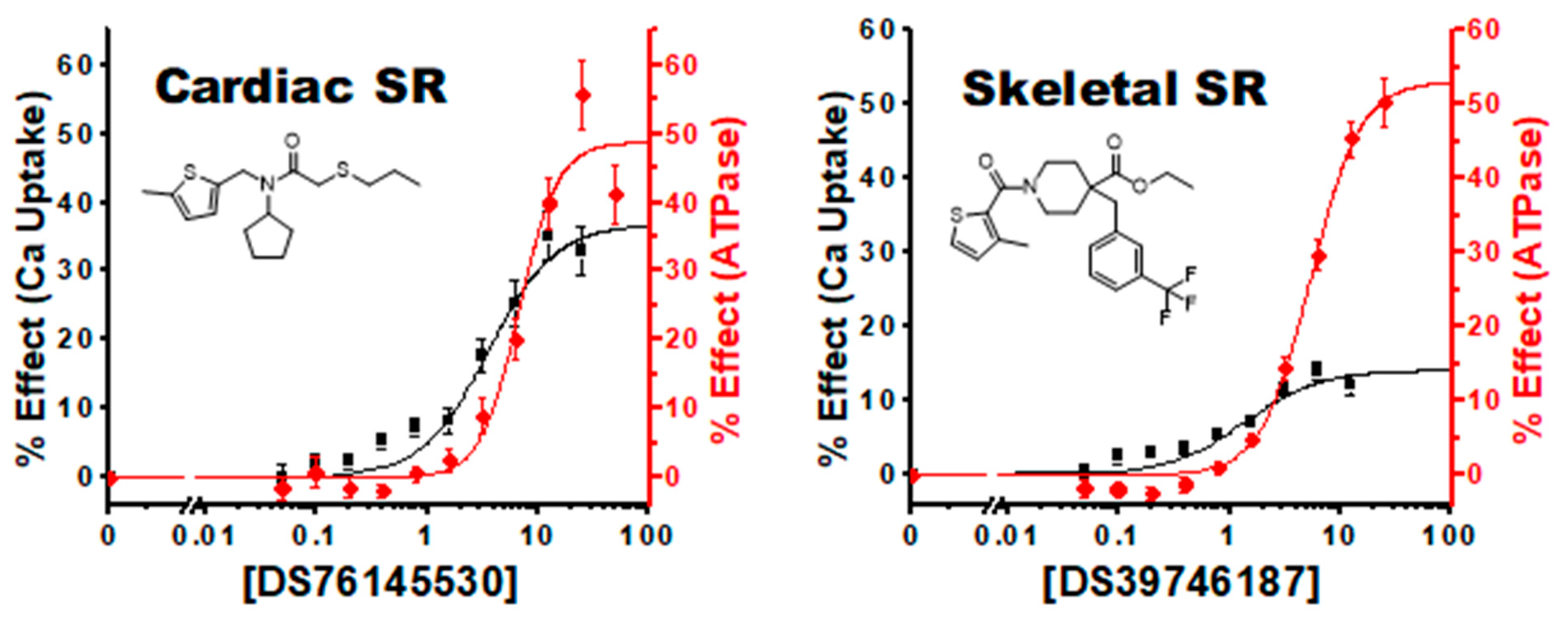
3.4. Characterization of Hit Compounds in Cardiac SR ATPase Assay
3.5. Characterization of Hit Compounds in Cardiac SR Ca Uptake Assays
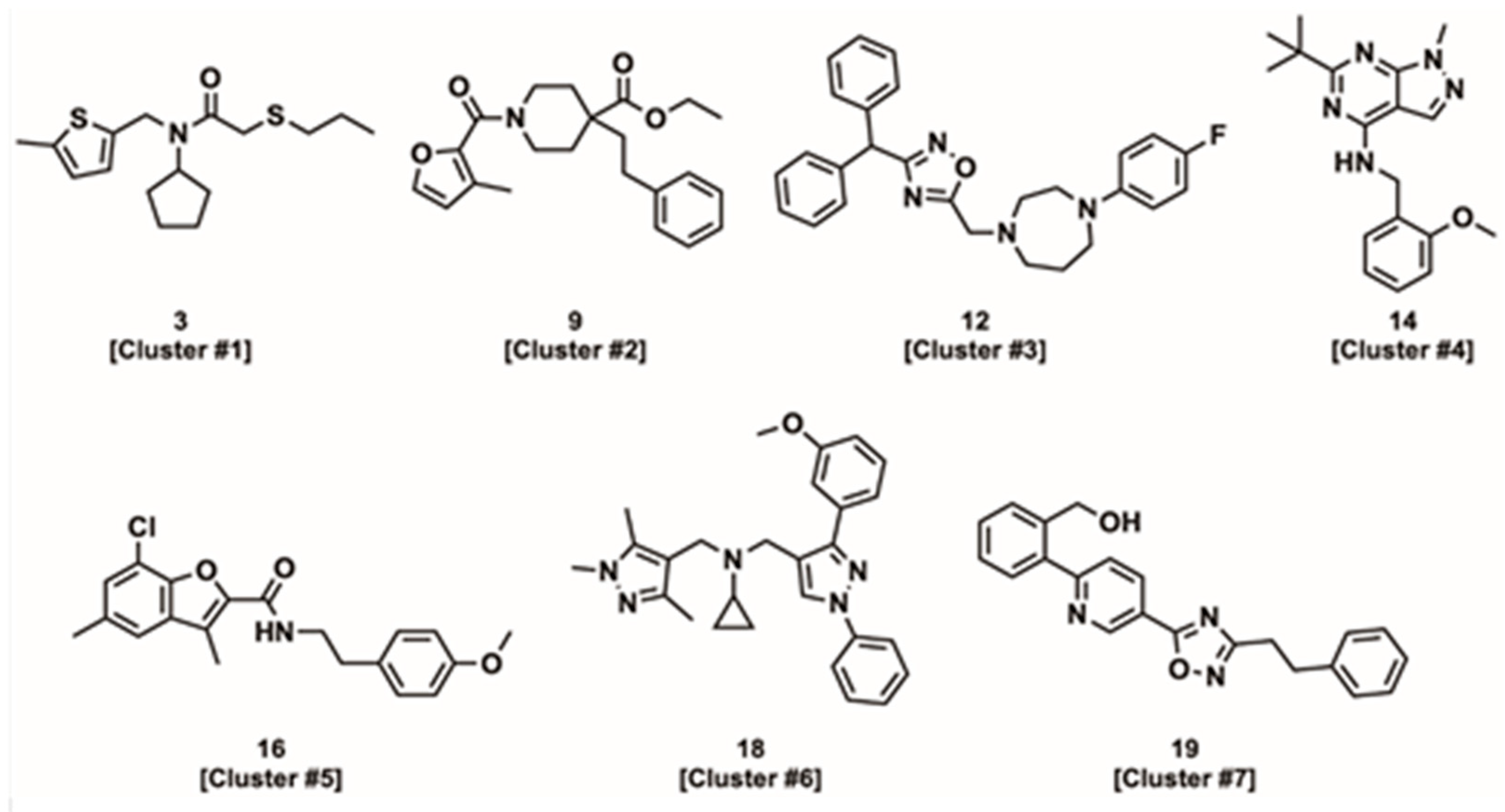
3.6. Compound Physico-Chemical Characteristics
4. Discussion
4.1. Robustness of HTS Assay
4.2. Compound Classifications: ATPase vs. Ca Uptake Activities
4.2.1. Linked Activating Compounds
4.2.2. Improved Coupling Compounds
4.2.3. Uncoupling Compounds
4.2.4. Tissue and Isoform Specificity
4.3. Study Limitations
4.4. Next Steps
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bers, D.M. Excitation-Contraction Coupling and Cardiac Contractile Force, 2nd ed.; Kluwer Academic Publishers: Dordrecht, The Netherlands; Boston, MA, USA, 2001. [Google Scholar]
- Haghighi, K.; Bidwell, P.; Kranias, E.G. Phospholamban interactome in cardiac contractility and survival: A new vision of an old friend. J. Mol. Cell. Cardiol. 2014, 77, 160–167. [Google Scholar] [CrossRef] [PubMed]
- Carafoli, E.; Krebs, J. Why calcium? How calcium became the best communicator. J. Biol. Chem. 2016, 291, 20849–20857. [Google Scholar] [CrossRef] [PubMed]
- Liao, R.; Helm, P.A.; Hajjar, R.J.; Saha, C.; Gwathmey, J.K. [Ca2+]i in human heart failure: A review and discussion of current areas of controversy. Yale J. Biol. Med. 1994, 67, 247–264. [Google Scholar] [PubMed]
- Schmidt, U.; Hajjar, R.J.; Helm, P.A.; Kim, C.S.; Doye, A.A.; Gwathmey, J.K. Contribution of abnormal sarcoplasmic reticulum ATPase activity to systolic and diastolic dysfunction in human heart failure. J. Mol. Cell. Cardiol. 1998, 30, 1929–1937. [Google Scholar] [CrossRef] [PubMed]
- Del Monte, F.; Hajjar, R.J.; Harding, S.E. Overwhelming evidence of the beneficial effects of SERCA gene transfer in heart failure. Circ. Res. 2001, 88, E66–E67. [Google Scholar] [CrossRef] [PubMed]
- Roti, G.; Carlton, A.; Ross, K.N.; Markstein, M.; Pajcini, K.; Su, A.H.; Perrimon, N.; Pear, W.S.; Kung, A.L.; Blacklow, S.C.; et al. Complementary genomic screens identify SERCA as a therapeutic target in NOTCH1 mutated cancer. Cancer Cell 2013, 23, 390–405. [Google Scholar] [CrossRef]
- Kang, S.; Dahl, R.; Hsieh, W.; Shin, A.; Zsebo, K.M.; Buettner, C.; Hajjar, R.J.; Lebeche, D. Small molecular allosteric activator of the sarco/endoplasmic reticulum Ca2+-ATPase (SERCA) attenuates diabetes and metabolic disorders. J. Biol. Chem. 2016, 291, 5185–5198. [Google Scholar] [CrossRef]
- Vallejo-Illarramendi, A.; Toral-Ojeda, I.; Aldanondo, G.; Lopez de Munain, A. Dysregulation of calcium homeostasis in muscular dystrophies. Expert Rev. Mol. Med. 2014, 16, e16. [Google Scholar] [CrossRef]
- Rouf, R.; Greytak, S.; Wooten, E.C.; Wu, J.; Boltax, J.; Picard, M.; Svensson, E.C.; Dillmann, W.H.; Patten, R.D.; Huggins, G.S. Increased FOG-2 in failing myocardium disrupts thyroid hormone-dependent SERCA2 gene transcription. Circ. Res. 2008, 103, 493–501. [Google Scholar] [CrossRef]
- Lipskaia, L.; Keuylian, Z.; Blirando, K.; Mougenot, N.; Jacquet, A.; Rouxel, C.; Sghairi, H.; Elaib, Z.; Blaise, R.; Adnot, S.; et al. Expression of sarco (endo) plasmic reticulum calcium ATPase (SERCA) system in normal mouse cardiovascular tissues, heart failure and atherosclerosis. Biochim. Biophys. Acta 2014, 1843, 2705–2718. [Google Scholar] [CrossRef]
- Bers, D.M.; Despa, S. Cardiac myocytes Ca2+ and Na+ regulation in normal and failing hearts. J. Pharmacol. Sci. 2006, 100, 315–322. [Google Scholar] [CrossRef] [PubMed]
- Schwinger, R.H.; Munch, G.; Bolck, B.; Karczewski, P.; Krause, E.G.; Erdmann, E. Reduced Ca(2+)-sensitivity of SERCA 2a in failing human myocardium due to reduced serin-16 phospholamban phosphorylation. J. Mol. Cell. Cardiol. 1999, 31, 479–491. [Google Scholar] [CrossRef] [PubMed]
- Sande, J.B.; Sjaastad, I.; Hoen, I.B.; Bokenes, J.; Tonnessen, T.; Holt, E.; Lunde, P.K.; Christensen, G. Reduced level of serine(16) phosphorylated phospholamban in the failing rat myocardium: A major contributor to reduced SERCA2 activity. Cardiovasc. Res. 2002, 53, 382–391. [Google Scholar] [CrossRef] [PubMed]
- Hoshijima, M.; Ikeda, Y.; Iwanaga, Y.; Minamisawa, S.; Date, M.O.; Gu, Y.; Iwatate, M.; Li, M.; Wang, L.; Wilson, J.M.; et al. Chronic suppression of heart-failure progression by a pseudophosphorylated mutant of phospholamban via in vivo cardiac rAAV gene delivery. Nat. Med. 2002, 8, 864–871. [Google Scholar] [CrossRef] [PubMed]
- Hadri, L.; Hajjar, R.J. Calcium cycling proteins and their association with heart failure. Clin. Pharmacol. Ther. 2011, 90, 620–624. [Google Scholar] [CrossRef]
- Jessup, M.; Greenberg, B.; Mancini, D.; Cappola, T.; Pauly, D.F.; Jaski, B.; Yaroshinsky, A.; Zsebo, K.M.; Dittrich, H.; Hajjar, R.J. Calcium Upregulation by Percutaneous Administration of Gene Therapy in Cardiac Disease (CUPID): A Phase 2 Trial of Intracoronary Gene Therapy of Sarcoplasmic Reticulum Ca2+-ATPase in Patients With Advanced Heart Failure. Circulation 2011, 124, 304–313. [Google Scholar] [CrossRef]
- Hulot, J.S.; Ishikawa, K.; Hajjar, R.J. Gene therapy for the treatment of heart failure: Promise postponed. Eur. Heart J. 2016, 37, 1651–1658. [Google Scholar] [CrossRef]
- Andersson, D.C.; Betzenhauser, M.J.; Reiken, S.; Meli, A.C.; Umanskaya, A.; Xie, W.; Shiomi, T.; Zalk, R.; Lacampagne, A.; Marks, A.R. Ryanodine receptor oxidation causes intracellular calcium leak and muscle weakness in aging. Cell Metab. 2011, 14, 196–207. [Google Scholar] [CrossRef]
- Lanner, J.T. Ryanodine receptor physiology and its role in disease. Adv. Exp. Med. Biol. 2012, 740, 217–234. [Google Scholar] [CrossRef]
- Wilmshurst, J.M.; Lillis, S.; Zhou, H.; Pillay, K.; Henderson, H.; Kress, W.; Muller, C.R.; Ndondo, A.; Cloke, V.; Cullup, T.; et al. RYR1 mutations are a common cause of congenital myopathies with central nuclei. Ann. Neurol. 2010, 68, 717–726. [Google Scholar] [CrossRef]
- Agrawal, A.; Suryakumar, G.; Rathor, R. Role of defective Ca(2+) signaling in skeletal muscle weakness: Pharmacological implications. J. Cell Commun. Signal. 2018, 12, 645–659. [Google Scholar] [CrossRef] [PubMed]
- Qaisar, R.; Bhaskaran, S.; Ranjit, R.; Sataranatarajan, K.; Premkumar, P.; Huseman, K.; Van Remmen, H. Restoration of SERCA ATPase prevents oxidative stress-related muscle atrophy and weakness. Redox Biol. 2019, 20, 68–74. [Google Scholar] [CrossRef] [PubMed]
- Mareedu, S.; Pachon, R.E.; Jayapalraj, T.; Fefelova, N.; Balakrishnan, R.; Niranjan, N.; Xie, L.H.; Babu, G.J. Sarcolipin haploinsufficiency prevents dystrophic cardiomyopathy in mdx mice. Am. J. Physiol. Heart Circ. Physiol. 2021, 320, H200–H210. [Google Scholar] [CrossRef] [PubMed]
- Umanskaya, A.; Santulli, G.; Xie, W.; Andersson, D.C.; Reiken, S.R.; Marks, A.R. Genetically enhancing mitochondrial antioxidant activity improves muscle function in aging. Proc. Natl. Acad. Sci. USA 2014, 111, 15250–15255. [Google Scholar] [CrossRef]
- Lawal, T.A.; Todd, J.J.; Meilleur, K.G. Ryanodine Receptor 1-Related Myopathies: Diagnostic and Therapeutic Approaches. Neurotherapeutics 2018, 15, 885–899. [Google Scholar] [CrossRef]
- Kushnir, A.; Todd, J.J.; Witherspoon, J.W.; Yuan, Q.; Reiken, S.; Lin, H.; Munce, R.H.; Wajsberg, B.; Melville, Z.; Clarke, O.B.; et al. Intracellular calcium leak as a therapeutic target for RYR1-related myopathies. Acta Neuropathol. 2020, 139, 1089–1104. [Google Scholar] [CrossRef]
- Volpatti, J.R.; Endo, Y.; Knox, J.; Groom, L.; Brennan, S.; Noche, R.; Zuercher, W.J.; Roy, P.; Dirksen, R.T.; Dowling, J.J. Identification of drug modifiers for RYR1-related myopathy using a multi-species discovery pipeline. eLife 2020, 9, e52946. [Google Scholar] [CrossRef]
- Wang, H.J.; Lee, C.S.; Yee, R.S.Z.; Groom, L.; Friedman, I.; Babcock, L.; Georgiou, D.K.; Hong, J.; Hanna, A.D.; Recio, J.; et al. Adaptive thermogenesis enhances the life-threatening response to heat in mice with an Ryr1 mutation. Nat. Commun. 2020, 11, 5099. [Google Scholar] [CrossRef]
- Schaaf, T.M.; Li, A.; Grant, B.D.; Peterson, K.; Yuen, S.; Bawaskar, P.; Kleinboehl, E.; Li, J.; Thomas, D.D.; Gillispie, G.D. Red-Shifted FRET Biosensors for High-Throughput Fluorescence Lifetime Screening. Biosensors 2018, 8, 99. [Google Scholar] [CrossRef]
- Rebbeck, R.T.; Essawy, M.M.; Nitu, F.R.; Grant, B.D.; Gillispie, G.D.; Thomas, D.D.; Bers, D.M.; Cornea, R.L. High-Throughput Screens to Discover Small-Molecule Modulators of Ryanodine Receptor Calcium Release Channels. SLAS Discov. 2017, 22, 176–186. [Google Scholar] [CrossRef]
- Gruber, S.J.; Cornea, R.L.; Li, J.; Peterson, K.C.; Schaaf, T.M.; Gillispie, G.D.; Dahl, R.; Zsebo, K.M.; Robia, S.L.; Thomas, D.D. Discovery of enzyme modulators via high-throughput time-resolved FRET in living cells. J. Biomol. Screen. 2014, 19, 215–222. [Google Scholar] [CrossRef] [PubMed]
- Lo, C.H.; Vunnam, N.; Lewis, A.K.; Chiu, T.L.; Brummel, B.E.; Schaaf, T.M.; Grant, B.D.; Bawaskar, P.; Thomas, D.D.; Sachs, J.N. An Innovative High-Throughput Screening Approach for Discovery of Small Molecules That Inhibit TNF Receptors. SLAS Discov. 2017, 22, 950–961. [Google Scholar] [CrossRef] [PubMed]
- Fruen, B.R.; Balog, E.M.; Schafer, J.; Nitu, F.R.; Thomas, D.D.; Cornea, R.L. Direct Detection of Calmodulin Tuning by Ryanodine Receptor Channel Targets Using a Ca(2+)-Sensitive Acrylodan-Labeled Calmodulin. Biochemistry 2005, 44, 278–284. [Google Scholar] [CrossRef] [PubMed]
- Schaaf, T.M.; Kleinboehl, E.; Yuen, S.L.; Roelike, L.N.; Svensson, B.; Thompson, A.R.; Cornea, R.L.; Thomas, D.D. Live-Cell Cardiac-Specific High-Throughput Screening Platform for Drug-Like Molecules that Enhance Ca(2+) Transport. Cells 2020, 9, 1170. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; James, Z.M.; Dong, X.; Karim, C.B.; Thomas, D.D. Structural and functional dynamics of an integral membrane protein complex modulated by lipid headgroup charge. J. Mol. Biol. 2012, 418, 379–389. [Google Scholar] [CrossRef]
- Zhang, J.H.; Chung, T.D.; Oldenburg, K.R. A Simple Statistical Parameter for Use in Evaluation and Validation of High Throughput Screening Assays. J. Biomol. Screen. 1999, 4, 67–73. [Google Scholar] [CrossRef]
- Lipinski, C.A.; Lombardo, F.; Dominy, B.W.; Feeney, P.J. Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings. Adv. Drug Deliv. Rev. 2001, 46, 3–26. [Google Scholar] [CrossRef]
- Veber, D.F.; Johnson, S.R.; Cheng, H.Y.; Smith, B.R.; Ward, K.W.; Kopple, K.D. Molecular properties that influence the oral bioavailability of drug candidates. J. Med. Chem. 2002, 45, 2615–2623. [Google Scholar] [CrossRef]
- Lovering, F.; Bikker, J.; Humblet, C. Escape from flatland: Increasing saturation as an approach to improving clinical success. J. Med. Chem. 2009, 52, 6752–6756. [Google Scholar] [CrossRef]
- Cornea, R.L.; Gruber, S.J.; Lockamy, E.L.; Muretta, J.M.; Jin, D.; Chen, J.; Dahl, R.; Bartfai, T.; Zsebo, K.M.; Gillispie, G.D.; et al. High-throughput FRET assay yields allosteric SERCA activators. J. Biomol. Screen. 2013, 18, 97–107. [Google Scholar] [CrossRef]
- Dyla, M.; Basse Hansen, S.; Nissen, P.; Kjaergaard, M. Structural dynamics of P-type ATPase ion pumps. Biochem. Soc. Trans. 2019, 47, 1247–1257. [Google Scholar] [CrossRef] [PubMed]
- Kirchberber, M.A.; Tada, M.; Katz, A.M. Phospholamban: A regulatory protein of the cardiac sarcoplasmic reticulum. Recent Adv. Stud. Cardiac. Struct. Metab. 1975, 5, 103–115. [Google Scholar] [PubMed]
- Racker, E.; Eytan, E. A coupling factor from sarcoplasmic reticulum required for the translocation of Ca2+ ions in a reconstituted Ca2+ATPase pump. J. Biol. Chem. 1975, 250, 7533–7534. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Inesi, G. Variable stoichiometric efficiency of Ca2+ and Sr2+ transport by the sarcoplasmic reticulum ATPase. J. Biol. Chem. 1995, 270, 4361–4367. [Google Scholar] [CrossRef]
- Reis, M.; Farage, M.; de Meis, L. Thermogenesis and energy expenditure: Control of heat production by the Ca(2+)-ATPase of fast and slow muscle. Mol. Membr. Biol. 2002, 19, 301–310. [Google Scholar] [CrossRef]
- Bal, N.C.; Periasamy, M. Uncoupling of sarcoendoplasmic reticulum calcium ATPase pump activity by sarcolipin as the basis for muscle non-shivering thermogenesis. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2020, 375, 20190135. [Google Scholar] [CrossRef]
- Rathod, N.; Bak, J.J.; Primeau, J.O.; Fisher, M.E.; Espinoza-Fonseca, L.M.; Lemieux, M.J.; Young, H.S. Nothing Regular about the Regulins: Distinct Functional Properties of SERCA Transmembrane Peptide Regulatory Subunits. Int. J. Mol. Sci. 2021, 22, 8891. [Google Scholar] [CrossRef]


| Skeletal SR | Cardiac SR | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Compound | ATPase Activity | Ca2+ Uptake Activity | ATPase Activity | Ca2+ Uptake Activity | |||||
| N, Cluster | DIVERSet ID | Percent Increase | EC50 | Percent Increase | EC50 | Percent Increase | EC50 | Percent Increase | EC50 |
| 1, 1 | 67200668 | 50 ± 25 | 40 ± 21 | 27 ± 3 | 28 ± 9 | 42 ± 12 | 31 ± 10 | 32 ± 2 | 37 ± 4 |
| 2, 1 | 75082844 | 36 ± 2 | 4.0 ± 0.5 | 15 ± 1 | 2.2 ± 0.4 | 33 ± 3 | 2.9 ± 0.6 | 12 ± 1 | 1.2 ± 0.2 |
| 3, 1 | 76145530 | 54 ± 2 | 7.3 ± 0.5 | 20 ± 1 | 3.4 ± 0.5 | 48 ± 4 | 6.7 ± 1.0 | 37 ± 3 | 3.5 ± 0.7 |
| 4, 1 | 81260867 | 53 ± 2 | 5.4 ± 0.4 | 14 ± 2 | 1.4 ± 0.5 | 58 ± 4 | 4.8 ± 0.6 | 24 ± 1 | 1.6 ± 0.3 |
| 5, 1 | 11966966 | 41 ± 3 | 9.0 ± 1.0 | 10 ± 1 | 1.4 ± 0.6 | 58 ± 6 | 7.7 ± 2 | 16 ± 3 | 1.0 ± 0.7 |
| 6, 1 | 25365366 | 45 ± 8 | 24 ± 6 | 9 ± 1 | 4 ± 1 | 63 ± 17 | 29 ± 12 | 17 ± 1 | 3.8 ± 0.6 |
| 7, 2 | 31221514 | 41 ± 3 | 3.6 ± 0.4 | 14 ± 2 | 1.4 ± 0.4 | 51 ± 3 | 2.9 ± 0.3 | 24 ± 3 | 1.3 ± 0.4 |
| 8, 2 | 39746187 | 45 ± 2 | 4.0 ± 0.4 | 13 ± 1 | 1.3 ± 0.4 | 43 ± 2 | 3.4 ± 0.3 | 18 ± 2 | 0.8 ± 0.3 |
| 9, 2 | 84875791 | 72 ± 2 | 13.3 ± 0.7 | 31 ± 7 | 8 ± 5 | 85 ± 5 | 11 ± 1 | 33 ± 3 | 4.0 ± 0.8 |
| 10, 3 | 19784159 | 57 ± 3 | 10 ± 1 | 11 ± 3 | 4 ± 2 | 55 ± 2 | 7.5 ± 0.4 | 16 ± 3 | 1.6 ± 0.7 |
| 11, 3 | 27035959 | 61 ± 2 | 7.2 ± 0.4 | 9.1 ± 0.8 | 1.2 ± 0.3 | 62 ± 2 | 6.4 ± 0.4 | 18 ± 2 | 1.2 ± 0.4 |
| 12, 3 | 60405307 | 41 ± 2 | 3.4 ± 0.4 | 6.5 ± 0.5 | 0.6 ± 0.2 | 29 ± 1 | 2.3 ± 0.3 | −10 ± 3 | 1.3 ± 0.1 |
| 13, 4 | 71721828 | 36 ± 4 | 3.2 ± 0.6 | 8 ± 2 | 1.1 ± 0.6 | 36 ± 4 | 2.9 ± 0.6 | 21 ± 10 | 1.8 ± 2 |
| 14, 4 | 32014423 | 25 ± 3 | 3.3 ± 0.6 | 8 ± 2 | 1.1 ± 0.6 | 56 ± 3 | 5.2 ± 0.5 | 28 ± 5 | 2.5 ± 0.9 |
| 15, 5 | 19396790 | 30 ± 1 | 30 ± 12 | -- | -- | 46 ± 6 | 16 ± 3 | 6 ± 5 | 5 ± 11 |
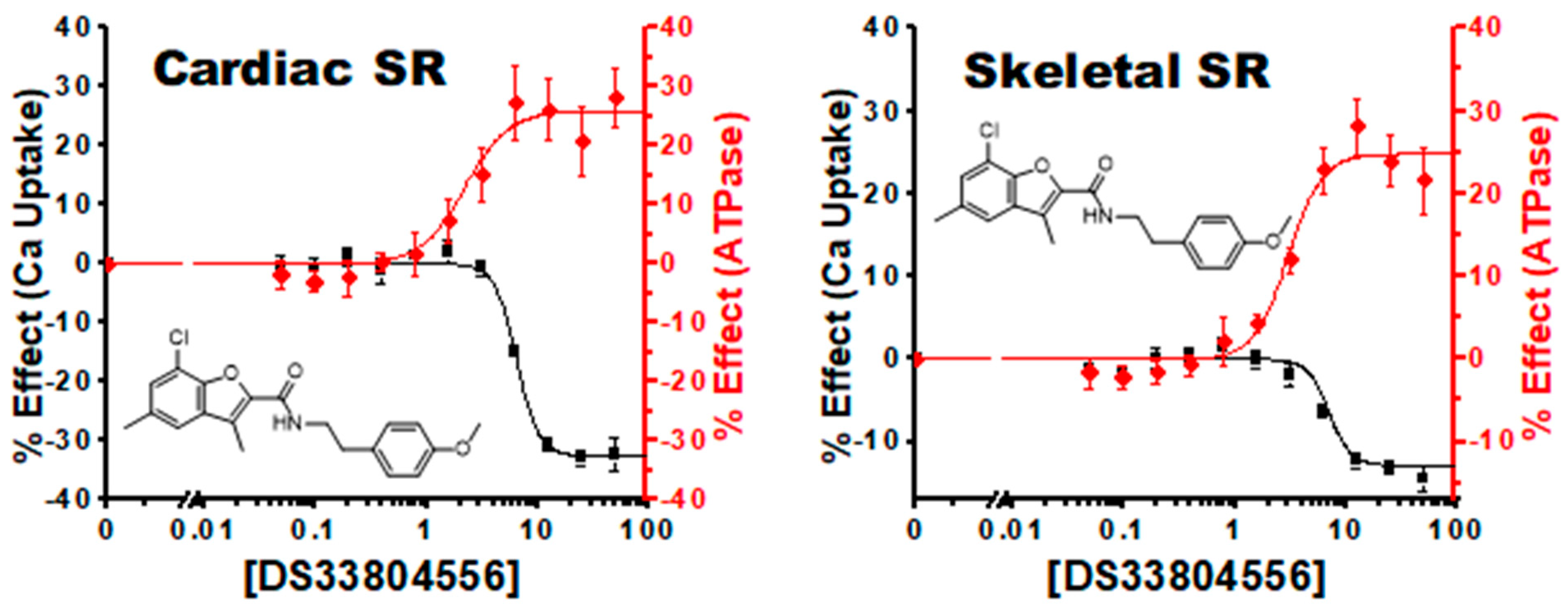
| 16, 5 | 33804556 | 25 ± 1 | 3.0 ± 0.3 | −10 ± 2 | 7 ± 13 | 26 ± 2 | 2.2 ± 0.4 | −27 ± 3 | 8.9 ± 2 |
| 17, 5 | 39779602 | 42 ± 4 | 16 ± 3 | -- | -- | 62 ± 5 | 9.4 ± 1 | -- | -- |
| 18, 6 | 81801810 | 39 ± 1 | 5.2 ± 0.3 | 8.9 ± 0.1 | 1.1 ± 0.3 | 37 ± 1 | 4.3 ± 0.3 | 12 ± 1 | 0.9 ± 0.2 |
| 19, 7 | 30616130 | 42 ± 2 | 7.3 ± 0.8 | 7.2 ± 0.1 | 1.8 ± 0.7 | 22 ± 2 | 6.3 ± 0.4 | 8 ± 1 | 2.4 ± 0.6 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bidwell, P.A.; Yuen, S.L.; Li, J.; Berg, K.; Rebbeck, R.T.; Aldrich, C.C.; Roopnarine, O.; Cornea, R.L.; Thomas, D.D. A Large-Scale High-Throughput Screen for Modulators of SERCA Activity. Biomolecules 2022, 12, 1789. https://doi.org/10.3390/biom12121789
Bidwell PA, Yuen SL, Li J, Berg K, Rebbeck RT, Aldrich CC, Roopnarine O, Cornea RL, Thomas DD. A Large-Scale High-Throughput Screen for Modulators of SERCA Activity. Biomolecules. 2022; 12(12):1789. https://doi.org/10.3390/biom12121789
Chicago/Turabian StyleBidwell, Philip A., Samantha L. Yuen, Ji Li, Kaja Berg, Robyn T. Rebbeck, Courtney C. Aldrich, Osha Roopnarine, Razvan L. Cornea, and David D. Thomas. 2022. "A Large-Scale High-Throughput Screen for Modulators of SERCA Activity" Biomolecules 12, no. 12: 1789. https://doi.org/10.3390/biom12121789
APA StyleBidwell, P. A., Yuen, S. L., Li, J., Berg, K., Rebbeck, R. T., Aldrich, C. C., Roopnarine, O., Cornea, R. L., & Thomas, D. D. (2022). A Large-Scale High-Throughput Screen for Modulators of SERCA Activity. Biomolecules, 12(12), 1789. https://doi.org/10.3390/biom12121789






