Biomolecular Strategies for Vascular Bundle Development to Improve Crop Yield
Abstract
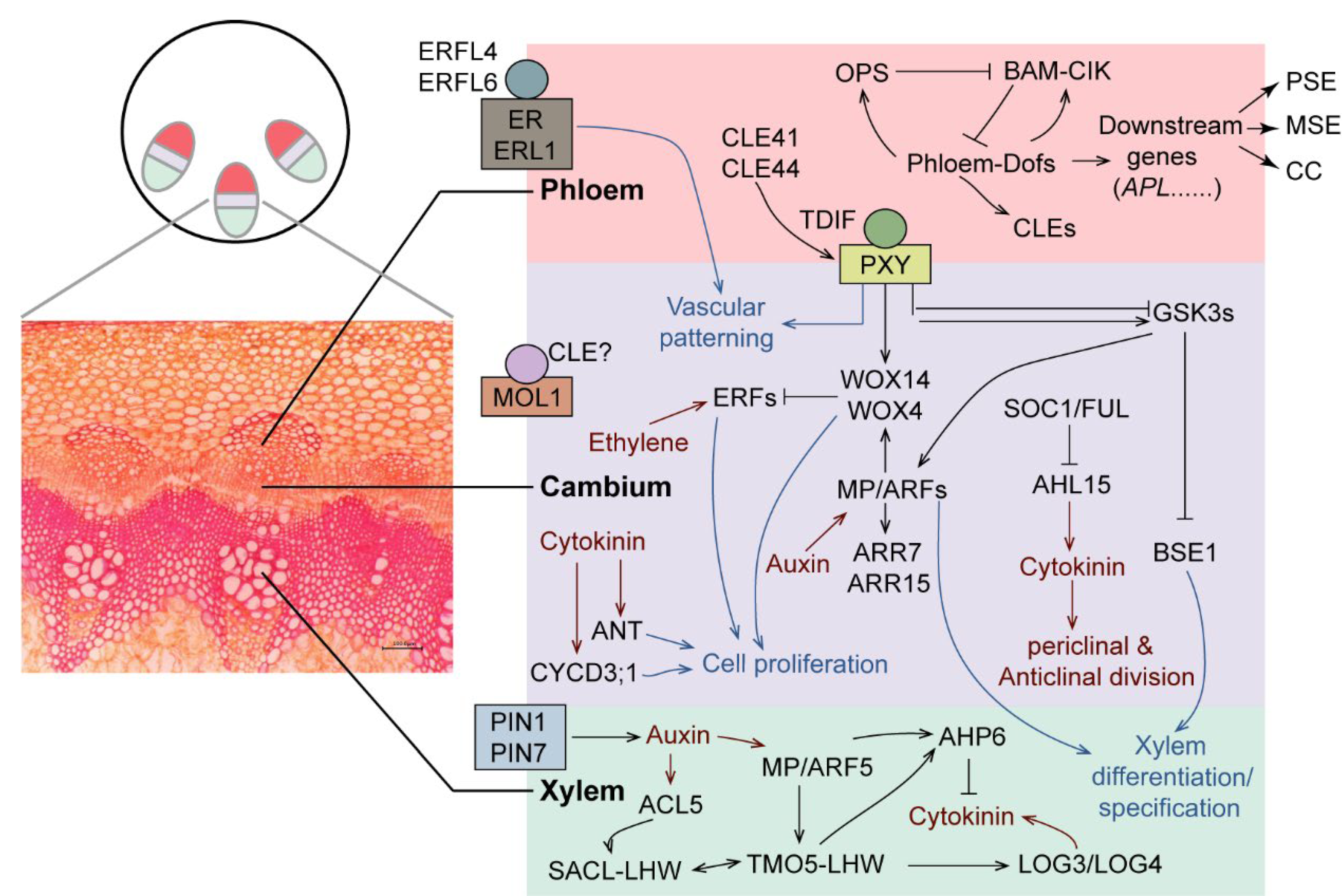
:1. Introduction
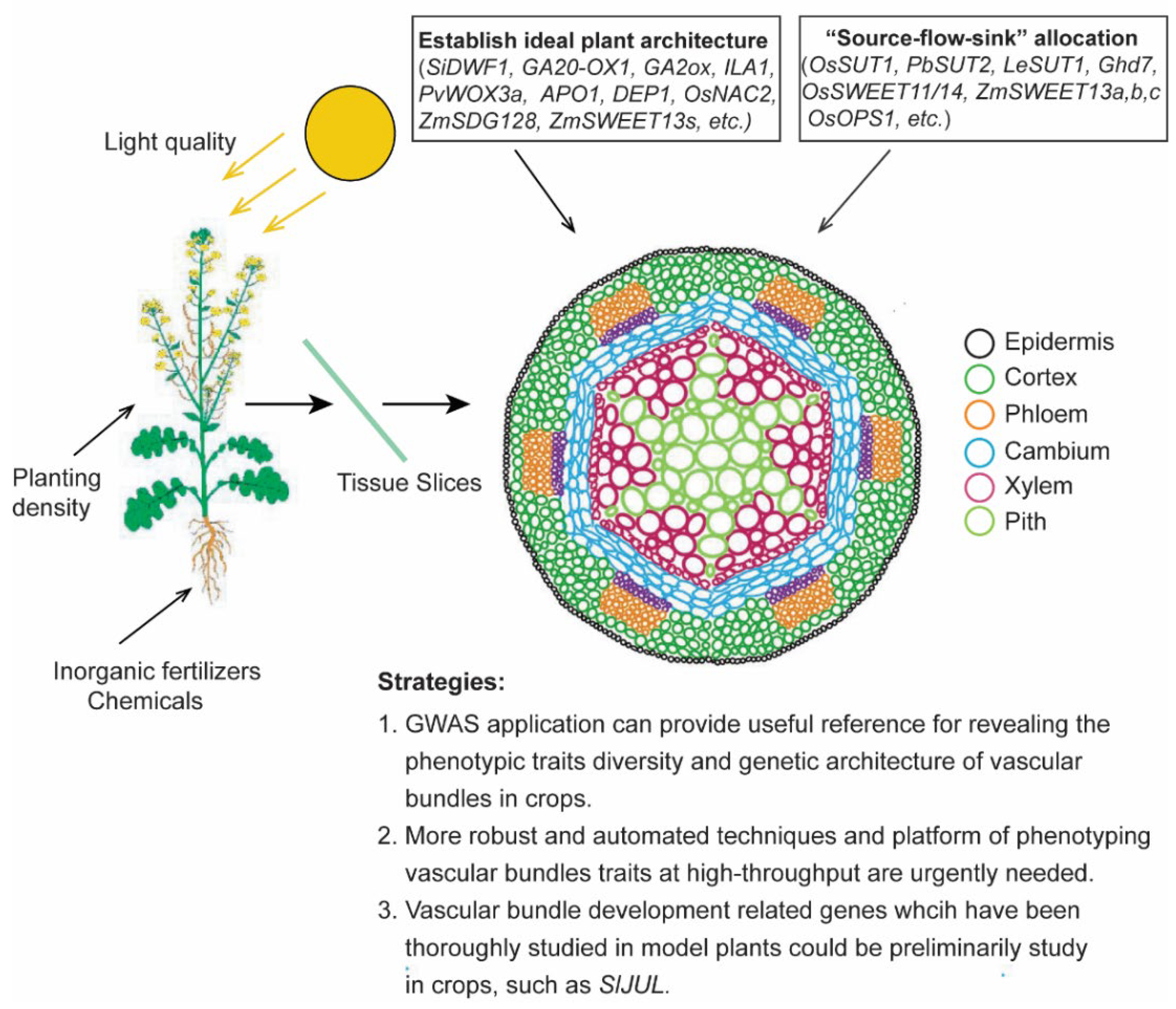
2. Advances in Vascular Bundle and “Source-Flow-Sink” Allocation
3. Vascular Bundles Development in Establishing Ideal Plant Architecture
4. External Environment Influences Vascular Bundle Development to Affect Crop Yield
5. Conclusions and Future Perspective
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Ray, D.K.; Mueller, N.D.; West, P.C.; Foley, J.A. Yield trends are insufficient to double global crop production by 2050. PLoS ONE 2013, 8, e66428. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.; Zhao, C.; Müller, C.; Wang, C.; Ciais, P.; Janssens, I.; Peñuelas, J.; Asseng, S.; Li, T.; Elliott, J.; et al. Emergent constraint on crop yield response to warmer temperature from field experiments. Nat. Sustain. 2020, 3, 908–916. [Google Scholar] [CrossRef]
- Voesenek, L.A.C.J.; Bailey-Serres, J. Flood adaptive traits and processes: An overview. New Phytol. 2015, 206, 57–73. [Google Scholar] [CrossRef] [PubMed]
- Bailey-Serres, J.; Parker, J.E.; Ainsworth, E.A.; Oldroyd, G.E.D.; Schroeder, J.I. Genetic strategies for improving crop yields. Nature 2019, 575, 109–118. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ismail, A.M.; Horie, T. Genomics, physiology, and molecular breeding approaches for improving salt tolerance. Annu. Rev. Plant Biol. 2017, 68, 405–434. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nelson, R.; Wiesner-Hanks, T.; Wisser, R.; Balint-Kurti, P. Navigating complexity to breed disease-resistant crops. Nat. Rev. Genet. 2018, 19, 21–33. [Google Scholar] [CrossRef]
- Transforming Our World: The 2030 Agenda for Sustainable Development (UN). Available online: https://sustainabledevelopment.un.org/post2015/transformingourworld (accessed on 22 June 2016).
- Ruonala, R.; Ko, D.; Helariutta, Y. Genetic Networks in Plant Vascular Development. Annu. Rev. Plant Biol. 2017, 51, 335–359. [Google Scholar] [CrossRef] [PubMed]
- Lucas, W.J.; Groover, A.; Lichtenberger, R.; Furuta, K.; Yadav, S.R.; Helariutta, Y.; He, X.Q.; Fukuda, H.; Kang, J.L.; Brady, S.M.; et al. The plant vascular system: Evolution, development and functions. J. Integr. Plant Biol. 2013, 55, 294–388. [Google Scholar]
- Scheres, B.J.G.; Wolkenfelt, H.; Willemsen, V.; Terlouw, M.; Lawson, E.; Dean, C.; Weisbeek, P. Embryonic origin of the Arabidopsis primary root and root meristem initials. Development 1994, 120, 2475–2487. [Google Scholar] [CrossRef]
- Xu, B.; Ohtani, M.; Yamaguchi, M.; Toyooka, K.; Wakazaki, M.; Sato, M.; Kubo, M.; Nakano, Y.; Sano, R.; Hiwatashi, Y.J.; et al. Contribution of NAC transcription factors to plant adaptation to land. Science 2014, 343, 1505–1508. [Google Scholar] [CrossRef]
- Fischer, U.; Kucukoglu, M.; Helariutta, Y.; Bhalerao, R.P. The Dynamics of Cambial Stem Cell Activity. Annu. Rev. Plant Biol. 2019, 70, 293–319. [Google Scholar] [CrossRef] [PubMed]
- Kondo, Y.; Nurani, A.M.; Saito, C.; Ichihashi, Y.; Saito, M.; Yamazaki, K.; Mitsuda, N.; Ohme-Takagi, M.; Fukuda, H. Vascular cell induction culture system using arabidopsis leaves (visual) reveals the sequential differentiation of sieve element-like cells. Plant Cell 2016, 28, 1250–1262. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miyashima, S.; Sebastian, J.; Lee, J.Y.; Helariutta, Y. Stem cell function during plant vascular development. EMBO J. 2013, 32, 178–193. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Birnbaum, K.; Shasha, D.E.; Wang, J.Y.; Jung, J.W.; Lambert, G.M.; Galbraith, D.W.; Benfey, P.N. A gene expression map of the Arabidopsis root. Science 2003, 302, 1956–1960. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brady, S.M.; Orlando, D.A.; Lee, J.Y.; Wang, J.Y.; Koch, J.; Dinneny, J.R.; Mace, D.; Ohler, U.; Benfey, P.N. A high-resolution root spatiotemporal map reveals dominant expression patterns. Science 2007, 318, 801–806. [Google Scholar] [CrossRef]
- Smit, M.E.; McGregor, S.R.; Sun, H.; Gough, C.; Bågman, A.M.; Soyars, C.L.; Kroon, J.T.; Gaudinier, A.; Williams, C.J.; Yang, X.Y.; et al. A PXY-Mediated Transcriptional Network Integrates Signaling Mechanisms to Control Vascular Development in Arabidopsis. Plant Cell 2020, 32, 319–335. [Google Scholar] [CrossRef] [Green Version]
- Wallner, E.S.; Lopez-Salmeron, V.; Belevich, I.; Poschet, G.; Jung, I.; Grunwald, K.; Sevilem, I.; Jokitalo, E.; Hell, R.; Helariutta, Y.; et al. Strigolactone- and Karrikin-Independent SMXL proteins are central regulators of phloem formation. Curr Biol. 2017, 27, 1241–1247. [Google Scholar] [CrossRef] [Green Version]
- Shi, D.; Lebovka, I.; Lopez-Salmeron, V.; Sanchez, P.; Greb, T. Bifacial cambium stem cells generate xylem and phloem during radial plant growth. Development 2019, 146, dev171355. [Google Scholar] [CrossRef] [Green Version]
- Wallner, E.S.; Tonn, N.; Shi, D.; Jouannet, V.; Greb, T. SUPPRESSOR OF MAX2 1-LIKE 5 promotes secondary phloem formation during radial stem growth. Plant J. 2020, 102, 903–915. [Google Scholar] [CrossRef] [Green Version]
- Fisher, K.; Turner, S. PXY, a receptor-like kinase essential for maintaining polarity during plant vascular-tissue development. Curr Biol. 2007, 17, 1061–1066. [Google Scholar] [CrossRef]
- Hirakawa, Y.; Shinohara, H.; Kondo, Y.; Inoue, A.; Nakanomyo, I.; Ogawa, M.; Sawa, S.; Ohashi-Ito, K.; Matsubayashi, Y.; Fukuda, H. Non-cell-autonomous control of vascular stem cell fate by a CLE peptide/receptor system. Proc. Natl. Acad. Sci. USA 2008, 105, 15208–15213. [Google Scholar] [CrossRef]
- Bonke, M.; Thitamadee, S.; Mahonen, A.P.; Hauser, M.T.; Helariutta, Y. APL regulates vascular tissue identity in Arabidopsis. Nature 2003, 426, 181–186. [Google Scholar] [CrossRef]
- Agusti, J.; Herold, S.; Schwarz, M.; Sanchez, P.; Ljung, K.; Dun, E.A.; Brewer, P.B.; Beveridge, C.A.; Sieberer, T.; Sehr, E.M.; et al. Strigolactone signaling is required for auxin-dependent stimulation of secondary growth in plants. Proc. Natl. Acad. Sci. USA 2011, 108, 20242–20247. [Google Scholar] [CrossRef] [Green Version]
- Roszak, P.; Heo, J.O.; Blob, B.; Toyokura, K.; Sugiyama, Y.; de Luis Balaguer, M.A.; Lau, W.W.Y.; Hamey, F.; Cirrone, J.; Madej, E.; et al. Cell-by-cell dissection of phloem development links a maturation gradient to cell specialization. Science 2021, 374, eaba5531. [Google Scholar] [CrossRef]
- Ren, S.H.; Song, X.F.; Chen, W.Q.; Lu, R.; Lucas, W.J.; Liu, C.M. CLE25 peptide regulates phloem initiation in Arabidopsis through a CLERK-CLV2 receptor complex. J. Integr. Plant Biol. 2019, 61, 1043–1061. [Google Scholar]
- Anne, P.; Amiguet-Vercher, A.; Brandt, B.; Kalmbach, L.; Geldner, N.; Hothorn, M.; Hardtke, C.S. CLERK is a novel receptor kinase required for sensing of root-active CLE peptides in Arabidopsis. Development 2018, 145, dev162354. [Google Scholar] [CrossRef] [Green Version]
- Depuydt, S.; Rodriguez-Villalon, A.; Santuari, L.; Wyser-Rmili, C.; Ragni, L.; Hardtke, C.S. Suppression of Arabidopsis protophloem differentiation and root meristem growth by CLE45 requires the receptor-like kinase BAM3. Proc. Natl. Acad. Sci. USA 2013, 110, 7074–7079. [Google Scholar] [CrossRef] [Green Version]
- Rodriguez-Villalon, A.; Gujas, B.; Kang, Y.H.; Breda, A.S.; Cattaneo, P.; Depuydt, S.; Hardtke, C.S. Molecular genetic framework for protophloem formation. Proc. Natl. Acad. Sci. USA 2014, 111, 11551–11556. [Google Scholar] [CrossRef] [Green Version]
- Miyashima, S.; Roszak, P.; Sevilem, I.; Toyokura, K.; Blob, B.; Heo, J.O.; Mellor, N.; Help-Rinta-Rahko, H.; Otero, S.; Smet, W.; et al. Mobile PEAR transcription factors integrate positional cues to prime cambial growth. Nature 2019, 565, 490–494. [Google Scholar] [CrossRef] [Green Version]
- Shi, D.; Jouannet, V.; Agustí, J.; Kaul, V.; Levitsky, V.; Sanchez, P.; Mironova, V.V.; Greb, T. Tissue-specific transcriptome profiling of the Arabidopsis inflorescence stem reveals local cellular signatures. Plant Cell 2021, 33, 200–223. [Google Scholar] [CrossRef]
- Qian, P.; Song, W.; Zaizen-Iida, M.; Kume, S.; Wang, G.; Zhang, Y.; Kinoshita-Tsujimura, K.; Chai, J.; Kakimoto, T. A Dof-CLE circuit controls phloem organization. Nat. Plants 2022, 8, 817–827. [Google Scholar] [CrossRef] [PubMed]
- Randall, R.S.; Miyashima, S.; Blomster, T.; Zhang, J.; Elo, A.; Karlberg, A.; Immanen, J.; Nieminen, K.; Lee, J.Y.; Kakimoto, T.; et al. AINTEGUMENTA and the D-type cyclin CYCD3;1 regulate root secondary growth and respond to cytokinins. Biol. Open 2015, 4, 1229–1236. [Google Scholar] [CrossRef] [PubMed]
- Collins, C.; Maruthi, N.M.; Jahn, C.E. CYCD3 D-type cyclins regulate cambial cell proliferation and secondary growth in Arabidopsis. J. Exp. Bot. 2015, 66, 4595–4606. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rahimi, A.; Karami, O.; Lestari, A.D.; de Werk, T.; Amakorová, P.; Shi, D.; Novák, O.; Greb, T.; Offringa, R. Control of cambium initiation and activity in Arabidopsis by the transcriptional regulator AHL15. Curr Biol. 2022, 32, 1764–1775.e3. [Google Scholar] [CrossRef] [PubMed]
- Hardtke, C.S.; Berleth, T. The Arabidopsis gene MONOPTEROS encodes a transcription factor mediating embryo axis formation and vascular development. EMBO J. 1998, 17, 1405–1411. [Google Scholar] [CrossRef] [PubMed]
- Schlereth, A.; Möller, B.; Liu, W.; Kientz, M.; Flipse, J.; Rademacher, E.H.; Weijers, D. MONOPTEROS controls embryonic root initiation by regulating a mobile transcription factor. Nature 2010, 464, 913–916. [Google Scholar] [CrossRef]
- De Rybel, B.; Adibi, M.; Breda, A.S.; Wendrich, J.R.; Smit, M.E.; Novák, O.; Yamaguchi, N.; Yoshida, S.; Van Isterdael, G.; Palovaara, J.; et al. Integration of growth and patterning during vascular tissue formation in Arabidopsis. Science 2014, 345, 1255215. [Google Scholar] [CrossRef]
- Katayama, H.; Iwamoto, K.; Kariya, Y.; Asakawa, T.; Kan, T.; Fukuda, H.; Ohashi-Ito, K. A negative feedback loop controlling bHLH complexes is involved in vascular cell division and differentiation in the root apical meristem. Curr. Biol. 2015, 25, 3144–3150. [Google Scholar] [CrossRef] [Green Version]
- Vera-Sirera, F.; De Rybel, B.; Úrbez, C.; Kouklas, E.; Pesquera, M.; Álvarez-Mahecha, J.C.; Minguet, E.G.; Tuominen, H.; Carbonell, J.; Borst, J.W.; et al. A bHLH-based feedback loop restricts vascular cell proliferation in plants. Dev. Cell 2015, 35, 432–443. [Google Scholar] [CrossRef] [Green Version]
- Ohashi-Ito, K.; Saegusa, M.; Iwamoto, K.; Oda, Y.; Katayama, H.; Kojima, M.; Sakakibara, H.; Fukuda, H. A bHLH complex activates vascular cell division via cytokinin action in root apical meristem. Curr. Biol. 2014, 24, 2053–2508. [Google Scholar] [CrossRef] [Green Version]
- Mähönen, A.P.; Bishopp, A.; Higuchi, M.; Nieminen, K.M.; Kinoshita, K.; Törmäkangas, K.; Ikeda, Y.; Oka, A.; Kakimoto, T.; Helariutta, Y. Cytokinin signaling and its inhibitor AHP6 regulate cell fate during vascular development. Science 2006, 311, 94–98. [Google Scholar] [CrossRef] [PubMed]
- Arsovski, A.A.; Zemke, J.E.; Haagen, B.D.; Kim, S.H.; Nemhauser, J.L. Phytochrome B regulates resource allocation in Brassica rapa. J. Exp. Bot. 2018, 69, 2837–2846. [Google Scholar] [CrossRef] [PubMed]
- Moreno, J.C.; Mi, J.; Agrawal, S.; Kössler, S.; Turečková, V.; Tarkowská, D.; Thiele, W.; Al-Babili, S.; Bock, R.; Schöttler, M.A. Expression of a carotenogenic gene allows faster biomass production by redesigning plant architecture and improving photosynthetic efficiency in tobacco. Plant J. 2020, 103, 1967–1984. [Google Scholar] [CrossRef] [PubMed]
- Cho, S.H.; Yoo, S.C.; Zhang, H.; Pandeya, D.; Koh, H.J.; Hwang, J.Y.; Kim, G.T.; Paek, N.C. The rice narrow leaf2 and narrow leaf3 loci encode WUSCHEL-related homeobox 3A (OsWOX3A) and function in leaf, spikelet, tiller and lateral root development. New Phytol. 2013, 198, 1071–1084. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Chang, W.; Li, X.; Yang, B.; Zhang, L.Y.; Xiao, Z.C.; Li, J.N.; Lu, K. Transcriptome and Small RNA Sequencing Reveal the Mechanisms Regulating Harvest Index in Brassica napus. Front. Plant Sci. 2022, 13, 855486. [Google Scholar] [CrossRef] [PubMed]
- Liao, S.; Yan, J.; Xing, H.; Tu, Y.; Zhao, H.; Wang, G. Genetic basis of vascular bundle variations in rice revealed by genome-wide association study. Plant Sci. 2021, 302, 110715. [Google Scholar] [CrossRef]
- Zhu, X.G.; Long, S.P.; Ort, D.R. Improving photosynthetic efficiency for greater yield. Annu Rev Plant Biol. 2010, 61, 235–261. [Google Scholar] [CrossRef] [Green Version]
- Long, S.P.; Marshall-Colon, A.; Zhu, X.G. Meeting the global food demand of the future by engineering crop photosynthesis and yield potential. Cell 2015, 161, 56–66. [Google Scholar] [CrossRef] [Green Version]
- Simkin, A.J.; López-Calcagno, P.E.; Raines, C.A. Feeding the world: Improving photosynthetic efficiency for sustainable crop production. J. Exp. Bot. 2019, 70, 1119–1140. [Google Scholar] [CrossRef] [Green Version]
- Faralli, M.; Lawson, T. Natural genetic variation in photosynthesis: An untapped resource to increase crop yield potential? Plant J. 2020, 101, 518–528. [Google Scholar] [CrossRef]
- Bihmidine, S.; Hunter, C.T., 3rd; Johns, C.E.; Koch, K.E.; Braun, D.M. Regulation of assimilate import into sink organs: Update on molecular drivers of sink strength. Front. Plant Sci. 2013, 4, 177. [Google Scholar] [CrossRef] [Green Version]
- Braun, D.M.; Wang, L.; Ruan, Y.L. Understanding and manipulating sucrose phloem loading, unloading, metabolism, and signalling to enhance crop yield and food security. J. Exp. Bot. 2014, 65, 1713–1735. [Google Scholar] [CrossRef]
- Fernie, A.R.; Bachem, C.W.B.; Helariutta, Y.; Neuhaus, H.E.; Prat, S.; Ruan, Y.L.; Stitt, M.; Sweetlove, L.J.; Tegeder, M.; Wahl, V.; et al. Synchronization of developmental, molecular and metabolic aspects of source-sink interactions. Nat. Plants 2020, 6, 55–66. [Google Scholar] [CrossRef] [PubMed]
- Wen, S.; Neuhaus, H.E.; Cheng, J.; Bie, Z. Contributions of sugar transporters to crop yield and fruit quality. J. Exp. Bot. 2022, 73, 2275–2289. [Google Scholar] [CrossRef]
- Wang, L.; Lu, Q.; Wen, X.; Lu, C. Enhanced sucrose loading improves rice yield by increasing grain size. Plant Physiol. 2015, 169, 2848–2862. [Google Scholar] [CrossRef] [Green Version]
- Wang, G.; Wu, Y.; Ma, L.; Lin, Y.; Hu, Y.; Li, M.; Li, W.; Ding, Y.; Chen, L. Phloem loading in rice leaves depends strongly on the apoplastic pathway. J. Exp. Bot. 2021, 72, 3723–3738. [Google Scholar] [CrossRef]
- Wang, L.F.; Qi, X.X.; Huang, X.S.; Xu, L.L.; Jin, C.; Wu, J.; Zhang, S.L. Overexpression of sucrose transporter gene PbSUT2 from Pyrus bretschneideri, enhances sucrose content in Solanum lycopersicum fruit. Plant Physiol. Biochem. 2016, 105, 150–161. [Google Scholar] [CrossRef]
- Hackel, A.; Schauer, N.; Carrari, F.; Fernie, A.R.; Grimm, B.; Kühn, C. Sucrose transporter LeSUT1 and LeSUT2 inhibition affects tomato fruit development in different ways. Plant J. 2006, 45, 180–192. [Google Scholar] [CrossRef]
- Lu, M.Z.; Snyder, R.; Grant, J.; Tegeder, M. Manipulation of sucrose phloem and embryo loading affects pea leaf metabolism, carbon and nitrogen partitioning to sinks as well as seed storage pools. Plant J. 2020, 101, 217–236. [Google Scholar] [CrossRef]
- Xu, Y.; Tao, Y.; Cheung, L.S.; Fan, C.; Chen, L.Q.; Xu, S.; Perry, K.; Frommer, W.B.; Feng, L. Structures of bacterial homologues of SWEET transporters in two distinct conformations. Nature 2014, 515, 448–452. [Google Scholar] [CrossRef] [Green Version]
- Fei, H.; Yang, Z.; Lu, Q.; Wen, X.; Zhang, Y.; Zhang, A.; Lu, C. OsSWEET14 cooperates with OsSWEET11 to contribute to grain filling in rice. Plant Sci. 2021, 306, 110851. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.; Zhang, D.; Miao, Q.; Yang, J.; Xuan, Y.; Hu, Y. Essential role of sugar transporter OsSWEET11 during the early stage of rice grain Filling. Plant Cell Physiol. 2017, 58, 863–873. [Google Scholar] [CrossRef]
- Bezrutczyk, M.; Hartwig, T.; Horschman, M.; Char, S.N.; Yang, J.; Yang, B.; Frommer, W.B.; Sosso, D. Impaired phloem loading in zmsweet13a,b,c sucrose transporter triple knock-out mutants in Zea mays. New Phytol. 2018, 218, 594–603. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mathan, J.; Singh, A.; Ranjan, A. Sucrose transport and metabolism control carbon partitioning between stem and grain in rice. J. Exp. Bot. 2021, 72, 4355–4372. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Hou, P.; Zhu, L.; Song, W.; Liu, H.; Huang, Y.; Wang, H.; Guo, J. Genome-wide association study of vascular bundle-related traits in maize stalk. Front. Plant Sci. 2021, 12, 699486. [Google Scholar] [CrossRef]
- Anne, P.; Azzopardi, M.; Gissot, L.; Beaubiat, S.; Hématy, K.; Palauqui, J.C. OCTOPUS Negatively Regulates BIN2 to Control Phloem Differentiation in Arabidopsis thaliana. Curr. Biol. 2015, 25, 2584–2590. [Google Scholar] [CrossRef] [Green Version]
- Ruiz Sola, M.A.; Coiro, M.; Crivelli, S.; Zeeman, S.C.; Schmidt Kjølner Hansen, S.; Truernit, E. OCTOPUS-LIKE 2, a novel player in Arabidopsis root and vascular development, reveals a key role for OCTOPUS family genes in root metaphloem sieve tube differentiation. New Phytol. 2017, 216, 1191–1204. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Han, E.; Peng, Y.; Wang, Y.; Wang, Y.; Geng, Z.; Xu, Y.; Geng, H.; Qian, Y.; Ma, S. Rice co-expression network analysis identifies gene modules associated with agronomic traits. Plant Physiol. 2022, 190, 1526–1542. [Google Scholar] [CrossRef]
- Nam, H.; Gupta, A.; Nam, H.; Lee, S.; Cho, H.S.; Park, C.; Park, S.; Park, S.J.; Hwang, I. JULGI-mediated increment in phloem transport capacity relates to fruit yield in tomato. Plant Biotechnol. J. 2022, 20, 1533–1545. [Google Scholar] [CrossRef]
- Donald, C.M. Breeding of crop ideotypes. Euphytica 1968, 17, 385–403. [Google Scholar] [CrossRef]
- Khush, G.S. Breaking the yield frontier of rice. Geo. J. 1995, 35, 329–332. [Google Scholar] [CrossRef]
- Fu, T.; Zhou, Y. Progress and future development of hybrid rapeseed in China. Eng. Sci. 2013, 11, 13–18. [Google Scholar]
- Li, H.; Li, J.; Song, J.; Zhao, B.; Guo, C.; Wang, B.; Zhang, Q.; Wang, J.; King, G.J.; Liu, K. An auxin signaling gene BnaA3.IAA7 contributes to improved plant architecture and yield heterosis in rapeseed. New Phytol. 2019, 222, 837–851. [Google Scholar] [CrossRef]
- Duvick, D.N. Genetic progress in yield of United States maize (Zea mays L.). Maydica 2005, 50, 193–202. [Google Scholar]
- Pan, Q.; Xu, Y.; Li, K.; Peng, Y.; Zhan, W.; Li, W.; Li, L.; Yan, J. The Genetic Basis of Plant Architecture in 10 Maize Recombinant Inbred Line Populations. Plant Physiol. 2017, 175, 858–873. [Google Scholar] [CrossRef] [Green Version]
- McGarry, R.C.; Prewitt, S.F.; Culpepper, S.; Eshed, Y.; Lifschitz, E.; Ayre, B.G. Monopodial and sympodial branching architecture in cotton is differentially regulated by the Gossypium hirsutum SINGLE FLOWER TRUSS and SELF-PRUNING orthologs. New Phytol. 2016, 212, 244–258. [Google Scholar] [CrossRef]
- Wang, Y.; Li, J. Molecular basis of plant architecture. Annu. Rev. Plant Biol. 2008, 59, 253–279. [Google Scholar] [CrossRef]
- Wang, B.; Smith, M.S.; Li, J. Genetic regulation of shoot architecture. Annu. Rev. Plant Biol. 2018, 69, 437–468. [Google Scholar] [CrossRef]
- Pierik, R.; Fankhauser, C.; Strader, L.C.; Sinha, N. Architecture and plasticity: Optimizing plant performance in dynamic environments. Plant Physiol. 2021, 187, 1029–1032. [Google Scholar] [CrossRef]
- Wang, H.; Zhang, H.; Ma, Q.; Wei, L.; Ju, M.; Li, C.; Duan, Y.; Miao, H. Optimization of EMS mutagenesis condition and screening of mutants in sesame. J. Henan Agri. Sci. 2017, 46, 36–41. [Google Scholar]
- Miao, H.; Li, C.; Duan, Y.; Wei, L.; Ju, M.; Zhang, H. Identification of a Sidwf1 gene controlling short internode length trait in the sesame dwarf mutant dw607. Theor. Appl. Genet. 2020, 133, 73–86. [Google Scholar] [CrossRef] [PubMed]
- Mauriat, M.; Moritz, T. Analyses of GA20ox- and GID1-over-expressing aspen suggest that gibberellins play two distinct roles in wood formation. Plant J. 2009, 58, 989–1003. [Google Scholar] [CrossRef] [PubMed]
- Dayan, J.; Schwarzkopf, M.; Avni, A.; Aloni, R. Enhancing plant growth and fiber production by silencing GA 2-oxidase. Plant Biotechnol. J. 2010, 8, 425–435. [Google Scholar] [CrossRef] [PubMed]
- Voorend, W.; Nelissen, H.; Vanholme, R.; De Vliegher, A.; Van Breusegem, F.; Boerjan, W.; Roldán-Ruiz, I.; Muylle, H.; Inzé, D. Overexpression of GA20-OXIDASE1 impacts plant height, biomass allocation and saccharification efficiency in maize. Plant Biotechnol. J. 2016, 14, 997–1007. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yan, J.; Liao, X.; He, R.; Zhong, M.; Feng, P.; Li, X.; Tang, D.; Liu, X.; Zhao, X. Ectopic expression of GA 2-oxidase 6 from rapeseed (Brassica napus L.) causes dwarfism, late flowering and enhanced chlorophyll accumulation in Arabidopsis thaliana. Plant Physiol. Biochem. 2017, 111, 10–19. [Google Scholar] [CrossRef]
- Wuddineh, W.A.; Mazarei, M.; Zhang, J.; Poovaiah, C.R.; Mann, D.G.; Ziebell, A.; Sykes, R.W.; Davis, M.F.; Udvardi, M.K.; Stewart, C.N., Jr. Identification and overexpression of gibberellin 2-oxidase (GA2ox) in switchgrass (Panicum virgatum L.) for improved plant architecture and reduced biomass recalcitrance. Plant Biotechnol. J. 2015, 13, 636–647. [Google Scholar] [CrossRef] [Green Version]
- Yang, R.; Wu, Z.; Bai, C.; Sun, Z.; Wang, M.; Huo, Y.; Zhang, H.; Wang, Y.; Zhou, H.; Dai, S.; et al. Overexpression of PvWOX3a in switchgrass promotes stem development and increases plant height. Hortic. Res. 2021, 8, 252. [Google Scholar] [CrossRef]
- Wang, J.; Zhu, J.; Lin, Q.; Li, X.; Teng, N.; Li, Z.; Li, B.; Zhang, A.; Lin, J. Effects of the stem structure and cell wall components on bending strength in wheat. Chin. Sci. Bull. 2006, 51, 815–823. [Google Scholar] [CrossRef] [Green Version]
- Packa, D.; Wiwart, M.; Suchowilska, E.; Bienkowska, E. Morpho-anatomical traits of two lowest internodes related to lodging resistance in selected genotypes of Triticum. Int. Agrophy. 2015, 29, 475–483. [Google Scholar] [CrossRef] [Green Version]
- Muszynska, A.; Guendel, A.; Melzer, M.; Tandron Moya, Y.A.; Röder, M.S.; Rolletschek, H.; Rutten, T.; Munz, E.; Melz, G.; Ortleb, S.; et al. A mechanistic view on lodging resistance in rye and wheat: A multiscale comparative study. Plant Biotechnol. J. 2021, 19, 2646–2661. [Google Scholar] [CrossRef]
- Zhou, F.; Huang, J.; Liu, W.; Deng, T.; Jia, Z. Multiscale simulation of elastic modulus of rice stem. Biosys. Eng. 2019, 187, 96–113. [Google Scholar] [CrossRef]
- Lee, S.; Zargar, O.; Reiser, C.; Li, Q.; Muliana, A.; Finlayson, S.A.; Gomez, F.E.; Pharr, M. Time-dependent mechanical behavior of sweet sorghum stems. J. Mech. Behav. Biomed. Mater. 2020, 106, 103731. [Google Scholar] [CrossRef]
- Wu, W.; Ma, B.L. The mechanical roles of the clasping leaf sheath in cereals: Two case studies from oat and wheat plants. J. Agron. Crop Sci. 2020, 206, 118–129. [Google Scholar] [CrossRef]
- Wojtowicz, T.; Grabowska-Joachimiak, A.; Zielinski, A. Analysis of morpho-anatomical stem properties determining its mechanical strength in selected rye cultivars. Int. Agrophy. 2020, 34, 123–131. [Google Scholar] [CrossRef]
- McClung, C.R. Making hunger yield. Science 2014, 344, 699–700. [Google Scholar] [CrossRef]
- Jiao, Y.; Wang, Y.; Xue, D.; Wang, J.; Yan, M.; Liu, G.; Dong, G.; Zeng, D.; Lu, Z.; Zhu, X.; et al. Regulation of OsSPL14 by OsmiR156 defines ideal plant architecture in rice. Nat. Genet. 2010, 42, 541–544. [Google Scholar] [CrossRef]
- Sang, D.; Chen, D.; Liu, G.; Liang, Y.; Huang, L.; Meng, X.; Chu, J.; Sun, X.; Dong, G.; Yuan, Y.; et al. Strigolactones regulate rice tiller angle by attenuating shoot gravitropism through inhibiting auxin biosynthesis. Proc. Natl. Acad. Sci. USA 2014, 111, 11199–11204. [Google Scholar] [CrossRef] [Green Version]
- Zhang, L.; Yu, H.; Ma, B.; Liu, G.; Wang, J.; Wang, J.; Gao, R.; Li, J.; Liu, J.; Xu, J.; et al. A natural tandem array alleviates epigenetic repression of IPA1 and leads to superior yielding rice. Nat. Commun. 2017, 8, 14789. [Google Scholar] [CrossRef] [Green Version]
- Terao, T.; Nagata, K.; Morino, K.; Hirose, T. A gene controlling the number of primary rachis branches also controls the vascular bundle formation and hence is responsible to increase the harvest index and grain yield in rice. Theor. Appl. Genet. 2010, 120, 875–893. [Google Scholar] [CrossRef]
- Muqaddasi, Q.H.; Brassac, J.; Koppolu, R.; Plieske, J.; Ganal, M.W.; Röder, M.S. TaAPO-A1, an ortholog of rice ABERRANT PANICLE ORGANIZATION 1, is associated with total spikelet number per spike in elite European hexaploid winter wheat (Triticum aestivum L.) varieties. Sci. Rep. 2019, 9, 13853. [Google Scholar] [CrossRef] [Green Version]
- Ikeda, K.; Nagasawa, N.; Nagato, Y. ABERRANT PANICLE ORGANIZATION 1 temporally regulates meristem identity in rice. Dev. Biol. 2005, 282, 349–360. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, L.; Hua, K.; Xu, R.; Zeng, D.; Wang, R.; Dong, G.; Zhang, G.; Lu, X.; Fang, N.; Wang, D.; et al. The LARGE2-APO1/APO2 regulatory module controls panicle size and grain number in rice. Plant Cell 2021, 33, 1212–1228. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Qian, Q.; Liu, Z.; Sun, H.; He, S.; Luo, D.; Xia, G.; Chu, C.; Li, J.; Fu, X. Natural variation at the DEP1 locus enhances grain yield in rice. Nat. Genet. 2009, 41, 494–497. [Google Scholar] [CrossRef] [PubMed]
- Jiang, D.; Chen, W.; Dong, J.; Li, J.; Yang, F.; Wu, Z.; Zhou, H.; Wang, W.; Zhuang, C. Overexpression of miR164b-resistant OsNAC2 improves plant architecture and grain yield in rice. J. Exp. Bot. 2018, 69, 1533–1543. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, S.Q.; Hu, J.; Guo, L.B.; Qian, Q.; Xue, H.W. Rice leaf inclination2, a VIN3-like protein, regulates leaf angle through modulating cell division of the collar. Cell Res. 2010, 20, 935–947. [Google Scholar] [CrossRef]
- Ning, J.; Zhang, B.; Wang, N.; Zhou, Y.; Xiong, L. Increased leaf angle1, a Raf-like MAPKKK that interacts with a nuclear protein family, regulates mechanical tissue formation in the Lamina joint of rice. Plant Cell 2011, 23, 4334–4347. [Google Scholar] [CrossRef] [Green Version]
- Wang, S.; Zhang, F.; Jiang, P.; Zhang, H.; Zheng, H.; Chen, R.; Xu, Z.; Ikram, A.U.; Li, E.; Xu, Z.; et al. SDG128 is involved in maize leaf inclination. Plant J. 2021, 108, 1597–1608. [Google Scholar] [CrossRef]
- Sangoi, L. Understanding plant density effects on maize growth and development: An important issue to maximize grain yield. Cienc. Rural. 2001, 31, 159–168. [Google Scholar] [CrossRef] [Green Version]
- Jin, L.; Cui, H.; Li, B.; Zhang, J.; Dong, S.; Liu, P. Effects of integrated agronomic management practices on yield and nitrogen efficiency of summer maize in North China. Field Crops Res. 2012, 134, 30–35. [Google Scholar] [CrossRef]
- Piao, L.; Qi, H.; Li, C.F.; Zhao, M. Optimized tillage practices and row spacing to improve grain yield and matter transport efficiency in intensive spring maize. Field Crops Res. 2016, 198, 258–268. [Google Scholar] [CrossRef]
- Mueller, N.D.; Gerber, J.S.; Johnston, M.; Ray, D.K.; Ramankutty, N.; Foley, J.A. Closing yield gaps through nutrient and water management. Nature 2012, 490, 254–257. [Google Scholar] [CrossRef]
- Ren, H.; Jiang, Y.; Zhao, M.; Qi, H.; Li, C. Nitrogen supply regulates vascular bundle structure and matter transport characteristics of spring maize under high plant density. Front Plant Sci. 2021, 11, 602739. [Google Scholar] [CrossRef]
- Zhou, W.; Lv, T.; Zhang, P.; Huang, Y.; Chen, Y.; Ren, W. Regular nitrogen application increases nitrogen utilization efficiency and grain yield in indica Hybrid Rice. Agron. J. 2016, 108, 1951–1961. [Google Scholar] [CrossRef]
- Zhou, W.; Lv, T.; Yang, Z.; Wang, T.; Fu, Y.; Chen, Y.; Hu, B.; Ren, W. Morphophysiological mechanism of rice yield increase in response to optimized nitrogen management. Sci. Rep. 2017, 7, 17226. [Google Scholar] [CrossRef] [Green Version]
- Pan, J.; Cui, K.; Wei, D.; Huang, J.; Xiang, J.; Nie, L. Relationships of non-structural carbohydrates accumulation and translocation with yield formation in rice recombinant inbred lines under two nitrogen levels. Physiol. Plant 2011, 141, 321–331. [Google Scholar] [CrossRef]
- Gasura, E.; Setimela, P.; Edema, R.; Gibson, P.T.; Okori, P.; Tarekegne, A. Exploiting grain-filling rate and effective grain-filling duration to improve grain yield of early-maturing maize. Crop Sci. 2013, 53, 2295–2303. [Google Scholar] [CrossRef] [Green Version]
- Xu, C.; Gao, Y.; Tian, B.; Ren, J.; Meng, Q.; Wang, P. Effects of EDAH, a novel plant growth regulator, on mechanical strength, stalk vascular bundles and grain yield of summer maize at high densities. Field Crops Res. 2017, 200, 71–79. [Google Scholar] [CrossRef]
- Chen, M.; Chory, J.; Fankhauser, C. Light signal transduction in higher plants. Annu. Rev. Genet. 2004, 38, 87–117. [Google Scholar] [CrossRef] [Green Version]
- Lee, K.M.; Lim, C.S.; Muneer, S.; Jeong, B.R. Functional vascular connections and light quality effects on tomato grafted unions. Sci. Hortic. 2016, 201, 306–317. [Google Scholar] [CrossRef]
- Bantis, F.; Panteris, E.; Dangitsis, C.; Carrera, E.; Koukounaras, A. Blue light promotes vascular reconnection, while red light boosts the physiological response and quality of grafted watermelon seedlings. Sci. Rep. 2021, 11, 21754. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, J.; Du, J.; Zhao, Y.; Lu, X.; Wen, W.; Gu, S.; Fan, J.; Wang, C.; Wu, S.; et al. Dissecting the phenotypic components and genetic architecture of maize stem vascular bundles using high-throughput phenotypic analysis. Plant Biotechnol. J. 2021, 19, 35–50. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.; Wang, M.; Chen, Y.; Nomura, K.; Hui, S.; Gui, J.; Zhang, X.; Wu, Y.; Liu, J.; Li, Q.; et al. An MKP-MAPK protein phosphorylation cascade controls vascular immunity in plants. Sci. Adv. 2022, 8, eabg8723. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chang, W.; Chen, H.; Jiao, G.; Dou, Y.; Liu, L.; Qu, C.; Li, J.; Lu, K. Biomolecular Strategies for Vascular Bundle Development to Improve Crop Yield. Biomolecules 2022, 12, 1772. https://doi.org/10.3390/biom12121772
Chang W, Chen H, Jiao G, Dou Y, Liu L, Qu C, Li J, Lu K. Biomolecular Strategies for Vascular Bundle Development to Improve Crop Yield. Biomolecules. 2022; 12(12):1772. https://doi.org/10.3390/biom12121772
Chicago/Turabian StyleChang, Wei, Hongqiao Chen, Guixiang Jiao, Yi Dou, Lin Liu, Cunmin Qu, Jiana Li, and Kun Lu. 2022. "Biomolecular Strategies for Vascular Bundle Development to Improve Crop Yield" Biomolecules 12, no. 12: 1772. https://doi.org/10.3390/biom12121772
APA StyleChang, W., Chen, H., Jiao, G., Dou, Y., Liu, L., Qu, C., Li, J., & Lu, K. (2022). Biomolecular Strategies for Vascular Bundle Development to Improve Crop Yield. Biomolecules, 12(12), 1772. https://doi.org/10.3390/biom12121772







