Noncoding RNAs Are Promising Therapeutic Targets for Diabetic Retinopathy: An Updated Review (2017–2022)
Abstract
1. Introduction
2. miRNAs
2.1. ECs
2.2. Pericytes
2.3. Retinal Ganglion Cells
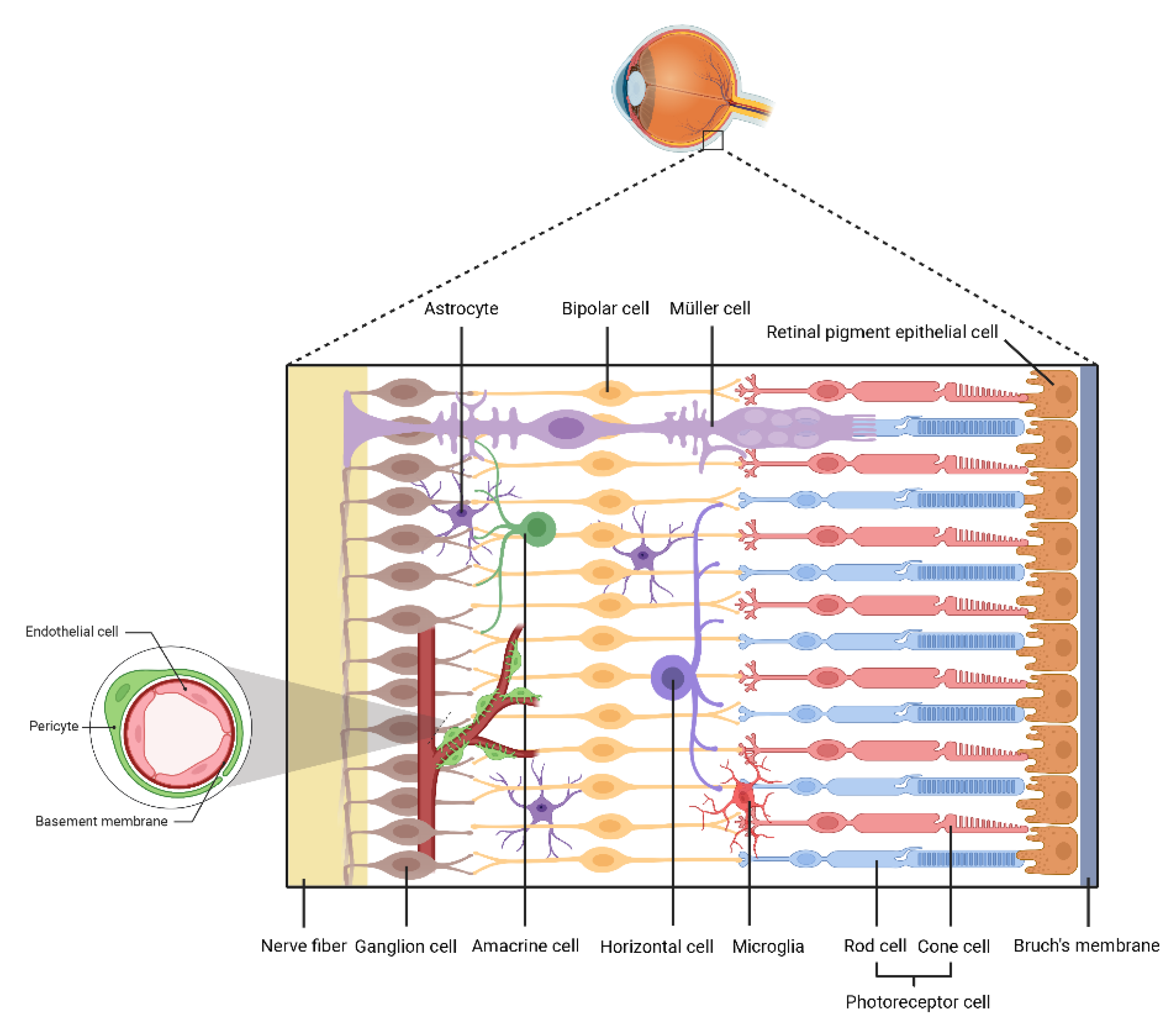
2.4. Glial Cells
2.5. RPE Cells
2.6. Blood and Tears
2.7. EVs
2.8. AH
3. circRNA
3.1. ECs
3.2. Pericytes
3.3. RPEs
3.4. Blood and Tears
3.5. VH
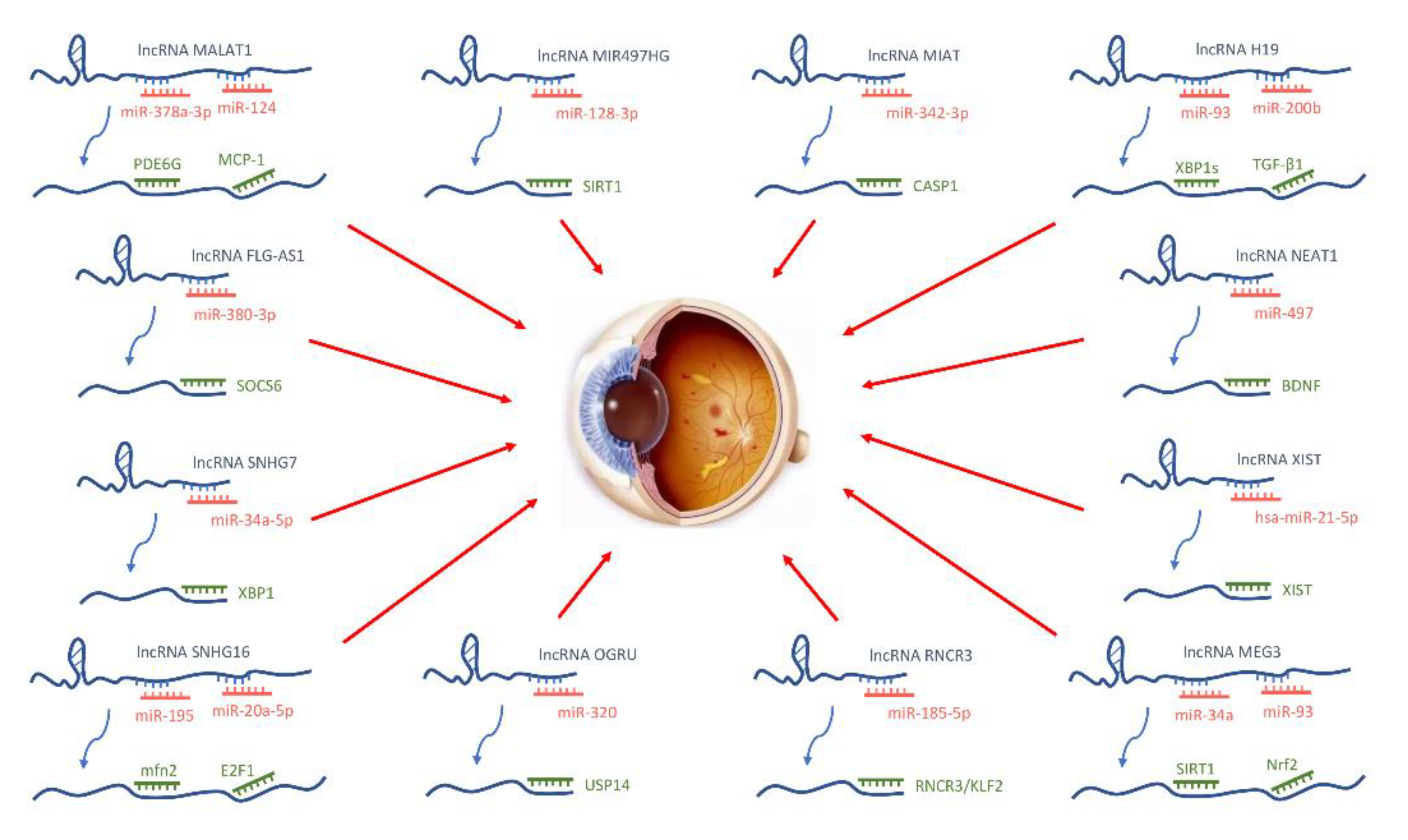
4. IncRNAs
4.1. ECs
4.2. Pericytes
4.3. Gangliocytes
4.4. Glial Cells
4.5. RPEs
4.6. Blood and Tears
4.7. Exosomes
5. Drug Regulation
5.1. Clinical Medicine
5.2. Preclinical Medicine
6. Perspective
7. Literature Search
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kollias, A.N.; Ulbig, M.W. Diabetic retinopathy: Early diagnosis and effective treatment. Dtsch. Arztebl. Int. 2010, 107, 75–83, quiz 84. [Google Scholar] [PubMed]
- Wang, W.; Lo, A.C.Y. Diabetic Retinopathy: Pathophysiology and Treatments. Int. J. Mol. Sci. 2018, 19, 1816. [Google Scholar] [CrossRef] [PubMed]
- Noma, H.; Yasuda, K.; Shimura, M. Involvement of Cytokines in the Pathogenesis of Diabetic Macular Edema. Int. J. Mol. Sci. 2021, 22, 3427. [Google Scholar] [CrossRef] [PubMed]
- Masland, R.H. The fundamental plan of the retina. Nat. Neurosci. 2001, 4, 877–886. [Google Scholar] [CrossRef] [PubMed]
- Antonetti, D.A.; Silva, P.S.; Stitt, A.W. Current understanding of the molecular and cellular pathology of diabetic retinopathy. Nat. Rev. Endocrinol. 2021, 17, 195–206. [Google Scholar] [CrossRef]
- Cheung, N.; Mitchell, P.; Wong, T.Y. Diabetic retinopathy. Lancet 2010, 376, 124–136. [Google Scholar] [CrossRef]
- Glassman, A.R.; Wells, J.A., 3rd; Josic, K.; Maguire, M.G.; Antoszyk, A.N.; Baker, C.; Beaulieu, W.T.; Elman, M.J.; Jampol, L.M.; Sun, J.K. Five-Year Outcomes after Initial Aflibercept, Bevacizumab, or Ranibizumab Treatment for Diabetic Macular Edema (Protocol T Extension Study). Ophthalmology 2020, 127, 1201–1210. [Google Scholar] [CrossRef]
- Fu, Z.; Wang, Z.; Liu, C.H.; Gong, Y.; Cakir, B.; Liegl, R.; Sun, Y.; Meng, S.S.; Burnim, S.B.; Arellano, I.; et al. Fibroblast Growth Factor 21 Protects Photoreceptor Function in Type 1 Diabetic Mice. Diabetes 2018, 67, 974–985. [Google Scholar] [CrossRef]
- Chang, X.; Zhu, G.; Cai, Z.; Wang, Y.; Lian, R.; Tang, X.; Ma, C.; Fu, S. miRNA, lncRNA and circRNA: Targeted Molecules Full of Therapeutic Prospects in the Development of Diabetic Retinopathy. Front. Endocrinol. (Lausanne) 2021, 12, 771552. [Google Scholar] [CrossRef]
- Chen, L.; Heikkinen, L.; Wang, C.; Yang, Y.; Sun, H.; Wong, G. Trends in the development of miRNA bioinformatics tools. Brief. Bioinform. 2019, 20, 1836–1852. [Google Scholar]
- Lima, R.T.; Busacca, S.; Almeida, G.M.; Gaudino, G.; Fennell, D.A.; Vasconcelos, M.H. MicroRNA regulation of core apoptosis pathways in cancer. Eur. J. Cancer 2011, 47, 163–174. [Google Scholar] [CrossRef] [PubMed]
- Sayed, D.; Abdellatif, M. MicroRNAs in development and disease. Physiol. Rev. 2011, 91, 827–887. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Sun, X.; Icli, B.; Feinberg, M.W. Emerging Roles for MicroRNAs in Diabetic Microvascular Disease: Novel Targets for Therapy. Endocr. Rev. 2017, 38, 145–168. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Qiu, F.; Zhou, K.; Matlock, H.G.; Takahashi, Y.; Rajala, R.V.S.; Yang, Y.; Moran, E.; Ma, J.X. Pathogenic Role of microRNA-21 in Diabetic Retinopathy Through Downregulation of PPARα. Diabetes 2017, 66, 1671–1682. [Google Scholar] [CrossRef]
- Bharadwaj, A.S.; Appukuttan, B.; Wilmarth, P.A.; Pan, Y.; Stempel, A.J.; Chipps, T.J.; Benedetti, E.E.; Zamora, D.O.; Choi, D.; David, L.L.; et al. Role of the retinal vascular endothelial cell in ocular disease. Prog. Retin. Eye Res. 2013, 32, 102–180. [Google Scholar]
- Gu, C.; Draga, D.; Zhou, C.; Su, T.; Zou, C.; Gu, Q.; Lahm, T.; Zheng, Z.; Qiu, Q. miR-590-3p Inhibits Pyroptosis in Diabetic Retinopathy by Targeting NLRP1 and Inactivating the NOX4 Signaling Pathway. Investig. Ophthalmol. Vis. Sci. 2019, 60, 4215–4223. [Google Scholar] [CrossRef]
- Zheng, Y.; Liu, Y.; Wang, L.; Xu, H.; Lu, Z.; Xuan, Y.; Meng, W.; Ye, L.; Fang, D.; Zhou, Y.; et al. MicroRNA-126 suppresses the proliferation and migration of endothelial cells in experimental diabetic retinopathy by targeting polo-like kinase 4. Int. J. Mol. Med. 2021, 47, 151–160. [Google Scholar] [CrossRef]
- Wang, Y.; Gao, L.; Li, Z.; Ma, X. MicroRNA-301a-3p promotes diabetic retinopathy via regulation of six-transmembrane epithelial antigen of prostate 4. Inflamm. Res. Off. J. Eur. Histamine Res. Soc. 2021, 70, 445–457. [Google Scholar] [CrossRef]
- Zhao, H.; He, Y. MiR-124-3p Suppresses the Dysfunction of High Glucose-Stimulated Endothelial Cells by Targeting G3BP2. Front. Genet. 2021, 12, 723625. [Google Scholar] [CrossRef]
- Yang, Y.; Liu, Y.; Li, Y.; Chen, Z.; Xiong, Y.; Zhou, T.; Tao, W.; Xu, F.; Yang, H.; Ylä-Herttuala, S.; et al. MicroRNA-15b Targets VEGF and Inhibits Angiogenesis in Proliferative Diabetic Retinopathy. J. Clin. Endocrinol. Metab. 2020, 105, 3404–3415. [Google Scholar] [CrossRef]
- Yu, B.; Xiao, M.; Yang, F.; Xiao, J.; Zhang, H.; Su, L.; Zhang, X.; Li, X. MicroRNA-431-5p encapsulated in serum extracellular vesicles as a biomarker for proliferative diabetic retinopathy. Int. J. Biochem. Cell Biol. 2021, 135, 105975. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Xu, H.; Zhao, H.; Sui, D. MicroRNA-135b-5p promotes endothelial cell proliferation and angiogenesis in diabetic retinopathy mice by inhibiting Von Hipp-el-Lindau and elevating hypoxia inducible factor α expression. J. Drug Target. 2021, 29, 300–309. [Google Scholar] [CrossRef] [PubMed]
- Duan, P.; Chen, S.; Zeng, Y.; Xu, H.; Liu, Y. Osteopontin Upregulates Col IV Expression by Repressing miR-29a in Human Retinal Capillary Endothelial Cells. Mol. Nucleic Acids 2020, 20, 242–251. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Song, C.; Wang, T.; Sun, L.; Qin, L.; Ju, J. miR-139-5p promotes neovascularization in diabetic retinopathy by regulating the phosphatase and tensin homolog. Arch. Pharmacal. Res. 2021, 44, 205–218. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Q.; Zhao, Y.; Sun, H.; Xu, J.; Li, W.; Gao, L. MiR-423-5p activated by E2F1 promotes neovascularization in diabetic retinopathy by targeting HIPK2. Diabetol. Metab. Syndr. 2021, 13, 152. [Google Scholar] [CrossRef]
- Xiao, Q.; Zhao, Y.; Xu, J.; Li, W.J.; Chen, Y.; Sun, H.J. NFE2/miR-423-5p/TFF1 axis regulates high glucose-induced apoptosis in retinal pigment epithelial cells. BMC Mol. Cell Biol. 2019, 20, 39. [Google Scholar] [CrossRef]
- Caporarello, N.; D’Angeli, F.; Cambria, M.T.; Candido, S.; Giallongo, C.; Salmeri, M.; Lombardo, C.; Longo, A.; Giurdanella, G.; Anfuso, C.D.; et al. Pericytes in Microvessels: From "Mural" Function to Brain and Retina Regeneration. Int. J. Mol. Sci. 2019, 20, 6351. [Google Scholar] [CrossRef]
- Bao, X.Y.; Cao, J. MiRNA-138-5p protects the early diabetic retinopathy by regulating NOVA1. Eur. Rev. Med. Pharmacol. Sci. 2019, 23, 7749–7756. [Google Scholar]
- Zhou, L.; Zhang, S.; Zhang, L.; Li, F.; Sun, H.; Feng, J. MiR-199a-3p inhibits the proliferation, migration, and invasion of endothelial cells and retinal pericytes of diabetic retinopathy rats through regulating FGF7 via EGFR/PI3K/AKT pathway. J. Recept. Signal Transduct. Res. 2021, 41, 19–31. [Google Scholar] [CrossRef]
- Xu, Y.; Xie, S.C.; Ma, Y.C. Low expression of microRNA-15b promotes the proliferation of retinal capillary endothelial cells and pericytes by up-regulating VEGFA in diabetic rats. Eur. Rev. Med. Pharmacol. Sci. 2019, 23, 6018–6025. [Google Scholar]
- Baden, T.; Berens, P.; Franke, K.; Román Rosón, M.; Bethge, M.; Euler, T. The functional diversity of retinal ganglion cells in the mouse. Nature 2016, 529, 345–350. [Google Scholar] [CrossRef] [PubMed]
- Parisi, V.; Oddone, F.; Ziccardi, L.; Roberti, G.; Coppola, G.; Manni, G. Citicoline and Retinal Ganglion Cells: Effects on Morphology and Function. Curr. Neuropharmacol. 2018, 16, 919–932. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Yang, Y.; Feng, Z. Suppression of microRNA-495 alleviates high-glucose-induced retinal ganglion cell apoptosis by regulating Notch/PTEN/Akt signaling. Biomed. Pharmacother. = Biomed. Pharmacother. 2018, 106, 923–929. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Cui, C.; Xu, H. Downregulation of miR-145-5p elevates retinal ganglion cell survival to delay diabetic retinopathy progress by targeting FGF5. Biosci. Biotechnol. Biochem. 2019, 83, 1655–1662. [Google Scholar] [CrossRef]
- Reichenbach, A.; Bringmann, A. Glia of the human retina. Glia 2020, 68, 768–796. [Google Scholar] [CrossRef]
- Luo, Y.; Dong, X.; Lu, S.; Gao, Y.; Sun, G.; Sun, X. Gypenoside XVII alleviates early diabetic retinopathy by regulating Müller cell apoptosis and autophagy in db/db mice. Eur. J. Pharmacol. 2021, 895, 173893. [Google Scholar] [CrossRef]
- Goldman, D. Müller glial cell reprogramming and retina regeneration. Nat. Rev. Neurosci. 2014, 15, 431–442. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, J.; Chen, X.; Yang, Y.; Wang, F.; Li, W.; Awuti, M.; Sun, Y.; Lian, C.; Li, Z.; et al. miR-365 promotes diabetic retinopathy through inhibiting Timp3 and increasing oxidative stress. Exp. Eye Res. 2018, 168, 89–99. [Google Scholar] [CrossRef]
- Liu, Y.; Yang, Q.; Fu, H.; Wang, J.; Yuan, S.; Li, X.; Xie, P.; Hu, Z.; Liu, Q. Müller glia-derived exosomal miR-9-3p promotes angiogenesis by restricting sphingosine-1-phosphate receptor S1P(1) in diabetic retinopathy. Mol. Nucleic Acids 2022, 27, 491–504. [Google Scholar] [CrossRef]
- Hwang, S.J.; Ahn, B.J.; Shin, M.W.; Song, Y.S.; Choi, Y.; Oh, G.T.; Kim, K.W.; Lee, H.J. miR-125a-5p attenuates macrophage-mediated vascular dysfunction by targeting Ninjurin1. Cell Death Differ. 2022, 29, 1199–1210. [Google Scholar] [CrossRef]
- Zhang, J.; Wu, L.; Chen, J.; Lin, S.; Cai, D.; Chen, C.; Chen, Z. Downregulation of MicroRNA 29a/b exacerbated diabetic retinopathy by impairing the function of Müller cells via Forkhead box protein O4. Diabetes Vasc. Dis. Res. 2018, 15, 214–222. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Stepicheva, N.A.; Ghosh, S.; Shang, P.; Chowdhury, O.; Daley, R.A.; Yazdankhah, M.; Gupta, U.; Hose, S.L.; Valapala, M.; et al. Reducing Akt2 in retinal pigment epithelial cells causes a compensatory increase in Akt1 and attenuates diabetic retinopathy. Nat. Commun. 2022, 13, 6045. [Google Scholar] [CrossRef]
- Ao, J.; Wood, J.P.; Chidlow, G.; Gillies, M.C.; Casson, R.J. Retinal pigment epithelium in the pathogenesis of age-related macular degeneration and photobiomodulation as a potential therapy? Clin. Exp. Ophthalmol. 2018, 46, 670–686. [Google Scholar] [CrossRef] [PubMed]
- Fu, S.H.; Lai, M.C.; Zheng, Y.Y.; Sun, Y.W.; Qiu, J.J.; Gui, F.; Zhang, Q.; Liu, F. MiR-195 inhibits the ubiquitination and degradation of YY1 by Smurf2, and induces EMT and cell permeability of retinal pigment epithelial cells. Cell Death Dis. 2021, 12, 708. [Google Scholar] [CrossRef]
- Liu, J.; Hou, Y.; Lin, L.; Yu, N.; Zhang, Y. MicroRNA-5195-3p alleviates high glucose-induced injury in human ARPE-19 cells by targeting GMFB. PLoS ONE 2021, 16, e0260071. [Google Scholar] [CrossRef] [PubMed]
- Sanguineti, R.; Puddu, A.; Nicolò, M.; Traverso, C.E.; Cordera, R.; Viviani, G.L.; Maggi, D. miR-126 Mimic Counteracts the Increased Secretion of VEGF-A Induced by High Glucose in ARPE-19 Cells. J. Diabetes Res. 2021, 2021, 6649222. [Google Scholar] [CrossRef]
- Shao, K.; Chen, G.; Xia, L.; Chen, C.; Huang, S. MicroRNA-139-5p Alleviates High Glucose-Triggered Human Retinal Pigment Epithelial Cell Injury by Targeting LIM-Only Factor 4. Mediat. Inflamm. 2021, 2021, 1629783. [Google Scholar] [CrossRef]
- Jadeja, R.N.; Jones, M.A.; Abdelrahman, A.A.; Powell, F.L.; Thounaojam, M.C.; Gutsaeva, D.; Bartoli, M.; Martin, P.M. Inhibiting microRNA-144 potentiates Nrf2-dependent antioxidant signaling in RPE and protects against oxidative stress-induced outer retinal degeneration. Redox Biol. 2020, 28, 101336. [Google Scholar] [CrossRef]
- Wang, M.; Zheng, H.; Zhou, X.; Zhang, J.; Shao, G. miR-122 promotes diabetic retinopathy through targeting TIMP3. Anim. Cells Syst. 2020, 24, 275–281. [Google Scholar] [CrossRef]
- Pastukh, N.; Meerson, A.; Kalish, D.; Jabaly, H.; Blum, A. Serum miR-122 levels correlate with diabetic retinopathy. Clin. Exp. Med. 2019, 19, 255–260. [Google Scholar] [CrossRef]
- Safi, H.; Safi, S.; Hafezi-Moghadam, A.; Ahmadieh, H. Early detection of diabetic retinopathy. Surv. Ophthalmol. 2018, 63, 601–608. [Google Scholar] [CrossRef] [PubMed]
- Kaštelan, S.; Orešković, I.; Bišćan, F.; Kaštelan, H.; Gverović Antunica, A. Inflammatory and angiogenic biomarkers in diabetic retinopathy. Biochem. Med. 2020, 30, 030502. [Google Scholar] [CrossRef]
- Platania, C.B.M.; Maisto, R.; Trotta, M.C.; D’Amico, M.; Rossi, S.; Gesualdo, C.; D’Amico, G.; Balta, C.; Herman, H.; Hermenean, A.; et al. Retinal and circulating miRNA expression patterns in diabetic retinopathy: An in silico and in vivo approach. Br. J. Pharmacol. 2019, 176, 2179–2194. [Google Scholar]
- Liu, H.N.; Cao, N.J.; Li, X.; Qian, W.; Chen, X.L. Serum microRNA-211 as a biomarker for diabetic retinopathy via modulating Sirtuin 1. Biochem. Biophys. Res. Commun. 2018, 505, 1236–1243. [Google Scholar] [CrossRef] [PubMed]
- Shi, R.; Chen, L.; Wang, W.; Deng, Y.; Liu, Y.; Zhou, H.; Lin, R. Plasma miR-26a-5p is a biomarker for retinal neurodegeneration of early diabetic retinopathy. Eye 2021, 35, 1587–1599. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Liu, X.; Zuo, Z. Regulatory role of miRNA-23a in diabetic retinopathy. Exp. Ther. Med. 2021, 22, 1477. [Google Scholar] [CrossRef] [PubMed]
- Greco, M.; Chiefari, E.; Accattato, F.; Corigliano, D.M.; Arcidiacono, B.; Mirabelli, M.; Liguori, R.; Brunetti, F.S.; Pullano, S.A.; Scorcia, V.; et al. MicroRNA-1281 as a Novel Circulating Biomarker in Patients With Diabetic Retinopathy. Front. Endocrinol. (Lausanne) 2020, 11, 528. [Google Scholar] [CrossRef]
- Alexandru, N.; Procopciuc, A.; Vîlcu, A.; Comariţa, I.K.; Bădilă, E.; Georgescu, A. Extracellular vesicles-incorporated microRNA signature as biomarker and diagnosis of prediabetes state and its complications. Rev. Endocr. Metab. Disord. 2021, 23, 309–332. [Google Scholar] [CrossRef]
- Gu, C.; Zhang, H.; Gao, Y. Adipose mesenchymal stem cells-secreted extracellular vesicles containing microRNA-192 delays diabetic retinopathy by targeting ITGA1. J. Cell Physiol. 2021, 236, 5036–5051. [Google Scholar] [CrossRef]
- Xu, Z.; Tian, N.; Li, S.; Li, K.; Guo, H.; Zhang, H.; Jin, H.; An, M.; Yu, X. Extracellular vesicles secreted from mesenchymal stem cells exert anti-apoptotic and anti-inflammatory effects via transmitting microRNA-18b in rats with diabetic retinopathy. Int. Immunopharmacol. 2021, 101 Pt B, 108234. [Google Scholar] [CrossRef]
- Martins, B.; Amorim, M.; Reis, F.; Ambrósio, A.F.; Fernandes, R. Extracellular Vesicles and MicroRNA: Putative Role in Diagnosis and Treatment of Diabetic Retinopathy. Antioxidants 2020, 9, 705. [Google Scholar] [CrossRef] [PubMed]
- Tiwari, A.; Singh, A.; Verma, S.; Stephenson, S.; Bhowmick, T.; Sangwan, V.S. Mini Review: Current Trends and Understanding of Exosome Therapeutic Potential in Corneal Diseases. Front. Pharmacol. 2021, 12, 684712. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Jiang, F.; Jiang, Y.; Wang, Y.; Li, Z.; Shi, X.; Zhu, Y.; Wang, H.; Zhang, Z. Roles of Exosomes in Ocular Diseases. Int. J. Nanomed. 2020, 15, 10519–10538. [Google Scholar] [CrossRef]
- Santovito, D.; Toto, L.; De Nardis, V.; Marcantonio, P.; D’Aloisio, R.; Mastropasqua, A.; De Cesare, D.; Bucci, M.; Paganelli, C.; Natarelli, L.; et al. Plasma microRNA signature associated with retinopathy in patients with type 2 diabetes. Sci. Rep. 2021, 11, 4136. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.C.; Xie, H.; Zhang, Y.C.; Meng, Q.H.; Xiong, M.M.; Jia, M.W.; Peng, F.; Tang, D.L. Exosomal miR-107 antagonizes profibrotic phenotypes of pericytes by targeting a pathway involving HIF-1α/Notch1/PDGFRβ/YAP1/Twist1 axis in vitro. Am. J. Physiol. Heart Circ. Physiol. 2021, 320, H520–H534. [Google Scholar] [CrossRef]
- Li, W.; Jin, L.; Cui, Y.; Nie, A.; Xie, N.; Liang, G. Bone marrow mesenchymal stem cells-induced exosomal microRNA-486-3p protects against diabetic retinopathy through TLR4/NF-κB axis repression. J. Endocrinol. Investig. 2021, 44, 1193–1207. [Google Scholar] [CrossRef]
- Li, W.; Jin, L.Y.; Cui, Y.B.; Xie, N. Human umbilical cord mesenchymal stem cells-derived exosomal microRNA-17-3p ameliorates inflammatory reaction and antioxidant injury of mice with diabetic retinopathy via targeting STAT1. Int. Immunopharmacol. 2021, 90, 107010. [Google Scholar] [CrossRef]
- Gu, S.; Liu, Y.; Zou, J.; Wang, W.; Wei, T.; Wang, X.; Zhu, L.; Zhang, M.; Zhu, J.; Xie, T.; et al. Retinal pigment epithelial cells secrete miR-202-5p-containing exosomes to protect against proliferative diabetic retinopathy. Exp. Eye Res. 2020, 201, 108271. [Google Scholar] [CrossRef]
- Kiel, J.W.; Hollingsworth, M.; Rao, R.; Chen, M.; Reitsamer, H.A. Ciliary blood flow and aqueous humor production. Prog. Retin. Eye Res. 2011, 30, 1–17. [Google Scholar] [CrossRef]
- Cho, H.; Hwang, M.; Hong, E.H.; Yu, H.; Park, H.H.; Koh, S.H.; Shin, Y.U. Micro-RNAs in the aqueous humour of patients with diabetic macular oedema. Clin. Exp. Ophthalmol. 2020, 48, 624–635. [Google Scholar] [CrossRef]
- Grieco, G.E.; Sebastiani, G.; Eandi, C.M.; Neri, G.; Nigi, L.; Brusco, N.; D’Aurizio, R.; Posarelli, M.; Bacci, T.; Benedetto, E.; et al. MicroRNA Expression in the Aqueous Humor of Patients with Diabetic Macular Edema. Int. J. Mol. Sci. 2020, 21, 7328. [Google Scholar] [CrossRef] [PubMed]
- Shafabakhsh, R.; Mirhosseini, N.; Chaichian, S.; Moazzami, B.; Mahdizadeh, Z.; Asemi, Z. Could circRNA be a new biomarker for pre-eclampsia? Mol. Reprod. Dev. 2019, 86, 1773–1780. [Google Scholar] [CrossRef]
- Cao, M.; Zhang, L.; Wang, J.H.; Zeng, H.; Peng, Y.; Zou, J.; Shi, J.; Zhang, L.; Li, Y.; Yoshida, S.; et al. Identifying circRNA-associated-ceRNA networks in retinal neovascularization in mice. Int. J. Med. Sci. 2019, 16, 1356–1365. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Hu, J.; Yu, Y. CircRNA Is a Rising Star in Researches of Ocular Diseases. Front. Cell Dev. Biol. 2020, 8, 850. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.J.; Chen, X.; Li, C.P.; Li, X.M.; Liu, C.; Liu, B.H.; Shan, K.; Jiang, Q.; Zhao, C.; Yan, B. Identification and Characterization of Circular RNAs as a New Class of Putative Biomarkers in Diabetes Retinopathy. Investig. Ophthalmol. Vis. Sci. 2017, 58, 6500–6509. [Google Scholar] [CrossRef]
- Zhou, H.R.; Kuang, H.Y. Circular RNAs: Novel target of diabetic retinopathy. Rev. Endocr. Metab. Disord. 2021, 22, 205–216. [Google Scholar] [CrossRef]
- Liu, C.; Yao, M.D.; Li, C.P.; Shan, K.; Yang, H.; Wang, J.J.; Liu, B.; Li, X.M.; Yao, J.; Jiang, Q.; et al. Silencing Of Circular RNA-ZNF609 Ameliorates Vascular Endothelial Dysfunction. Theranostics 2017, 7, 2863–2877. [Google Scholar] [CrossRef]
- Wang, F.; Zhang, M. Circ_001209 aggravates diabetic retinal vascular dysfunction through regulating miR-15b-5p/COL12A1. J. Transl. Med. 2021, 19, 294. [Google Scholar] [CrossRef]
- Zou, J.; Liu, K.C.; Wang, W.P.; Xu, Y. Circular RNA COL1A2 promotes angiogenesis via regulating miR-29b/VEGF axis in diabetic retinopathy. Life Sci. 2020, 256, 117888. [Google Scholar] [CrossRef]
- Zeng, Q.; Liu, J. Silencing circ_0001879 inhibits the proliferation and migration of human retinal microvascular endothelial cells under high-glucose conditions via modulating miR-30-3p. Gene 2020, 760, 144992. [Google Scholar] [CrossRef]
- Liu, G.; Zhou, S.; Li, X.; Ding, X.; Tian, M. Inhibition of hsa_circ_0002570 suppresses high-glucose-induced angiogenesis and inflammation in retinal microvascular endothelial cells through miR-1243/angiomotin axis. Cell Stress Chaperones 2020, 25, 767–777. [Google Scholar] [CrossRef] [PubMed]
- Zhu, K.; Hu, X.; Chen, H.; Li, F.; Yin, N.; Liu, A.L.; Shan, K.; Qin, Y.W.; Huang, X.; Chang, Q.; et al. Downregulation of circRNA DMNT3B contributes to diabetic retinal vascular dysfunction through targeting miR-20b-5p and BAMBI. EBioMedicine 2019, 49, 341–353. [Google Scholar] [CrossRef]
- Hu, J.; Dziumbla, S.; Lin, J.; Bibli, S.I.; Zukunft, S.; de Mos, J.; Awwad, K.; Fromel, T.; Jungmann, A.; Devraj, K.; et al. Inhibition of soluble epoxide hydrolase prevents diabetic retinopathy. Nature 2017, 552, 248–252. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Q.; Liu, C.; Li, C.P.; Xu, S.S.; Yao, M.D.; Ge, H.M.; Sun, Y.N.; Li, X.M.; Zhang, S.J.; Shan, K.; et al. Circular RNA-ZNF532 regulates diabetes-induced retinal pericyte degeneration and vascular dysfunction. J. Clin. Investig. 2020, 130, 3833–3847. [Google Scholar] [CrossRef]
- Liu, C.; Ge, H.M.; Liu, B.H.; Dong, R.; Shan, K.; Chen, X.; Yao, M.D.; Li, X.M.; Yao, J.; Zhou, R.M.; et al. Targeting pericyte-endothelial cell crosstalk by circular RNA-cPWWP2A inhibition aggravates diabetes-induced microvascular dysfunction. Proc. Natl. Acad. Sci. USA 2019, 116, 7455–7464. [Google Scholar] [CrossRef]
- Zhang, Y.; Zheng, L.; Xu, H.; Ling, L. Circ_0084043 Facilitates High Glucose-Induced Retinal Pigment Epithelial Cell Injury by Activating miR-128-3p/TXNIP-Mediated Wnt/β-Catenin Signaling Pathway. J. Cardiovasc. Pharmacol. 2021, 78, e112–e121. [Google Scholar] [CrossRef]
- Li, Y.; Cheng, T.; Wan, C.; Cang, Y. circRNA_0084043 contributes to the progression of diabetic retinopathy via sponging miR-140-3p and inducing TGFA gene expression in retinal pigment epithelial cells. Gene 2020, 747, 144653. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Li, F.F.; Wang, S.M. Circ-ITCH restrains the expression of MMP-2, MMP-9 and TNF-α in diabetic retinopathy by inhibiting miR-22. Exp. Mol. Pathol. 2021, 118, 104594. [Google Scholar] [CrossRef]
- Sun, H.; Kang, X. hsa_circ_0041795 contributes to human retinal pigment epithelial cells (ARPE 19) injury induced by high glucose via sponging miR-646 and activating VEGFC. Gene 2020, 747, 144654. [Google Scholar] [CrossRef]
- Liang, G.H.; Luo, Y.N.; Wei, R.Z.; Yin, J.Y.; Qin, Z.L.; Lu, L.L.; Ma, W.H. CircZNF532 knockdown protects retinal pigment epithelial cells against high glucose-induced apoptosis and pyroptosis by regulating the miR-20b-5p/STAT3 axis. J. Diabetes Investig. 2021, 13, 781–795. [Google Scholar] [CrossRef]
- Zhu, Z.; Duan, P.; Song, H.; Zhou, R.; Chen, T. Downregulation of Circular RNA PSEN1 ameliorates ferroptosis of the high glucose treated retinal pigment epithelial cells via miR-200b-3p/cofilin-2 axis. Bioengineered 2021, 12, 12555–12567. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Wang, J.; Qian, H.; Wu, Y.; Zhang, Z.; Hu, Z.; Xie, P. Serum Exosomal Circular RNA Expression Profile and Regulative Role in Proliferative Diabetic Retinopathy. Front. Genet. 2021, 12, 719312. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Liu, B.; Ma, Y.; Chen, H.; Wu, J.; Wang, J. Discovery and validation of hsa_circ_0001953 as a potential biomarker for proliferative diabetic retinopathy in human blood. Acta Ophthalmol. 2021, 99, 306–313. [Google Scholar] [CrossRef] [PubMed]
- Monteiro, J.P.; Santos, F.M.; Rocha, A.S.; Castro-de-Sousa, J.P.; Queiroz, J.A.; Passarinha, L.A.; Tomaz, C.T. Vitreous humor in the pathologic scope: Insights from proteomic approaches. Proteomics. Clin. Appl. 2015, 9, 187–202. [Google Scholar] [CrossRef]
- Nawaz, I.M.; Rezzola, S.; Cancarini, A.; Russo, A.; Costagliola, C.; Semeraro, F.; Presta, M. Human vitreous in proliferative diabetic retinopathy: Characterization and translational implications. Prog. Retin. Eye Res. 2019, 72, 100756. [Google Scholar]
- He, M.; Wang, W.; Yu, H.; Wang, D.; Cao, D.; Zeng, Y.; Wu, Q.; Zhong, P.; Cheng, Z.; Hu, Y.; et al. Comparison of expression profiling of circular RNAs in vitreous humour between diabetic retinopathy and non-diabetes mellitus patients. Acta Diabetol. 2020, 57, 479–489. [Google Scholar] [CrossRef]
- Guo, J.; Xiao, F.; Ren, W.; Zhu, Y.; Du, Q.; Li, Q.; Li, X. Circular Ribonucleic Acid circFTO Promotes Angiogenesis and Impairs Blood-Retinal Barrier Via Targeting the miR-128-3p/Thioredoxin Interacting Protein Axis in Diabetic Retinopathy. Front. Mol. Biosci. 2021, 8, 685466. [Google Scholar] [CrossRef]
- Bridges, M.C.; Daulagala, A.C.; Kourtidis, A. LNCcation: lncRNA localization and function. J. Cell Biol. 2021, 220, e202009045. [Google Scholar] [CrossRef]
- Sun, X.; Wong, D. Long non-coding RNA-mediated regulation of glucose homeostasis and diabetes. Am. J. Cardiovasc. Dis. 2016, 6, 17–25. [Google Scholar]
- Wang, Y.; Huang, L.; Wang, Y.; Luo, W.; Li, F.; Xiao, J.; Qin, S.; Wang, Z.; Song, X.; Wang, Y.; et al. Single-cell RNA-sequencing analysis identifies host long noncoding RNA MAMDC2-AS1 as a co-factor for HSV-1 nuclear transport. Int. J. Biol. Sci. 2020, 16, 1586–1603. [Google Scholar] [CrossRef]
- Kitagawa, M.; Kitagawa, K.; Kotake, Y.; Niida, H.; Ohhata, T. Cell cycle regulation by long non-coding RNAs. Cell Mol. Life Sci. 2013, 70, 4785–4794. [Google Scholar] [CrossRef] [PubMed]
- Ballarino, M.; Morlando, M.; Fatica, A.; Bozzoni, I. Non-coding RNAs in muscle differentiation and musculoskeletal disease. J. Clin. Investig. 2016, 126, 2021–2030. [Google Scholar] [CrossRef] [PubMed]
- Gong, Q.; Su, G. Roles of miRNAs and long noncoding RNAs in the progression of diabetic retinopathy. Biosci. Rep. 2017, 37, BSR20171157. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Gao, X.; Liu, J.; Wang, J.; Zhang, Y.; Zhang, T.; Zhang, H. Effect of intravitreal conbercept treatment on the expression of Long Noncoding RNAs and mRNAs in Proliferative Diabetic Retinopathy Patients. Acta Ophthalmol. 2019, 97, e902–e912. [Google Scholar] [CrossRef]
- Zhang, R.; Ma, X.; Jiang, L.; Xia, W.; Li, H.; Zhao, N.; Cui, X.; Zhang, N.; Zhou, H.; Xu, S. Decreased lncRNA SNHG16 Accelerates Oxidative Stress Induced Pathological Angiogenesis in Human Retinal Microvascular Endothelial Cells by Regulating miR-195/mfn2 Axis. Curr. Pharm. Des. 2021, 27, 3047–3060. [Google Scholar] [CrossRef]
- Yang, J.; Yang, F.J.; Wang, Y.G.; Su, G.F.; Miao, X. LncRNA MIR497HG inhibits proliferation and migration of retinal endothelial cells under high-level glucose treatment via miRNA-128-3p/SIRT1 axis. Eur. Rev. Med. Pharmacol. Sci. 2020, 24, 5871–5877. [Google Scholar]
- Thomas, A.A.; Biswas, S.; Feng, B.; Chen, S.; Gonder, J.; Chakrabarti, S. lncRNA H19 prevents endothelial-mesenchymal transition in diabetic retinopathy. Diabetologia 2019, 62, 517–530. [Google Scholar] [CrossRef]
- He, Y.; Dan, Y.; Gao, X.; Huang, L.; Lv, H.; Chen, J. DNMT1-mediated lncRNA MEG3 methylation accelerates endothelial-mesenchymal transition in diabetic retinopathy through the PI3K/Akt/mTOR signaling pathway. Am. J. Physiol. Endocrinol. Metab. 2021, 320, E598–E608. [Google Scholar] [CrossRef]
- Sehgal, P.; Mathew, S.; Sivadas, A.; Ray, A.; Tanwar, J.; Vishwakarma, S.; Ranjan, G.; Shamsudheen, K.V.; Bhoyar, R.C.; Pateria, A.; et al. LncRNA VEAL2 regulates PRKCB2 to modulate endothelial permeability in diabetic retinopathy. EMBO J. 2021, 40, e107134. [Google Scholar] [CrossRef]
- Li, X. lncRNA MALAT1 promotes diabetic retinopathy by upregulating PDE6G via miR-378a-3p. Arch. Physiol. Biochem. 2021, 1–9. Available online: https://www.tandfonline.com/doi/abs/10.1080/13813455.2021.1985144 (accessed on 29 October 2022). [CrossRef]
- Zhang, D.; Qin, H.; Leng, Y.; Li, X.; Zhang, L.; Bai, D.; Meng, Y.; Wang, J. LncRNA MEG3 overexpression inhibits the development of diabetic retinopathy by regulating TGF-β1 and VEGF. Exp. Ther. Med. 2018, 16, 2337–2342. [Google Scholar] [CrossRef] [PubMed]
- Shan, K.; Li, C.P.; Liu, C.; Liu, X.; Yan, B. RNCR3: A regulator of diabetes mellitus-related retinal microvascular dysfunction. Biochem. Biophys. Res. Commun. 2017, 482, 777–783. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Ma, X.; Lin, W.; Xu, Q.; Zhou, H.; Kuang, H. Long noncoding RNA MIAT regulates primary human retinal pericyte pyroptosis by modulating miR-342-3p targeting of CASP1 in diabetic retinopathy. Exp. Eye Res. 2021, 202, 108300. [Google Scholar] [CrossRef]
- Liu, J.Y.; Yao, J.; Li, X.M.; Song, Y.C.; Wang, X.Q.; Li, Y.J.; Yan, B.; Jiang, Q. Pathogenic role of lncRNA-MALAT1 in endothelial cell dysfunction in diabetes mellitus. Cell Death Dis. 2014, 5, e1506. [Google Scholar] [CrossRef] [PubMed]
- Li, C.P.; Wang, S.H.; Wang, W.Q.; Song, S.G.; Liu, X.M. Long Noncoding RNA-Sox2OT Knockdown Alleviates Diabetes Mellitus-Induced Retinal Ganglion Cell (RGC) injury. Cell Mol. Neurobiol. 2017, 37, 361–369. [Google Scholar] [CrossRef]
- Zhou, R.R.; Li, H.B.; You, Q.S.; Rong, R.; You, M.L.; Xiong, K.; Huang, J.F.; Xia, X.B.; Ji, D. Silencing of GAS5 Alleviates Glaucoma in Rat Models by Reducing Retinal Ganglion Cell Apoptosis. Hum. Gene Ther. 2019, 30, 1505–1519. [Google Scholar] [CrossRef]
- Ge, Y.; Zhang, R.; Feng, Y.; Li, H. Mbd2 Mediates Retinal Cell Apoptosis by Targeting the lncRNA Mbd2-AL1/miR-188-3p/Traf3 Axis in Ischemia/Reperfusion Injury. Mol. Nucleic Acids 2020, 19, 1250–1265. [Google Scholar] [CrossRef]
- Zhang, J.; Chen, C.; Zhang, S.; Chen, J.; Wu, L.; Chen, Z. LncRNA XIST restrains the activation of Müller cells and inflammation in diabetic retinopathy via stabilizing SIRT1. Autoimmunity 2021, 54, 504–513. [Google Scholar] [CrossRef]
- Liu, C.; Li, C.P.; Wang, J.J.; Shan, K.; Liu, X.; Yan, B. RNCR3 knockdown inhibits diabetes mellitus-induced retinal reactive gliosis. Biochem. Biophys. Res. Commun. 2016, 479, 198–203. [Google Scholar] [CrossRef]
- Dong, N.; Xu, B.; Shi, H. Long noncoding RNA MALAT1 acts as a competing endogenous RNA to regulate Amadori-glycated albumin-induced MCP-1 expression in retinal microglia by a microRNA-124-dependent mechanism. Inflamm. Res. Off. J. Eur. Histamine Res. Soc. 2018, 67, 913–925. [Google Scholar] [CrossRef]
- Li, X.J. Long non-coding RNA nuclear paraspeckle assembly transcript 1 inhibits the apoptosis of retina Müller cells after diabetic retinopathy through regulating miR-497/brain-derived neurotrophic factor axis. Diabetes Vasc. Dis. Res. 2018, 15, 204–213. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.Q.; Zhou, H.J. LncRNA MALAT1 promotes high glucose-induced inflammatory response of microglial cells via provoking MyD88/IRAK1/TRAF6 signaling. Sci. Rep. 2018, 8, 8346. [Google Scholar] [CrossRef] [PubMed]
- Wan, P.; Su, W.; Zhang, Y.; Li, Z.; Deng, C.; Li, J.; Jiang, N.; Huang, S.; Long, E.; Zhuo, Y. LncRNA H19 initiates microglial pyroptosis and neuronal death in retinal ischemia/reperfusion injury. Cell Death Differ. 2020, 27, 176–191. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.Q.; Zheng, Y.Y.; Zhou, H.J.; Zhang, X.X.; Wu, P.; Zhu, S.M. LncRNA-Fendrr protects against the ubiquitination and degradation of NLRC4 protein through HERC2 to regulate the pyroptosis of microglia. Mol. Med. (Camb. Mass.) 2021, 27, 39. [Google Scholar] [CrossRef]
- Dong, Y.; Wan, G.; Peng, G.; Yan, P.; Qian, C.; Li, F. Long non-coding RNA XIST regulates hyperglycemia-associated apoptosis and migration in human retinal pigment epithelial cells. Biomed. Pharmacother. = Biomed. Pharmacother. 2020, 125, 109959. [Google Scholar] [CrossRef] [PubMed]
- Luo, R.; Jin, H.; Li, L.; Hu, Y.X.; Xiao, F. Long Noncoding RNA MEG3 Inhibits Apoptosis of Retinal Pigment Epithelium Cells Induced by High Glucose via the miR-93/Nrf2 Axis. Am. J. Pathol. 2020, 190, 1813–1822. [Google Scholar] [CrossRef]
- Tong, P.; Peng, Q.H.; Gu, L.M.; Xie, W.W.; Li, W.J. LncRNA-MEG3 alleviates high glucose induced inflammation and apoptosis of retina epithelial cells via regulating miR-34a/SIRT1 axis. Exp. Mol. Pathol. 2019, 107, 102–109. [Google Scholar] [CrossRef]
- Luo, R.; Xiao, F.; Wang, P.; Hu, Y.X. lncRNA H19 sponging miR-93 to regulate inflammation in retinal epithelial cells under hyperglycemia via XBP1s. Inflamm. Res. Off. J. Eur. Histamine Res. Soc. 2020, 69, 255–265. [Google Scholar] [CrossRef]
- Cheng, Y.; Zhu, Y.; Ma, L. LncRNA LINC00673 is Downregulated in Diabetic Retinopathy and Regulates the Apoptosis of Retinal Pigment Epithelial Cells via Negatively Regulating p53. Diabetes Metab. Syndr. Obes. Targets Ther. 2021, 14, 4233–4240. [Google Scholar] [CrossRef]
- Zhang, X.; Shi, E.; Yang, L.; Fu, W.; Hu, F.; Zhou, X. LncRNA AK077216 is downregulated in diabetic retinopathy and inhibited the apoptosis of retinal pigment epithelial cells by downregulating miR-383. Endocr. J. 2019, 66, 1011–1016. [Google Scholar] [CrossRef]
- Fu, S.; Zheng, Y.; Sun, Y.; Lai, M.; Qiu, J.; Gui, F.; Zeng, Q.; Liu, F. Suppressing long noncoding RNA OGRU ameliorates diabetic retinopathy by inhibition of oxidative stress and inflammation via miR-320/USP14 axis. Free Radic. Biol. Med. 2021, 169, 361–381. [Google Scholar] [CrossRef] [PubMed]
- Zeng, F.; Luo, G.; Lu, Y.; Zhang, Z.; Zhou, Y.; Chen, Y.; Zhou, Z. Long non-coding RNA VIM Antisense RNA 1 (VIM-AS1) sponges microRNA-29 to participate in diabetic retinopathy. Acta Diabetol. 2020, 57, 1111–1116. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Guo, C.; Chen, Y.; Yu, F. Long non-coding RNA SNHG16 regulates E2F1 expression by sponging miR-20a-5p and aggravating proliferative diabetic retinopathy. Can. J. Physiol. Pharmacol. 2021, 99, 1207–1216. [Google Scholar] [CrossRef] [PubMed]
- Ji, X.; Sun, J.; Wang, Z. High level of lncRNA NR2F1-AS1 predict the onset and progression of diabetic retinopathy in type 2 diabetes. Exp. Eye Res. 2022, 219, 109069. [Google Scholar] [CrossRef]
- Luo, R.; Li, L.; Xiao, F.; Fu, J. LncRNA FLG-AS1 Mitigates Diabetic Retinopathy by Regulating Retinal Epithelial Cell Inflammation, Oxidative Stress, and Apoptosis via miR-380-3p/SOCS6 Axis. Inflammation 2022, 45, 1936–1949. [Google Scholar] [CrossRef]
- Cao, X.; Xue, L.D.; Di, Y.; Li, T.; Tian, Y.J.; Song, Y. MSC-derived exosomal lncRNA SNHG7 suppresses endothelial-mesenchymal transition and tube formation in diabetic retinopathy via miR-34a-5p/XBP1 axis. Life Sci. 2021, 272, 119232. [Google Scholar] [CrossRef]
- Guo, J.; Zhou, P.; Liu, Z.; Dai, F.; Pan, M.; An, G.; Han, J.; Du, L.; Jin, X. The Aflibercept-Induced MicroRNA Profile in the Vitreous of Proliferative Diabetic Retinopathy Patients Detected by Next-Generation Sequencing. Front. Pharmacol. 2021, 12, 781276. [Google Scholar] [CrossRef]
- Li, H.L.; Hao, G.M.; Tang, S.J.; Sun, H.H.; Fang, Y.S.; Pang, X.; Liu, H.; Ji, Q.; Wang, X.R.; Tian, J.Y.; et al. HuoXue JieDu formula improves diabetic retinopathy in rats by regulating microRNAs. J. Ethnopharmacol. 2021, 268, 113616. [Google Scholar] [CrossRef]
- Alzahrani, S.; Ajwah, S.M.; Alsharif, S.Y.; Said, E.; El-Sherbiny, M.; Zaitone, S.A.; Al-Shabrawey, M.; Elsherbiny, N.M. Isoliquiritigenin downregulates miR-195 and attenuates oxidative stress and inflammation in STZ-induced retinal injury. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2020, 393, 2375–2385. [Google Scholar] [CrossRef]
- Peng, Q.H.; Tong, P.; Gu, L.M.; Li, W.J. Astragalus polysaccharide attenuates metabolic memory-triggered ER stress and apoptosis via regulation of miR-204/SIRT1 axis in retinal pigment epithelial cells. Biosci. Rep. 2020, 40, BSR20192121. [Google Scholar] [CrossRef]
- Liu, P.; Peng, Q.H.; Tong, P.; Li, W.J. Astragalus polysaccharides suppresses high glucose-induced metabolic memory in retinal pigment epithelial cells through inhibiting mitochondrial dysfunction-induced apoptosis by regulating miR-195. Mol. Med. (Camb. Mass.) 2019, 25, 21. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Zhang, Z.; Song, C.; Sun, L.; Sui, X.; Qu, Q.; Liu, J. Astragaloside IV protects retinal pigment epithelial cells from apoptosis by upregulating miR-128 expression in diabetic rats. Int. J. Mol. Med. 2020, 46, 340–350. [Google Scholar] [CrossRef] [PubMed]
- Dai, C.; Jiang, S.; Chu, C.; Xin, M.; Song, X.; Zhao, B. Baicalin protects human retinal pigment epithelial cell lines against high glucose-induced cell injury by up-regulation of microRNA-145. Exp. Mol. Pathol. 2019, 106, 123–130. [Google Scholar] [CrossRef] [PubMed]
- Xue, L.P.; Fu, X.L.; Hu, M.; Zhang, L.W.; Li, Y.D.; Peng, Y.L.; Ding, P. Rg1 inhibits high glucose-induced mesenchymal activation and fibrosis via regulating miR-2113/RP11-982M15.8/Zeb1 pathway. Biochem. Biophys. Res. Commun. 2018, 501, 827–832. [Google Scholar] [CrossRef] [PubMed]
- Shi, Q.; Chen, X.; Sun, G.; Wang, L.; Cui, L. Ginsenoside Rg1 protects human retinal pigment epithelial ARPE-19 cells from toxicity of high glucose by up-regulation of miR-26a. Life Sci. 2019, 221, 152–158. [Google Scholar] [CrossRef]
- Zeng, K.; Wang, Y.; Yang, N.; Wang, D.; Li, S.; Ming, J.; Wang, J.; Yu, X.; Song, Y.; Zhou, X.; et al. Resveratrol Inhibits Diabetic-Induced Müller Cells Apoptosis through MicroRNA-29b/Specificity Protein 1 Pathway. Mol. Neurobiol. 2017, 54, 4000–4014. [Google Scholar] [CrossRef] [PubMed]
- Tu, Y.; Zhu, M.; Wang, Z.; Wang, K.; Chen, L.; Liu, W.; Shi, Q.; Zhao, Q.; Sun, Y.; Wang, X.; et al. Melatonin inhibits Müller cell activation and pro-inflammatory cytokine production via upregulating the MEG3/miR-204/Sirt1 axis in experimental diabetic retinopathy. J. Cell Physiol. 2020, 235, 8724–8735. [Google Scholar] [CrossRef]
- Deng, W.; Huang, D.; Xie, H.; Wang, L.; Shen, Q.; Zeng, R.; Huang, Y.; Li, J.; Yang, B. Danhong injection represses diabetic retinopathy and nephropathy advancement in diabetic mice by upregulating microRNA-30d-5p and targeting JAK1. Bioengineered 2022, 13, 8187–8200. [Google Scholar] [CrossRef]
- Liu, S.; Fang, Y.; Yu, J.; Chang, X. Hawthorn polyphenols reduce high glucose-induced inflammation and apoptosis in ARPE-19 cells by regulating miR-34a/SIRT1 to reduce acetylation. J. Food Biochem. 2021, 45, e13623. [Google Scholar] [CrossRef]
- Wang, Y.; Yan, H. MicroRNA-126 contributes to Niaspan treatment induced vascular restoration after diabetic retinopathy. Sci. Rep. 2016, 6, 26909. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, M.; Li, Q.; Jin, M.; Wang, Z.; Zhang, X.; Sun, X.; Luo, Y. Noncoding RNAs Are Promising Therapeutic Targets for Diabetic Retinopathy: An Updated Review (2017–2022). Biomolecules 2022, 12, 1774. https://doi.org/10.3390/biom12121774
Wang M, Li Q, Jin M, Wang Z, Zhang X, Sun X, Luo Y. Noncoding RNAs Are Promising Therapeutic Targets for Diabetic Retinopathy: An Updated Review (2017–2022). Biomolecules. 2022; 12(12):1774. https://doi.org/10.3390/biom12121774
Chicago/Turabian StyleWang, Mengchen, Qiaoyu Li, Meiqi Jin, Zhen Wang, Xuelian Zhang, Xiaobo Sun, and Yun Luo. 2022. "Noncoding RNAs Are Promising Therapeutic Targets for Diabetic Retinopathy: An Updated Review (2017–2022)" Biomolecules 12, no. 12: 1774. https://doi.org/10.3390/biom12121774
APA StyleWang, M., Li, Q., Jin, M., Wang, Z., Zhang, X., Sun, X., & Luo, Y. (2022). Noncoding RNAs Are Promising Therapeutic Targets for Diabetic Retinopathy: An Updated Review (2017–2022). Biomolecules, 12(12), 1774. https://doi.org/10.3390/biom12121774





