Anticancer, Antimicrobial, and Antioxidant Activities of Organodiselenide-Tethered Methyl Anthranilates
Abstract
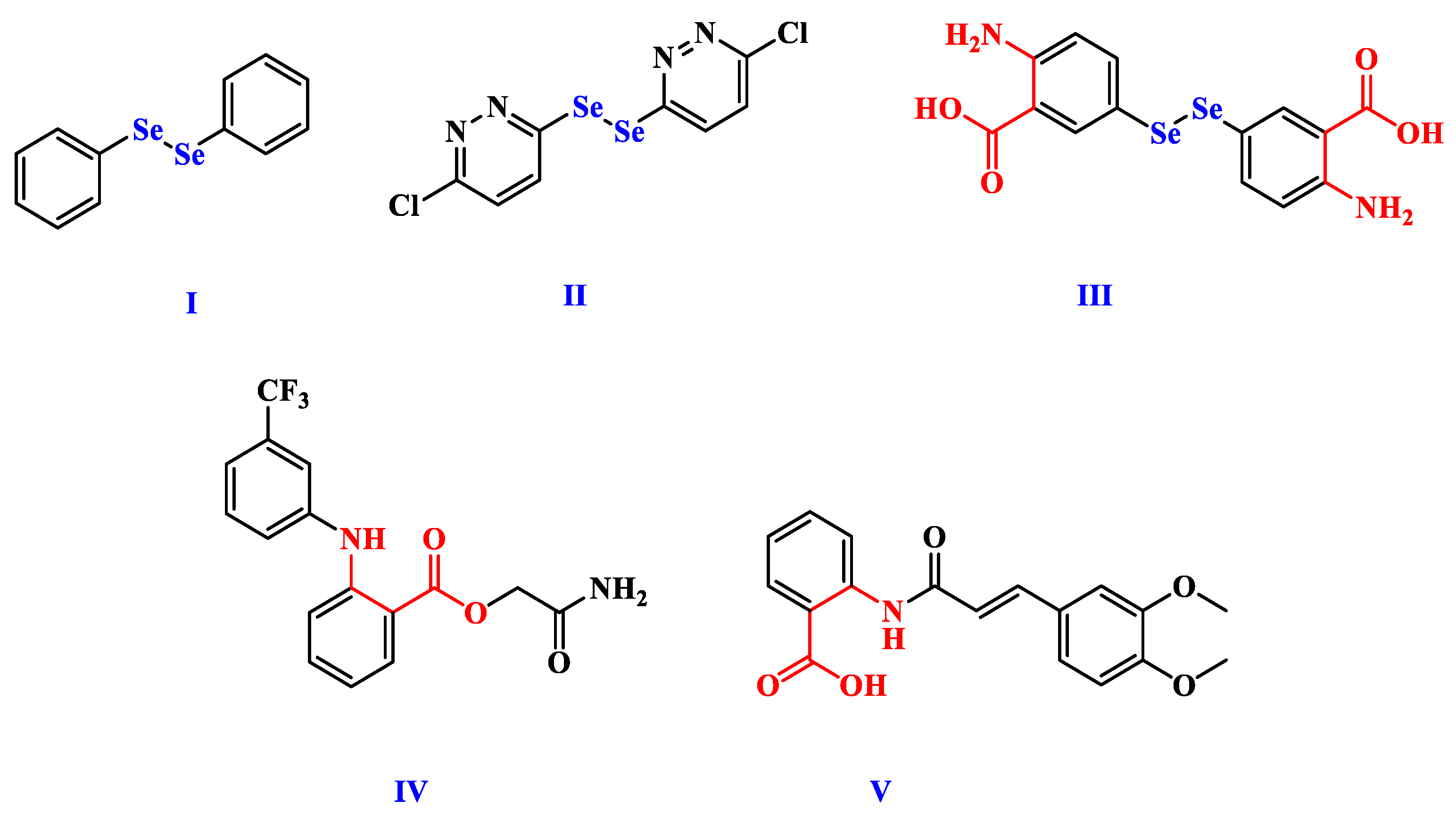
1. Introduction
2. Materials and Methods
2.1. Material and Methods
2.2. Chemistry
2.3. The Biological Assays
2.3.1. The Anticancer Activity
2.3.2. The Antimicrobial Activity
2.3.3. The Antioxidant Activity
3. Results and Discussion
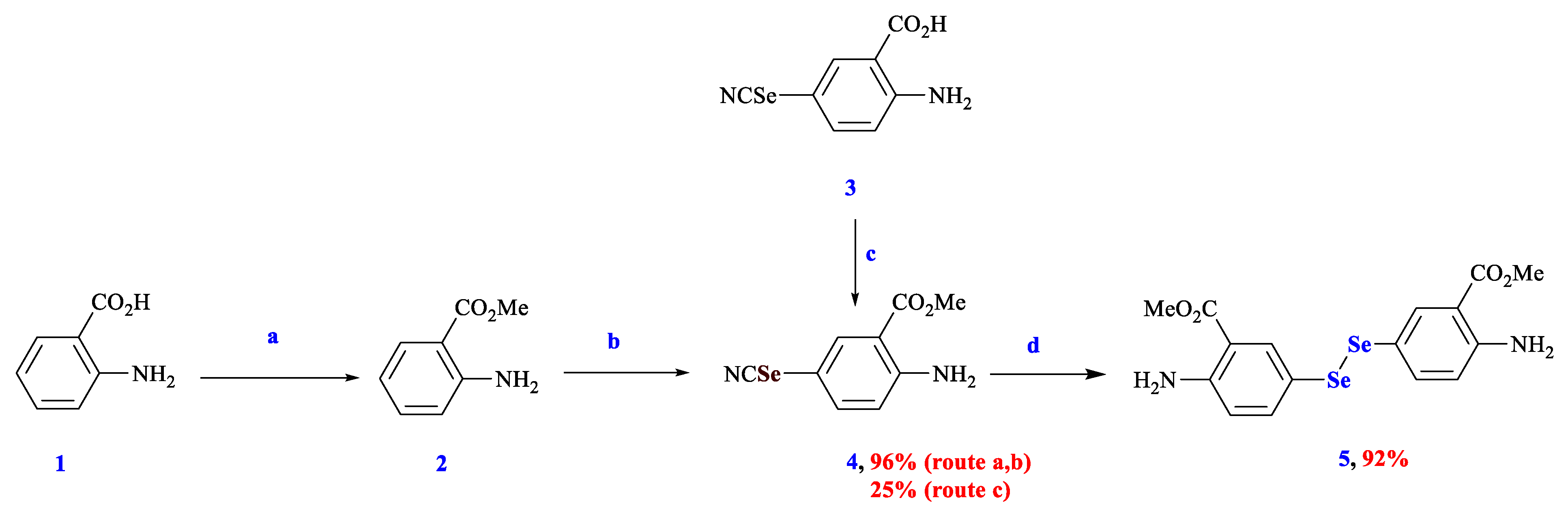
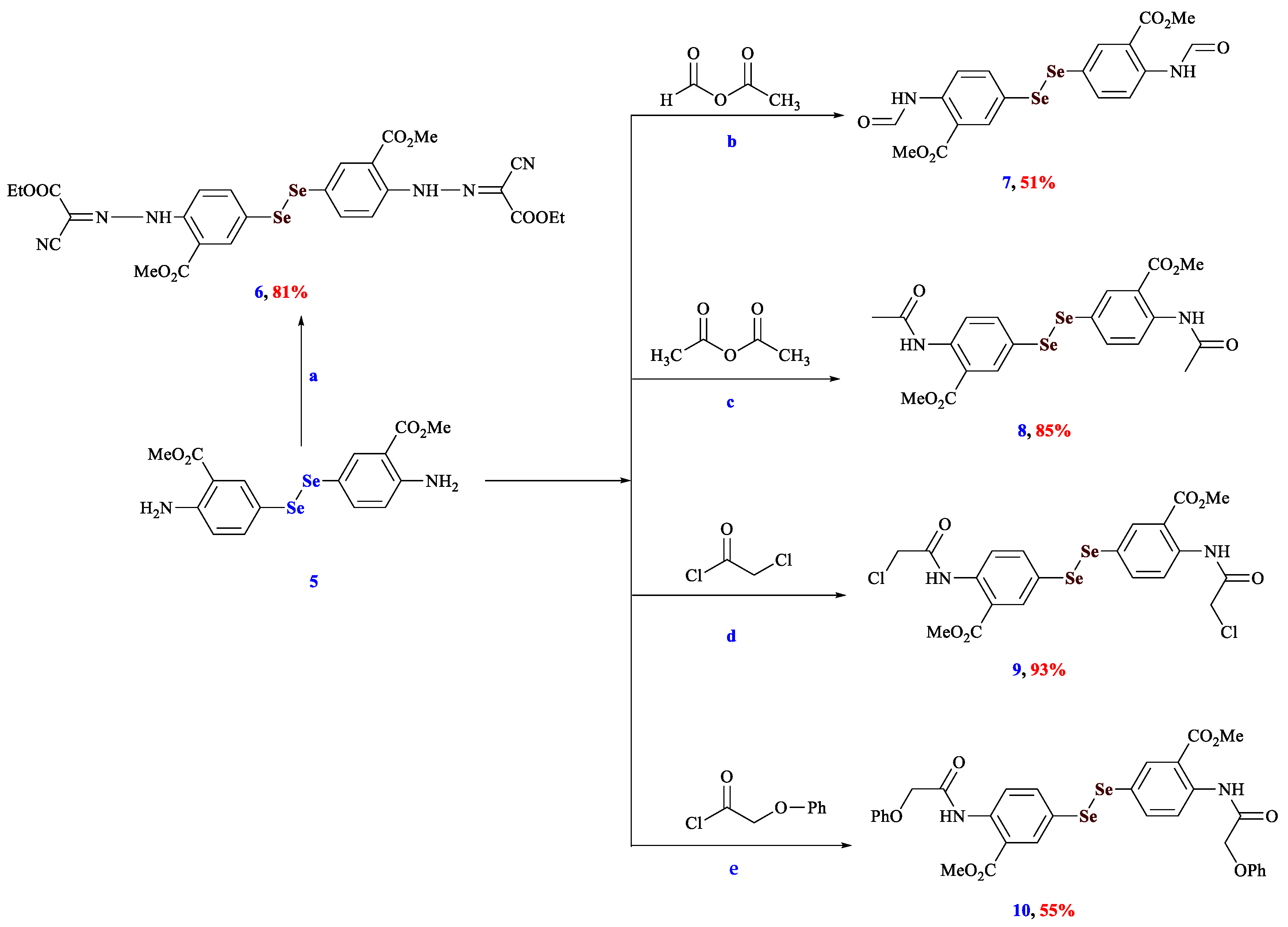
3.1. Synthesis
3.2. Biology
3.2.1. Cytotoxicity of OSe2-tethered Anthranilic Acid Hybrids
3.2.2. Evaluation of the Antimicrobial Activities of the OSe Compounds
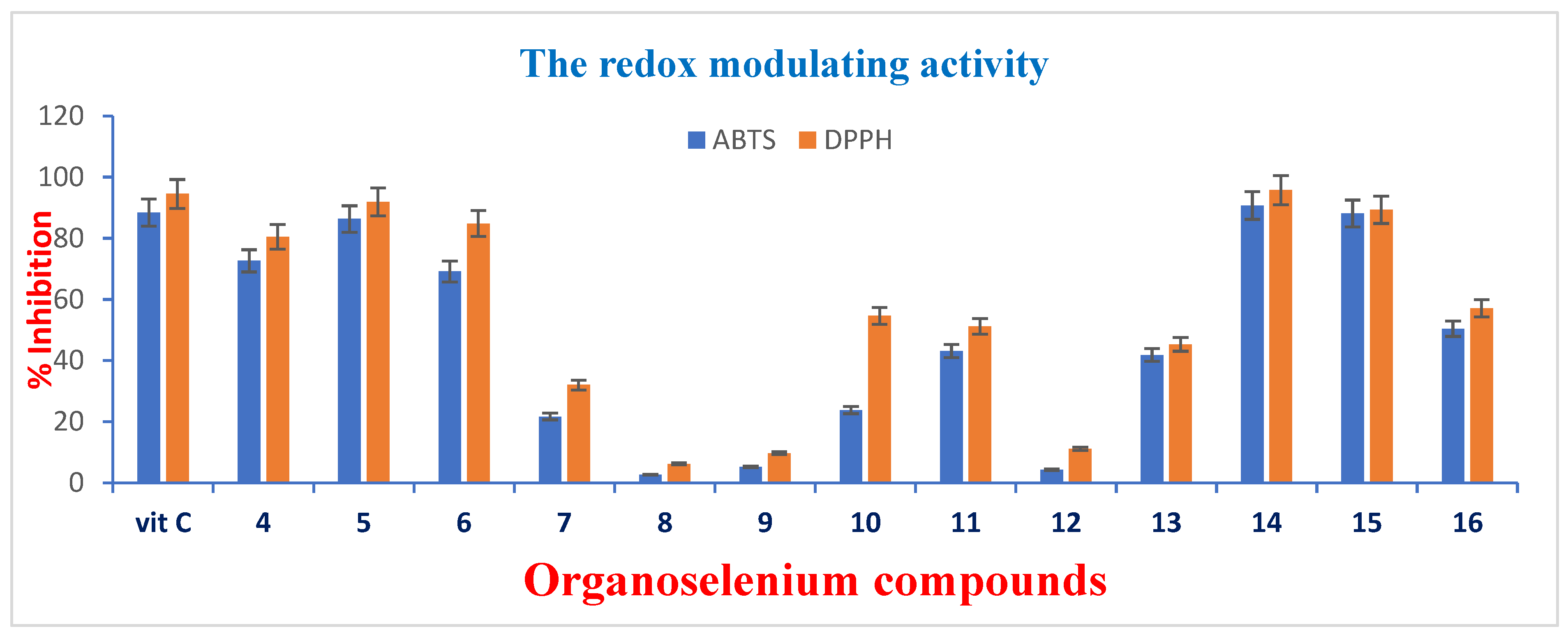
3.2.3. The Antioxidant Properties of the OSe Compounds
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bevinakoppamath, S.; Saleh Ahmed, A.M.; Ramachandra, S.C.; Vishwanath, P.; Prashant, A. Chemopreventive and Anticancer Property of Selenoproteins in Obese Breast Cancer. Front. Pharmacol. 2021, 12, 618172. [Google Scholar] [CrossRef] [PubMed]
- Nogueira, C.W.; Barbosa, N.V.; Rocha, J.B.T. Toxicology and pharmacology of synthetic organoselenium compounds: An update. Arch. Toxicol. 2021, 95, 1179–1226. [Google Scholar] [CrossRef] [PubMed]
- Kalimuthu, K.; Keerthana, C.K.; Mohan, M.; Arivalagan, J.; Christyraj, J.; Firer, M.A.; Choudry, M.H.A.; Anto, R.J.; Lee, Y.J. The emerging role of selenium metabolic pathways in cancer: New therapeutic targets for cancer. J. Cell. Biochem. 2022, 123, 532–542. [Google Scholar] [CrossRef] [PubMed]
- Radomska, D.; Czarnomysy, R.; Radomski, D.; Bielawska, A.; Bielawski, K. Selenium as a Bioactive Micronutrient in the Human Diet and Its Cancer Chemopreventive Activity. Nutrients 2021, 13, 1649. [Google Scholar] [CrossRef]
- Radomska, D.; Czarnomysy, R.; Radomski, D.; Bielawski, K. Selenium compounds as novel potential anticancer agents. Int. J. Mol. Sci. 2021, 22, 1009. [Google Scholar] [CrossRef] [PubMed]
- Lenardão, E.J.; Santi, C.; Sancineto, L. New Frontiers in Organoselenium Compounds; Springer: Berlin/Heidelberg, Germany, 2018. [Google Scholar]
- Shaaban, S.; Negm, A.; Sobh, M.A.; Wessjohann, L.A. Organoselenocyanates and symmetrical diselenides redox modulators: Design, synthesis and biological evaluation. Eur. J. Med. Chem. 2015, 97, 190–201. [Google Scholar] [CrossRef] [PubMed]
- Shaaban, S.; Arafat, M.A.; Hamama, W.S. Vistas in the Domain of Organoselenocyanates; Michigan Publishing: Ann Arbor, MI, USA, 2014. [Google Scholar]
- Plano, D.; Baquedano, Y.; Moreno-Mateos, D.; Font, M.; Jimenez-Ruiz, A.; Palop, J.A.; Sanmartin, C. Selenocyanates and diselenides: A new class of potent antileishmanial agents. Eur. J. Med. Chem. 2011, 46, 3315–3323. [Google Scholar] [CrossRef]
- de Oliveira, I.M.; Degrandi, T.H.; Jorge, P.M.; Saffi, J.; Rosa, R.M.; Guecheva, T.N.; Henriques, J.A. Dicholesteroyl diselenide: Cytotoxicity, genotoxicity and mutagenicity in the yeast Saccharomyces cerevisiae and in Chinese hamster lung fibroblasts. Mutat. Res. Genet. Toxicol. Environ. Mutagen. 2014, 763, 1–11. [Google Scholar] [CrossRef]
- Prasher, P.; Sharma, M. Medicinal chemistry of anthranilic acid derivatives: A mini review. Drug Dev. Res. 2021, 82, 945–958. [Google Scholar] [CrossRef] [PubMed]
- Congiu, C.; Cocco, M.T.; Lilliu, V.; Onnis, V. New potential anticancer agents based on the anthranilic acid scaffold. Synthesis and evaluation of biological activity. J. Med. Chem. 2005, 48, 8245–8252. [Google Scholar] [CrossRef] [PubMed]
- Kachanov, V.A.; Slabko, Y.O.; Baranova, V.O.; Shilova, V.E.; Kaminskii, A.V. Triselenium dicyanide from malononitrile and selenium dioxide. One-pot synthesis of selenocyanates. Tetrahedron Lett. 2004, 45, 4461–4463. [Google Scholar] [CrossRef]
- El-Senduny, F.F.; Shabana, S.M.; Rösel, D.; Brabek, J.; Althagafi, I.; Angeloni, G.; Manolikakes, G.; Shaaban, S. Urea-functionalized organoselenium compounds as promising anti-HepG2 and apoptosis-inducing agents. Future Med. Chem. 2021, 13, 1655–1677. [Google Scholar] [CrossRef] [PubMed]
- Shaaban, S.; Zarrouk, A.; Vervandier-Fasseur, D.; Al-Faiyz, Y.S.; El-Sawy, H.; Althagafi, I.; Andreoletti, P.; Cherkaoui-Malki, M. Cytoprotective organoselenium compounds for oligodendrocytes. Arab. J. Chem. 2021, 14, 103051. [Google Scholar] [CrossRef]
- Sak, M.; Al-Faiyz, Y.S.; Elsawy, H.; Shaaban, S. Novel organoselenium redox modulators with potential anticancer, antimicrobial, and antioxidant activities. Antioxidants 2022, 11, 1231. [Google Scholar] [CrossRef] [PubMed]
- Shaaban, S.; Sasse, F.; Burkholz, T.; Jacob, C. Sulfur, selenium and tellurium pseudopeptides: Synthesis and biological evaluation. Bioorg. Med. Chem. 2014, 22, 3610–3619. [Google Scholar] [CrossRef] [PubMed]
- Shaaban, S.; Negm, A.; Ashmawy, A.M.; Ahmed, D.M.; Wessjohann, L.A. Combinatorial synthesis, in silico, molecular and biochemical studies of tetrazole-derived organic selenides with increased selectivity against hepatocellular carcinoma. Eur. J. Med. Chem. 2016, 122, 55–71. [Google Scholar] [CrossRef]
- Abdel-Motaal, M.; Almohawes, K.; Tantawy, M.A. Antimicrobial evaluation and docking study of some new substituted benzimidazole-2yl derivatives. Bioorg. Chem. 2020, 101, 103972. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Motaal, M.; Nabil, A. Biological activity of some newly synthesized hydrazone derivatives derived from (dicyclopropylmethylene) hydrazone. Eur. Chem. Bull. 2018, 7, 280–287. [Google Scholar] [CrossRef]
- Shaaban, S.; Negm, A.; Sobh, M.A.; Wessjohann, L.A. Expeditious Entry to Functionalized Pseudo-peptidic Organoselenide Redox Modulators via Sequential Ugi/SN Methodology. Anticancer Agents Med. Chem. 2016, 16, 621–632. [Google Scholar] [CrossRef] [PubMed]
- Shaaban, S.; El-Lateef, H.M.A.; Khalaf, M.M.; Gouda, M.; Youssef, I. One-Pot Multicomponent Polymerization, Metal-, and Non-Metal-Catalyzed Synthesis of Organoselenium Compounds. Polymers 2022, 14, 2208. [Google Scholar] [CrossRef] [PubMed]
- Refaay, D.A.; Ahmed, D.M.; Mowafy, A.M.; Shaaban, S. Evaluation of novel multifunctional organoselenium compounds as potential cholinesterase inhibitors against Alzheimer’s disease. Med. Chem. Res. 2022, 31, 894–904. [Google Scholar] [CrossRef]
- Shaaban, S.; Negm, A.; Ibrahim, E.E.; Elrazak, A.A. Hepatocellular carcinoma chemotherapeutic agents: Efficacy and mode of action. Onc. Rev. 2014, 8, 25. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Shaaban, S.; Adam, M.S.S.; El-Metwaly, N.M. Novel organoselenium-based N-mealanilic acid and its zinc (II) chelate: Catalytic, anticancer, antimicrobial, antioxidant, and computational assessments. J. Mol. Liq. 2022, 363, 119907. [Google Scholar] [CrossRef]
- Shaaban, S.; Shabana, S.M.; Al-Faiyz, Y.S.; Manolikakes, G.; El-Senduny, F.F. Enhancing the chemosensitivity of HepG2 cells towards cisplatin by organoselenium pseudopeptides. Bioorg. Chem. 2021, 109, 104713. [Google Scholar] [CrossRef] [PubMed]
- Abd El-Lateef, H.M.; Shaaban, S.; Khalaf, M.M.; Toghan, A.; Shalabi, K. Synthesis, experimental, and computational studies of water soluble anthranilic organoselenium compounds as safe corrosion inhibitors for J55 pipeline steel in acidic oilfield formation water. Colloids Surf. A Physicochem. Eng. Asp. 2021, 625, 126894. [Google Scholar] [CrossRef]
- Naithani, R. Organoselenium compounds in cancer chemoprevention. Mini Rev. Med. Chem. 2008, 8, 657–668. [Google Scholar] [CrossRef] [PubMed]
- Mugesh, G.; du Mont, W.W.; Sies, H. Chemistry of biologically important synthetic organoselenium compounds. Chem. Rev. 2001, 101, 2125–2179. [Google Scholar] [CrossRef] [PubMed]
- Shaaban, S.; Negm, A.; Ibrahim, E.E.; Elrazak, A.A. Chemotherapeutic agents for the treatment of hepatocellular carcinoma: Efficacy and mode of action. Oncol. Rev. 2014, 8, 246. [Google Scholar] [CrossRef][Green Version]
- Gouda, M.; Ferjani, H.; Abd El-Lateef, H.M.; Khalaf, M.M.; Shaaban, S.; Yousef, T.A. A Competition between Hydrogen, Stacking, and Halogen Bonding in N-(4-((3-Methyl-1, 4-dioxo-1, 4-dihydronaphthalen-2-yl) selanyl) phenyl) acetamide: Structure, Hirshfeld Surface Analysis, 3D Energy Framework Approach, and DFT Calculation. Int. J. Mol. Sci. 2022, 23, 2716. [Google Scholar] [CrossRef] [PubMed]
- Shaaban, S.; Ferjani, H.; Yousef, T.; Abdel-Motaal, M. Supramolecular Self-Assembly Built by Hydrogen, Stacking and Br · Br Interactions in 4-((4-Bromobenzyl) Selanyl) Aniline: Structure, Hirshfeld Surface Analysis, 3D Energy Framework Approach and Global Reactivity Descriptors. J. Inorg. Organomet. Polym. Mater. 2022, 32, 1878–1890. [Google Scholar] [CrossRef]
- Shaaban, S.; Ashmawy, A.M.; Negm, A.; Wessjohann, L.A. Synthesis and biochemical studies of novel organic selenides with increased selectivity for hepatocellular carcinoma and breast adenocarcinoma. Eur. J. Med. Chem. 2019, 179, 515–526. [Google Scholar] [CrossRef] [PubMed]
- Metwally, M.A.; Shaaban, S.; Abdel-Wahab, B.F.; El-Hiti, G.A. 3-Acetylindoles: Synthesis, reactions and biological activities. Curr. Org. Chem. 2009, 13, 1475. [Google Scholar] [CrossRef]
- Shaaban, S.; Gaffer, E.H.; Alshahd, M.; Elmorsy, S.S. Cytotoxic Symmetrical Thiazolediselenides with Increased Selectivity Against MCF-7 Breast Cancer Cells. Int. J. Res. Dev. Pharm. Life Sci. 2015, 4, 1654–1668. [Google Scholar]
- Shaaban, S.; Gaffer, E.H.; Jabar, Y.; Elmorsy, S.S. Cytotoxic naphthalene based-symmetrical diselenides with increased selectivity against MCF-7 breast cancer cells. Int. J. Pharm. 2015, 5, 738–746. [Google Scholar]
- Soriano-Garcia, M. Organoselenium compounds as potential therapeutic and chemopreventive agents: A review. Curr. Med. Chem. 2004, 11, 1657–1669. [Google Scholar] [CrossRef] [PubMed]
- Tian, X.; Schaich, K.M. Effects of molecular structure on kinetics and dynamics of the trolox equivalent antioxidant capacity assay with ABTS+•. J. Agric. Food Chem. 2013, 61, 5511–5519. [Google Scholar] [CrossRef] [PubMed]

| Compounds | MCF7 a | HepG2 a | WI38 a | ||
|---|---|---|---|---|---|
| IC50 (µM) a | TI c | IC50 (µM) a | TI c | IC50 (µM) a | |
| Adriamycin | 4.17 ± 0.2 | 1.6 | 4.50 ± 0.2 | 1.5 | 6.72 ± 0.5 |
| 4 | 23.45 ± 1.8 | 2.3 | 11.21 ± 1.0 | 4.8 | 52.62 ± 3.1 |
| 5 | 9.89 ± 0.7 | 3.6 | 7.08 ± 0.6 | 5 | 35.91 ± 2.3 |
| 6 | 15.92 ± 1.2 | 2.7 | 16.22 ± 1.2 | 2.6 | 42.45 ± 2.6 |
| 7 | 79.01 ± 4.2 | - | 83.67 ± 4.4 | - | 45.73 ± 2.7 |
| 8 | - b | - | - b | - | - b |
| 9 | - b | - | - b | - | - b |
| 10 | 53.49 ± 3.0 | - | 81.48 ± 4.2 | - | 38.23 ± 2.5 |
| 11 | 55.10 ± 3.2 | - | 43.70 ± 2.6 | - | - b |
| 12 | - b | - | - b | - | - b |
| 13 | 76.24 ± 4.0 | - | 78.53 ± 3.9 | - | 47.03 ± 2.8 |
| 14 | 5.64 ± 0.3 | 11 | 3.57 ± 0.1 | 17 | 62.38 ± 3.6 |
| 15 | 12.76 ± 0.9 | 2 | 6.48 ± 0.4 | 3.9 | 25.81 ± 1.8 |
| 16 | 50.81 ± 2.9 | 1.4 | 30.62 ± 2.4 | 2.3 | 71.90 ± 4.1 |
| Compound | E. coli | S. aureus | C. Albicans | |||
|---|---|---|---|---|---|---|
| ZID (mm) a | IA% b | ZID (mm) a | IA% b | ZID (mm) a | IA% b | |
| 4 | 14 | 60.9 | 16 | 76.2 | 18 | 75.0 |
| 5 | 19 | 82.6 | 18 | 85.7 | 21 | 87.5 |
| 6 | 16 | 69.6 | 16 | 76.2 | 17 | 70.8 |
| 7 | - | - | 3 | 14.3 | 4 | 16.7 |
| 8 | - | - | - | - | - | - |
| 9 | - | - | - | - | - | - |
| 10 | 5 | 21.7 | 8 | 38.1 | 5 | 20.8 |
| 11 | 5 | 21.7 | 7 | 33.3 | 10 | 41.7 |
| 12 | - | - | - | - | - | - |
| 13 | - | - | 4 | 19.0 | 5 | 20.8 |
| 14 | 21 | 91.3 | 19 | 90.5 | 24 | 100 |
| 15 | 17 | 73.9 | 18 | 85.7 | 22 | 91.7 |
| 16 | 6 | 26.1 | 9 | 42.8 | 13 | 54.2 |
| Ampicillin | 23 | 100 | 21 | 100 | - | - |
| Clotrimazole | - | - | - | - | 24 | 100 |
| Compounds | MIC (μM) | ||
|---|---|---|---|
| E. coli | S. aureus | C. Albicans | |
| 14 | 0.5 | 2 | 2 |
| Ampicillin | 0.5 | 1 | - |
| Clotrimazole | - | - | 2 |
| Compounds | IC50 (µM) | |
|---|---|---|
| DPPH | ABTS | |
| Vitamin C | 19.18 ± 0.13 | 28.16 ± 0.19 |
| 14 | 18.67 ± 0.12 | 29.14 ± 0.20 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Al-Abdallah, B.; Al-Faiyz, Y.S.; Shaaban, S. Anticancer, Antimicrobial, and Antioxidant Activities of Organodiselenide-Tethered Methyl Anthranilates. Biomolecules 2022, 12, 1765. https://doi.org/10.3390/biom12121765
Al-Abdallah B, Al-Faiyz YS, Shaaban S. Anticancer, Antimicrobial, and Antioxidant Activities of Organodiselenide-Tethered Methyl Anthranilates. Biomolecules. 2022; 12(12):1765. https://doi.org/10.3390/biom12121765
Chicago/Turabian StyleAl-Abdallah, Batool, Yasair S. Al-Faiyz, and Saad Shaaban. 2022. "Anticancer, Antimicrobial, and Antioxidant Activities of Organodiselenide-Tethered Methyl Anthranilates" Biomolecules 12, no. 12: 1765. https://doi.org/10.3390/biom12121765
APA StyleAl-Abdallah, B., Al-Faiyz, Y. S., & Shaaban, S. (2022). Anticancer, Antimicrobial, and Antioxidant Activities of Organodiselenide-Tethered Methyl Anthranilates. Biomolecules, 12(12), 1765. https://doi.org/10.3390/biom12121765








