Abstract
Multiple myeloma (MM) is a clonal plasma cell tumor originating from a post-mitotic lymphoid B-cell lineage. Bortezomib(BTZ), a first-generation protease inhibitor, has increased overall survival, progression-free survival, and remission rates in patients with MM since its clinical approval in 2003. However, the use of BTZ is challenged by the malignant features of MM and drug resistance. Polyphenols, classified into flavonoid and non-flavonoid polyphenols, have potential health-promoting activities, including anti-cancer. Previous preclinical studies have demonstrated the anti-MM potential of some dietary polyphenols. Therefore, these dietary polyphenols have the potential to be alternative therapies in anti-MM treatment regimens. This systematic review examines the synergistic effects of flavonoids and non-flavonoid polyphenols on the anti-MM impacts of BTZ. Preclinical studies on flavonoids and non-flavonoid polyphenols-BTZ synergism in MM were collected from PubMed, Web of Science, and Embase published between 2008 and 2020. 19 valid preclinical studies (Published from 2008 to 2020) were included in this systematic review. These studies demonstrated that eight flavonoids (icariin, icariside II, (-)-epigallocatechin-3-gallate, scutellarein, wogonin, morin, formononetin, daidzin), one plant extract rich in flavonoids (Punica granatum juice) and four non-flavonoid polyphenols (silibinin, resveratrol, curcumin, caffeic acid) synergistically enhanced the anti-MM effect of BTZ. These synergistic effects are mediated through the regulation of cellular signaling pathways associated with proliferation, apoptosis, and drug resistance. Given the above, flavonoids and non-flavonoid polyphenols can benefit MM patients by overcoming the challenges faced in BTZ treatment. Despite the positive nature of this preclinical evidence, some additional investigations are still needed before proceeding with clinical studies. For this purpose, we conclude by providing some suggestions for future research directions.
1. Introduction
Multiple myeloma(MM) is a clonal plasma cell tumor originating from the post-germinal lymphoid B cell line. It has an incidence of approximately 2.1 out of 100,000 people worldwide [1]. Its incidence varies notably among different races, with older individuals at higher risk [2]. Moreover, it often leads to poor outcomes in low and middle-income countries due to the lack of specialized health care, diagnosis, and advanced treatments. The 5-year survival rate for MM patients treated in Nigeria is only 7.6% [3].
At present, internal medicine intervention predominates in MM therapy. Standard treatment options include (1) induction therapy with a combination of injectable proteasome inhibitors(i.e., bortezomib(BTZ)), oral immunomodulators(i.e., lenalidomide), and dexamethasone; and (2) autologous stem cell transplantation(ASCT) with subsequent maintenance lenalidomide [4]. According to the clinical evidence, Velcade, Revlimid, and Dexamethasone (VRD)-based pre-ASCT induction therapy enhances the prognosis of MM patients [5,6]. Nevertheless, these interventions remain challenged by MM’s malignant qualities. For example, ASCT is not recommended for MM patients who are over the age of 65 or who have severe comorbid conditions like renal or pulmonary impairment, cardiac disease, or hepatic disease [7]. Most significantly, intrinsic clonal heterogeneity and genomic instability of plasma cells influence both inherent and acquired drug resistance [8]. For most MM patients, after several remissions and relapses, drug resistance develops into almost all available therapies [9]. Additionally, these MM medications could also have adverse effects. BTZ, for instance, may have adverse effects on the cardiovascular system, bone marrow suppression, and peripheral neuropathy [10]. Therefore, in order to overcome these difficulties, alternative and supportive therapies are required.
In recent years, natural dietary compounds have attracted the attention of researchers. They have a wide range of biological activities, such as anti-inflammatory, antioxidant, anti-aging, immune enhancement, as well as anti-tumor [11,12,13,14,15]. Among them, flavonoids, a class of polyphenolic plant secondary metabolites, are of great interest. Through inhibition of cell proliferation, stimulation of apoptosis, targeting of cancer stem cells, inhibition of angiogenesis and metastasis, and chemotherapy sensitization, flavonoids have anti-cancer effects [16]. In addition, the cancer prevention and treatment potential of some non-flavonoid polyphenols are equally noteworthy [17,18]. Jöhrer and Pojero reviewed the tumor activity inhibition and targeting cancer effects of some natural compounds (including polyphenols) in MM in preclinical trials, respectively [19,20]. As mentioned earlier, the main challenge MM drug interventions face is drug resistance. Therefore, exploring possible alternative treatments to overcome it is clinically valuable. This systematic review focuses on the synergistic anti-MM effects of flavonoids, other non-flavonoid polyphenols, and BTZ.
2. Research Methodology
The systematic review adheres to the guidance of the Preferred Reporting Items for Reference Systems Evaluation and Meta-Analysis (PRISMA) statement [21]. Preclinical studies on flavonoids or non-flavonoid polyphenols -bortezomib synergistic potentiation of anti-MM effects were collected by searching PubMed, Web of Science(WOS), and Embase for the keywords “flavonoid” or “polyphenol,” “bortezomib” or “Velcade,” and “Multiple myeloma” or “Myeloma, Plasma-Cell.”
Searching with the search formula “Multiple myeloma” or “Myeloma, plasma cell” (subject) and “Bortezomib or Velcade” (all fields) and “flavonoids or polyphenols” (all fields) were put into WOS, 33 publications were found. Performing a search at PubMed with the search formula: ((Multiple myeloma or “Myeloma, Plasma-Cell”) AND (Bortezomib or Velcade)) AND (flavonoids or polyphenols), 38 publications were retrieved. Searching in Embase with the search terms: ((Multiple myeloma or “Myeloma, Plasma-Cell”) AND (Bortezomib or Velcade)) AND (flavonoids or polyphenols), 144 publications were retrieved. The filters had not been applied in any of the above three databases. Endnote 20 was used to check for duplicates automatically and by hand for 215 publications, and 41 duplicates were taken out. The titles and abstracts of 174 publications were reviewed by two independent reviewers (Kaixi Ding and Wei Jiang). In case no consensus was reached, a third independent reviewer made the final decision. 121 publications that were not relevant to the topic of this review were removed.
The remaining 53 publications were then read in full by two independent reviewers. According to the inclusion and exclusion criteria: (1) treatment with flavonoid compounds or non-flavonoid polyphenolic compounds or plant extracts containing polyphenols; (2) animal studies or cell cultures; (3) interventions in animals or cell models of multiple myeloma; (4) studies included flavonoid compounds or non-flavonoid polyphenolic compounds or plant extracts containing polyphenols and BTZ synergistic anti-MM effects or mechanisms (5) Excluding review literature, meta-analyses, case reports, editorials, abstracts and conference proceedings and other types of literature. A total of 38 publications were discharged after a detailed review by the reviewers. In addition, two reviewers agreed that four publications with relevant citation searches from the research and the review literature should be added as secondary sources.
Eventually, 19 valid articles (Published from 2008 to 2020) were included in this review (Figure 1) (Table 1).
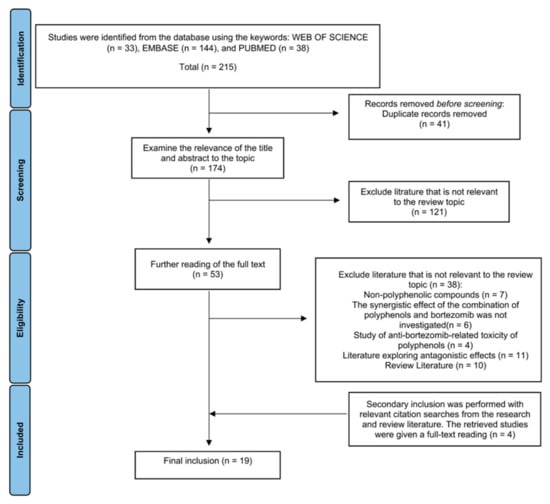
Figure 1.
Diagram of the process for including and excluding the literature for this review.

Table 1.
General information on the 19 publications included in this systematic review.
3. Mechanisms of MM
MM’s onset, progression, metastasis, and osteolytic destruction are associated with the regulation of multiple signaling pathways (Figure 2). Throughout the development of MM, rapid cell proliferation was mainly regulated by PI3K/Akt, Ras/Raf/MEK/Erk, JAK/STAT, Wnt/β-catenin, and RANK/RANKL/OPG signal pathways [41]. Uncontrolled cell proliferation was attributed to the inhibition of normal cell cycle regulation (e.g., FOXO1 and Cyclin D1), and the upregulation of downstream key anti-apoptotic factors (e.g., Bcl2, Bcl-xl, Mcl-1, and Caspase) [42]. Angiogenesis, mainly promoted by VEGF, provides oxygen and nutrients for tumor proliferation. Additionally, the activation of the Wnt/-catenin and RANK/RANKL/OPG signal pathways is primarily responsible for osteolytic bone disease in MM, which is brought on by an increase in osteoclast activity [41].
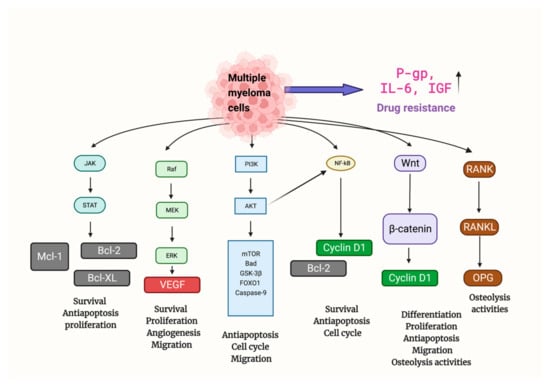
Figure 2.
Multiple signaling pathways are involved in the tumor formation and progression of MM. Mechanisms promoting anti-apoptosis, proliferation, angiogenesis, migration, and drug resistance were upregulated, while mechanisms related to apoptosis and normal cell cycle regulation were suppressed. Abbreviations: Bad: BCL2 associated agonist of cell death; Bcl2: B-cell lymphoma 2; Bcl-xl: B-cell lymphoma-extra-large; FOXO1: Forkhead box protein O1; GSK-3β: Glycogen synthase kinase-3 beta; JAK/STAT: Janus kinases/signal transducer and activator of transcription proteins; Mcl-1: Myeloid cell leukemia 1; MEK/Erk: MAP kinase–ERK kinase/extracellular-signal-regulated kinases; TOR: mammalian target of rapamycin; PI3K/Akt: Phosphoinositide 3-kinases/Protein Kinase B; RANK/RANKL/OPG: Receptor activator of nuclear factor κB/Receptor activator of nuclear factor kappa-Β ligand/Osteoprotegerin; Wnt: Wingless and Int-1.
Finally, MM cells can further develop drug resistance, and this may be related to mechanisms such as the upregulation of permeability-glycoprotein(P-gp) (leading to reduced drug aggregation in vivo). IL-6 and insulin-like growth factor (IGF), two cytokines produced by bone marrow mesenchymal stem cells, also encourage drug resistance in MM cells(activating specific signaling pathways that lead to drug resistance) [43].
4. Bortezomib in MM Therapy
An important class of medications for the treatment of MM is protease inhibitors. Based on targeted inhibition of the 26S proteasome, protease inhibitors are crucial in the pathogenesis and proliferation of MM [44]. BTZ, the first protease inhibitor, approved for clinical use in 2003, has resulted in gains in overall survival, progression-free survival, and remission rates in patients with MM (Figure 3) [45]. Its main anti-cancer mechanism is the inhibition of the chymotrypsin-like site of the 20S protein hydrolysis core within the 26S proteasome, which induces cell cycle arrest and apoptosis [46]. The main signaling mechanisms of BTZ-induced apoptosis in MM cells are NF-κB blockade and JNK activation. In addition, BTZ stabilizes various tumor suppressor proteins, such as P53, inhibiting MM cell cycle progression [47]. Currently, the drug is commonly used in MM patients in first-line, relapsed, and/or refractory settings [48]. However, drug resistance in MM therapy is the main challenge currently faced when using BTZ [43].
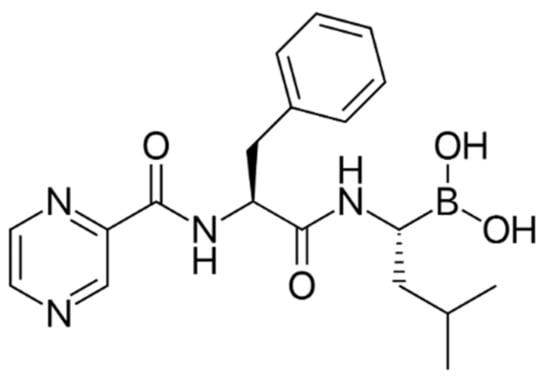
Figure 3.
Chemical structure of bortezomib, an anti-MM Protease inhibitor.
5. Exploration of Synergistic Drug Combinations in MM Therapy
When two or more medications are combined to improve their therapeutic effects, this is referred to as drug synergism [49]. For various diseases, including MM, appropriate drug combinations can reduce drug resistance or maximize efficacy [50,51,52,53]. For instance, the previously mentioned VRD is currently an effective and well-tolerated pre-ASCT induction protocol, benefiting newly diagnosed MM patients [6]. BTZ, as a clinically used protease inhibitor, is a cornerstone of VRD. However, significant obstacles to using BTZ to treat MM are drug resistance and the malignancy of MM. To cross these obstacles, researchers have investigated the BTZ resistance-reducing effects of various drug combinations and their synergistic anti-MM effects, such as daratumumab (anti-CD38 antibody), BTZ, and dexamethasone (CASTOR trial), TAK-243 (novel and specific UAE inhibitor) and BTZ, decitabine (epigenetic modulator) and BTZ [54,55,56]. Additionally, recent studies have demonstrated that some naturally occurring polyphenolic compounds have anti-MM activity. These polyphenolic compounds also have the potential to reduce BTZ resistance. Given the low toxicity of natural polyphenolic compounds, their synergistic combination with BTZ may offer MM patients extra therapeutic options [19,20].
6. Flavonoids and Non-Flavonoid Polyphenols in MM Therapy
Polyphenols are a class of phytochemicals divided into flavonoids and non-flavonoids. A group of compounds known as flavonoids consists of two benzene rings connected by phenolic hydroxyl groups by a central three-carbon atom. They are secondary metabolites in fruits, vegetables, and other herbal plants. The main flavonoid subgroup includes flavones, flavonols, flavan-3-ols, anthocyanins, isoflavones, and chalcones (Figure 4) [57]. The main non-flavonoids contained phenolic, hydroxycinnamic, lignans, stilbenes, and tannins [58]. Based on the results of this systematic review, various polyphenols, including flavonols(icariin, icariside II), flavan-3-ols((-)-epigallocatechin-3-gallate), flavone(scutellarein, wogonin, morin), isoflavone(formononetin, daidzin), plant extracts rich in flavonoids(punica granatum juice) and non-flavonoid polyphenols(silibinin, resveratrol, curcumin, caffeic acid) exerted anti-MM synergistic effects in combination with BTZ in vivo and/or in vitro(Table 2) (Figure 5).
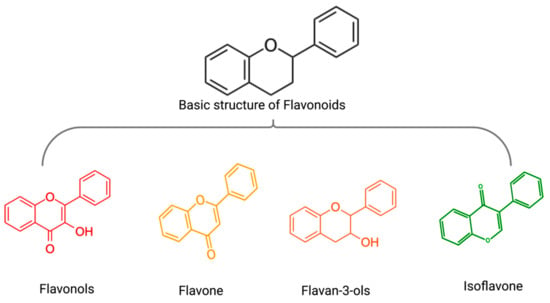
Figure 4.
The basic structure of flavonoids(black) and the general structure of flavonols (red), flavone (yellow), flavan-3-ols (orange), isoflavone (green).

Table 2.
Classes and sources of 13 polyphenols (9 flavonoids and 4 non-flavonoids) that synergize with BTZ against MM.
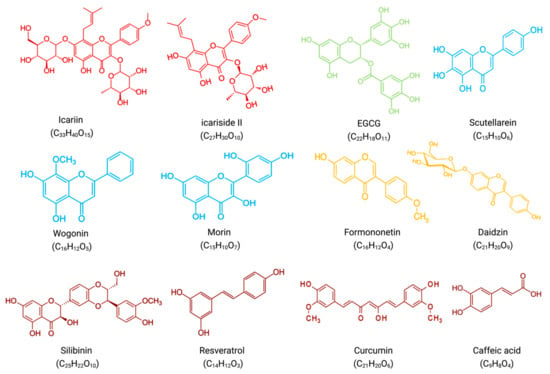
Figure 5.
Molecular formulae and chemical structures of flavonoid compounds and non-flavonoid polyphenolic compounds(excluding PGJ, a plant extract rich in flavonoids). Flavonols(icariin, icariside II) have a red chemical formula. Flavan-3-ol (EGCG) is green in color. The formula of flavones (scutellarein, wogonin, morin) is blue. The chemical formula of isoflavones (formononetin, daidzin) is yellow. Non-flavonoid polyphenols (silibinin, resveratrol, curcumin, caffeic acid) have a brown chemical formula.
Flavonols are a class of flavonoids with a 3-hydroxyflavonoid backbone. Two flavonols are of interest in synergistic anti-MM therapy. Icariin, an active ingredient in the stem and leaves of Epimedium, exerts significant anti-tumor effects on a variety of human tumor cells [22]. Similarly, icariside II, isolated from E. koreanum, inhibits the proliferation and induces apoptosis of many human cancer cells, such as MM, breast cancer, and prostate cancer [23].
Flavan-3-ols are flavan derivatives with a 2-phenyl-3,4-dihydro-2H-chroman-3-ol backbone. One flavan-3-ol is of interest in synergistic anti-MM therapy. (-)-epigallocatechin-3-gallate(EGCG) belongs to the category of catechins, commonly found in green tea. Catechins have anti-mutagenic, tumor progression-inhibiting, apoptosis-promoting, and antioxidant effects in various solid tumors [59,62].
Flavones are a class of flavonoids with a 2-phenylchromen-4-one backbone. Three flavones are of interest in synergistic anti-MM therapy. Scutellarein, obtained from S. baicalensis or S. barbata, exerts anticancer activity against various tumors, including breast, lymphoma, colorectal, lung, and liver cancers [24,25]. Similarly, wogonin, an active monoflavone from Scutellaria baicalensis with anti-angiogenic activity, is a potential anti-cancer drug with low toxicity [26]. Morin, isolated from members of the mulberry family, such as mulberry figs and old figs, has anti-proliferative activity against some tumors, such as oral squamous cell carcinoma, leukemia, and colorectal cancer [27].
In contrast to flavones, isoflavones have a chemical structure based on a 3-phenylchromium-4-one backbone. Two isoflavones are of interest in synergistic anti-MM therapy. Formononetin, mainly isolated from the roots of Astragalus membranaceus, Trifolium pratense, Glycyrrhiza glabra, and Pueraria lobate, has anti-inflammatory, antioxidant, antiviral, neuroprotective, wound healing, and antitumor biological activities [28,29]. Daidzin can be isolated from Pueraria lobate. It has demonstrated anticancer activity in preventing and treating breast and prostate cancers [30].
In addition, Punica granatum juice(PGJ), one plant extract rich in flavonoids, is also of attention. The drug activity of PGJ is related to many flavonoid phytoactive components, including the anthocyanins catechin, quercetin, kaempferol, apigenin, and lignan. The anticancer effects of PGJ are widely used to prevent and treat colon, lung, skin, and prostate cancers [31].
Finally, polyphenols other than flavonoids belong to non-flavonoid polyphenols. Four non-flavonoid polyphenols are of interest in synergistic anti-MM therapy. Silibinin, an extract of Milk Thistle, exerts very high antioxidant and antitumor properties [32,60]. Resveratrol is widely found in grapes, berries, and peanuts and has anticancer activity against most human cancers [61]. Another non-flavonoid polyphenol, curcumin, is present in turmeric root. It exerts anti-inflammatory, anti-atherosclerotic, and anti-tumor pharmacological effects [33]. In addition, caffeic acid, an active component of honeybee propolis, has cytotoxic, apoptosis, and anti-proliferation effects [34].
7. Synergistic Effects of Flavonoids and Bortezomib in Anti-MM
Nine flavonoids (icariin, icariside II, EGCG, scutellarein, wogonin, morin, formononetin, daidzin) plus a flavonoid-rich plant extract (PGJ) synergize with BTZ in anti-MM by regulating proliferation, apoptosis, and drug resistance-related signaling pathways (Figure 6) (Table 3).
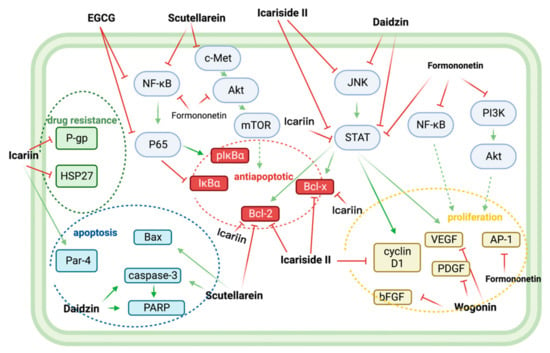
Figure 6.
Nine flavonoids, icariin, icariside II, EGCG, scutellarein, wogonin, morin, formononetin, daidzin, and isoginkgetin, together exerted multiple synergistic BTZ anti-MM effects. Icariin synergistically improved the drug sensitivity of MM to BTZ by reducing the expression of drug resistance proteins HSP27 and P-gp. EGCG, scutellarein, icariin, icariside II, and formononetin down-regulated the downstream anti-apoptotic proteins Bcl2, BclXl, pIκBα by inhibiting NF-κB, c-Met/Akt/mTOR, and JAK/STAT pathways, thereby synergistically reversing anti-apoptosis. Icariside II, formononetin, and Wogonin, with BTZ synergistically, exerted antiproliferative effects by downregulating VEGF, PDGF, bFGF, cyclin D1, and AP-1. Finally, scutellarein, Daidzin, and Icariin enhanced BTZ-related apoptosis by upregulating the expression of Par-4, Bax, caspase-3, and PARP. Abbreviations: AP-1: Activator protein 1; Bax: bcl-2-like protein 4; Bcl2: B-cell lymphoma 2; Bcl-xl: B-cell lymphoma-extra-large; bFGF: basic fibroblast growth factor; c-Met: tyrosine-protein kinase Met; NF-κB/P56: Nuclear factor-kappa-B/p65; HSP27: Heat shock protein 27; JAK/STAT: Janus kinases/signal transducer and activator of transcription proteins; IκBα: nuclear factor of kappa light polypeptide gene enhancer in B-cells inhibitor, alpha; TOR: mammalian target of rapamycin; PARP: Poly (ADP-ribose) polymerase; Par-4: Prostate apoptosis response-4; PDGF: Platelet-derived growth factor; P-gp: P-glycoprotein; PI3K/Akt: Phosphoinositide 3-kinases/Protein Kinase B.

Table 3.
Mechanistic anti-MM effects of flavonoid-BTZ combinations, as demonstrated in vitro and in vivo.
7.1. Icariin and Bortezomib
In KM3/BTZ-resistant cells, icariin increased the sensitivity of KM3/BTZ cells to BTZ and partially reversed the drug resistance. Its effect on changing drug resistance may be mediated by upregulating the expression of pro-apoptotic cytokine Par-4, decreasing the expression of drug resistance proteins HSP27 and P-gp [22]. Another study found that the combination of icariin and BTZ promoted apoptosis in U266 cells by blocking the JAK/STAT pathway and lowering the expression of anti-apoptotic proteins like Bcl-2, Bcl-xl, and Survivin. This combination also delayed the cell cycle progression, as shown by the result that more U266 cells were in the G0/G1 phase [23].
7.2. Icariside II and Bortezomib
While icariside II enhanced the apoptotic effect of BTZ in U266 cells, it also inhibited the JAK/STAT pathway and down-regulated the expression of STAT3 target genes Bcl-2, Bcl-xl, Survivin, cyclin D1, COX-2, and VEGF, beneficially inhibiting proliferation and promoting apoptosis [35].
7.3. EGCG and Bortezomib
EGCG, a catechin in green tea, and BTZ synergistically inhibited KM3 cell growth and induced apoptosis by inhibiting NF-κB/P65 expression, down-regulating pIκBα, and up-regulating IκBα expression [36].
7.4. Scutellarein and Bortezomib
In vitro, scutellarein alone time-dependently reduced cell viability and significantly induced apoptosis in MM.1R and IM-9 cells. In vivo, dual intervention with scutellarein and BTZ significantly reduced xenograft tumor burden in nude mice with no significant effect on mouse body weight. In parallel, protein expression levels of some apoptotic markers were altered, such as active caspase-3 (upregulated), Bax (upregulated), and Bcl-2 (downregulated) [25]. In another in vivo study, scutellarein eliminated MM cell resistance to BTZ through multiple mechanistic pathways. This result was caused by the HDAC/miR-34a-mediated epigenetic regulation of the c-Met/Akt/mTOR pathway and the NF-κB-mediated activation of the apoptosis cascade [24].
7.5. Wogonin and Bortezomib
Wogonin and BTZ synergistically inhibited the secretion levels of pro-angiogenic factors VEGF, PDGF, and bFGF in RPMI 8226 cells. The angiogenesis-inhibitory effect of wogonin may be related to its inhibition of the c-Myc/HIF-1α signaling axis [26].
7.6. Morin and Bortezomib
Morin potentiates BTZ-induced apoptosis in U266 cells, from 18.7% to 51.2%, by inhibiting the STAT3 pathway, leading to downregulation of STAT3-dependent gene expressions, such as XIAP, cFLIP, Mcl-1, Survivin, Bcl2, BclXl, and c-IAP-2. And morin’s inhibition of STAT3 phosphorylation was more pronounced than other flavonols (galangin, kaempferol, quercetin, and myricetin) with different positions and numbers of its hydroxyl groups on the B-loop [27].
7.7. Formononetin and Bortezomib
In vitro, formononetin and BTZ enhanced STAT3 inhibition and promoted the death of U266 cells [28]. Subsequently, a follow-up study by the same research group found that formononetin and BTZ exert synergistic enhancement of anti-proliferation and pro-apoptosis by blocking the activation of NF-κB, PI3K/AKT, and AP-1 [29].
7.8. Daidzin and Bortezomib
Daidzin synergistically increased the apoptotic and cytotoxic effects of BTZ by inhibiting the activation of STAT3 and its upstream kinases (JAK1, JAK2, and c-Src). In addition, this drug combination increased caspase-3 activation and PARP cleavage, leading to the downregulation of the expression of various oncogenic apoptotic proteins [30].
7.9. Plant Extracts and Bortezomib
In vitro, PGJ inhibited angiogenesis, microvascular growth outside of aortic rings, cell migration, and invasion in MM cells. In addition, After BTZ exposure, PGJ intervention increased the cytotoxic effects on U266 cells [31].
8. Synergistic Effects of Non-Flavonoid Polyphenols and Bortezomib in Anti-MM
Four flavonoids (silibinin, resveratrol, curcumin, caffeic acid) synergize with BTZ in anti-MM effect by regulating proliferation, apoptosis, and drug resistance-related signaling pathways (Table 4).

Table 4.
Mechanistic anti-MM effects of non-flavonoid polyphenols-BTZ combinations, as demonstrated in vitro and in vivo.
8.1. Silibinin and Bortezomib
In combination with low concentrations of BTZ, silibinin increased the cytotoxic effect of BTZ by increasing the expression of activated caspases, which promoted apoptosis [32].
8.2. Resveratrol and Bortezomib
In vitro, resveratrol induced apoptosis in MM144 cells by upregulating the Fas/CD95 signaling pathway and caspase-8 and caspase-10. Moreover, when used in combination, resveratrol, and BTZ highly enhanced U266 cell apoptosis [37].
8.3. Curcumin and Bortezomib
Curcumin enhances the apoptotic effect of BTZ on MM cells by regulating multiple signaling pathways (Figure 7). The bone marrow microenvironment, in which bone marrow stromal cells (BMSCs) interact with MM, influences the survival and growth of MM cells. In vitro, curcumin inhibited the activation of JAK/STAT and MAPK pathways in U266 cells after treatment with BMSCs cell supernatant. Additionally, curcumin and BTZ co-treatment efficiently prevented IL-6-induced STAT3 and Erk phosphorylation, increased PARP cleavage, and decreased pro-caspase-3 levels. Through these mechanisms mentioned above, this combination inhibited the growth of U266 cells and promoted apoptosis [38]. After that, in another trial, curcumin enhanced the inhibitory effect of BTZ on NF-κB activation in U266 cells, leading to increased apoptosis. In vivo, the combination group was more potent than the BTZ group in reducing tumor volume [39]. Moreover, curcumin increased the expression of cleaved caspase-3 protein by inhibiting the Notch1 signaling pathway, which increased the drug sensitivity of RPMI-8226 cells and U266 cells to BTZ [33].
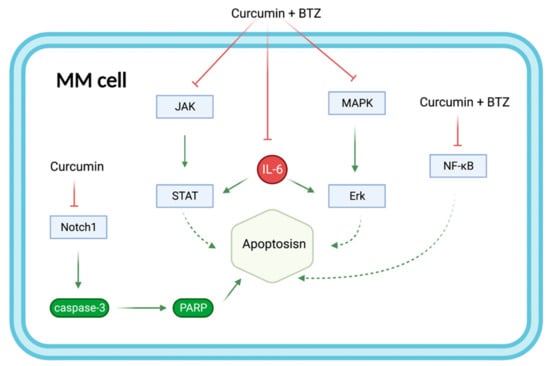
Figure 7.
The combination of curcumin and BTZ synergistically enhances the effect on MM apoptosis by regulating multiple signaling pathways. Curcumin+BTZ synergistically enhanced apoptosis through potent inhibition of IL-6-induced JNK/STAT and MAPK/ERK, as well as NF-κB. Additional inhibition of the Notch1 signaling pathway by curcumin increased the expression of caspase3 protein and PARP and therefore enhanced apoptosis. Abbreviations: BTZ: bortezomib; NF-κB: Nuclear factor-kappa-B; MAPK/ERK: mitogen-activated protein kinases/extracellular signal-regulated kinases; MM: Multiple myeloma; Notch1: Neurogenic locus notch homolog protein 1; JAK/STAT: Janus kinases/signal transducer and activator of transcription proteins; PARP: Poly (ADP-ribose) polymerase.
A class of novel curcumin analogs known as amino acid adducts of curcumin improved the proteasomal inhibitory effect of BTZ on MM cells and they enhanced the proliferation inhibition and apoptosis induction of BTZ. Notably, similar to curcumin, the twelfth curcumin analog increased PARP and caspase-3 cleavage, enhancing BTZ-induced apoptosis. The water-soluble, highly bioavailable twelfth curcumin analog has the potential to replace curcumin in anti-MM therapy in the future [40].
8.4. Caffeic acid and Bortezomib
In vitro, caffeic acid alone inhibits NF-κB-binding activity and IL-6 levels, which are closely linked to apoptosis and growth of tumor cells. Moreover, the combination of caffeic acid and BTZ synergistically increased cytotoxic and anti-proliferative effects on ARH-77 cells [34].
9. Conclusions and Future Directions
Although treatment options for MM continue to be optimized, the prognosis remains unsatisfactory. The aggressiveness and drug resistance of malignant tumors hinder the current treatment with protease inhibitors—especially BTZ. In this regard, flavonoids and non-flavonoid polyphenols are potential supportive therapies to address some of the challenges faced in BTZ treatment. Flavonoids(icariin, icariside II, EGCG, scutellarein, wogonin, morin, formononetin, daidzin), plant extract rich in flavonoids(PGJ), and non-flavonoid polyphenols(silibinin, resveratrol, curcumin, caffeic acid) combined with BTZ demonstrate the synergistic anti-MM effect. These synergistic anti-MM effects were achieved by anti-proliferative, pro-apoptotic, and anti-drug resistance. Based on those pieces of evidence, flavonoids, plant extract rich in flavonoids, and non-flavonoid polyphenols may benefit patients with MM, especially by overcoming the challenges faced in BTZ therapy.
According to some other relevant preclinical evidence, some polyphenols alone have anti-MM activity, such as isoginkgetin (a biflavone) and virola oleifera (a polyphenol-rich plant extract) [63,64]. Their synergistic effects, in combination with BTZ for the treatment of MM, need to be further explored. In addition, most of the synergistic anti-MM impacts of polyphenols + BTZ are only verified at the cellular level, such as icariin, icariside, EGCG, wogonin, morin, daidzin, PGJ, silibinin, and resveratrol. Their anti-MM synergistic effects in combination with BTZ need further clinically relevant animal models for validation. These polyphenols + BTZ exert synergistic effects through modulation of MM-related signaling pathways such as NF-κB, PI3K/Akt, Ras/Raf/MEK/Erk, JAK/STAT, and Wnt/β-catenin. However, the signaling pathways of each combination of anti-MM are still rudimentary, and more research is needed to refine the signaling pathway network. Although these results show promise for a synergistic MM treatment using these polyphenolic compounds and BTZ, the evidence at this time is only preclinical. Therefore, further clinical studies must demonstrate these synergistic effects. Moreover, preclinical studies on the anti-MM synergistic effects of these polyphenols with two other clinically available protease inhibitors, carfilzomib, and estazomib, need to be conducted to provide more options for clinical combination use. Adverse effects of protease inhibitor use are another challenge in the treatment of MM, although not within the scope of the review. Some polyphenols have the ameliorative potential for the non-tumor toxicity of protease inhibitors. For instance, rutin mitigates carfilzomib-induced cardiotoxicity by inhibiting NF-κB, mast gene expression, and reducing oxidative stress [65]. Resveratrol, by activating SIRT1, improves BTZ mechanical nociceptive sensitization [66]. For the comprehensive management of MM, additional studies on the antagonism of polyphenols against protease inhibitor-associated toxicity are also of clinical value. Finally, BTZ could alleviate MM-associated bone disease by regulating the RANK/RANKL/OPG signaling pathway [67]. By concentrating on the modulation of the RANK/RANKL/OPG signaling pathway, it is possible to investigate whether combining polyphenols and BTZ has beneficial effects on MM-associated bone disease.
Some polyphenols contain catechol moieties, such as EGCG, quercetin, and myricetin. Notably, they reduce the protease inhibitory activity of BTZ by forming stable cyclic boronic esters, thereby decreasing the anti-MM effect of BTZ [68,69]. A representative potential BTZ antagonistic polyphenol, EGCG, demonstrated dose-dependent inhibition of BTZ antitumor activity [62]. An in vitro study found that, by activating Wnt/β-catenin, EGCG antagonized the antitumor effect of BTZ [70]. Therefore, clinical staff members should be cautious about BTZ drug interactions with these polyphenols in clinical use. Last but not least, the problem of bioavailability is an important reason why polyphenols do not work as well as they could in the body. In the future, Low bioavailability polyphenols will eventually need to be combined with new drug delivery systems or other derivatives to utilize their anticancer capabilities fully.
Author Contributions
Conceptualization, K.D. and H.J.; methodology, K.D. and W.J.; software, W.J.; validation, K.D.; writing—original draft preparation, K.D.; writing—review and editing, K.D. and W.J.; visualization, W.J.; supervision, M.L. and H.J.; project administration, M.L. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Agreed to publish.
Data Availability Statement
Not applicable.
Acknowledgments
The authors would like to thank Xueping Li of the department of geriatrics, Hospital of Chengdu University of Traditional Chinese Medicine for critically reviewing the manuscript.
Conflicts of Interest
The authors declare no competing interest.
References
- Ludwig, H.; Novis Durie, S.; Meckl, A.; Hinke, A.; Durie, B. Multiple Myeloma Incidence and Mortality Around the Globe; Interrelations Between Health Access and Quality, Economic Resources, and Patient Empowerment. Oncologist 2020, 25, e1406–e1413. [Google Scholar] [CrossRef] [PubMed]
- Kazandjian, D. Multiple myeloma epidemiology and survival: A unique malignancy. Semin. Oncol. 2016, 43, 676–681. [Google Scholar] [CrossRef] [PubMed]
- Cowan, A.J.; Allen, C.; Barac, A.; Basaleem, H.; Bensenor, I.; Curado, M.P.; Foreman, K.; Gupta, R.; Harvey, J.; Hosgood, H.D.; et al. Global Burden of Multiple Myeloma: A Systematic Analysis for the Global Burden of Disease Study 2016. JAMA Oncol. 2018, 4, 1221–1227. [Google Scholar] [CrossRef] [PubMed]
- Cowan, A.J.; Green, D.J.; Kwok, M.; Lee, S.; Coffey, D.G.; Holmberg, L.A.; Tuazon, S.; Gopal, A.K.; Libby, E.N. Diagnosis and Management of Multiple Myeloma. JAMA: J. Am. Med. Assoc. 2022, 327, 464–477. [Google Scholar] [CrossRef] [PubMed]
- Kumar, L.; Gundu, N.; Kancharia, H.; Sahoo, R.K.; Malik, P.S.; Sharma, A.; Gupta, R.; Sharma, O.; Biswas, A.; Kumar, R.; et al. Multiple Myeloma—Effect of Induction Therapy on Transplant Outcomes. Clin. Lymphoma Myeloma Leuk. 2020, 21, 80–90.e5. [Google Scholar] [CrossRef]
- Rosiñol, L.; Oriol, A.; Rios, R.; Sureda, A.; Blanchard, M.J.; Hernández, M.T.; Martínez-Martínez, R.; Moraleda, J.M.; Jarque, I.; Bargay, J.; et al. Bortezomib, lenalidomide, and dexamethasone as induction therapy prior to autologous transplant in multiple myeloma. Blood 2019, 134, 1337–1345. [Google Scholar] [CrossRef]
- Facon, T.; Venner, C.P.; Bahlis, N.J.; Offner, F.; White, D.J.; Karlin, L.; Benboubker, L.; Rigaudeau, S.; Rodon, P.; Voog, E.; et al. Oral ixazomib, lenalidomide, and dexamethasone for transplant-ineligible patients with newly diagnosed multiple myeloma. Blood 2021, 137, 3616–3628. [Google Scholar] [CrossRef]
- Al Hadidi, S.; Yellapragada, S. Treatment Options for Relapsed and Refractory Multiple Myeloma: A Luxury or a Challenge? JAMA Oncol. 2021, 7, 1449. [Google Scholar] [CrossRef]
- Kastritis, E.; Terpos, E.; Dimopoulos, M.A. How I treat relapsed multiple myeloma. Blood 2022, 139, 2904–2917. [Google Scholar] [CrossRef]
- Pancheri, E.; Guglielmi, V.; Wilczynski, G.M.; Malatesta, M.; Tonin, P.; Tomelleri, G.; Nowis, D.; Vattemi, G. Non-Hematologic Toxicity of Bortezomib in Multiple Myeloma: The Neuromuscular and Cardiovascular Adverse Effects. Cancers 2020, 12, 2540. [Google Scholar] [CrossRef]
- Argyropoulou, A.; Aligiannis, N.; Trougakos, I.P.; Skaltsounis, A.-L. Natural compounds with anti-ageing activity. Nat. Prod. Rep. 2013, 30, 1412–1437. [Google Scholar] [CrossRef] [PubMed]
- Haque, A.; Brazeau, D.; Amin, A.R. Perspectives on natural compounds in chemoprevention and treatment of cancer: An update with new promising compounds. Eur. J. Cancer 2021, 149, 165–183. [Google Scholar] [CrossRef] [PubMed]
- Mrityunjaya, M.; Pavithra, V.; Neelam, R.; Janhavi, P.; Halami, P.M.; Ravindra, P.V. Immune-Boosting, Antioxidant and Anti-inflammatory Food Supplements Targeting Pathogenesis of COVID-19. Front. Immunol. 2020, 11, 570122. [Google Scholar] [CrossRef] [PubMed]
- Neha, K.; Haider, R.; Pathak, A.; Yar, M.S. Medicinal prospects of antioxidants: A review. Eur. J. Med. Chem. 2019, 178, 687–704. [Google Scholar] [CrossRef] [PubMed]
- Rondanelli, M.; Faliva, M.A.; Miccono, A.; Naso, M.; Nichetti, M.; Riva, A.; Guerriero, F.; De Gregori, M.; Peroni, G.; Perna, S. Food pyramid for subjects with chronic pain: Foods and dietary constituents as anti-inflammatory and antioxidant agents. Nutr. Res. Rev. 2018, 31, 131–151. [Google Scholar] [CrossRef] [PubMed]
- Forni, C.; Rossi, M.; Borromeo, I.; Feriotto, G.; Platamone, G.; Tabolacci, C.; Mischiati, C.; Beninati, S. Flavonoids: A Myth or a Reality for Cancer Therapy? Molecules 2021, 26, 3583. [Google Scholar] [CrossRef]
- Maiuolo, J.; Gliozzi, M.; Carresi, C.; Musolino, V.; Oppedisano, F.; Scarano, F.; Nucera, S.; Scicchitano, M.; Bosco, F.; Macri, R.; et al. Nutraceuticals and Cancer: Potential for Natural Polyphenols. Nutrients 2021, 13, 3834. [Google Scholar] [CrossRef]
- Zhou, Y.; Zheng, J.; Li, Y.; Xu, D.-P.; Li, S.; Chen, Y.-M.; Li, H.-B. Natural Polyphenols for Prevention and Treatment of Cancer. Nutrients 2016, 8, 515. [Google Scholar] [CrossRef]
- Jöhrer, K.; Çiçek, S. Multiple Myeloma Inhibitory Activity of Plant Natural Products. Cancers 2021, 13, 2678. [Google Scholar] [CrossRef]
- Pojero, F.; Poma, P.; Spanò, V.; Montalbano, A.; Barraja, P.; Notarbartolo, M. Targeting multiple myeloma with natural polyphenols. Eur. J. Med. Chem. 2019, 180, 465–485. [Google Scholar] [CrossRef]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G.; the PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. BMJ 2009, 339, b2535. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.Y.; Li, Z.J.; Chen, X.; Huang, X.R.; Fang, Z.Q.; Zhang, J.G.; Fang, J. Icaritin Reverses Multidrug Resistance of Multiple Myeloma Cell Line KM3/BTZ. Zhongguo Shi Yan Xue Ye Xue Za Zhi 2017, 25, 1690–1695. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.-H.; Ahn, K.S.; Jeong, S.-J.; Kwon, T.-R.; Jung, J.H.; Yun, S.-M.; Han, I.; Lee, S.-G.; Kim, D.K.; Kang, M.; et al. Janus activated kinase 2/signal transducer and activator of transcription 3 pathway mediates icariside II-induced apoptosis in U266 multiple myeloma cells. Eur. J. Pharmacol. 2011, 654, 10–16. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Zheng, Y.; Zhang, W.; Hou, L.; Gao, Y. Scutellarin circumvents chemoresistance, promotes apoptosis, and represses tumor growth by HDAC/miR-34a-mediated down-modulation of Akt/mTOR and NF-κB-orchestrated signaling pathways in multiple myeloma. Int. J. Clin. Exp. Pathol. 2020, 13, 212–219. [Google Scholar] [PubMed]
- Shi, L.; Wu, Y.; Lv, D.L.; Feng, L. Scutellarein selectively targets multiple myeloma cells by increasing mitochondrial superoxide production and activating intrinsic apoptosis pathway. Biomed. Pharmacother. 2018, 109, 2109–2118. [Google Scholar] [CrossRef]
- Fu, R.; Chen, Y.; Wang, X.-P.; An, T.; Tao, L.; Zhou, Y.-X.; Huang, Y.-J.; Chen, B.-A.; Li, Z.-Y.; You, Q.-D.; et al. Wogonin inhibits multiple myeloma-stimulated angiogenesis via c-Myc/VHL/HIF-1α signaling axis. Oncotarget 2015, 7, 5715–5727. [Google Scholar] [CrossRef]
- Gupta, S.C.; Phromnoi, K.; Aggarwal, B.B. Morin inhibits STAT3 tyrosine 705 phosphorylation in tumor cells through activation of protein tyrosine phosphatase SHP1. Biochem. Pharmacol. 2012, 85, 898–912. [Google Scholar] [CrossRef]
- Kim, C.; Lee, S.-G.; Yang, W.M.; Arfuso, F.; Um, J.-Y.; Kumar, A.P.; Bian, J.; Sethi, G.; Ahn, K.S. Formononetin-induced oxidative stress abrogates the activation of STAT3/5 signaling axis and suppresses the tumor growth in multiple myeloma preclinical model. Cancer Lett. 2018, 431, 123–141. [Google Scholar] [CrossRef]
- Kim, C.; Lee, J.H.; Ko, J.-H.; Chinnathambi, A.; Alharbi, S.A.; Shair, O.H.; Sethi, G.; Ahn, K.S. Formononetin Regulates Multiple Oncogenic Signaling Cascades and Enhances Sensitivity to Bortezomib in a Multiple Myeloma Mouse Model. Biomolecules 2019, 9, 262. [Google Scholar] [CrossRef]
- Yang, M.H.; Jung, S.H.; Chinnathambi, A.; Alahmadi, T.A.; Alharbi, S.A.; Sethi, G.; Ahn, K.S. Attenuation of STAT3 Signaling Cascade by Daidzin Can Enhance the Apoptotic Potential of Bortezomib against Multiple Myeloma. Biomolecules 2019, 10, 23. [Google Scholar] [CrossRef]
- Tibullo, D.; Caporarello, N.; Giallongo, C.; Anfuso, C.D.; Genovese, C.; Arlotta, C.; Puglisi, F.; Parrinello, N.L.; Bramanti, V.; Romano, A.; et al. Antiproliferative and Antiangiogenic Effects of Punica granatum Juice (PGJ) in Multiple Myeloma (MM). Nutrients 2016, 8, 611. [Google Scholar] [CrossRef] [PubMed]
- Geraldes, C.; Goncalves, A.; Alves, R.; Roque, A.; Manuel, J.; Costa, N.; Sarmento-Ribeiro, A. New Targeted Drugs in Multiple Myeloma Therapy—in Vitro Studies. Clin. Lymphoma Myeloma Leuk. 2015, 15, e254. [Google Scholar] [CrossRef]
- Ge, X.-P.; Hong, Y.-T.; Li, Y.-L.; Li, B.-Z. Curcumin Increases the Chemosensitivity of Multiple Myeloma to Bortezomib by Inhibiting the Notch1 Signaling Pathway. Zhongguo Shi Yan Xue Ye Xue Za Zhi 2019, 27, 464–471. [Google Scholar]
- Altayli, E.; Koru, Ö.; ÖNGÜRÜ, Ö.; Ide, T.; Acikel, C.; Sarper, M.; Elci, M.P.; Sağkan, R.I.; Astarci, E.; Tok, D.; et al. An in vitro and in vivo investigation of the cytotoxic effects of caffeic acid (3,4-dihydroxycinnamic acid) phenethyl ester and bortezomib in multiple myeloma cells. Turk. J. Med. Sci. 2015, 45, 38–46. [Google Scholar] [CrossRef] [PubMed]
- Jung, Y.Y.; Lee, J.H.; Nam, D.; Narula, A.S.; Namjoshi, O.A.; Blough, B.E.; Um, J.-Y.; Sethi, G.; Ahn, K.S. Anti-myeloma Effects of Icariin Are Mediated Through the Attenuation of JAK/STAT3-Dependent Signaling Cascade. Front. Pharmacol. 2018, 9, 531. [Google Scholar] [CrossRef]
- Wang, Q.; Li, J.; Gu, J.; Huang, B.; Zhao, Y.; Zheng, D.; Ding, Y.; Zeng, L. Potentiation of (−)-epigallocatechin-3-gallate-induced apoptosis by bortezomib in multiple myeloma cells. Acta Biochim. et Biophys. Sin. 2009, 41, 1018–1026. [Google Scholar] [CrossRef] [PubMed]
- Reis-Sobreiro, M.; Gajate, C.; Mollinedo, F. Involvement of mitochondria and recruitment of Fas/CD95 signaling in lipid rafts in resveratrol-mediated antimyeloma and antileukemia actions. Oncogene 2009, 28, 3221–3234. [Google Scholar] [CrossRef]
- Park, J.; Ayyappan, V.; Bae, E.-K.; Lee, C.; Kim, B.-S.; Kim, B.K.; Lee, Y.-Y.; Ahn, K.-S.; Yoon, S.-S. Curcumin in combination with bortezomib synergistically induced apoptosis in human multiple myeloma U266 cells. Mol. Oncol. 2008, 2, 317–326. [Google Scholar] [CrossRef]
- Sung, B.; Kunnumakkara, A.B.; Sethi, G.; Anand, P.; Guha, S.; Aggarwal, B.B. Curcumin circumvents chemoresistance in vitro and potentiates the effect of thalidomide and bortezomib against human multiple myeloma in nude mice model. Mol. Cancer Ther. 2009, 8, 959–970. [Google Scholar] [CrossRef]
- Chan, T.H.; Mujtaba, T.; Kanwar, J.; Wan, S.B.; Dou, Q.P. Sensitizing human multiple myeloma cells to the proteasome inhibitor bortezomib by novel curcumin analogs. Int. J. Mol. Med. 2011, 29, 102–106. [Google Scholar] [CrossRef]
- Hu, J.; Hu, W.-X. Targeting signaling pathways in multiple myeloma: Pathogenesis and implication for treatments. Cancer Lett. 2018, 414, 214–221. [Google Scholar] [CrossRef] [PubMed]
- Anderson, K.C.; Carrasco, R.D. Pathogenesis of Myeloma. Annu. Rev. Pathol. Mech. Dis. 2011, 6, 249–274. [Google Scholar] [CrossRef] [PubMed]
- Robak, P.; Drozdz, I.; Szemraj, J.; Robak, T. Drug resistance in multiple myeloma. Cancer Treat. Rev. 2018, 70, 199–208. [Google Scholar] [CrossRef] [PubMed]
- Gandolfi, S.; Laubach, J.P.; Hideshima, T.; Chauhan, D.; Anderson, K.C.; Richardson, P.G. The proteasome and proteasome inhibitors in multiple myeloma. Cancer Metastasis Rev. 2017, 36, 561–584. [Google Scholar] [CrossRef]
- Scott, K.; Hayden, P.J.; Will, A.; Wheatley, K.; Coyne, I. Bortezomib for the treatment of multiple myeloma. Cochrane Database Syst. Rev. 2016, 4, CD010816. [Google Scholar] [CrossRef]
- Tan, C.R.C.; Abdul-Majeed, S.; Cael, B.; Barta, S.K. Clinical Pharmacokinetics and Pharmacodynamics of Bortezomib. Clin. Pharmacokinet. 2018, 58, 157–168. [Google Scholar] [CrossRef]
- Thibaudeau, T.A.; Smith, D.M. A Practical Review of Proteasome Pharmacology. Pharmacol. Rev. 2019, 71, 170–197. [Google Scholar] [CrossRef]
- Manasanch, E.E.; Orlowski, R.Z. Proteasome inhibitors in cancer therapy. Nat. Rev. Clin. Oncol. 2017, 14, 417–433. [Google Scholar] [CrossRef]
- Tallarida, R.J. Drug synergism: Its detection and applications. J. Pharmacol. Exp. Ther. 2001, 298, 865–872. Available online: https://pubmed.ncbi.nlm.nih.gov/11504778/ (accessed on 31 September 2001).
- Coates, A.R.M.; Hu, Y.; Holt, J.; Yeh, P. Antibiotic combination therapy against resistant bacterial infections: Synergy, rejuvenation and resistance reduction. Expert Rev. Anti-Infect. Ther. 2020, 18, 5–15. [Google Scholar] [CrossRef]
- Guieu, B.; Jourdan, J.-P.; Dreneau, A.; Willand, N.; Rochais, C.; Dallemagne, P. Desirable drug–drug interactions or when a matter of concern becomes a renewed therapeutic strategy. Drug Discov. Today 2020, 26, 315–328. [Google Scholar] [CrossRef] [PubMed]
- Ianevski, A.; Giri, A.K.; Aittokallio, T. SynergyFinder 2.0: Visual analytics of multi-drug combination synergies. Nucleic Acids Res. 2020, 48, W488–W493. [Google Scholar] [CrossRef] [PubMed]
- Rashid, M.B.M.A.; Toh, T.B.; Hooi, L.; Silva, A.; Zhang, Y.; Tan, P.F.; Teh, A.L.; Karnani, N.; Jha, S.; Ho, C.-M.; et al. Optimizing drug combinations against multiple myeloma using a quadratic phenotypic optimization platform (QPOP). Sci. Transl. Med. 2018, 10, eaan0941. [Google Scholar] [CrossRef]
- Jin, Y.; Xu, L.; Wu, X.; Feng, J.; Shu, M.; Gu, H.; Gao, G.; Zhang, J.; Dong, B.; Chen, X. Synergistic Efficacy of the Demethylation Agent Decitabine in Combination With the Protease Inhibitor Bortezomib for Treating Multiple Myeloma Through the Wnt/β-Catenin Pathway. Oncol. Res. Featur. Preclin. Clin. Cancer Ther. 2019, 27, 729–737. [Google Scholar] [CrossRef]
- Palumbo, A.; Chanan-Khan, A.; Weisel, K.; Nooka, A.K.; Masszi, T.; Beksac, M.; Spicka, I.; Hungria, V.; Munder, M.; Mateos, M.V.; et al. Daratumumab, Bortezomib, and Dexamethasone for Multiple Myeloma. N. Engl. J. Med. 2016, 375, 754–766. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, J.; Shirazi, F.; Singh, R.K.; Kuiatse, I.; Wang, H.; Lee, H.C.; Berkova, Z.; Berger, A.; Hyer, M.; Chattopadhyay, N.; et al. Ubiquitin-activating enzyme inhibition induces an unfolded protein response and overcomes drug resistance in myeloma. Blood 2019, 133, 1572–1584. [Google Scholar] [CrossRef] [PubMed]
- Tzanova, M.; Atanasov, V.; Yaneva, Z.; Ivanova, D.; Dinev, T. Selectivity of Current Extraction Techniques for Flavonoids from Plant Materials. Processes 2020, 8, 1222. [Google Scholar] [CrossRef]
- Di Lorenzo, C.; Colombo, F.; Biella, S.; Stockley, C.; Restani, P. Polyphenols and Human Health: The Role of Bioavailability. Nutrients 2021, 13, 273. [Google Scholar] [CrossRef]
- Lu, X.; Saeed, M.E.M.; Hegazy, M.-E.F.; Kampf, C.J.; Efferth, T. Chemopreventive Property of Sencha Tea Extracts towards Sensitive and Multidrug-Resistant Leukemia and Multiple Myeloma Cells. Biomolecules 2020, 10, 1000. [Google Scholar] [CrossRef]
- Tuli, H.S.; Mittal, S.; Aggarwal, D.; Parashar, G.; Parashar, N.C.; Upadhyay, S.K.; Barwal, T.S.; Jain, A.; Kaur, G.; Savla, R.; et al. Path of Silibinin from diet to medicine: A dietary polyphenolic flavonoid having potential anti-cancer therapeutic significance. Semin. Cancer Biol. 2020, 73, 196–218. [Google Scholar] [CrossRef]
- Rauf, A.; Imran, M.; Butt, M.S.; Nadeem, M.; Peters, D.G.; Mubarak, M.S. Resveratrol as an anti-cancer agent: A review. Crit. Rev. Food Sci. Nutr. 2018, 58, 1428–1447. [Google Scholar] [CrossRef] [PubMed]
- Bannerman, B.; Xu, L.; Jones, M.; Tsu, C.; Yu, J.; Hales, P.; Monbaliu, J.; Fleming, P.; Dick, L.; Manfredi, M.; et al. Preclinical evaluation of the antitumor activity of bortezomib in combination with vitamin C or with epigallocatechin gallate, a component of green tea. Cancer Chemother. Pharmacol. 2011, 68, 1145–1154. [Google Scholar] [CrossRef] [PubMed]
- Francisco, V.; Ruiz-Fernández, C.; González-Rodríguez, M.; Cordero-Barreal, A.; Pino, J.; Viñuela, J.E.; Lago, F.; Conde, J.; Gómez, R.; Carvalho, G.R.; et al. Evaluation of Virola oleifera activity in musculoskeletal pathologies: Inhibition of human multiple myeloma cells proliferation and combination therapy with dexamethasone or bortezomib. J. Ethnopharmacol. 2021, 272, 113932. [Google Scholar] [CrossRef] [PubMed]
- Tsalikis, J.; Abdel-Nour, M.; Farahvash, A.; Sorbara, M.T.; Poon, S.; Philpott, D.J.; Girardin, S.E. Isoginkgetin, a Natural Biflavonoid Proteasome Inhibitor, Sensitizes Cancer Cells to Apoptosis via Disruption of Lysosomal Homeostasis and Impaired Protein Clearance. Mol. Cell. Biol. 2019, 39, e00489-18. [Google Scholar] [CrossRef] [PubMed]
- Imam, F.; Al-Harbi, N.O.; Al-Harbia, M.M.; Korashy, H.M.; Ansari, M.A.; Sayed-Ahmed, M.M.; Nagi, M.N.; Iqbal, M.; Anwer, K.; Kazmi, I.; et al. Rutin Attenuates Carfilzomib-Induced Cardiotoxicity Through Inhibition of NF-κB, Hypertrophic Gene Expression and Oxidative Stress. Cardiovasc. Toxicol. 2015, 17, 58–66. [Google Scholar] [CrossRef]
- Chen, K.; Fan, J.; Luo, Z.-F.; Yang, Y.; Xin, W.-J.; Liu, C.-C. Reduction of SIRT1 epigenetically upregulates NALP1 expression and contributes to neuropathic pain induced by chemotherapeutic drug bortezomib. J. Neuroinflamm. 2018, 15, 292. [Google Scholar] [CrossRef]
- Accardi, F.; Toscani, D.; Costa, F.; Aversa, F.; Giuliani, N. The Proteasome and Myeloma-Associated Bone Disease. Calcif. Tissue Res. 2017, 102, 210–226. [Google Scholar] [CrossRef]
- Golden, E.B.; Lam, P.Y.; Kardosh, A.; Gaffney, K.; Cadenas, E.; Louie, S.G.; Petasis, N.; Chen, T.C.; Schönthal, A.H. Green tea polyphenols block the anticancer effects of bortezomib and other boronic acid–based proteasome inhibitors. Blood 2009, 113, 5927–5937. [Google Scholar] [CrossRef]
- Sak, K. Dietary Flavonoids with Catechol Moiety Inhibit Anticancer Action of Bortezomib: What about the other Boronic Acid-Based Drugs? Curr. Cancer Drug Targets 2022, 22, 741–748. [Google Scholar] [CrossRef]
- Qiu, X.; Wu, X.; He, W. (−)-Epigallocatechin-3-gallate plays an antagonistic role in the antitumor effect of bortezomib in myeloma cells via activating Wnt/β-catenin signaling pathway. Adv. Clin. Exp. Med. 2022, 31, 789–794. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).