Gelatin-Based Electrospun Nanofibers Cross-Linked Using Horseradish Peroxidase for Plasmid DNA Delivery
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Plasmids
2.3. Cell Lines
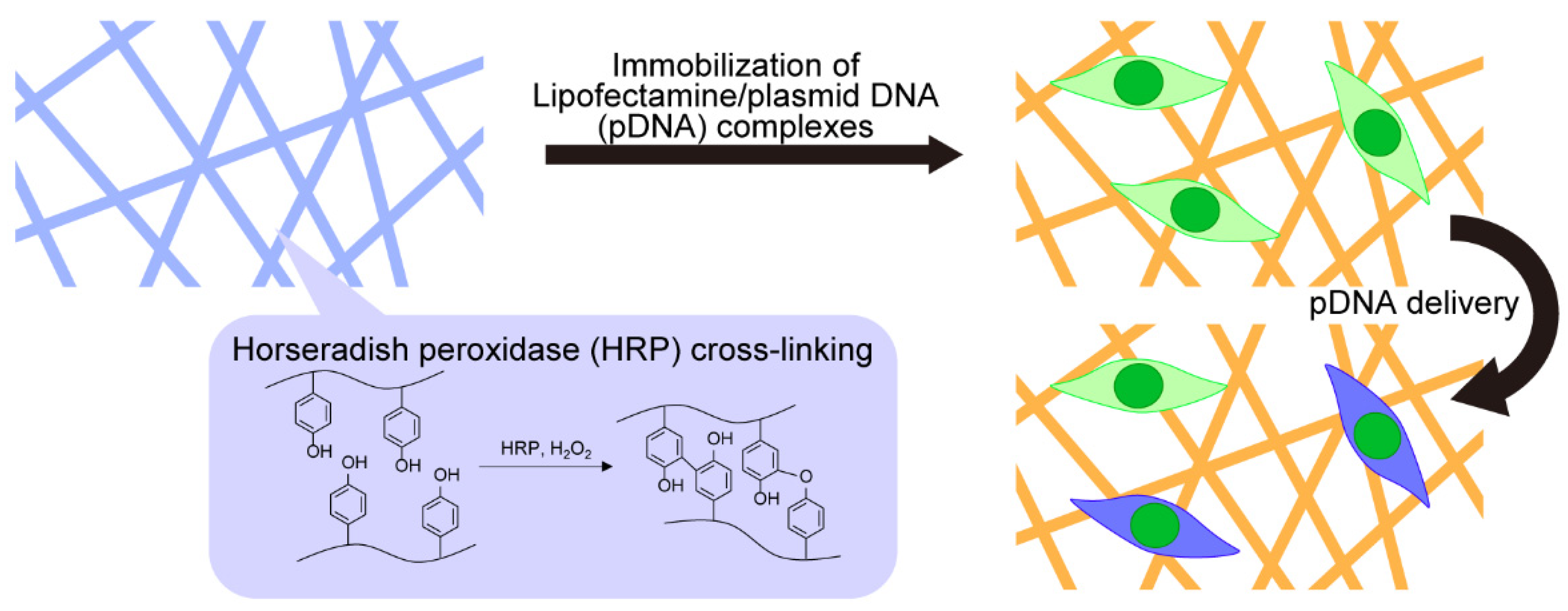
2.4. Fabrication of Gelatin-Ph Nanofibers through Electrospinning
2.5. Insolubilization of Gelatin-Ph Nanofibers
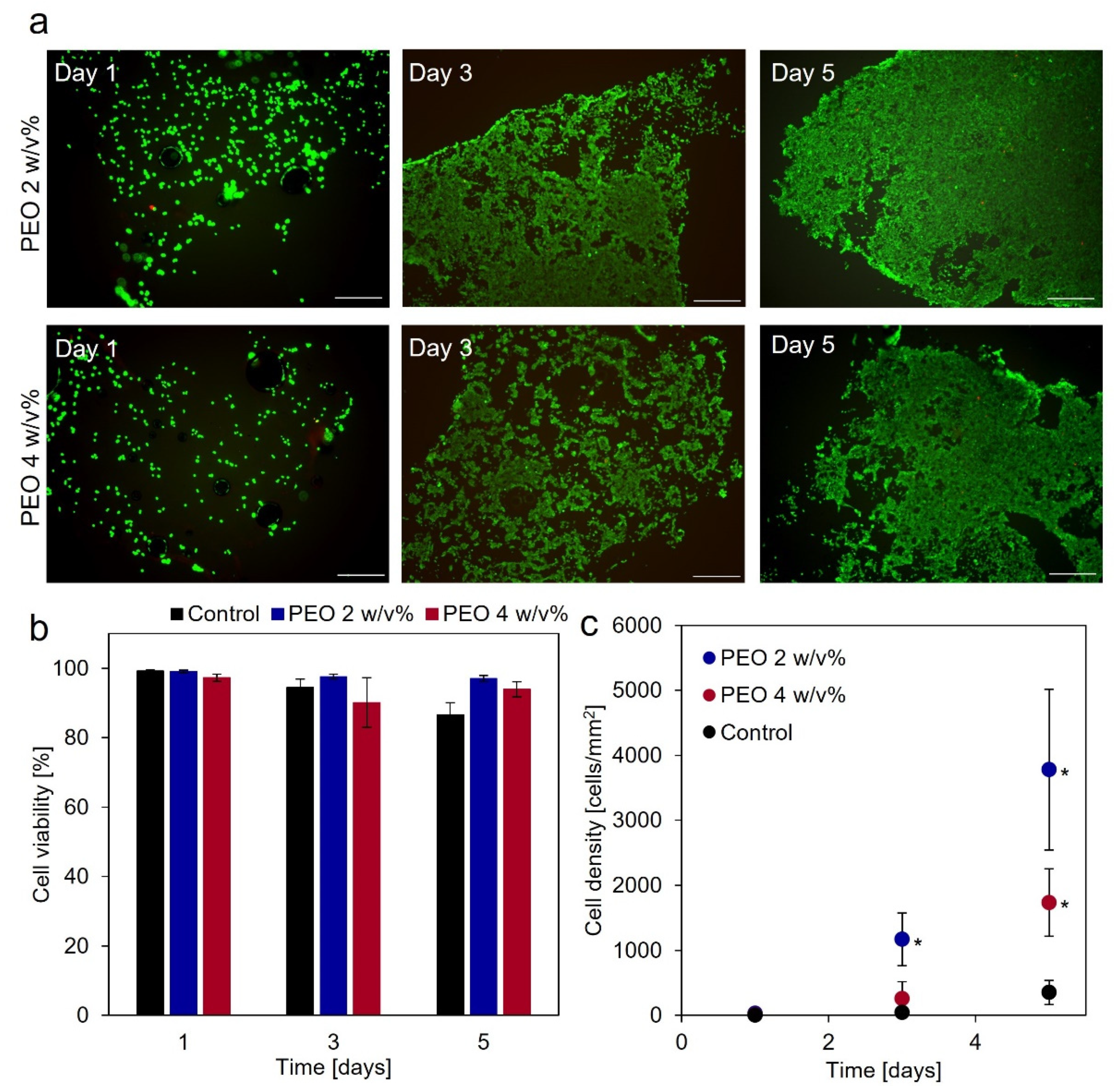
2.6. Cytocompatibility of Gelatin-Ph Nanofibers
2.7. Immobilization of Lipofectamine/pDNA Complexes on Gelatin-Ph Nanofibers
2.8. Transfection Using Gelatin-Ph Nanofibers with Lipofectamine/pDNA Complexes
2.9. Genome-Editing Using Gelatin-Ph Nanofibers with Lipofectamine/pDNA Complexes
3. Results and Discussion
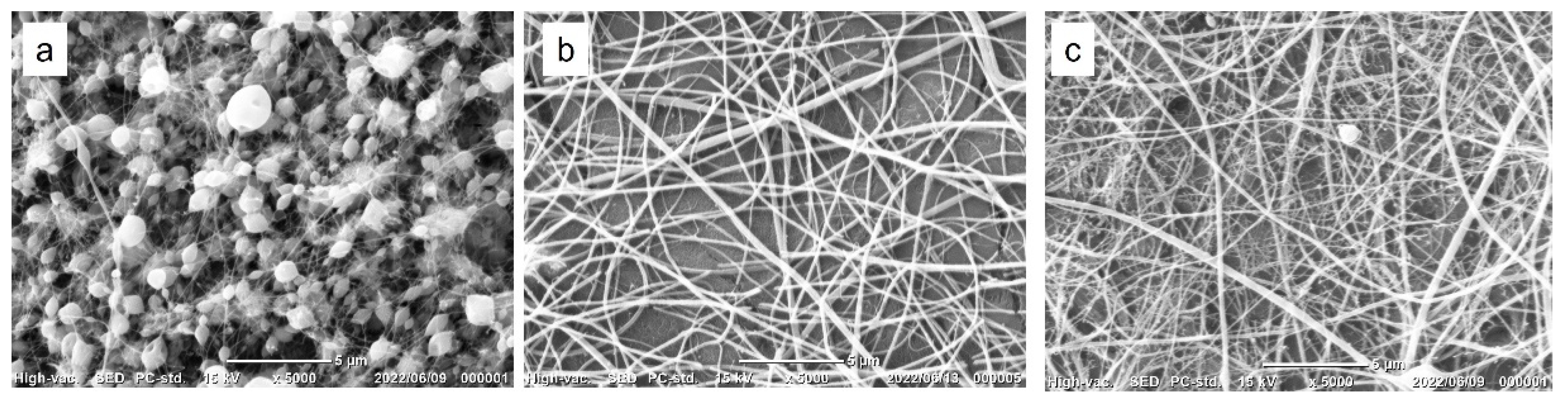
3.1. Fabrication of Gelatin-Ph Nanofibers
3.2. Insolubilization of Gelatin-Ph Nanofibers
3.3. Cytocompatibility of Gelatin-Ph Nanofibers
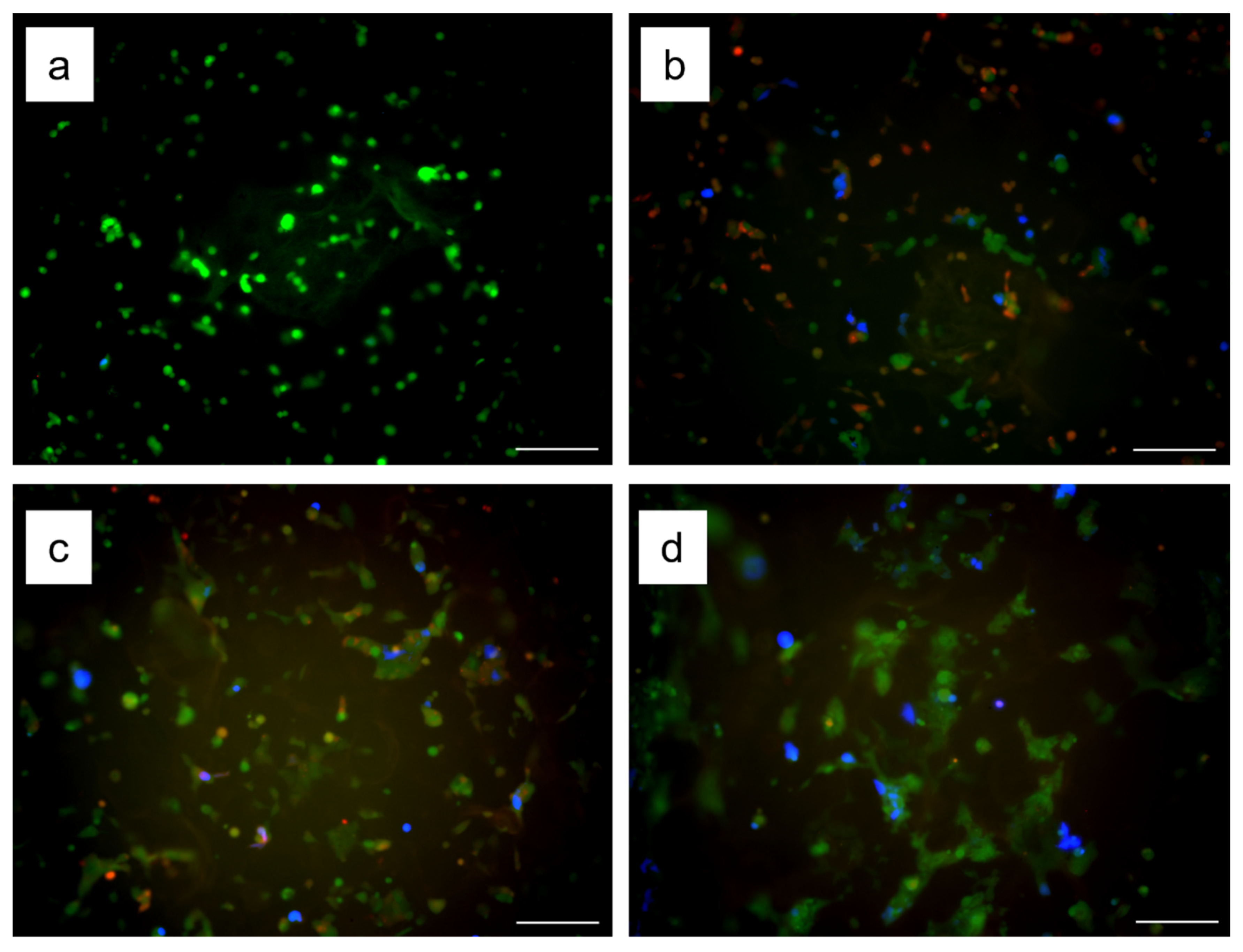
3.4. Transfection Using Gelatin-Ph Nanofibers with Lipofectamine/pDNA Complexes
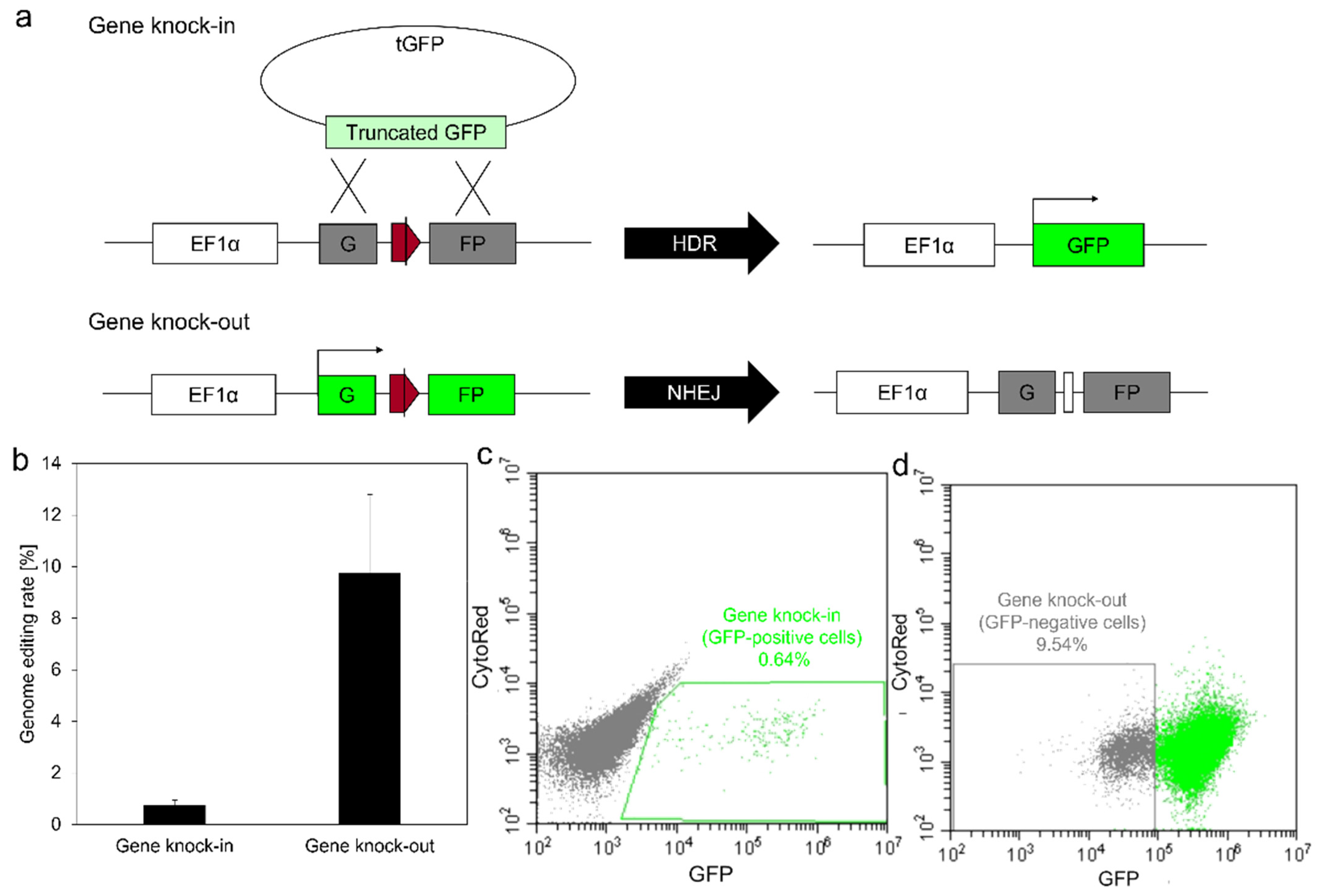
3.5. Genome Editing Using Gelatin-Ph Nanofibers with Lipofectamine/pDNA Complexes
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wu, P.; Chen, H.; Jin, R.; Weng, T.; Ho, J.K.; You, C.; Zhang, L.; Wang, X.; Han, C. Non-viral gene delivery systems for tissue repair and regeneration. J. Transl. Med. 2018, 16, 29. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Sun, L.; Gu, Z.; Li, W.; Guo, L.; Ma, S.; Guo, L.; Zhang, W.; Han, B.; Chang, J. N-carboxymethyl chitosan/sodium alginate composite hydrogel loading plasmid DNA as a promising gene activated matrix for in-situ burn wound treatment. Bioact. Mater. 2022, 15, 330–342. [Google Scholar] [CrossRef] [PubMed]
- Dunbar, C.E.; High, K.A.; Joung, J.K.; Kohn, D.B.; Ozawa, K.; Sadelain, M. Gene therapy comes of age. Science 2018, 359, eaan4672. [Google Scholar] [CrossRef]
- Torres-Vanegas, J.D.; Cruz, J.C.; Reyes, L.H. Delivery Systems for Nucleic Acids and Proteins: Barriers, Cell Capture Pathways and Nanocarriers. Pharmaceutics 2021, 13, 428. [Google Scholar] [CrossRef]
- Nayerossadat, N.; Maedeh, T.; Ali, P.A. Viral and nonviral delivery systems for gene delivery. Adv. Biomed. Res. 2012, 1, 27. [Google Scholar] [CrossRef]
- Abd Elhameed, H.A.H.; Ungor, D.; Igaz, N.; Gopisetty, M.K.; Kiricsi, M.; Csapo, E.; Gyurcsik, B. High Molecular Weight Poly(ethylenimine)-Based Water-Soluble Lipopolymer for Transfection of Cancer Cells. Macromol. Biosci. 2020, 20, e2000040. [Google Scholar] [CrossRef]
- Rohiwal, S.S.; Dvorakova, N.; Klima, J.; Vaskovicova, M.; Senigl, F.; Slouf, M.; Pavlova, E.; Stepanek, P.; Babuka, D.; Benes, H.; et al. Polyethylenimine based magnetic nanoparticles mediated non-viral CRISPR/Cas9 system for genome editing. Sci. Rep. 2020, 10, 4619. [Google Scholar] [CrossRef]
- Rai, R.; Alwani, S.; Badea, I. Polymeric Nanoparticles in Gene Therapy: New Avenues of Design and Optimization for Delivery Applications. Polymers 2019, 11, 745. [Google Scholar] [CrossRef]
- Ramamoorth, M.; Narvekar, A. Non viral vectors in gene therapy- an overview. J. Clin. Diagn. Res. 2015, 9, GE01–GE06. [Google Scholar] [CrossRef]
- Duran-Mota, J.A.; Yani, J.Q.; Almquist, B.D.; Borros, S.; Oliva, N. Polyplex-Loaded Hydrogels for Local Gene Delivery to Human Dermal Fibroblasts. ACS Biomater. Sci. Eng. 2021, 7, 4347–4361. [Google Scholar] [CrossRef]
- Furuno, K.; Suzuki, K.; Sakai, S. Gelatin nanofiber mats with Lipofectamine/plasmid DNA complexes for in vitro genome editing. Colloids Surf. B Biointerfaces 2022, 216, 112561. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Wu, Y.; Zhou, Y.; Wu, J.; Wang, X.; Qu, Y.; Wang, Y.; Zhang, Y.; Yu, Q. Photothermally Activated Electrospun Nanofiber Mats for High-Efficiency Surface-Mediated Gene Transfection. ACS Appl. Mater. Interfaces 2020, 12, 7905–7914. [Google Scholar] [CrossRef] [PubMed]
- Anup, N.; Chavan, T.; Chavan, S.; Polaka, S.; Kalyane, D.; Abed, S.N.; Venugopala, K.N.; Kalia, K.; Tekade, R.K. Reinforced electrospun nanofiber composites for drug delivery applications. J. Biomed. Mater. Res. A 2021, 109, 2036–2064. [Google Scholar] [CrossRef] [PubMed]
- He, S.; Fang, J.; Zhong, C.; Ren, F.; Wang, M. Controlled pVEGF delivery via a gene-activated matrix comprised of a peptide-modified non-viral vector and a nanofibrous scaffold for skin wound healing. Acta Biomater. 2022, 140, 149–162. [Google Scholar] [CrossRef]
- Li, K.; Feng, L.; Shen, J.; Zhang, Q.; Liu, Z.; Lee, S.T.; Liu, J. Patterned substrates of nano-graphene oxide mediating highly localized and efficient gene delivery. ACS Appl. Mater. Interfaces 2014, 6, 5900–5907. [Google Scholar] [CrossRef]
- Yang, M.; Yang, C.; Zhang, Y.; Yan, X.; Ma, Y.; Zhang, Y.; Cao, Y.; Xu, Q.; Tu, K.; Zhang, M. An oral pH-activated “nano-bomb” carrier combined with berberine by regulating gene silencing and gut microbiota for site-specific treatment of ulcerative colitis. Biomater. Sci. 2022, 10, 1053–1067. [Google Scholar] [CrossRef]
- Streeter, B.W.; Xue, J.; Xia, Y.; Davis, M.E. Electrospun Nanofiber-Based Patches for the Delivery of Cardiac Progenitor Cells. ACS Appl. Mater. Interfaces 2019, 11, 18242–18253. [Google Scholar] [CrossRef]
- Lee, S.; Jin, G.; Jang, J.H. Electrospun nanofibers as versatile interfaces for efficient gene delivery. J. Biol. Eng. 2014, 8, 30. [Google Scholar] [CrossRef]
- Erencia, M.; Cano, F.; Tornero, J.A.; Fernandes, M.M.; Tzanov, T.; Macanas, J.; Carrillo, F. Electrospinning of gelatin fibers using solutions with low acetic acid concentration: Effect of solvent composition on both diameter of electrospun fibers and cytotoxicity. J. Appl. Polym. Sci. 2015, 132, 42115. [Google Scholar] [CrossRef]
- Chen, X.; Feng, B.; Zhu, D.Q.; Chen, Y.W.; Ji, W.; Ji, T.J.; Li, F. Characteristics and toxicity assessment of electrospun gelatin/PCL nanofibrous scaffold loaded with graphene in vitro and in vivo. Int. J. Nanomed. 2019, 14, 3669–3678. [Google Scholar] [CrossRef]
- Sisson, K.; Zhang, C.; Farach-Carson, M.C.; Chase, D.B.; Rabolt, J.F. Evaluation of cross-linking methods for electrospun gelatin on cell growth and viability. Biomacromolecules 2009, 10, 1675–1680. [Google Scholar] [CrossRef] [PubMed]
- Akhshabi, S.; Biazar, E.; Singh, V.; Keshel, S.H.; Geetha, N. The effect of the carbodiimide cross-linker on the structural and biocompatibility properties of collagen-chondroitin sulfate electrospun mat. Int. J. Nanomed. 2018, 13, 4405–4416. [Google Scholar] [CrossRef] [PubMed]
- Furuno, K.; Wang, J.; Suzuki, K.; Nakahata, M.; Sakai, S. Gelatin-Based Electrospun Fibers Insolubilized by Horseradish Peroxidase-Catalyzed Cross-Linking for Biomedical Applications. ACS Omega 2020, 5, 21254–21259. [Google Scholar] [CrossRef] [PubMed]
- Sakai, S.; Nakahata, M. Horseradish Peroxidase Catalyzed Hydrogelation for Biomedical, Biopharmaceutical, and Biofabrication Applications. Chem. Asian J. 2017, 12, 3098–3109. [Google Scholar] [CrossRef]
- Sakai, S.; Hirose, K.; Taguchi, K.; Ogushi, Y.; Kawakami, K. An injectable, in situ enzymatically gellable, gelatin derivative for drug delivery and tissue engineering. Biomaterials 2009, 30, 3371–3377. [Google Scholar] [CrossRef]
- Sakai, S.; Kawakami, K. Synthesis and characterization of both ionically and enzymatically cross-linkable alginate. Acta Biomater. 2007, 3, 495–501. [Google Scholar] [CrossRef]
- Sakai, S.; Mochizuki, K.; Qu, Y.; Mail, M.; Nakahata, M.; Taya, M. Peroxidase-catalyzed microextrusion bioprinting of cell-laden hydrogel constructs in vaporized ppm-level hydrogen peroxide. Biofabrication 2018, 10, 045007. [Google Scholar] [CrossRef]
- Suzuki, K.; Tsunekawa, Y.; Hernandez-Benitez, R.; Wu, J.; Zhu, J.; Kim, E.J.; Hatanaka, F.; Yamamoto, M.; Araoka, T.; Li, Z.; et al. In vivo genome editing via CRISPR/Cas9 mediated homology-independent targeted integration. Nature 2016, 540, 144–149. [Google Scholar] [CrossRef]
- Kikugawa, K.; Kato, T.; Hayasaka, A. Formation of dityrosine and other fluorescent amino acids by reaction of amino acids with lipid hydroperoxides. Lipids 1991, 26, 922–929. [Google Scholar] [CrossRef]
- Marquez, L.A.; Dunford, H.B. Kinetics of oxidation of tyrosine and dityrosine by myeloperoxidase compounds I and II. Implications for lipoprotein peroxidation studies. J. Biol. Chem. 1995, 270, 30434–30440. [Google Scholar] [CrossRef]
- Righi, T.M.; Almeida, R.S.; d’Ávila, M.A. Electrospinning of Gelatin/PEO Blends: Influence of Process Parameters in the Nanofiber Properties. Macromol. Symp. 2012, 319, 230–234. [Google Scholar] [CrossRef]
- Subrahmanya, T.M.; Arshad, A.B.; Lin, P.T.; Widakdo, J.; Makari, H.K.; Austria, H.F.M.; Hu, C.C.; Lai, J.Y.; Hung, W.S. A review of recent progress in polymeric electrospun nanofiber membranes in addressing safe water global issues. RSC Adv. 2021, 11, 9638–9663. [Google Scholar] [CrossRef]
- Desai, K.; Kit, K.; Li, J.; Zivanovic, S. Morphological and surface properties of electrospun chitosan nanofibers. Biomacromolecules 2008, 9, 1000–1006. [Google Scholar] [CrossRef] [PubMed]
- Babanejad, N.; Kandalam, U.; Ahmad, R.; Omidi, Y.; Omidian, H. Abuse-deterrent properties and cytotoxicity of poly(ethylene oxide) after thermal tampering. Int. J. Pharm. 2021, 600, 120481. [Google Scholar] [CrossRef] [PubMed]
- Amores de Sousa, M.C.; Rodrigues, C.A.V.; Ferreira, I.A.F.; Diogo, M.M.; Linhardt, R.J.; Cabral, J.M.S.; Ferreira, F.C. Functionalization of Electrospun Nanofibers and Fiber Alignment Enhance Neural Stem Cell Proliferation and Neuronal Differentiation. Front. Bioeng. Biotechnol. 2020, 8, 580135. [Google Scholar] [CrossRef]
- Rasti Boroojeni, F.; Mashayekhan, S.; Abbaszadeh, H.A.; Ansarizadeh, M.; Khoramgah, M.S.; Rahimi Movaghar, V. Bioinspired Nanofiber Scaffold for Differentiating Bone Marrow-Derived Neural Stem Cells to Oligodendrocyte-Like Cells: Design, Fabrication, and Characterization. Int. J. Nanomed. 2020, 15, 3903–3920. [Google Scholar] [CrossRef]
- Azarmi, S.; Huang, Y.; Chen, H.; McQuarrie, S.; Abrams, D.; Roa, W.; Finlay, W.H.; Miller, G.G.; Lobenberg, R. Optimization of a two-step desolvation method for preparing gelatin nanoparticles and cell uptake studies in 143B osteosarcoma cancer cells. J. Pharm. Pharm. Sci. 2006, 9, 124–132. [Google Scholar]
- Chesnoy, S.; Huang, L. Structure and function of lipid-DNA complexes for gene delivery. Annu. Rev. Biophys. Biomol. Struct. 2000, 29, 27–47. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Furuno, K.; Suzuki, K.; Sakai, S. Gelatin-Based Electrospun Nanofibers Cross-Linked Using Horseradish Peroxidase for Plasmid DNA Delivery. Biomolecules 2022, 12, 1638. https://doi.org/10.3390/biom12111638
Furuno K, Suzuki K, Sakai S. Gelatin-Based Electrospun Nanofibers Cross-Linked Using Horseradish Peroxidase for Plasmid DNA Delivery. Biomolecules. 2022; 12(11):1638. https://doi.org/10.3390/biom12111638
Chicago/Turabian StyleFuruno, Kotoko, Keiichiro Suzuki, and Shinji Sakai. 2022. "Gelatin-Based Electrospun Nanofibers Cross-Linked Using Horseradish Peroxidase for Plasmid DNA Delivery" Biomolecules 12, no. 11: 1638. https://doi.org/10.3390/biom12111638
APA StyleFuruno, K., Suzuki, K., & Sakai, S. (2022). Gelatin-Based Electrospun Nanofibers Cross-Linked Using Horseradish Peroxidase for Plasmid DNA Delivery. Biomolecules, 12(11), 1638. https://doi.org/10.3390/biom12111638








