Polysaccharide Based Polymers Produced by Scabby Cankered Cactus Pear (Opuntia ficus-indica L.) Infected by Neofusicoccum batangarum: Composition, Structure, and Chemico-Physical Properties †
Abstract
:1. Introduction
2. Materials and Methods
2.1. General Chemica Procedures
2.2. Plant Material
2.3. Isolation of the Polysaccharide Gel
2.4. Acid Hydrolysis of Crude and Purified EPS
2.5. Gel-Filtration Chromatography
2.6. Derivatization of Monosaccharides and the HPLC Analysis of the Aldose Enantiomers Derivative
2.7. Gel Permeation Chromatography
2.8. Attenuated Total Reflection Fourier Transform Infrared Spectroscopy (FTIR-ATR)
2.9. Degree of Methoxylation (DM) Measurement
2.10. Thermogravimetric Analysis (TGA)
2.11. Scanning Electron Microscopy (SEM)
3. Results and Discussion
3.1. Isolation and Chemical Characterization of Polysaccharides
3.2. Gel Permeation Chromatography (GPC)
3.3. Attenuated Total Reflection Fourier Transform Infrared Spectroscopy (FTIR-ATR)
3.4. Scanning Electron Microscopy (SEM)
3.5. Thermogravimetric Analysis (TGA)
3.6. NMR Spectroscopic Characterization of the Milky Exudate (EPSC)
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Graniti, A.; Durbin, R.D.; Ballio, A. Phytotoxins and Plant Pathogenesis; Springer: Berlin/Heidelberg, Germany, 1989. [Google Scholar]
- Graniti, A. Phytotoxins and their involvement in plant diseases. Introduction. Experientia 1991, 47, 751–755. [Google Scholar] [CrossRef]
- Evidente, A.; Motta, A. Phytotoxins from fungi, pathogenic for agrarian, forestal and weedy plants. In Bioactive Compounds from Natural Sources; Tringali, C., Ed.; Francis and Taylor: London, UK, 2001; pp. 473–526. [Google Scholar]
- Berestetskiy, A.O. A review of fungal phytotoxins: From basic studies to practical use. Appl. Biochem. Microbiol. 2008, 44, 453–465. [Google Scholar] [CrossRef]
- Evidente, A.; Cimmino, A.; Masi, M. Phytotoxins produced by pathogenic fungi of agrarian plants. Phytochem. Rev. 2019, 18, 843–870. [Google Scholar] [CrossRef]
- Konno, K. Plant latex and other exudates as plant defense systems: Roles of various defense chemicals and proteins contained therein. Phytochemistry 2011, 72, 1510–1530. [Google Scholar] [CrossRef] [PubMed]
- Abarca, L.F.S.; Klinkhamer, P.G.; Choi, Y.H. Plant latex, from ecological interests to bioactive chemical resources. Planta Med. 2019, 85, 856–868. [Google Scholar]
- Agrawal, A.A.; Konno, K. Latex: A model for understanding mechanisms, ecology, and evolution of plant defense against herbivory. Annu. Rev. Ecol. Evol. Syst. 2009, 40, 311–331. [Google Scholar] [CrossRef] [Green Version]
- Bhosale, R.R.; Osmani, R.A.M.; Moin, A. Natural gums and mucilages: A review on multifaceted excipients in pharmaceutical science and research. Int. J. Pharm. Phytochem. Res. 2014, 15, 901–912. [Google Scholar]
- Malsawmtluangi, C.; Thanzami, K.; Lalhlenmawia, H.; Selvan, V.; Palanisamy, S.; Kandasamy, R.; Pachuau, L. Physicochemical characteristics and antioxidant activity of Prunus cerasoides D. Don gum exudates. Int. J. Biol. Macromol. 2014, 69, 192–199. [Google Scholar] [CrossRef] [PubMed]
- Osemwegie, O.O.; Adetunji, C.O.; Ayeni, E.A.; Adejobi, O.I.; Arise, R.O.; Nwonuma, C.O.; Oghenekaro, A.O. Exopolysaccharides from bacteria and fungi: Current status and perspectives in Africa. Helion 2020, 6, e04205. [Google Scholar] [CrossRef]
- Jahr, H.R.; Bahro, R.; Eichenlaub, R. Exopolysaccharides from phytopathogenic bacteria. In Progress in Botany; Esser, K., Ed.; Springer: Berlin/Heidelberg, Germany, 1999; Volume 60, pp. 119–138. [Google Scholar]
- Hayashi, K.; Senuma, W.; Kai, K.; Kiba, A.; Ohnishi, K.; Hikichi, Y. Major exopolysaccharide, EPS I, is associated with the feedback loop in the quorum sensing of Ralstonia solanacearum strain OE1-1. Mol. Plant Pathol. 2019, 20, 1740–1747. [Google Scholar] [CrossRef] [Green Version]
- Spalding, D.H.; Brueh, G.W.; Foster, R.J. Possible role of pectinolytic enzymes and polysaccharide in pathogenesis by Cephalosporium gramineum in wheat. Phytopathology 1961, 51, 227–235. [Google Scholar]
- McWain, P.; Gregory, G.F. A neutral mannan from Ceratocystis fagacearum culture filtrate. Phytochemistry 1972, 11, 2609–2612. [Google Scholar] [CrossRef]
- Strobel, G.A.; Van Alfen, N.; Hapner, K.D.; McNeil, M.; Albersheim, P. Some phytotoxic glycopeptides from Ceratocystis ulmi, the dutch elm disease pathogen. Biochem. Biophys. Acta 1978, 538, 60–75. [Google Scholar] [CrossRef]
- Corsaro, M.M.; De Castro, C.; Evidente, A.; Lanzetta, R.; Molinaro, A.; Parrilli, M.; Sparapano, L. Phytotoxic extracellular polysaccharide fractions from Cryphonectria parasitica (Murr.) Barr strains. Carbohydr. Polym. 1998, 37, 167–172. [Google Scholar] [CrossRef]
- Corsaro, M.M.; De Castro, C.; Evidente, A.; Lanzetta, R.; Molinaro, A.; Mugnai, L.; Parrilli, M.; Surico, G. Chemical structure of two phytotoxic exopolysaccharides produced by Phomopsis foeniculi. Carbohydr. Res. 1998, 308, 349–357. [Google Scholar] [CrossRef]
- Cimmino, A.; Cinelli, T.; Evidente, M.; Masi, M.; Mugnai, L.; Silva, M.A.; Evidente, A. Phytotoxic fungal exopolysaccharides produced by fungi involved in grapevine trunk diseases. Nat. Prod. Commun. 2016, 11, 1481–1484. [Google Scholar] [CrossRef]
- Andolfi, A.; Cimmino, A.; Evidente, A.; Iannaccone, M.; Capparelli, R.; Mugnai, L.; Surico, G. A new flow cytometry technique to identify Phaeomoniella chlamydospora exopolysaccharides and study mechanisms of esca grapevine foliar symptoms. Plant Dis. 2009, 93, 680–684. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Masi, M.; Aloi, F.; Nocera, P.; Cacciola, S.O.; Surico, G.; Evidente, A. Phytotoxic metabolites isolated from Neufusicoccum batangarum, the causal agent of the scabby canker of cactus pear (Opuntia ficus-indica L.). Toxins 2020, 12, 126. [Google Scholar] [CrossRef] [Green Version]
- Chaouch, M.A.; Hafsa, J.; Rihouey, C.; Le Cerf, D.; Majdoub, H. Effect of pH during extraction on the antioxidant and antiglycated activities of polysaccharides from Opuntia ficus-indica. J. Food Biochem. 2016, 40, 316–325. [Google Scholar] [CrossRef]
- Salehi, E.; Emam-Djomeh, Z.; Askari, G.; Fathi, M. Opuntia ficus indica fruit gum: Extraction, characterization, antioxidant activity and functional properties. Carbohydr. Polym. 2019, 206, 565–572. [Google Scholar] [CrossRef] [PubMed]
- Di Lorenzo, F.; Silipo, A.; Molinaro, A.; Parrilli, M.; Schiraldi, C.; D’Agostino, A.; Izzo, E.; Rizza, L.; Bonina, A.; Lanzetta, R. The polysaccharide and low molecular weight components of Opuntia ficus indica cladodes: Structure and skin repairing properties. Carbohydr. Polym. 2017, 157, 128–136. [Google Scholar] [CrossRef] [PubMed]
- Catanzano, O.; d’Ayala, G.G.; D’Agostino, A.; Di Lorenzo, F.; Schiraldi, C.; Malinconico, M.; Lanzetta, R.; Bonina, F.; Laurienzo, P. PEG-crosslinked-chitosan hydrogel films for in situ delivery of Opuntia ficus-indica extract. Carbohydr. Polym. 2021, 264, 117987. [Google Scholar] [CrossRef] [PubMed]
- Thurber, K.R.; Tycko, R. Measurement of sample temperatures under magic-angle spinning from the chemical shift and spin-lattice relaxation rate of 79Br in KBr powder. J. Magn. Reson. 2009, 19, 84–87. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tanaka, T.; Nakashima, T.; Ueda, T.; Tomii, K.; Kouno, I. Facile discrimination of aldose enantiomers by reversed-phase HPLC. Chem. Pharm. Bull. 2007, 55, 899–901. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lu, Y.; He, Y.; Yang, M.; Fan, B.Y. Arvensic acids K and L, components of resin glycoside fraction from Convolvulus arvensis. Nat. Prod. Res. 2021, 35, 2303–2307. [Google Scholar] [CrossRef] [PubMed]
- Conte, C.; Dal Poggetto, G.; Schiano Di Cola, V.; Russo, A.; Ungaro, F.; Russo, G.; Laurienzo, P.; Quaglia, F. Pegylated cationic nanoassemblies based on triblock copolymers to combine siRNA therapeutics with anticancer drugs. Biomater. Sci. 2021, 9, 6251–6265. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.-L.; Gao, J.; Yin, Y.-Y.; Jin, Z.-Y.; Xu, X.-M.; Chen, H.-Q. Extraction optimization by response surface methodology of mucilage polysaccharide from the peel of Opuntia dillenii haw. fruits and their physicochemical properties. Carbohydr. Polym. 2016, 151, 381–391. [Google Scholar] [CrossRef]
- Madera-Santana, T.J.; Vargas-Rodríguez, L.; Núñez-Colín, C.A.; González-García, G.; Peña-Caballero, V.; Núñez-Gastélum, J.A.; Gallegos-Vázquez, C.; Rubén Rodríguez-Núñezcan, J. Mucilage from cladodes of Opuntia spinulifera Salm-Dyck: Chemical, morphological, structural and thermal characterization. J. Food 2018, 16, 650–657. [Google Scholar] [CrossRef] [Green Version]
- Kazy, S.K.; Sar, P.; Singh, S.P.; Sen, A.K.; Souza, S.F.D. Extracellular polysaccharides of a copper-sensitive and a copper-resistant Pseudomonas aeruginosa strain: Synthesis, chemical nature and copper binding. World J. Microbiol. Biotechnol. 2004, 18, 583–588. [Google Scholar] [CrossRef]
- Roya, R.R.; Kulcinskaja, S.E.; Ron, E.Y.C.; Björnsdóttir, S.; Friðjónsson, O.H.; Hreggviðsson, G.O.; Nordberg Karlsson, E. Evaluation of the production of exopolysaccharides by two strains of the thermophilic bacterium Rhodothermus marinus. Carbohydr. Polym. 2017, 156, 1–8. [Google Scholar]
- Barrett, A.J.; Rawlings, N.D.; O’Brien, E.A. The MEROPS database as a protease information system. J. Struct. Biol. 2001, 134, 95–102. [Google Scholar] [CrossRef]
- Zannini, D.; Dal Poggetto, G.; Malinconico, M.; Santagata, G.; Immirzi, B. Citrus pomace biomass as a source of pectin and lignocellulose fibers: From waste to upgraded biocomposites for mulching applications. Polymers 2021, 13, 1280. [Google Scholar] [CrossRef]
- Luque Castellane, T.C.; Campanharo, J.C.; Colnago, L.A.; Duarte Coutinho, I.; Mendes Lopes, E.; Franco Lemos, M.V.; Macedo Lemos, E.G. Characterization of new exopolysaccharide production by Rhizobium tropici during growth on hydrocarbon substrate. Int. J. Biol. Macromol. 2017, 96, 361–369. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Shi, J.; Yang, X.; Nan, B.; Liu, Y.; Wang, Z. Chemical and physical characteristics and antioxidant activities of the exopolysaccharide produced by Tibetan kefir grains during milk fermentation. Int. Dairy J. 2015, 43, 15–21. [Google Scholar] [CrossRef]
- Kalegowda, P.; Chauhan, A.S.; Nanjaraj, U.S.M. Opuntia dillenii (Ker-Gawl) Haw cladode mucilage: Physico-chemical, rheological and functional behavior. Carbohydr. Polym. 2017, 157, 1057–1064. [Google Scholar] [CrossRef] [PubMed]
- Bhuiyan, N.H.; Selvaraj, G.; Wei, Y.; King, J. Role of lignification in plant defense. Plant Signal Behav. 2009, 4, 158–159. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- El Nokab, M.E.H.; Van Der Wel, P.C. Use of solid-state NMR spectroscopy for investigating polysaccharide-based hydrogels: A review. Carbohydr. Polym. 2020, 240, 116276. [Google Scholar] [CrossRef] [PubMed]
- Kang, X.; Zhao, W.; Widanage, M.C.D.; Kirui, A.; Ozdenvar, U.; Wang, T. CCMRD: A solid-state NMR database for complex carbohydrates. J. Biomol. NMR 2020, 74, 239–245. [Google Scholar] [CrossRef]
- Hall, L.D.; Sukumar, S.; Sullivan, G.R. Two-dimensional J spectroscopy: 1H NMR spectra of mono-and di-saccharides. J. Chem. Soc. Chem. Commun. 1979, 292–294. [Google Scholar] [CrossRef]
- Bubb, W.A. NMR spectroscopy in the study of carbohydrates: Characterizing the structural complexity. Concepts Magn. Reson. Part A Educ. J. 2003, 19, 1–19. [Google Scholar] [CrossRef]
- Brown, G.D.; Bauer, J.; Osborn, H.M.; Kuemmerle, R. A solution NMR approach to determine the chemical structures of carbohydrates using the hydroxyl groups as starting points. ACS Omega 2018, 3, 17957–17975. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.Q.; Yin, J.Y.; Nie, S.P.; Xie, M.Y. A review of NMR analysis in polysaccharide structure and conformation: Progress, challenge and perspective. Food Res. Int. 2021, 143, 110290. [Google Scholar]

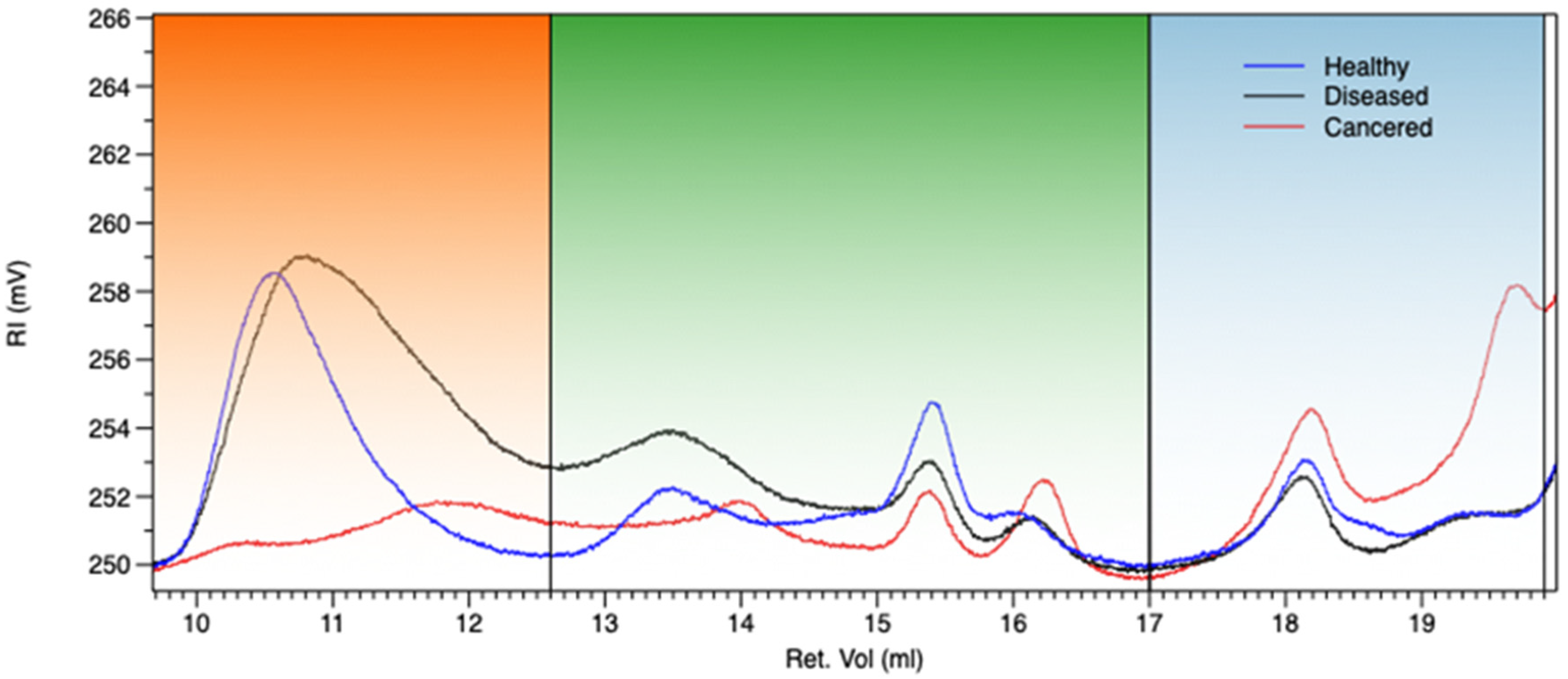
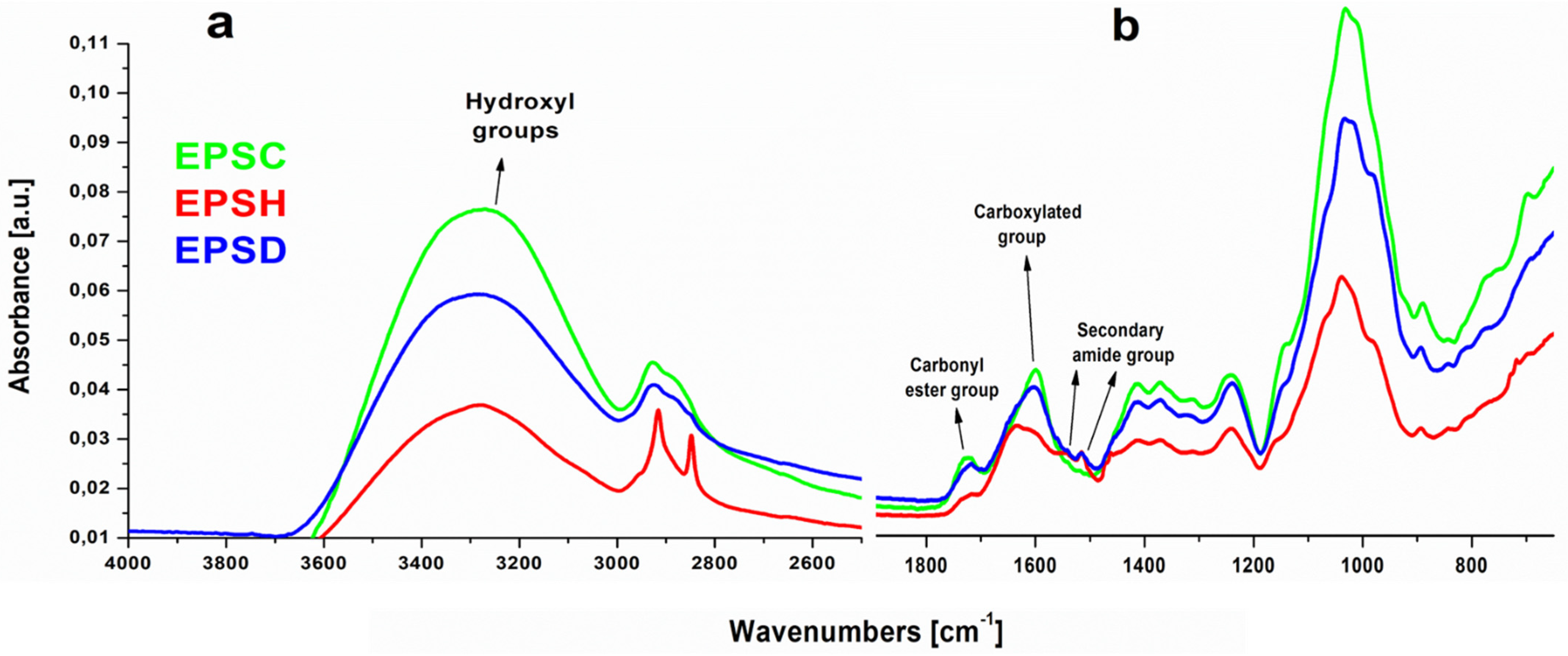

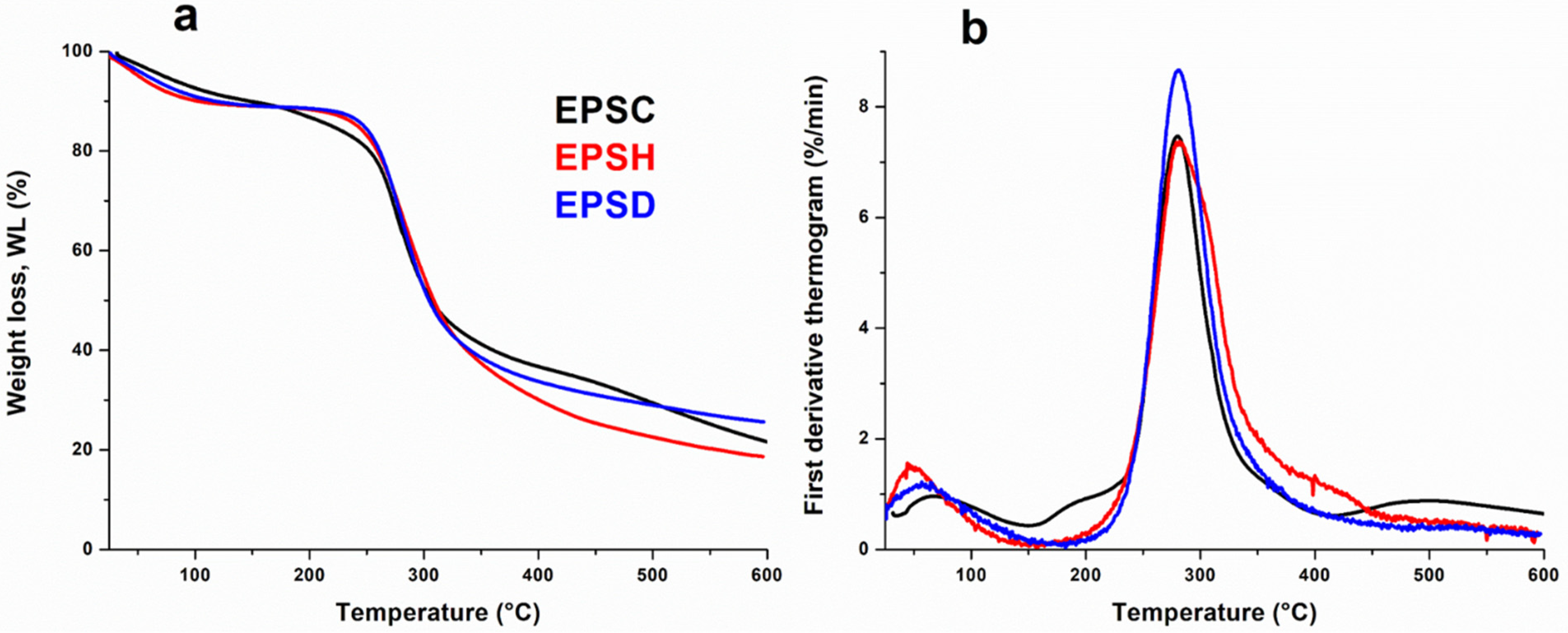
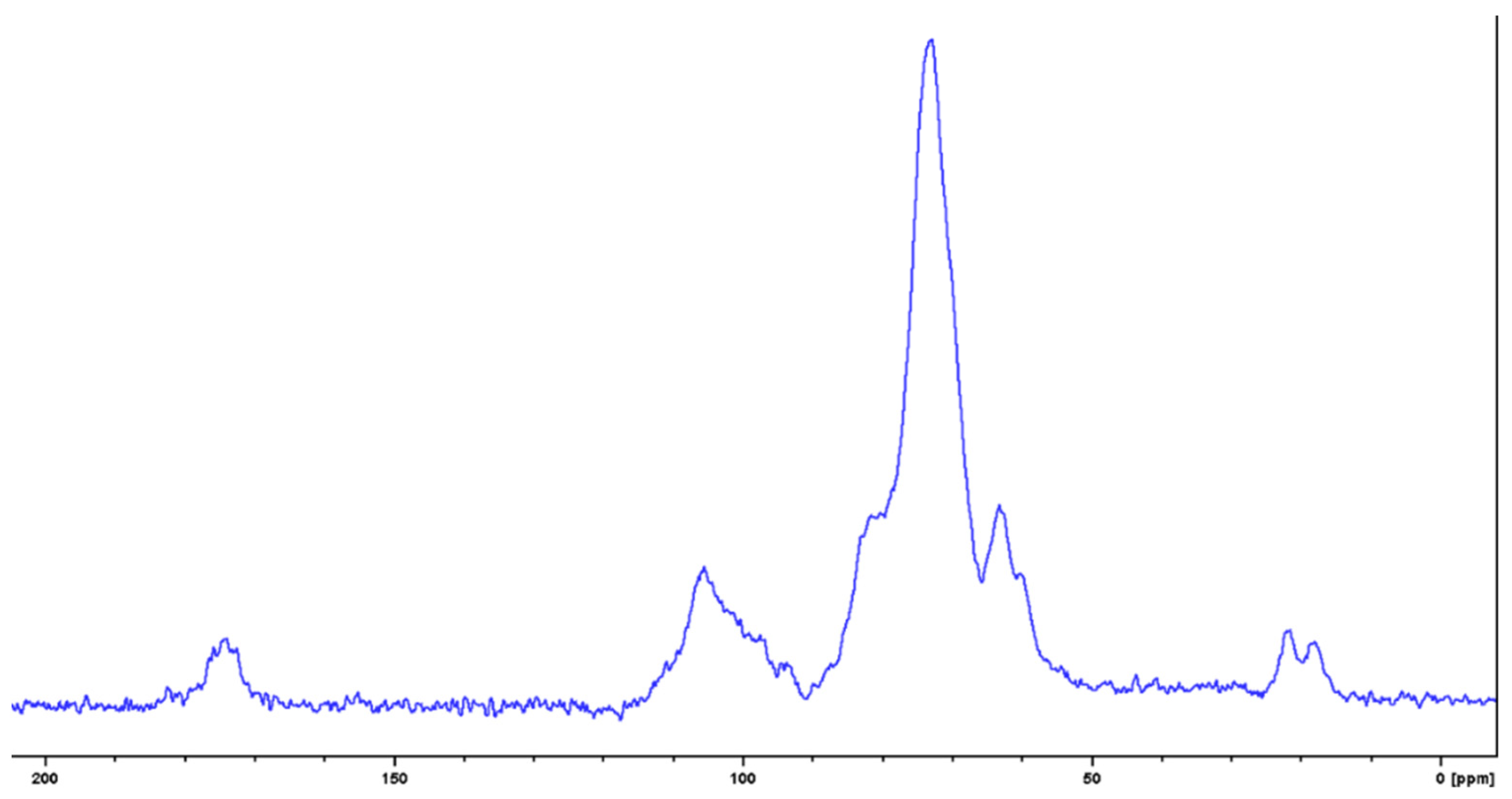


| Sample | Mw(1) 9.0–12.6 (MDa) | Mn(1) 9.0–12.6 (MDa) | Mw(2) 12.6–17.0 (kDa) | Mn(2) 12.6–17.0 (kDa) | Mw(3) 17–19.8 (kDa) | Mn(3) 17–19.8 (kDa) | IV (1) | IV (2) | IV (3) | %Wt (1) | %Wt (2) | %Wt (3) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| EPSH | 4.3 | 1.4 | 35.5 | 24.9 | 2.5 | 2.3 | 4.91 | 0.19 | 0.05 | 81 | 12 | 7.3 |
| EPSD | 2.8 | 1.3 | 113 | 26 | 1.4 | 1.2 | 4.89 | 0.28 | 0.08 | 47.0 | 38.7 | 14.2 |
| EPSC | 2.9 | 2.6 | 103 | 62 | 7.6 | 7.2 | 3.4 | 0.04 | 0.01 | 54.4 | 17.6 | 28.0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Santagata, G.; Cimmino, A.; Poggetto, G.D.; Zannini, D.; Masi, M.; Emendato, A.; Surico, G.; Evidente, A. Polysaccharide Based Polymers Produced by Scabby Cankered Cactus Pear (Opuntia ficus-indica L.) Infected by Neofusicoccum batangarum: Composition, Structure, and Chemico-Physical Properties. Biomolecules 2022, 12, 89. https://doi.org/10.3390/biom12010089
Santagata G, Cimmino A, Poggetto GD, Zannini D, Masi M, Emendato A, Surico G, Evidente A. Polysaccharide Based Polymers Produced by Scabby Cankered Cactus Pear (Opuntia ficus-indica L.) Infected by Neofusicoccum batangarum: Composition, Structure, and Chemico-Physical Properties. Biomolecules. 2022; 12(1):89. https://doi.org/10.3390/biom12010089
Chicago/Turabian StyleSantagata, Gabriella, Alessio Cimmino, Giovanni Dal Poggetto, Domenico Zannini, Marco Masi, Alessandro Emendato, Giuseppe Surico, and Antonio Evidente. 2022. "Polysaccharide Based Polymers Produced by Scabby Cankered Cactus Pear (Opuntia ficus-indica L.) Infected by Neofusicoccum batangarum: Composition, Structure, and Chemico-Physical Properties" Biomolecules 12, no. 1: 89. https://doi.org/10.3390/biom12010089
APA StyleSantagata, G., Cimmino, A., Poggetto, G. D., Zannini, D., Masi, M., Emendato, A., Surico, G., & Evidente, A. (2022). Polysaccharide Based Polymers Produced by Scabby Cankered Cactus Pear (Opuntia ficus-indica L.) Infected by Neofusicoccum batangarum: Composition, Structure, and Chemico-Physical Properties. Biomolecules, 12(1), 89. https://doi.org/10.3390/biom12010089










