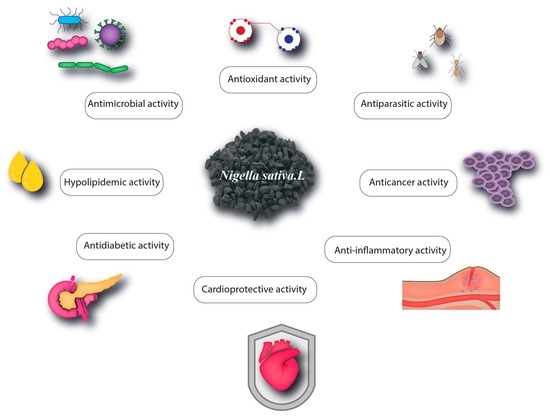
Nigella sativa L. Phytochemistry and Pharmacological Activities: A Review (2019–2021)
Abstract
:1. Introduction
2. Methodology of Research
3. Nigella sativa Phytochemistry
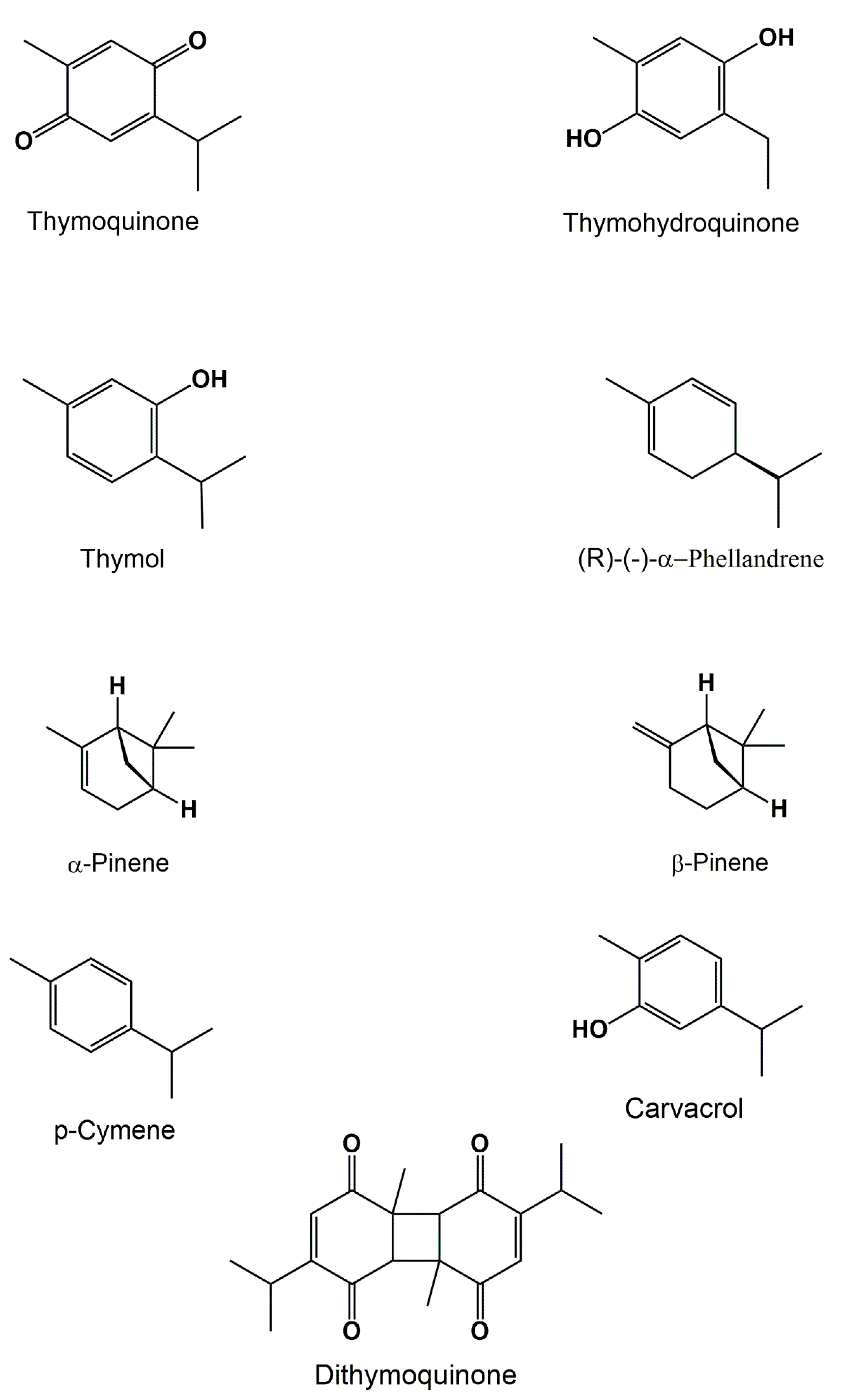
3.1. Volatile Compounds
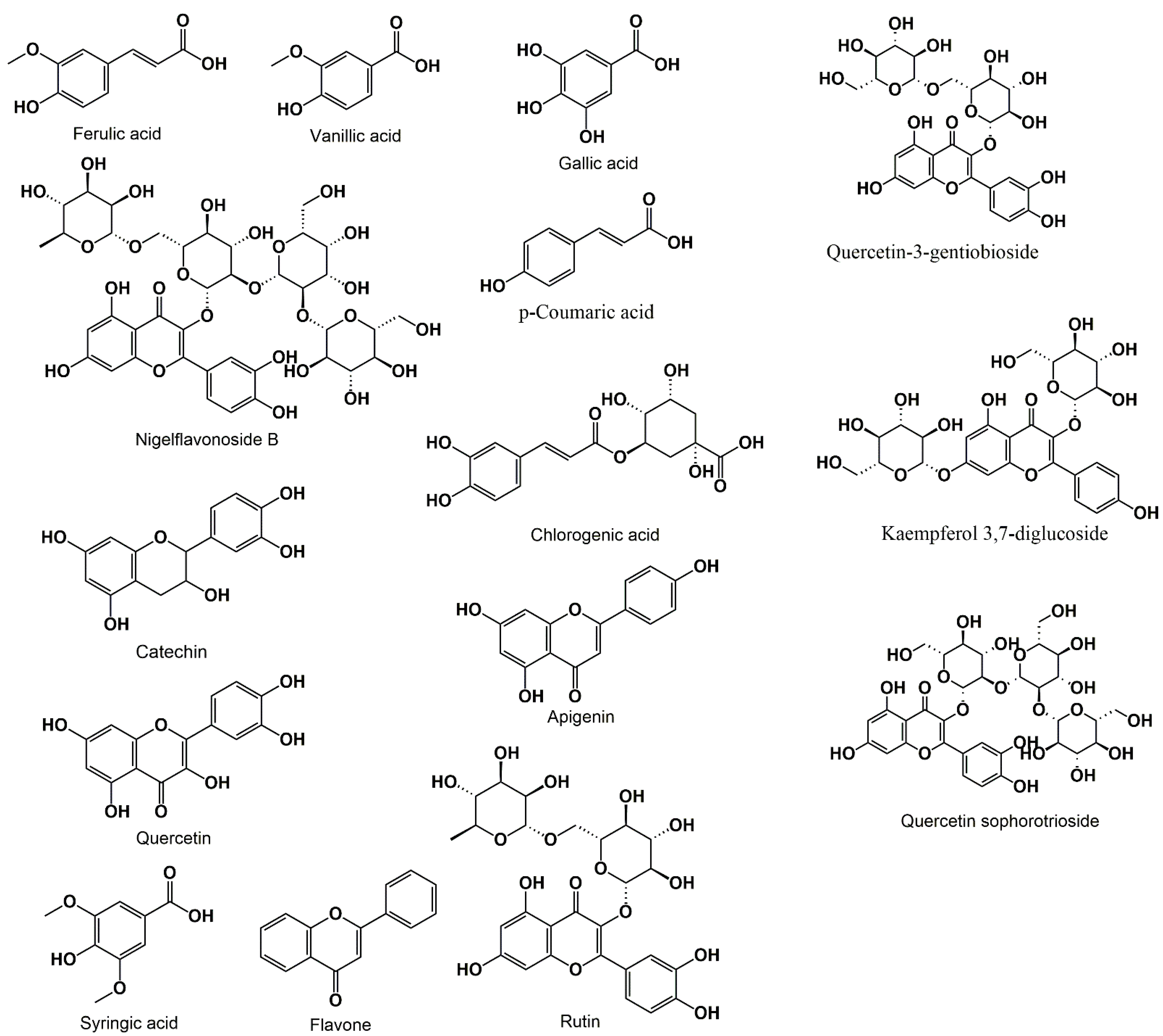
3.2. Phenolic Acids and Flavonoids
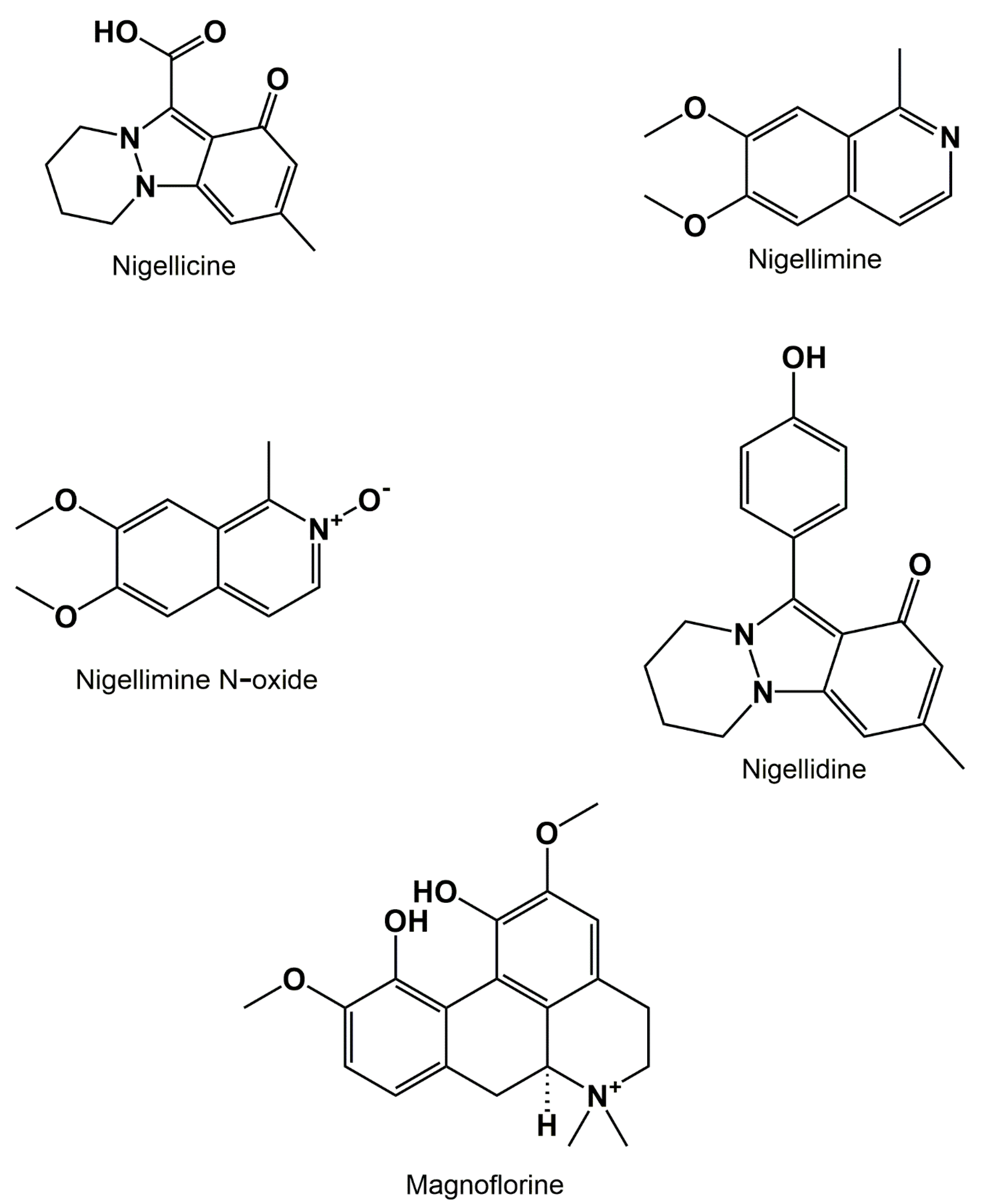
3.3. Alkaloids
3.4. Saponins
3.5. Fatty Acids
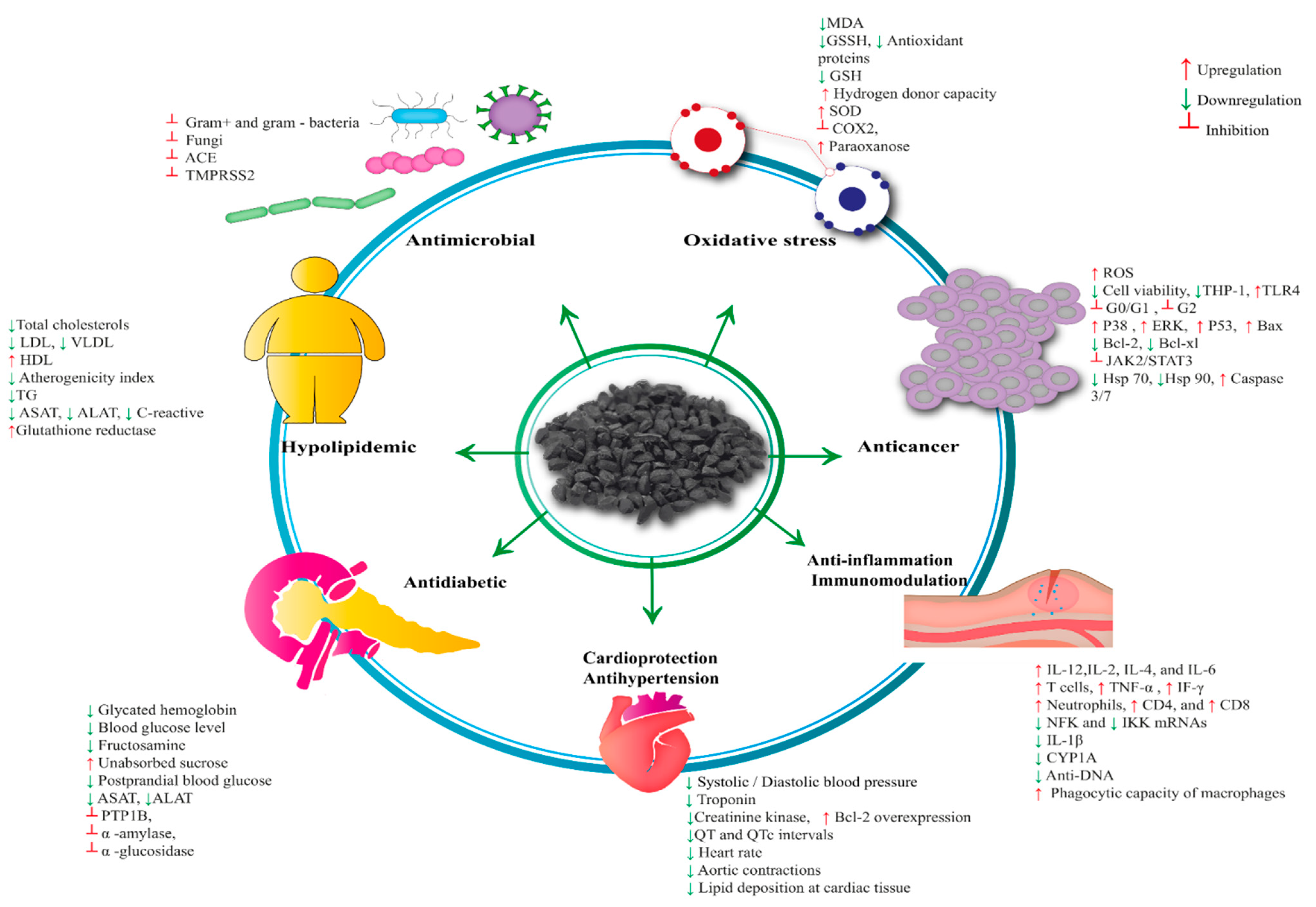
4. Pharmacological Properties of Nigella sativa
4.1. Antioxidant Activity
4.1.1. In Vitro
4.1.2. In Vivo
4.2. Antimicrobial Activity/Antibacterial Activity
4.2.1. In Vitro
4.2.2. In Vivo
4.3. Antiviral Activity
4.4. Antifungal Activity
In Vivo
4.5. Antiparasitic
4.6. Anticancer Activity
4.7. Anti-Inflammatory and Immunomodulatory Activity
4.8. Cardioprotective and Antihypertensive Activity
4.9. Antidiabetic Activity
4.10. Anti-Obesity and Dyslipidemic Activity
4.11. Toxicology
5. Conclusions and Future Perspectives
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviation
References
- Gharby, S.; Harhar, H.; Guillaume, D.; Roudani, A.; Boulbaroud, S.; Ibrahimi, M.; Ahmad, M.; Sultana, S.; Hadda, T.B.; Chafchaouni-Moussaoui, I.; et al. Chemical investigation of Nigella sativa L. seed oil produced in Morocco. J. Saudi Soc. Agric. Sci. 2015, 14, 172–177. [Google Scholar] [CrossRef] [Green Version]
- Shabana, A.; El-Menyar, A.; Asim, M.; Al-Azzeh, H.; Al Thani, H. Cardiovascular benefits of black cumin (Nigella sativa). Cardiovasc. Toxicol. 2013, 13, 9–21. [Google Scholar] [CrossRef] [PubMed]
- Kehili, N.; Saka, S.; Aouacheri, O. L’effet phytoprotecteur de la nigelle (Nigella sativa) contre la toxicité induite par le cadmium chez les rats. Phytothérapie 2018, 16, 194–203. [Google Scholar] [CrossRef]
- Medhi, H. Contribution à l’étude de la graine de nigelle ou cumin noir Nigella sativa L. Master’s Thesis, Aix-Marseille Université, Marseille, France, 2019. [Google Scholar]
- Ghedira, K. La nigelle cultivée: Nigella sativa L. (Ranunculaceae). Phytotherapie 2006, 4, 220–226. [Google Scholar] [CrossRef]
- Fakchich, J.; Elachouri, M. An overview on ethnobotanico-pharmacological studies carried out in Morocco, from 1991 to 2015: Systematic review (part 1). J. Ethnopharmacol. 2021, 267, 113200. [Google Scholar] [CrossRef] [PubMed]
- Dalli, M.; Azizi, S.; Kandsi, F.; Gseyra, N. Evaluation of the in vitro antioxidant activity of different extracts of Nigella sativa L. seeds, and the quantification of their bioactive compounds. Mater. Today Proc. 2021, 45, 7259–7263. [Google Scholar] [CrossRef]
- Dalli, M.; Azizi, S.; Benouda, H.; Azghar, H.A.; Tahri, M.; Boufalja, B.; Maleb, A.; Gseyra, N. Molecular Composition and Antibacterial Effect of Five Essential Oils Extracted from Nigella sativa L. Seeds against Multidrug-Resistant Bacteria: A Comparative Study. Evid.-Based Complement. Altern. Med. 2021, 2021, 6643765. [Google Scholar] [CrossRef] [PubMed]
- Shahid, M.A.; Rahim, A.; Chowdhury, M.A.; Kashem, M.A. Development of antibacterial nanofibrous wound dressing and conceptual reaction mechanism to deactivate the viral protein by Nigella sativa extract. Adv. Tradit. Med. 2021. [Google Scholar] [CrossRef]
- Hwang, J.R.; Cartron, A.M.; Khachemoune, A. A review of Nigella sativa plant-based therapy in dermatology. Int. J. Dermatol. 2021, 60, e493–e499. [Google Scholar] [CrossRef] [PubMed]
- Alhmied, F.; Alammar, A.; Alsultan, B.; Alshehri, M.; Pottoo, F.H. Molecular Mechanisms of Thymoquinone as Anticancer Agent. Comb. Chem. High Throughput Screen. 2021, 24, 1644–1653. [Google Scholar] [CrossRef] [PubMed]
- Hossain, M.S.; Sharfaraz, A.; Dutta, A.; Ahsan, A.; Masud, M.A.; Ahmed, I.A.; Goh, B.H.; Urbi, Z.; Sarker, M.M.R.; Ming, L.C. A review of ethnobotany, phytochemistry, antimicrobial pharmacology and toxicology of Nigella sativa L. Biomed. Pharmacother. 2021, 143, 112182. [Google Scholar] [CrossRef]
- Majdalawieh, A.F.; Fayyad, M.W. Recent advances on the anti-cancer properties of Nigella sativa, a widely used food additive. J. Ayurveda Integr. Med. 2016, 7, 173–180. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kulyar, M.F.-E.-A.; Li, R.; Mehmood, K.; Waqas, M.; Li, K.; Li, J. Potential influence of Nagella sativa (Black cumin) in reinforcing immune system: A hope to decelerate the COVID-19 pandemic. Phytomedicine 2021, 85, 153277. [Google Scholar] [CrossRef] [PubMed]
- Ansary, J.; Giampieri, F.; Forbes-Hernandez, T.Y.; Regolo, L.; Quinzi, D.; Gracia Villar, S.; Garcia Villena, E.; Tutusaus Pifarre, K.; Alvarez-Suarez, J.M.; Battino, M.; et al. Nutritional Value and Preventive Role of Nigella sativa L. and Its Main Component Thymoquinone in Cancer: An Evidenced-Based Review of Preclinical and Clinical Studies. Molecules 2021, 26, 2108. [Google Scholar] [CrossRef] [PubMed]
- Hannan, M.; Rahman, M.; Sohag, A.A.M.; Uddin, M.; Dash, R.; Sikder, M.H.; Timalsina, B.; Munni, Y.A.; Sarker, P.P.; Alam, M.; et al. Black cumin (Nigella sativa L.): A comprehensive review on phytochemistry, health benefits, molecular pharmacology, and safety. Nutrients 2021, 13, 1784. [Google Scholar] [CrossRef]
- Kabir, Y.; Akasaka-Hashimoto, Y.; Kubota, K.; Komai, M. Volatile compounds of black cumin (Nigella sativa L.) seeds cultivated in Bangladesh and India. Heliyon 2020, 6, e05343. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.P.P.K. Advances in Biochemistry of Medicinal Plants. In Biochemistry and Therapeutic Uses of Medicinal Plants; Discovery Publishing House Pvt. Ltd.: New Delhi, India, 2017. [Google Scholar]
- Dalli, M.; Daoudi, N.E.; Azizi, S.; Benouda, H.; Bnouham, M.; Gseyra, N. Chemical Composition Analysis Using HPLC-UV/GC-MS and Inhibitory Activity of Different Nigella sativa Fractions on Pancreatic α-Amylase and Intestinal Glucose Absorption. BioMed Res. Int. 2021, 2021, 9979419. [Google Scholar] [CrossRef] [PubMed]
- Parveen, A.; Farooq, M.A.; Kyunn, W.W. A new oleanane type saponin from the aerial parts of nigella sativa with anti-oxidant and anti-diabetic potential. Molecules 2020, 25, 2171. [Google Scholar] [CrossRef]
- Atta-ur-Rahman, S.M. Isolation and structure determination of nigellicine, a novel alkaloid from the seeds of nigella sativa. Tetrahedron Lett. 1985, 26, 2759–2762. [Google Scholar] [CrossRef]
- Atta-ur-Rahman, S.M.; Zaman, K. Nigellimine: A new isoquinoline alkaloid from the seeds of nigella sativa. J. Nat. Prod. 1992, 55, 676–678. [Google Scholar] [CrossRef]
- Atta-ur-Rahman, S.M.; Hasan, S.S.; Choudhary, M.I.; Ni, C.Z.; Clardy, J. Nigellidine—A new indazole alkaloid from the seeds of Nigella sativa. Tetrahedron Lett. 1995, 36, 1993–1996. [Google Scholar] [CrossRef]
- Makkar, H.P.S.; Siddhuraju, P.; Becker, K. Plant Secondary Metabolites; Humana Press: Totowa, NJ, USA, 2007. [Google Scholar]
- Taşkin, M.K.; Çalişkan, Ö.A.; Anil, H.; Abou-Gazar, H.; Khan, I.A.; Bedir, E. Triterpene saponins from Nigella sativa L. Turk. J. Chem. 2005, 29, 561–569. [Google Scholar]
- Takruri, H.R.H.; Dameh, M.A.F. Study of the nutritional value of black cumin seeds (Nigella sativa L). J. Sci. Food Agric. 1998, 76, 404–410. [Google Scholar] [CrossRef]
- Tiji, S.; Benayad, O.; Berrabah, M.; El Mounsi, I.; Mimouni, M. Phytochemical Profile and Antioxidant Activity of Nigella sativa L. Growing in Morocco. Sci. World J. 2021, 2021, 6623609. [Google Scholar] [CrossRef] [PubMed]
- Nickavar, F.; Mojab, B.; Javidnia, K.; Amoli Roodgar, M.A. Chemical Composition of the Fixed and Volatile Oils of Nigella sativa L. from Iran. Z. Naturforsch.-Sect. C J. Biosci. 2003, 58, 629–631. [Google Scholar] [CrossRef] [Green Version]
- Mahmoud, H.S.; Almallah, A.A.; L-Hak, H.N.G.E.; Aldayel, T.S.; Abdelrazek, H.M.A.; Khaled, H.E. The effect of dietary supplementation with Nigella sativa (black seeds) mediates immunological function in male Wistar rats. Sci. Rep. 2021, 11, 7542. [Google Scholar] [CrossRef] [PubMed]
- Bocsan, V.S.; Pop, I.C.; Sabin, R.M.; Sarkandy, O.; Boarescu, E.; Roşian, P.M.; Leru, Ş.H.; Chedea, P.M.; Socaci, S.A.; Buzoianu, A.D. Comparative protective effect of nigella sativa oil and vitis vinifera seed oil in an experimental model of isoproterenol-induced acute myocardial ischemia in rats. Molecules 2021, 26, 3221. [Google Scholar] [CrossRef]
- Bilto, Y.Y.; Alabdallat, N.G.; Atoom, A.M.; Khalaf, N.A. Effects of commonly used medicinal herbs in Jordan on erythrocyte oxidative stress oxidative. J. Pharm. Pharmacogn. Res. 2021, 9, 422–434. [Google Scholar]
- Farshori, A.A.; Saquib, N.N.; Siddiqui, Q.; Al-Oqail, M.A.; Al-Sheddi, M.M.; Al-Massarani, E.S.; Al-Khedhairy, S.M. Protective effects of Nigella sativa extract against H2O2-induced cell death through the inhibition of DNA damage and cell cycle arrest in human umbilical vein endothelial cells (HUVECs). J. Appl. Toxicol. 2021, 41, 820–831. [Google Scholar] [CrossRef]
- Liang, J.; Lian, L.; Wang, X.; Li, L. Thymoquinone, extract from Nigella sativa seeds, protects human skin keratinocytes against UVA-irradiated oxidative stress, inflammation and mitochondrial dysfunction. Mol. Immunol. 2021, 135, 21–27. [Google Scholar] [CrossRef] [PubMed]
- Rathi, B.; Devanesan, S.; AlSalhi, M.S.; Singh, A.J.R. In-vitro free radical scavenging effect and cytotoxic analysis of Black Cummins and Honey formulation. Saudi J. Biol. Sci. 2021, 28, 1576–1581. [Google Scholar] [CrossRef] [PubMed]
- Adebayo-Tayo, B.C.; Briggs-Kamara, A.I.; Salaam, A.M. Phytochemical composition, antioxidant, antimicrobial potential and gc-ms analysis of crude and partitioned fractions of Nigella sativa seed extract. Acta Microbiol. Bulg. 2021, 37, 34–45. [Google Scholar]
- Bonesi, R.; Saab, M.; Tenuta, A.M.; Leporini, M.C.; Saab, M.; Loizzo, M.J.; Tundis, M.R. Screening of traditional Lebanese medicinal plants as antioxidants and inhibitors of key enzymes linked to type 2 diabetes. Plant Biosyst. 2020, 154, 656–662. [Google Scholar] [CrossRef]
- Babar, Z.M.; Azizi, W.M.; Ichwan, S.J.A.; Ahmed, Q.U.; Azad, A.K.; Mawa, I. A simple method for extracting both active oily and water soluble extract (WSE) from Nigella sativa (L.) seeds using a single solvent system. Nat. Prod. Res. 2019, 33, 2266–2270. [Google Scholar] [CrossRef]
- Jan, K.; Ahmad, M.; Rehman, S.; Gani, A.; Khaqan, K. Effect of roasting on physicochemical and antioxidant properties of kalonji (Nigella sativa) seed flour. J. Food Meas. Charact. 2019, 13, 1364–1372. [Google Scholar] [CrossRef]
- Vesa, S.C.; Chedea, V.S.; Bocsan, I.C.; Ancut, S.; Buzoianu, A.D. Nigella sativa’s Anti-Inflammatory and Antioxidative Effects in Experimental Inflammation. Antioxidants 2020, 9, 921. [Google Scholar]
- Nehar, S.; Rani, P.; Kumar, C. Evaluation of genoprotective and antioxidative potentiality of ethanolic extract of N. sativa seed in streptozotocin induced diabetic albino rats. Vegetos 2021, 34, 453–459. [Google Scholar] [CrossRef]
- Alkis, H.; Demir, E.; Taysi, M.R.; Sagir, S.; Taysi, S. Effects of Nigella sativa oil and thymoquinone on radiation-induced oxidative stress in kidney tissue of rats. Biomed. Pharmacother. 2021, 139, 111540. [Google Scholar] [CrossRef]
- Badger-Emeka, L.I.; Emeka, P.M.; Ibrahim, H.I.M. A Molecular Insight into the Synergistic Mechanism of Nigella sativa (Black Cumin) with Β-Lactam Antibiotics against Clinical Isolates of Methicillin-Resistant Staphylococcus aureus. Appl. Sci. 2021, 11, 3206. [Google Scholar] [CrossRef]
- Arif, P.L.; Saqib, S.; Mubashir, H.; Malik, M.; Mukhtar, S.I.; Saqib, A.; Ullah, S.; Show, S. Comparison of Nigella sativa and Trachyspermum ammi via experimental investigation and biotechnological potential. Chem. Eng. Process.-Process Intensif. 2021, 161, 108313. [Google Scholar] [CrossRef]
- Alizadeh-naini, M.; Yousefnejad, H.; Hejazi, N. The beneficial health effects of Nigella sativa on Helicobacter pylori eradication, dyspepsia symptoms, and quality of life in infected patients: A pilot study. Phyther. Res. 2020, 34, 1367–1376. [Google Scholar] [CrossRef]
- Raveesha, K.A. Raveesha Antibacterial activity and time-kill assay of Terminalia catappa L. And Nigella sativa L. And selected human pathogenic bacteria. J. Pure Appl. Microbiol. 2021, 15, 285–299. [Google Scholar]
- Dera, A.A.; Ahmad, I.; Rajagopalan, P.; Al Shahrani, M.; Saif, A.; Alshahrani, M.Y.; Alraey, Y.; Alamri, A.M.; Alasmari, S.; Makkawi, M.; et al. Synergistic efficacies of thymoquinone and standard antibiotics against multi-drug resistant isolates. Saudi Med. J. 2021, 42, 196–204. [Google Scholar] [CrossRef]
- Habib, N.; Choudhry, S. HPLC Quantification of Thymoquinone Extracted from Nigella sativa L. (Ranunculaceae) Seeds and Antibacterial Activity of Its Extracts against Bacillus Species. Evid.-Based Complement. Altern. Med. 2021, 2021, 6645680. [Google Scholar] [CrossRef] [PubMed]
- Tada, T.; Nakayama-Imaohji, A.; Yamasaki, H.; Elahi, H.; Nagao, M.; Yagi, T.; Ishikawa, H.; Shibuya, M.; Kuwahara, K. Effect of thymoquinone on Fusobacterium nucleatum-associated biofilm and inflammation. Mol. Med. Rep. 2020, 22, 643–650. [Google Scholar] [CrossRef] [PubMed]
- Hal, A.M.; El-Barbary, M.I. Effect of Nigella sativa oil and ciprofloxacin against bacterial infection on gene expression in Nile tilapia (Oreochromis niloticus) blood. Aquaculture 2021, 532, 736071. [Google Scholar] [CrossRef]
- Widdatallah, M.O.; Mohamed, R.; Alrasheid, A.A.; Widatallah, A.A.; Yassin, H.A.; Eltilib, L.F.; Abdel, S.H.; Ahmed, S. Green Synthesis of Silver Nanoparticles Using Nigella sativa Seeds and Evaluation of Their Antibacterial Activity. Adv. Nanopart. 2020, 9, 41–48. [Google Scholar] [CrossRef] [Green Version]
- Nazarparvar, M.; Shakeri, A.; Ranjbariyan, A. Chemical composition and antimicrobial activity against food poisoning of alcoholic extract of Nigella sativa L. Biointerface Res. Appl. Chem. 2020, 10, 6991–7001. [Google Scholar]
- Yasmin, S.; Nawaz, K.; Anjum, M.; Ashraf, A.A.; Basra, I.; Mehmood, M.A.R.; Khan, A.; Malik, F. Phytochemical analysis and in vitro activity of essential oils of selected plants against Salmonella enteritidis and Salmonella gallinarum of poultry origin. Pak. Vet. J. 2020, 40, 139–144. [Google Scholar] [CrossRef]
- Mosolygó, G.; Mouwakeh, T.; Ali, A.; Kincses, M.H.; Mohácsi-Farkas, A.; Kiskó, C.; Spengler, G. Bioactive compounds of Nigella sativa essential oil as antibacterial agents against Chlamydia trachomatis D. Microorganisms 2019, 7, 370. [Google Scholar] [CrossRef] [Green Version]
- Nayef, Y.A.; Zangana, B.S. Effect of essential oils on chemical composition and microbial load of minced and frozen stored chicken meat. Biochem. Cell. Arch. 2020, 20, 841–846. [Google Scholar]
- Hetta, H.F.; Meshaal, A.K.; Algammal, A.M.; Yahia, R.; Makharita, R.R.; Marraiki, N.; Shah, M.A.; Hassan, H.A.M.; Batiha, G.E.S. In-vitro antimicrobial activity of essential oils and spices powder of some medicinal plants against bacillus species isolated from raw and processed meat. Infect. Drug Resist. 2020, 13, 4367–4378. [Google Scholar] [CrossRef]
- Barashkova, A.S.; Sadykova, V.S.; Salo, V.A.; Zavriev, S.K.; Rogozhin, E.A. Nigellothionins from black cumin (Nigella sativa l.) seeds demonstrate strong antifungal and cytotoxic activity. Antibiotics 2021, 10, 166. [Google Scholar] [CrossRef] [PubMed]
- Pournajafian, M.; Naseri, A.; Fata, A.; Rakhshandeh, A.; Afzal-Aghaee, H. The antifungal effects of hydroalcoholic extracts of Nigella sativa and urtica dioica on fungal agents in comparison with amphotericin B. J. Isfahan Med. Sch. 2021, 39, 198–205. [Google Scholar]
- Rusda, M.; Adenin, I.; Siregar, M.F.G.; Rambe, A.Y.M.; Sudewo, Y. Therapeutic effect of 48 h after Nigella sativa extract administration on female wistar rats vaginal candidiasis model: An experimental study. Open Access Maced. J. Med. Sci. 2021, 9, 6–8. [Google Scholar] [CrossRef]
- Aftab, A.; Yousaf, Y.; Javaid, A.; Riaz, N.; Younas, A.; Rashid, M.; Shamsher, B.; Arif, A. Antifungal activity of vegetative methanolic extracts of Nigella sativa against Fusarium oxysporum and Macrophomina phaseolina and its phytochemical profiling by GC-MS analysis. Int. J. Agric. Biol. 2019, 21, 569–576. [Google Scholar]
- Koshak, A.E.; Koshak, E.A.; Mobeireek, A.F.; Badawi, M.A.; Wali, S.O.; Malibary, H.M.; Atwah, A.F.; Alhamdan, M.M.; Almalki, R.A.; Madani, T.A. Complementary Therapies in Medicine Nigella sativa for the treatment of COVID-19: An open-label randomized controlled clinical trial. Complement. Ther. Med. 2021, 61, 102769. [Google Scholar] [CrossRef] [PubMed]
- Kadil, Y.; Mouhcine, M.; Filali, H. In Silico Investigation of the SARS-CoV2 Protease with Thymoquinone, the Major Constituent of Nigella sativa. Curr. Drug Discov. Technol. 2021, 18, 570–573. [Google Scholar] [CrossRef]
- Sommer, A.P.; Försterling, H.D.; Sommer, K.E. Tutankhamun’s Antimalarial Drug for COVID-19. Drug Res. 2021, 71, 4–9. [Google Scholar] [CrossRef]
- Khan, S.A. Combating COVID-19: The role of drug repurposing and medicinal plants. J. Infect. Public Health 2021, 14, 495–503. [Google Scholar] [CrossRef] [PubMed]
- Mir, S.A.; Firoz, A.; Alaidarous, M.; Alshehri, B.; Dukhyil, A.A.B.; Banawas, S.; Alsagaby, S.A.; Alturaiki, W.; Bhat, G.A.; Kashoo, F.; et al. Identification of SARS-CoV-2 RNA-dependent RNA polymerase inhibitors from the major phytochemicals of Nigella sativa: An in silico approach. Saudi J. Biol. Sci. 2021. [Google Scholar] [CrossRef]
- Yadav, P.K.; Jaiswal, A.; Singh, R.K. In silico study on spice-derived antiviral phytochemicals against SARS-CoV-2 TMPRSS2 target. J. Biomol. Struct. Dyn. 2021, 1–11. [Google Scholar] [CrossRef]
- Ahmad, S.; Abbasi, H.W.; Shahid, S.; Gul, S.; Abbasi, S.W. Molecular docking, simulation and MM-PBSA studies of nigella sativa compounds: A computational quest to identify potential natural antiviral for COVID-19 treatment. J. Biomol. Struct. Dyn. 2021, 39, 4225–4233. [Google Scholar] [CrossRef] [PubMed]
- Sumaryada, T.; Pramudita, C.A. Molecular docking evaluation of some indonesian’s popular herbals for a possible COVID-19 treatment. Biointerface Res. Appl. Chem. 2021, 11, 9827–9835. [Google Scholar]
- El-Sayed, S.A.E.S.; Rizk, M.A.; Yokoyama, N.; Igarashi, I. Evaluation of the in vitro and in vivo inhibitory effect of thymoquinone on piroplasm parasites. Parasites Vectors 2019, 12, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Dagtas, A.S.; Griffin, R.J. Nigella sativa extract kills pre-malignant and malignant oral squamous cell carcinoma cells. J. Herb. Med. 2021, 29, 100473. [Google Scholar] [CrossRef] [PubMed]
- El-Obeid, A.; Alajmi, H.; Harbi, M.; Yahya, W.B.; Al-Eidi, H.; Alaujan, M.; Haseeb, A.; Trivilegio, T.; Alhallaj, A.; Alghamdi, S.; et al. Distinct anti-proliferative effects of herbal melanin on human acute monocytic leukemia thp-1 cells and embryonic kidney hek293 cells. BMC Complement. Med. Ther. 2020, 20, 154. [Google Scholar] [CrossRef]
- Al-Obeed, O.; El-Obeid, A.S.; Matou-Nasri, S.; Vaali-Mohammed, M.A.; AlHaidan, Y.; Elwatidy, M.; Al Dosary, H.; Alehaideb, Z.; Alkhayal, K.; Haseeb, A.; et al. Herbal melanin inhibits colorectal cancer cell proliferation by altering redox balance, inducing apoptosis, and modulating MAPK signaling. Cancer Cell Int. 2020, 20, 126. [Google Scholar] [CrossRef] [Green Version]
- Franco-Ramos, R.S.; López-Romero, C.A.; Torres-Ortega, H.; Oseguera-Herrera, D.; Lamoreaux-Aguayo, J.P.; Molina-Noyola, D.; Juárez-Vázquez, C.I.; Torres-Bugarín, O. Evaluation of anti-cytotoxic and anti-genotoxic effects of Nigella sativa through a micronucleus test in balb/c mice. Nutrients 2020, 12, 1317. [Google Scholar] [CrossRef]
- Al-Mutairi, A.; Rahman, A.; Rao, M.S. Low Doses of Thymoquinone and Ferulic Acid in Combination Effectively Inhibit Proliferation of Cultured MDA-MB 231 Breast Adenocarcinoma Cells. Nutr. Cancer 2021, 73, 282–289. [Google Scholar] [CrossRef]
- Chae, I.G.; Song, N.Y.; Kim, D.H.; Lee, M.Y.; Park, J.M.; Chun, K.S. Thymoquinone induces apoptosis of human renal carcinoma Caki-1 cells by inhibiting JAK2/STAT3 through pro-oxidant effect. Food Chem. Toxicol. 2020, 139, 111253. [Google Scholar] [CrossRef]
- Hu, X.; Lin, M.; Zhu, W.; Zheng, Y.; Zhang, Q.; Wu, G.; Qiu, Y. Potential Cytotoxicity, Pharmacokinetics, and Excretion Properties of Sapindoside B from the Seeds of Nigella sativa var hispidula. Planta Med. 2020, 86, 356–363. [Google Scholar] [CrossRef] [PubMed]
- Silva, A.F.C.; Haris, P.I.; Serralheiro, M.L.; Pacheco, R. Mechanism of action and the biological activities of Nigella sativa oil components. Food Biosci. 2020, 38, 100783. [Google Scholar] [CrossRef]
- Pathiranage, V.C.; Thabrew, I.; Samarakoon, S.R.; Tennekoon, K.H.; Rajagopalan, U.; Ediriweera, M.K. Evaluation of anticancer effects of a pharmaceutically viable extract of a traditional polyherbal mixture against non-small-cell lung cancer cells. J. Integr. Med. 2020, 18, 242–252. [Google Scholar] [CrossRef]
- Kordestani, Z.; Shahrokhi-Farjah, M.; Rouholamini, S.E.Y.; Saberi, A. Reduced ikk/nf-kb expression by Nigella sativa extract in breast cancer. Middle East J. Cancer 2020, 11, 150–158. [Google Scholar]
- Zhang, B.; Ting, W.J.; Gao, J.; Kang, Z.F.; Huang, C.Y.; Weng, Y.J. Erk phosphorylation reduces the thymoquinone toxicity in human hepatocarcinoma. Environ. Toxicol. 2021, 36, 1990–1998. [Google Scholar] [CrossRef]
- Mutabagani, A.; El-Mahdy, S.A. A study of the anti-inflammatory activity of Nigella sativa L. and thymoquinone in rats. Saudi Pharm. J. 1997, 5, 110–113. [Google Scholar]
- Gholamnezhad, Z.; Boskabady, M.H.; Hosseini, M. The effect of chronic supplementation of Nigella sativa on splenocytes response in rats following treadmill exercise. Drug Chem. Toxicol. 2021, 44, 487–492. [Google Scholar] [CrossRef]
- El-Shanshory, M.; Hablas, N.M.; Aboonq, M.S.; Fakhreldin, A.R.; Attia, M.; Arafa, W.; Mariah, R.A.; Baghdadi, H.; Ayat, M.; Zolaly, M.; et al. Nigella sativa improves anemia, enhances immunity and relieves iron overload-induced oxidative stress as a novel promising treatment in children having beta-thalassemia major. J. Herb. Med. 2019, 16, 100245. [Google Scholar] [CrossRef]
- Hakim, A.S.; Abouelhag, H.A.; Abdou, A.M.; Fouad, E.A.; Khalaf, D.D. Assessment of Immunomodulatory Effects of Black Cumin Seed (Nigella sativa) Extract on Macrophage Activity in Vitro. Int. J. Vet. Sci. 2019, 5, 44–47. [Google Scholar]
- Guritno, T.; Barlianto, W.; Wulandari, D.; Amru, W.A. Effect Nigella sativa extract for balancing immune response in pristane induced lupus mice model. J. Appl. Pharm. Sci. 2021, 11, 146–152. [Google Scholar]
- Liang, Q.; Dong, J.; Wang, S.; Shao, W.; Ahmed, A.F.; Zhang, Y.; Kang, W. Immunomodulatory effects of Nigella sativa seed polysaccharides by gut microbial and proteomic technologies. Int. J. Biol. Macromol. 2021, 184, 483–496. [Google Scholar] [CrossRef] [PubMed]
- Shoaei-Hagh, P.; Kamelan Kafi, F.; Najafi, S.; Zamanzadeh, M.; Heidari Bakavoli, A.; Ramezani, J.; Soltanian, S.; Asili, J.; Hosseinzadeh, H.; Eslami, S.; et al. A randomized, double-blind, placebo-controlled, clinical trial to evaluate the benefits of Nigella sativa seeds oil in reducing cardiovascular risks in hypertensive patients. Phytother. Res. 2021, 35, 4388–4400. [Google Scholar] [CrossRef]
- El-Kader, M.A. Evaluation of azithromycin induced cardiotoxicity in male albino rats and the possible protective role of Nigella sativa oil. Egypt. J. Histol. 2020, 43, 465–476. [Google Scholar] [CrossRef]
- Altun, E.; Avci, E.; Yildirim, T.; Yildirim, S. Protective effect of Nigella sativa oil on myocardium in streptozotocin-induced diabetic rats. Acta Endocrinol. 2019, 15, 289–294. [Google Scholar]
- Razmpoosh, E.; Safi, S.; Nadjarzadeh, A.; Fallahzadeh, H.; Abdollahi, N.; Mazaheri, M.; Nazari, M.; Salehi-Abargouei, A. The effect of Nigella sativa supplementation on cardiovascular risk factors in obese and overweight women: A crossover, double-blind, placebo-controlled randomized clinical trial. Eur. J. Nutr. 2021, 60, 1863–1874. [Google Scholar] [CrossRef]
- Alam, M.A.; Jardan, Y.A.B.; Raish, M.; Al-Mohizea, A.M.; Ahad, A.; Al-Jenoobi, F.I. Effect of Nigella sativa and Fenugreek on the Pharmacokinetics and Pharmacodynamics of Amlodipine in Hypertensive Rats. Curr. Drug Metab. 2020, 21, 318–325. [Google Scholar] [CrossRef]
- Hosseinzadeh, H.; Tafaghodi, M.; Mosavi, M.J.; Taghiabadi, E. Effect of Aqueous and Ethanolic Extracts of Nigella sativa Seeds on Milk Production in Rats. JAMS J. Acupunct. Meridian Stud. 2013, 6, 18–23. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pei, Z.W.; Guo, Y.; Zhu, H.L.; Dong, M.; Zhang, Q.; Wang, F. Thymoquinone Protects against Hyperlipidemia-Induced Cardiac Damage in Low-Density Lipoprotein Receptor-Deficient (LDL-R-/-) Mice via Its Anti-inflammatory and Antipyroptotic Effects. BioMed Res. Int. 2020, 2020, 4878704. [Google Scholar] [CrossRef]
- Al Asoom, L.I.; Al-Hariri, M.T. Cardiac Inotropic Effect of Long-Term Administration of Oral Thymoquinone. Evid.-Based Complement. Altern. Med. 2019, 2019, 8575136. [Google Scholar] [CrossRef]
- Al Asoom, L.I. Molecular Mechanisms of Nigella sativa—And Nigella sativa Exercise-Induced Cardiac Hypertrophy in Rats. Evid.-Based Complement. Altern. Med. 2021, 2021, 5553022. [Google Scholar] [CrossRef]
- Mousavi, S.M.; Abbasnezhad, A.; Mohebbati, R.; Kianmehr, M.; Ghorbani, M. Comparative effects of Glibenclamide and Nigella sativa on aortic contractile and dilation response in diabetic rat. Acta Med. Mediterr. 2020, 36, 2213–2219. [Google Scholar]
- Jardan, Y.A.B.; Ahad, A.; Raish, M.; Alam, M.A.; Al-Mohizea, A.M.; Al-Jenoobi, F.I. Effects of garden cress, fenugreek and black seed on the pharmacodynamics of metoprolol: An herb-drug interaction study in rats with hypertension. Pharm. Biol. 2021, 59, 1088–1097. [Google Scholar] [CrossRef]
- Varghese, L.N.; Mehrotra, N. α-Amylase inhibitory activity of microencapsulated Nigella sativa L. and herb- drug interaction: An in vitro analysis. Ann. Phytomed. Int. J. 2020, 9, 107–112. [Google Scholar] [CrossRef]
- Hamdan, A.; Haji Idrus, R.; Mokhtar, M.H. Effects of Nigella sativa on type-2 diabetes mellitus: A systematic review. Int. J. Environ. Res. Public Health 2020, 17, 4911. [Google Scholar] [CrossRef]
- Vijayakumar, S.; Divya, M.; Vaseeharan, B.; Chen, J.; Biruntha, M.; Silva, L.P.; Durán-Lara, E.F.; Shreema, K.; Ranjan, S.; Dasgupta, N. Biological Compound Capping of Silver Nanoparticle with the Seed Extracts of Blackcumin (Nigella sativa): A Potential Antibacterial, Antidiabetic, Anti-inflammatory, and Antioxidant. J. Inorg. Organomet. Polym. Mater. 2021, 31, 624–635. [Google Scholar] [CrossRef]
- Ali, S.M.; Chen, P.; Sheikh, S.; Ahmad, A.; Ahmad, M.; Paithankar, M.; Desai, B.; Patel, P.; Khan, M.; Chaturvedi, A.; et al. Thymoquinone with Metformin Decreases Fasting, Post Prandial Glucose, and HbA1c in Type 2 Diabetic Patients. Drug Res. 2021, 71, 302–306. [Google Scholar] [CrossRef] [PubMed]
- Ida, M.; Ahmad, M.; Dwi, A.N. Fruit, Trigonella foenum -graecum and Nigella sativa L. Seeds Using In vitro and In vivo Assay. Trop. J. Nat. Prod. Res. 2020, 4, 801–805. [Google Scholar]
- Rao, A.S.; Hegde, S.; Pacioretty, L.M.; Debenedetto, J.; Babish, J.G. Nigella sativa and Trigonella foenum-graecum Supplemented Chapatis Safely Improve HbA1c, Body Weight, Waist Circumference, Blood Lipids, and Fatty Liver in Overweight and Diabetic Subjects: A Twelve-Week Safety and Efficacy Study. J. Med. Food 2020, 23, 905–919. [Google Scholar] [CrossRef]
- Ibrahim, H.A.E.; Hashem, M.A.; Mohamed, N.E.; El-Rahman, A.A.A. Assessment of ameliorative effects of zingiber officinale and Nigella sativa on streptozotocin-induced diabetic rats. Adv. Anim. Vet. Sci. 2020, 8, 1211–1219. [Google Scholar] [CrossRef]
- Hannan, J.M.A.; Ansari, P.; Haque, A.; Sanju, A.; Huzaifa, A.; Rahman, A.; Ghosh, A.; Azam, S. Nigella sativa stimulates insulin secretion from isolated rat islets and inhibits the digestion and absorption of (CH2O)n in the gut. Biosci. Rep. 2019, 39, BSR20190723. [Google Scholar] [CrossRef] [Green Version]
- Pelegrin, S.; Galtier, F.; Chalançon, A.; Gagnol, J.P.; Barbanel, A.M.; Pélissier, Y.; Larroque, M.; Lepape, S.; Faucanié, M.; Gabillaud, I.; et al. Effects of Nigella sativa seeds (black cumin) on insulin secretion and lipid profile: A pilot study in healthy volunteers. Br. J. Clin. Pharmacol. 2019, 85, 1607–1611. [Google Scholar] [CrossRef] [PubMed]
- Ramdan, B.; Ramdan, R.; El Karbane, M.; El Maadoudi, M.; Ben Mrid, R.; Nhiri, M. Anti-glycation study of hydro-alcohol and aqueous extracts of Moroccan plant species. Int. J. Res. Pharm. Sci. 2019, 10, 826–837. [Google Scholar] [CrossRef]
- Bonab, S.B.; Tofighi, A. Effect of 8 weeks aerobic training and nigella supplement on insulin resistance, lipid profile and plasma level of hba1c in type 2 diabetic rats. J. Adv. Med. Biomed. Res. 2019, 27, 20–29. [Google Scholar] [CrossRef]
- Muhsin, S.M.; Mahmood, R.I.; Abdul-Lattif, R.F.; Sabrei, D.A. Hypoglycemic and hypolipidemic properties of three plants extract in Alloxan induced diabetic rats. Plant Arch. 2019, 19, 1558–1563. [Google Scholar]
- Hadi, M.A. E4An ultrastructural study by transmission electron microscope of exocrine pancreatic cells in diabetic rats treated with herbal combination. Baghdad Sci. J. 2019, 16, 966–977. [Google Scholar] [CrossRef] [Green Version]
- Aboul-Mahasen, L.M.; Alshali, R.A. The possible protective effects of virgin olive oil and Nigella sativa seeds on the biochemical and histopathological changes in pancreas of hyperlipidaemic rats. Folia Morphol. 2019, 78, 762–772. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abbasnezhad, A.; Niazmand, S.; Mahmoudabady, M.; Rezaee, S.A.; Soukhtanloo, M.; Mosallanejad, R.; Hayatdavoudi, P. Nigella sativa L. seed regulated eNOS, VCAM-1 and LOX-1 genes expression and improved vasoreactivity in aorta of diabetic rat. J. Ethnopharmacol. 2019, 228, 142–147. [Google Scholar] [CrossRef] [PubMed]
- Shari, F.H.; Ramadhan, H.H.; Mohammed, R.N.; Al-Bahadily, D.C. Hypolipidemic and antioxidant effects of fenugreek- Nigella sativa combination on diabetic patients in Iraq. Syst. Rev. Pharm. 2020, 11, 911–915. [Google Scholar]
- Zaoui, A.; Cherrah, Y.; Mahassini, N.; Alaoui, K.; Amarouch, H.; Hassar, M. Acute and chronic toxicity of Nigella sativa fixed oil. Phytomedicine 2002, 9, 69–74. [Google Scholar] [CrossRef] [Green Version]
- Mashayekhi-Sardoo, H.; Rezaee, R.; Karimi, G. An overview of in vivo toxicological profile of thymoquinone. Toxin Rev. 2020, 39, 115–122. [Google Scholar] [CrossRef]
- Badary, O.A.; Al-Shabanah, O.A.; Nagi, M.N.; Al-Bekairi, A.M.; Elmazar, M.M.A. Acute and subchronic toxicity of thymoquinone in mice. Drug Dev. Res. 1998, 44, 56–61. [Google Scholar] [CrossRef]
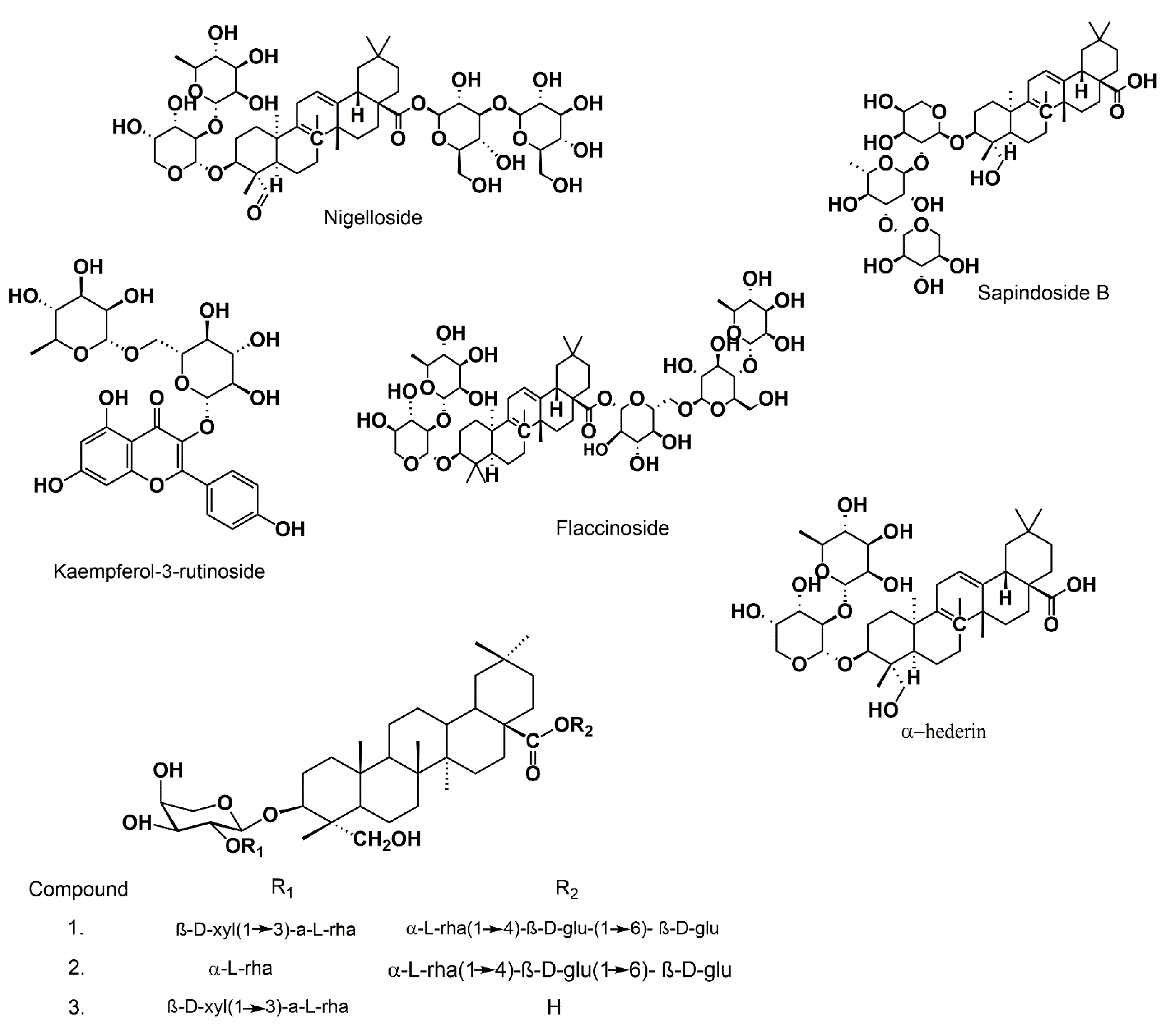

| Extract/Compound | Methods | Test | Results | Reference |
|---|---|---|---|---|
| NS seeds | ||||
| NS supplementation (30 and 50 g/kg BW) | In vitro | DPPH (antiradical scavenging activity) | IC50 = 1.367 mg TE/g | [29] |
| In vivo (Wistar rats) | Total antioxidant capacity (TAC) |
| ||
| NSO (5 mg/mL) | In vitro | DPPH | IC50 = 12.713 mM T/100 g | [30] |
|
| [36,39] | ||
| In vivo | Wistar rats | ↓ MDA ↓ GSSH ↑ Hydrogen donor capacity | ||
| MeOH extract (0.2/0.4/0.6 and 0.8 mg/mL) | In vitro | Erythrocytes exposed to H2O2 | ↓ MDA ↓ Antioxidant enzymes ↓ GSH Anti-hemolytic activity | [31] |
| EtOH extract (10, 30 and 50 µg/mL) | In vitro | Human umbilical vein endothelial cells H2O2 | ↑ GSH level ↓ Lipid peroxidation | [32,40] |
| In vivo | Wistar rats | ↓ DNA damages ↓ Lipid peroxidation ↑ SOD | ||
| TQ (6 and 12 µM) (50 mg/kg/day) | In vitro | Irradiation HaCaT keratinocytes cells by the UVA | Inhibition of the cyclooxygenase 2 (COX2) via the activation of NrF2/ARE pathway. | [33,41] |
| TQ (6 and 12 µM) (50 mg/kg/day) | In vivo | Irradiation of Sprague Dawley rat kidney tissue | Arylesterase (not significant) ↑ Paraoxonase Sulfhydryl groups (not significant) ↑ Ceruloplasmin ↓ Lipid hydroxide | [33,41] |
| Aqueous extract + honey (25–125 mg/mL) | In vitro | DPPH | IC50 = 20 mg/mL | [34] |
| Aqueous extract (0.2/0.4/0.6 and 0.8 mg/mL) | In vivo | Human healthy subjects | ↓ MDA (not significant) ↑ SOD (Not significant) ↑ GSH level | [31] |
| MeOH extract (100 µg/mL et 1000 µg/mL) | In vitro |
|
| [35] |
| Water-soluble extract | In vitro | DPPH | IC50 = 33.32 mg/mL | [37] |
| In vitro | DPPH | Inhibition percentage of DPPH 87.76%, 86.14%, 87.11% respectively, for the different prepared seeds | [38] |
| FRAP | The ferric reducing power percentage, 80.07%, 83.46%, 85.09% respectively for the different preparations | |||
| Aerial part | ||||
| Magnoflorine (25, 50, 75 and 100 µM for DPPH test) (50, 100, 125 and 250 µM for ABTS test) | In vitro |
|
| [20] |
| Nigelflavonoside (25, 50, 75 and 100 µM for DPPH test) (50, 100, 125 and 250 µM for ABTS test) | In vitro |
|
| |
| Quercetin sphorotrioside (25, 50, 75 and 100 µM for DPPH test) (50, 100, 125 and 250 µM for ABTS test) | In vitro |
|
| |
| Kaempferol-3,7-diglucoside (25, 50, 75 and 100 µM for DPPH test) (50, 100, 125 and 250 µM for ABTS test) | In vitro |
|
| |
| Rutin (25, 50, 75 and 100 µM for DPPH test) (50, 100, 125 and 250 µM for ABTS test) | In vitro |
|
| |
| Extract/Fraction | Method | Bacterial Strains | Results | Reference |
|---|---|---|---|---|
| Antibacterial Activity | ||||
| NS supplementation + quadritherapy (2 g/day) | in vivo Clinical study on unhealthy volunteers | Helicobacter pylori |
| [44] |
| MeOH fraction | in vitro Agar diffusion method |
| All tested bacteria showed a susceptibility toward the methanolic fraction | [35] |
| NS + ATB (Augmentin®) (5 and 7.5 µg/mL for NS, 10 and 20 mg for Augmentin®) | in vitro Agar diffusion method | MRSA | Potentiation of the ATB activity, plus a membrane deformation | [42] |
| Aqueous extract | in vitro Agar diffusion method |
| MIC at 100 µg/mL | [43] |
| Hexane extract (100 mg/mL) | in vitro Agar diffusion method |
| Inhibitory activity on the tested strains that was characterized by an inhibition diameter between 11.25 to 19 mm. | [45] |
| TQ (50 µg/mL) (1.25, 2.5 and 6 mg/µL) (1 µg/mL) | in vitro Microdilution technique, and agar diffusion method |
|
| [46,47,48] |
| Inhibition of biofilm formation at a concentration of 0.1% | |||
| NSO (1.25, 2.5 and 5 mg/µL) (7% mL/kg diet) | in vitro Agar diffusion method |
| A bacterial inhibition at a concentration of 5 µg/mL. | [47] |
| in vivo | Injection of bacterial strains to Oreochromis niloticus
| ↓ CYP1A | [49] | |
| Aqueous extract (20 mg/mL) | in vitro Agar diffusion method |
|
| [50] |
| n-butanol extract (1.5, 2 and 2.5 µL/mL) | in vitro Microdilution method |
| A strong activity with an MIC value ranging from 0.25 to 1 µL/mL | [51] |
| Inactive | |||
| Essential oil (100 mg/mL) (0.1%) (0.25 and 0.5%) | in vitro Agar diffusion |
| Inactive | [52,53] |
| in vitro On infected Hela cells | Chlamydia trachomatis | IC50 = 0.009% v/v | ||
| in vitro On Stocked boilers meat | Bacillus spp. | ↓ Total number of bacteria and also of the cold-resistant bacteria Total inhibition of bacterial growth | [54,55] | |
| in vitro Microdilution technique |
| An inhibitory potential with an MIC ranging from 3 to 20 µL/mL and an MBC value varies between (3 to 40 µL/mL). | [8] | |
| Carvacrol (3.12, 6.25, 12.5, 25, 50 and 100 µM) | in vitro On infected hela cells | Chlamydia trachomatis | IC50 = 6.25 µM | [48,56] |
| Thymol (3.12, 6.25, 12.5, 25, 50 and 100 µM) (0.1%) | in vitro On infected hela cells | Chlamydia trachomatis | IC50 = 6.25 µM | |
| in vitro | Fusobacterium nucleatum associated to Actinomyces naeslundii | Inhibition of biofilm formation at a concentration of 0.1% | ||
| Cymene (3.12, 6.25, 12.5, 25, 50 and 100 µM) | in vitro On infected hela cells | Chlamydia trachomatis | IC50 = 3.12 µM | |
| Antifungal activity | ||||
| NS Seeds | ||||
| MeOH extract | in vitro Agar diffusion method |
|
| [35] |
| EtOH extract | in vitro Microdilution method |
|
| [57] |
| n-butanol extract (1.25, 2 and 2.5 µL/mL) | in vitro Microdilution method |
| MIC value between 0.125 and 0.5 µL/mL | [51] |
| Sodium carboxymethylcellulose NS extract (5 mg/mL at a dose 6.6 mL/kg) | in vivo | Injection of Candida albicans to female rats | Significant decrease of colonies at a dose of 6.6 mL/kg | [58] |
| Hexane, Ethyl acetate, MeOH, Chloroform extracts (Obtained by methanolic extract fractionation) | in vitro Agar diffusion method |
|
| [35] |
| Nigellothionines (concentrations ranging from 0.5 to 64 mg/mL) | in vitro Agar diffusion method |
| The zone inhibition ranged from 11 to 12.7 mm, and the MIC was 0.77 µM | [56] |
| Aerial part | ||||
(concentrations ranging from 1.562 to 200 mg/mL) | Antifungal bioassay |
| The different fractions exhibited a biomass reduction at 50 mg/mL, except for the aqueous fraction that had a low activity | [59] |
| Extract/Compound | Method | Cell Lines | Results | Reference |
|---|---|---|---|---|
| Aqueous extract (250, 500, 1000 mg/mL) | In vitro | Brine shrimp assay on artemia salina | Cytotoxic effect with an IC50 value equal to 284.9 mg/mL | [43] |
| NS + honey (concentrations ranging between 10 and 70 μg/mL) | In vitro | Ovarian cancer cells PA-1 | Inhibition of the cell proliferation in a dose-dependent manner. | [34] |
| Aqueous extract prepared with mimicking the chewing process (1.25, 2.5 and 5%) | In vitro | Mouse squamous cell carcinoma cells SCC VII | 5% of the diluted extract induced inhibition of cancer cell growth. | [69] |
| α-hederin (5, 10, 20, 40 and 80 μg/mL) | In vitro | Mouse squamous cell carcinoma cells SCC VII | The α-hederin alone at a dose of 20 µg/mL induced an antiproliferative effect. | |
| Nigellothionines (concentrations ranging from 0.1–50 μM) | In vitro |
|
| [56] |
| NS melanin (concentrations ranging from 7.8–500 μg/mL) (concentrations ranging from 5–200 μg/mL) | In vitro | THP-1 cells a human monocytic cell line |
| [70,71] |
|
| |||
|
| |||
| NSO (500 mg/kg/day) | In vivo | Balb/c mice | ↓ Polychromatic erythrocyte (PCE) at 500 mg/kg of NSO, and NSO + Cisplatin which indicated a bone marrow recovery. | [72] |
| TQ (1, 10 and 25 µM) | In vitro |
|
| [73,74] |
| Renal human cancer cells CaKi-1 |
| |||
| Ferulic acid (250, 350 and 450 µM) | In vitro | Breast cancer cells MDA-MB 231 |
| |
| TQ + ferulic acid (“25 µM + 250 µM”, “50 µM + 350 µM”, “50 µM + 450 µM”, “100 µM + 350 µM” and “100 µM + 450 (“25 µM + 250 µM” respectively) | 25 µM + 250 µM antiproliferative effect | |||
| Sapindoside B (1, 5, 10, 20 and 50 µM) | In vitro | HCT116, AGS, and on HCC-LM3 | IC50 that was lower than 10 µM | [75] |
| A549, H1299, H460, HGC27, and HepG2 | IC50 ranging from 11.93 to 20.05 µM | |||
| Gastric cancer cells MGC830 | Inactive | |||
| NS virgin oil rich with volatile compounds (concentrations ranging from 0.46 to 3.09 mg/mL | In vitro |
| LC50 1.6 µg/mL and 1.3 µg/mL for MCF5 and A325. | [76] |
| Virgin oil without volatile compounds | No effect even at high doses | |||
| Combination of (NS, Hemidesmus indicus, and smilax glabra) ethyl acetate extract (25, 50, 100, 200 and 400 µg/mL) | In vitro | Lung cancer cells NCI-H292 |
| [77] |
| Hydroalcoholic extract (100, 200, 400, 600 and 800 µg/mL) | In vitro | MCF-5 | IC50 value noted was 3.29 mg/mL | [78] |
| Extract/Compound | Method | Test type | Results | Reference |
|---|---|---|---|---|
| NSO (1, 2 and 4 mL/kg BW for the acute phase, 4 mL/kg BW for the subacute phase) | In vivo | Inflammation caused by carrageenan on the rat’s path | Suppression of the edema | [39,80] |
| Acute treatment and subacute |
| |||
| NS supplementation (200 mg/kg BW) (2 g/day added to foods or drinks) | In vivo | Injection of 10% PHA phytohemagglutinin to rats |
| [81] |
| Clinical study on patients with β-thalassemia | (2 g/d) for 3 months induced:
| [82,83] | ||
| Hydroalcoholic extract | In vivo | Oreochromis niloticus |
| [49] |
| Ethanolic extract (1.2, 2.4 and 4.8 g/kg BW/day) | In vivo | On lupus mice |
| [81,84] |
| NS polysaccharides (NSSP) (0.1 mL/10 g) | In vivo | Injection cyclophosphamide CTX to mice | Protection of thymus and spleen against CTX-induced damage
| [85] |
| TQ | In vitro | Human monocytic leukemia cell line THP-1 |
| [48] |
| Extract/Compound | Method | Test | Results | Reference |
|---|---|---|---|---|
| NSO (2.5 mL/day) (0.4 mL/100 g BW) (4 mL/kg BW/day) (400 mg/kg BW/day) | In vivo | Clinical on patients suffering from hypertension (8 weeks treatment) | ↓ Systolic and Diastolic blood pressure | [86] |
| In vivo | Pre-treatment of rats during 14 days by a dose of 4 mL/kg/day pursued with isoproterenol injection |
| [30,87,88] | |
| In vivo | Cotreatment of rats using Azithromycin® and NSO |
| ||
| In vivo | Female rats with streptozotocin-induced diabetes |
| ||
| NSsupplementation (2000 mg/day) | In vivo | Clinical study on healthy obese and overweight subjects (2 g/day) |
| [89] |
| NS + amlodipine | In vivo | Hypertensive rats |
| [90] |
| EtOH extract (400 mg/kg) | Ex vivo | Aortic ring |
| [91] |
| TQ (50 mg/kg BW/day) (10 mg/kg BW/day) | In vivo | Mice | ↓ Lipid deposition at the cardiac tissue | [92,93] |
| In vivo | Rats |
|
| Extract/Fraction/Compound | Method | Test Type | Results | Reference |
|---|---|---|---|---|
| NS Seeds | ||||
| NS supplementation (5 g/day) (1 g/day for 4 weeks) (1.5 mg/kg BW) | In vivo | Glycated hemoglobin (5 g/day of black cumin for 6 months) | ↓ Glycated hemoglobin | [98] |
|
| [105,110] | ||
| Hydroacetone extract (concentrations ranging between 156.25 and 2000 µg/mL) | In vitro | α-amylase | IC50 = 314.4 µg/mL | [97] |
| n-hexane fraction (0.45, 0.9 and 1.82 mg/mL) | In vitro | α -amylase | IC50 = 0.760 mg/mL | [19] |
| EtOH fraction (0.45, 0.9 and 1.82 mg/mL) | IC50 = 0.255 mg/mL | |||
| MeOH fraction (0.45, 0.9 and 1.82 mg/mL) | IC50 = 0.103 mg/mL | |||
| Aqueous fraction (0.45, 0.9 and 1.82 mg/mL) | IC50 = 0.310 mg/mL | |||
| Dichloromethane fraction (0.45, 0.9 and 1.82 mg/mL) | IC50 = 1.330 mg/mL | |||
| Hydroalcoholic combination (70%) of Morinda citrifolia, Trigonella foenum-graecum, and NS (15.625, 31.25, 62.5, 125 and 250 μg/mL) (3 doses: 70/70/280, 140/70/140 and 70/140/140 mg/kg BW) | In vitro | α -amylase | 140/70/140 mg/kg was the most effective among the all doses with an IC50 = 35.7 µg/mL | [101] |
| In vivo (rats) | ↓ blood glucose level in rats | |||
| n-hexane extract (400 mg/kg BW/day) | In vitro |
|
| [36,107] |
| Ethanolic extract (300 mg/kg BW/day) (1.5, 3.5 and 10 mg/mL) | In vivo | Rats with streptozotocin-induced diabetes | 300 mg/kg of the NS induced:
| [103,106] |
| In vitro | Glycation of bovine serum albumin | Antiglycation effect at different concentrations | ||
| MeOH extract (Glucose tolerance/Residual gut sucrose content/Intestinal glucose absorption/Gut motility/Intestinal disaccharidase enzyme activity: 500 mg/kg BW) (Insulin secretion from isolated islets: 25, 50, 100 and 200 μg/mL) | In vivo | Long–Evans rats |
| [104] |
| Aqueous extract (1.5, 3.5 and 10 mg/mL) | In vitro | Glycation of bovine serum albumin | Antiglycation effect at different concentrations | [106] |
| Trigonella foenum-graecum, NS, Zingiber officinale, Olea europea, Fraxinus ssp (water 80%–MeOH 20%) (concentrations of extracts graded from 10–100 mg/kg BW) | In vivo | Rats |
| [109] |
| NS + fenugreek (4.7 and 0.75 g powdered NS and fenugreek respectively seed/day) | In vivo | Clinical study on overweight, type 2 diabetes, and overweight + type 2 diabetes |
| [102] |
| TQ (50 or 100 mg) with metformin (1000 mg) | In vivo | Clinical study on volunteers |
| [100] |
| Aerial part | ||||
| Hederagenin, |
In vitro (12.5, 25, 50 and 100 µM) |
|
| [20] |
| Flaccidoside III, | ||||
| Quercetin-3-gentiobiosides, | ||||
| Magnoflorin, | ||||
| Nigelflavonoside B, | ||||
| Nigelloside, | ||||
| Quercetin sphorotrioside, | ||||
| Kaempferol-3, 7-diglucoside, | ||||
| Kaempferol 3-O- rutinoside | ||||
| Rutin | ||||
| 3-O-[α-L-Rhamnopyranosyl-(1-2)-α-l-arabinopyranpsyl] hederagenin |
| IC50 of 91.3 ± 2.5µM | ||
| Extract/Compound | Method | Test Type | Results | Reference |
|---|---|---|---|---|
| NS Seeds | ||||
| NSO (0.4 mL/100 g BW) | In vivo | Rats |
| [30] |
| NS supplementation (2 g/day) | In vivo | Obese and overweight healthy women |
| [89] |
| NS+ fenugreek (4.7:0.75 g/day respectively) | In vivo | overweight, type 2 diabetes, and overweight + type 2 diabetes volunteers |
| [102] |
| TQ (50 mg/kg BW/day | In vivo | Mice |
| [92] |
| Hydroalcoholic extract (100, 200, and 400 mg/kg) | In vivo | Rats | Six weeks treatment
| [111] |
| NS + fenugreek (2 g:10 g/d) | In vivo | Volunteer patients with type 2 diabetes |
| [112] |
| Artemisia sieberi, NS, and Teucrium polium (150 mg/kg BW) | In vivo | Rats |
| [108] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dalli, M.; Bekkouch, O.; Azizi, S.-e.; Azghar, A.; Gseyra, N.; Kim, B. Nigella sativa L. Phytochemistry and Pharmacological Activities: A Review (2019–2021). Biomolecules 2022, 12, 20. https://doi.org/10.3390/biom12010020
Dalli M, Bekkouch O, Azizi S-e, Azghar A, Gseyra N, Kim B. Nigella sativa L. Phytochemistry and Pharmacological Activities: A Review (2019–2021). Biomolecules. 2022; 12(1):20. https://doi.org/10.3390/biom12010020
Chicago/Turabian StyleDalli, Mohammed, Oussama Bekkouch, Salah-eddine Azizi, Ali Azghar, Nadia Gseyra, and Bonglee Kim. 2022. "Nigella sativa L. Phytochemistry and Pharmacological Activities: A Review (2019–2021)" Biomolecules 12, no. 1: 20. https://doi.org/10.3390/biom12010020
APA StyleDalli, M., Bekkouch, O., Azizi, S.-e., Azghar, A., Gseyra, N., & Kim, B. (2022). Nigella sativa L. Phytochemistry and Pharmacological Activities: A Review (2019–2021). Biomolecules, 12(1), 20. https://doi.org/10.3390/biom12010020