Abstract
The reduction-oxidation (redox) system consists of the coupling and coordination of various electron gradients that are generated thanks to serial reduction-oxidation enzymatic reactions. These reactions happen in every cell and produce radical oxidants that can be mainly classified into reactive oxygen species (ROS) and reactive nitrogen species (RNS). ROS and RNS modulate cell-signaling pathways and cellular processes fundamental to normal cell function. However, overproduction of oxidative species can lead to oxidative stress (OS) that is pathological. Oxidative stress is a main contributor to diabetic kidney disease (DKD) onset. In the kidney, the proximal tubular cells require a high energy supply to reabsorb proteins, metabolites, ions, and water. In a diabetic milieu, glucose-induced toxicity promotes oxidative stress and mitochondrial dysfunction, impairing tubular function. Increased glucose level in urine and ROS enhance the activity of sodium/glucose co-transporter type 2 (SGLT2), which in turn exacerbates OS. SGLT2 inhibitors have demonstrated clear cardiovascular benefits in DKD which may be in part ascribed to the generation of a beneficial equilibrium between oxidant and antioxidant mechanisms.
1. Introduction: The Redox System
In general terms, the reduction-oxidation (redox) system consists of the coupling and coordination of various electron gradients that are generated by serial reduction-oxidation enzymatic reactions. These reduction-oxidation reactions happen in fundamental biological processes in every cell [1] and lead to the production of radical oxidants or free radicals [2]. Free radicals are in fact oxygen or nitrogen metabolites that contain an unpaired electron and are thus partially reduced [3]. These free radicals have strong oxidizing capacity and can be classified into reactive oxygen species (ROS) and reactive nitrogen species (RNS) [4] (Table 1).

Table 1.
Main known reactive oxygen species (ROS) and reactive nitrogen species (RNS).
The redox system is especially important during energy metabolism to generate adenosine triphosphate (ATP). The most important source of energy is glucose which is transformed into pyruvate via glycolysis. Pyruvate enters the tricarboxylic acid or Krebs cycle and reduces nicotinamide adenine dinucleotide (NAD) to NADH. Further, fatty acids (FA) can undergo β-oxidation to generate acetyl-CoA which then enters the Krebs cycle [5,6]. The NADH produced during the Krebs cycle is oxidized again in the mitochondrial respiratory chain [7]. The complexes that constitute the mitochondrial respiratory chain are NADH dehydrogenase (complex I), succinate dehydrogenase (complex II), cytochrome c reductase (complex III), cytochrome c oxidase (complex IV), and ATP synthase (complex V). These enzymes are able to oxidize NADH, starting the electron transport across the mitochondrial membrane and generating an electrochemical potential that allows complex V to generate ATP. Additionally, the electrons transported from complex I, through II and III, to complex IV are used to reduce oxygen to water [8,9]. ROS are generated as a byproduct of electron transfer. Therefore, mitochondria are considered the main source of ROS [10]. Approximately, 1–2% of the O2 consumed in the mitochondria is incompletely metabolized [11]. Here, the predominant origin of ROS is the respiratory chain (mainly complexes I and III), necessary for ATP synthesis. However, other mitochondrial enzymes have been reported to be sources of ROS, such as pyruvate dehydrogenase, α-ketoglutarate dehydrogenase, or succinate dehydrogenase, which are a part of the Krebs cycle [12]. Additionally, p66shc, an adaptor protein with proapoptotic activity, is involved in the production of ROS [13]. In vitro and in vivo experiments have shown that this protein can generate ROS by oxidizing cytochrome C [14,15]. The NADPH oxidase (NOX) family of enzymes are another source of ROS. NOX are multiunit enzymes that use NADPH as an electron donor to reduce oxygen, leading to the generation of superoxide. There are seven members of the NOX family, NOX1–5 and DUOX1 and 2. NOX enzymes are expressed in different tissues and differ in the domains that constitute them, for example, DUOX1 and 2 have an additional peroxidase domain at their N-terminal end [16,17] (Figure 1).
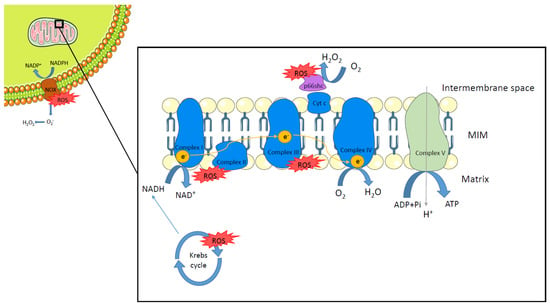
Figure 1.
Sources of reactive oxygen species. Reactive oxygen species (ROS) are generated at different sites within the cell. Respiratory chain complexes I and III (in blue) produce ROS as a byproduct of electron transfer in mitochondria. Several enzymes involved in the Krebs cycle are also reported as sources of ROS. p66shc (in purple), by oxidizing cytochrome c (Cyt C), is able to generate ROS within the mitochondria intermembrane space. The NADPH oxidase (NOX) family of enzymes (in brown) are another important source of ROS by reducing oxygen to generate superoxide. MIM: mitochondria inner membrane.
Regarding RNS, these comprise nitric oxide (NO) and its derivatives, such as peroxynitrite (ONOO−), dinitrogen trioxide (N2O3), dinitrogen tetraoxide (N2O4), nitrogen dioxide (NO2), or S-nitrosothiols (RSNO) [18,19] (Table 1). NO is produced from the metabolism of L-arginine [20]. The enzyme responsible for its synthesis is nitric oxide synthase (NOS) that converts L-arginine into L-citrulline, forming NO during the reaction. There are three forms of NOS: endothelial NOS (eNOS), neuronal NOS (nNOS) and inducible NOS (iNOS). eNOS and nNOS are constitutively expressed while iNOS is only expressed under specific stimuli like infection or trauma. The different NOS isoforms are expressed in different types of cells in which NO is involved in signaling and regulation of several cellular functions [21]. In mitochondria, NO reacts with complex III of the respiratory chain, inhibiting the electron transfer and enhancing the production of O2−. O2− is highly reactive and may combine with NO, leading to the formation of peroxynitrite (ONOO−). ONOO− is a powerful RNS that can irreversibly inhibit electron transport, which is pathological [18,22,23,24].
Both ROS and RNS are necessary for normal cell function as they modulate cell-signaling pathways and cellular processes. However, overproduction of any of them is pathological since it can damage macromolecules like proteins, lipids, or DNA [25]. DNA damage can lead to defective complexes I and III, which may result in a shutdown of mitochondrial energy production [26] and further increase ROS levels [27,28]. This eventually ends up causing mitochondrial damage and apoptosis [29,30]. Therefore, to regulate the generated ROS, there is a network of antioxidant systems to alleviate this stress. Superoxide dismutase (SOD) is an enzyme that contributes to the elimination of some oxidants. According to its subcellular location, we can differentiate between SOD1 (in the cytosol and mitochondrial intermembrane space), SOD2 (in the mitochondrial matrix), and SOD3 (in the extracellular matrix) [31]. SOD enzymes convert the superoxide radical into hydrogen peroxide [32,33] that is finally detoxified by catalase or glutathione peroxidase [34,35]. Catalases decompose H2O2 into water and oxygen, and glutathione peroxidases reduce H2O2 to water [36]. Glutathione has also a role in the antioxidant system, as it can either react directly with ROS and RNS or act as a cofactor for various enzymes helping the cell to maintain its redox status [37]. Further, exogenous and synthetic antioxidants can also be effective to prevent oxidative stress [38,39].
In this review, we will focus on oxidative stress-mediated mechanisms of kidney tubular dysfunction. Further, the contribution of the sodium/glucose co-transporter type 2 (SGLT2) in diabetic kidney disease (DKD) onset and the antioxidant potential of the SGLT2 inhibitors will be discussed.
2. Oxidative Stress-Induced Tubular Impairment
The tubular system has a very precise and highly regulated transport capacity between the tubule lumen and the bloodstream. A broad range of tubular transporters contribute to ion, amino acid, glucose, and other solute reabsorption. Therefore, tubular cells require a sufficient energy supply to sustain the high rate of exchange within the two compartments. Tubular cells are the most energy-demanding cells of the body after cardiac cells [40]. Their metabolism is highly dependent on oxygen consumption to produce ATP by oxidative phosphorylation. The oxidation of a glucose molecule provides a high energy yield (30–32 ATP) and is used as a substrate in every cell. However, in the kidney, each section of the tubule has its own preferences in terms of energy substrates, including amino acids, ketone bodies, or FA. Strikingly, FAs are an important energy fuel in tubular cells, providing 106–129 ATP through β-oxidation within the mitochondria [41]. Proximal tubular cells (PTC), located at the first section of the tubular system (segments S1, S2, and S3), are particularly energy-consuming, as 80% of the biomolecules filtered by the glomerulus are reabsorbed by these cells [42,43]. In PTC, ATP generation relies mostly on oxidative phosphorylation of FA rather than of glucose. However, under pathological conditions, such as acute kidney injury (AKI), which is associated with hypoxia [44], PTCs undergo a metabolic shift towards glycolysis [45].
As mentioned above, mitochondrial metabolism is a source of ROS that directly modulates cell-signaling pathways, protein function through post-translational modifications, and influences cell survival, proliferation, and apoptosis [46]. However, increased ROS production results in oxidative stress that causes damage to lipids, proteins, and nucleic acids, eventually disrupting cellular homeostasis. Overall, elevated ROS levels can lead to mitochondrial dysfunction, bioenergetics defects, altered gene expression, and cell death. PTCs have a high mitochondrial density that makes them susceptible to ROS-induced cell damage [42]. In vitro experiments performed using the HK-2 cell line (human PTCs) demonstrated an increase of oxidative stress, mitochondrial destabilization, DNA damage, apoptosis, and cell senescence when treated with hydrogen peroxide (H2O2) [47]. Aragno et al. showed that rats subjected to unilateral ischemia/reperfusion (I/R) injury exhibit high hydrogen peroxide levels in the cytosol obtained from kidney homogenate and high levels of nitrite/nitrate in serum compared to control and sham-operated animals. Consequently, the proximal tubules showed dilation and cell debris and cast formation in the lumen. These effects were attenuated in rats supplemented with dehydroepiandrosterone (DHEA), a steroid with antioxidant properties [48]. Beyond the respiratory chain, there are other sources of oxidants in the kidney. NOX enzymes have been recognized as a primary source of ROS in the kidney, especially NOX4. NOX4-derived H2O2 mediates several cell functions, but excessive levels can induce inflammation, apoptosis, fibrosis, and cell damage. NOX4 has constitutive activity, hence, the amount of H2O2 depends on the expression level. As a result, it can be damaging or beneficial depending on its abundance at the given time [49]. Normally, the levels of ROS increase in some AKI and chronic kidney disease (CKD) models, secondary to the overexpression of NOX4 [50]. Upregulation of renal NOX4 plays an important role in several pathologies, like DKD, hypertensive nephropathy, and polycystic kidney disease by increasing ROS levels and mitochondrial damage [51,52,53,54,55]. It has been described that mitochondrial ROS and NOX-produced ROS can enhance damage and depolarization of the mitochondrial membrane potential [46,56,57]. The total absence of NOX4 has also been reported to be pathological since it results in fibrosis and oxidative stress [58]. This could be in part related to the fact that H2O2 generated by NOX4 enhances nuclear factor erythroid 2-related factor 2 (Nrf2) stability, a regulator of antioxidant activity [59].
Excessive ROS production can damage mitochondria [60]. Mitochondrial damage has been recognized as a main contributor to tubular necrosis and apoptosis. Tubular cell necrosis involves disruption of respiration complexes, loss of mitochondrial membrane potential, and mitochondrial membrane transition, while apoptosis is caused by mitochondrial outer membrane permeabilization and release of apoptogenic factors [61]. Mitochondrial damage secondary to necrosis or apoptosis is especially problematic for the tubular cells as it may compromise its normal function and ultimately lead to renal dysfunction. Despite the effects of oxidative stress in kidney impairment having been studied extensively [42,46], its complexity and interconnection with a broad range of metabolic and cellular pathways make it puzzling to decipher its full implications in the onset, progression, and development of renal pathology.
3. Oxidative Stress in Diabetic Kidney Disease: Contribution of Sodium Glucose Co-Transporter Type 2 (SGLT2)
Albeit glucose consumption is low in the proximal tubule (except for segment S3 [62]), PTCs are primarily responsible for its reabsorption via the sodium/glucose co-transporter type 2 (SGLT2) [44]. SGLT2 is located at the brush border of PTCs in the S1 and S2 segments of the proximal tubule, where 90% of the total filtered glucose is reabsorbed [63]. Its transport is coupled with sodium (Na+/Glucose, 1:1) and it accounts for 5.7% of total renal Na+ reabsorption in healthy individuals [64]. Although SGLT2 activity has a role in maintaining the intrarenal osmolarity, the main sodium exchanger at the tubular level is the Na+/H+ Exchanger-3 (NHE3). Interestingly, NHE3 and SGLT2 have been shown to be interdependent [65]. Therefore, SGLT2 activity has a huge impact on both glucose and sodium balance. The Na+ gradient created by the sodium-potassium pump (Na+/K+-ATPase), located in the basolateral membrane of PTC, is harnessed by SGLT2 to transport glucose and sodium across the plasma membrane down-gradient [63]. At the same time, the basolateral glucose transporter, GLUT2, maintains the glucose gradient by passively easing its backflow into the bloodstream [66].
SGLT2 plays a crucial role in glucose transport and intrarenal osmolarity. For that reason, the dysfunction of this co-transporter, which happens in diabetes, has a direct effect on intrarenal glucose metabolism and also on the redox environment. In diabetic patients, SGLT2 is overactivated, leading to increased glucose and sodium reabsorption [67]. The deleterious effects in kidney function and fluid homeostasis include the alteration of tubular-glomerular feedback (a self-regulated system of the kidney) and the increase of the glomerular filtration rate and the tubular reabsorption capacity. Systematically, the plasma volume and blood pressure increase, leading to hypertension, a common feature of these patients [68]. At the molecular level, this has drastic consequences for the activity of other transporters located in the tubule, such as NHE3, which is sensitive to small changes in sodium concentration. Packer et al. reviewed that Na+/H+ Exchanger-1 (NHE1) in the heart and vasculature and NHE3—the kidney isoform—are upregulated in heart failure and type 2 diabetes mellitus (T2DM) [69]. Additionally, when sodium levels drop in the luminal filtrate, Na+/K+-ATPase activity increases to restore the sodium gradient. As this transporter is ATP-dependent, its overactivation implies an increase in energy demand and oxygen consumption. It is worth mentioning that under normal conditions, 60% of kidney ATP consumption is intended for sodium uptake which is mostly attributed to basal Na+/K+-ATPase activity [70]. Therefore, the energy consumption of this transporter cannot be underestimated, and its impact on mitochondrial function should be further explored. The enhanced mitochondrial phosphorylation produces substantial accumulation of ROS products. In addition, it has been described that interleukin 6 (IL-6) activates SGLT2 through ROS in cultured tubular cells [71], which suggests that an inflammatory context would further promote ROS generation and SGLT2 overactivation. Thus, SGLT2 activity, oxidative stress, and inflammation actively contribute to the onset of DKD. By definition, DKD is a microvascular complication of diabetes mellitus that affects the kidney. These patients are diagnosed by the presence of persistent albuminuria, starting at first stage by hyperfiltration and ending with a progressive decrease in GFR at later stages. The Renal Pathology Society divide DKD pathology into glomerular, tubulointerstitial, and vascular lesions [72]. Focusing on the tubule, tubular membrane thickening, interstitial fibrosis, and tubular atrophy are usually observed. Coughlan et al. mapped the changes in mitochondrial adaptation in experimental rat’s kidney mitochondria at 4, 8, 16, and 32 weeks after induction of diabetes by streptozotocin intravenous injection [73]. The authors demonstrated that DKD begins with decreased ATP production in the renal cortex, mitochondrial fragmentation, which is accompanied by increased ROS, and early renal hyperfiltration. Their results suggest that mitochondrial dynamics and bioenergetic function worsen over time, preceding the development of albuminuria and histological lesions. An in vitro experiment with HK-2 cells found out that under high glucose conditions, HK-2 cells were susceptible to mitochondrial fragmentation. These effects were ameliorated with a SGLT2 inhibitor (empagliflozin), by regulating proteins of mitochondrial fission and fusion [74]. Mitochondrial fusion and fission are essential for mitochondrial maintenance but also for mitochondrial DNA (mtDNA) integrity, which can participate in the regulation of cell survival, metabolic processes, and redox-sensitive signals [75]. In addition, high glucose conditions increase superoxide formation in mitochondria, which combined with NO (released by endothelial cells under stress) generates peroxynitrite [76]. Peroxynitrite is a cytotoxic oxidant that induces eNOS uncoupling, restricting vascular relaxation, and promoting diabetic vascular complications. As mentioned before, NOX enzymes have been shown to mediate ROS production. NOX-derived ROS regulate many physiological mechanisms of the kidney, including tubular-glomerular feedback, glucose metabolism and transport, kidney hemodynamics, and electrolyte transport [77]. NOX4 is the major source of ROS production in podocytes, and it is upregulated under high-glucose conditions [78]. For this reason, NOX4 has been largely linked to DKD [53,79]. Induction of NOX4 in the Akita model of DKD results in the emergence of the typical structural changes seen in the diabetic kidney: glomerular hyperfiltration, mesangial matrix accumulation, glomerular membrane thickening, albuminuria, and podocyte loss [53]. In addition, metabolomic analysis performed on these mice revealed alterations in tubular energy metabolism with the accumulation Krebs cycle-related urinary metabolites [53]. The Krebs cycle is the central axis of energy production in mitochondria, thus, disproportion of the cycle substrates leads to mitochondrial dysfunction in advanced DKD [80]. Accordingly, a cross-sectional study in patients with and without DKD found that injured tubular cells showed several signs of mitochondrial damage, including (1) changes in mitochondrial dynamics, (2) decreased mtDNA copy number, and (3) increased release of mtDNA into the extracellular space [81]. Paradoxically, Cao et al. reported that plasma mtDNA content was decreased in patients with T2DM with clinically significant proteinuria (24 h urinary protein level >0.5 g) but not mild proteinuria (24 h urinary protein level ≤0.5 g), however, mtDNA abundance was increased in the urine of the former [82]. They propose that hyperglycemia reduces intracellular mtDNA and facilitates its extracellular release, so that circulating mtDNA may be filtered by the kidneys and end up in the urine [82]. In concordance, Kafaji et al. reported that a decreased mtDNA copy number in blood was associated with the severity and the presence of DKD [83]. Their group found out that mtDNA was lower in diabetic patients with macroalbuminuria than in patients with microalbuminuria or normalbuminuria. Therefore, altered mtDNA content can predict the occurrence of DKD and the oxidative stress environment [84]. Interestingly, release of mtDNA under cellular stress has been previously considered as a damage-associated molecular pattern (DAMP) [85]. mtDNA can be recognized by Toll-like receptor 9 (TLR9) and cystosolic Cgas-stimulator of interferon genes (STING) and activate the inflammasome, leading to kidney inflammation and fibrosis [86]. In this line, recent evidence showed an increase in macrophage infiltration in a mouse model of type 2 diabetes [87] and in human progressive DKD [88], which correlates with the disease state and renal injury. Other than the direct effect of mtDNA as DAMP, oxidative stress can induce inflammation in the kidney by stimulating cytokine production [89]. ROS derivates, which participate in cellular signaling, can activate the transcription factors nuclear factor kappa B (NFκB) and activator protein-1 (AP-1), promoting the transcription of cytokines, growth factors, and extracellular membrane proteins [89] (Figure 2).
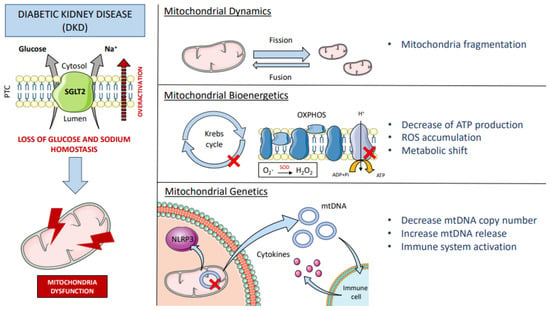
Figure 2.
Mitochondrial dysfunction in DKD. The sodium/glucose co-transporter type 2 (SGLT2) is located in the apical membrane of the proximal tubular cells (PTC) of the kidney and is responsible for glucose and sodium uptake. Diabetic patients have overactivation of SGLT2 that alters glucose and sodium homeostasis, which directly affects mitochondrial function at different levels; imbalance of fission and fusion together with mitochondrial fragmentation (mitochondrial dynamics). Alteration and accumulation of Krebs cycle substrates, uncoupling of the oxidative phosphorylation chain (OXPHOS) with generation of ROS (e.g., formation of H2O2 by the enzyme superoxide dismutase (SOD)), leading to ATP depletion, oxidative stress, and a metabolic shift to oxygen-independent energy sources (mitochondrial bioenergetics). Mitochondrial DNA (mtDNA) is also damaged, leading to a reduction in mtDNA copy number and increased release of mtDNA into the cytosol, which activates the NLR family pyrin domain-containing 3 (NLRP3) inflammasome, and into the extracellular space, triggering the recruitment of immune cells and the onset of the inflammatory response (mitochondrial genetics). DKD: diabetic kidney disease; ROS: reactive oxygen species; ADP: adenosine diphosphate; ATP: adenosine triphosphate; NLR: NOD-like receptor.
Adding the immune response to the theme above reaffirms that mitochondrial dysfunction and redox imbalance lead to the emergence of the main precipitating factors: altered metabolism, oxidative stress, and inflammation. These three elements combine and reciprocally feed back into each other. As in the chicken-and-egg dilemma, determining which of these events triggers kidney disease in the first place entails a great difficulty. However, it is clear that pharmacological approaches focused on blocking/stimulating the involved pathways mentioned in this section and targeting the SGLT2 transporter are of particular interest.
4. Antioxidant Properties of SGLT2 Inhibitors
SGLT2 inhibitors (SGLT2is) are hypoglycemic drugs that target SGLT2 producing glycosuria. Consequently, SGLT2is decrease blood glucose in an insulin-independent manner, improving insulin resistance in diabetes [90,91]. To date, different SGLT2is, such as canagliflozin, dapagliflozin, ertugluflozin, and empagliflozin, have been approved to treat T2DM and DKD. Large clinical trials have demonstrated that these drugs (mainly canagliflozin, dapagliflozin, and empagliflozin) delay the progression of DKD on top of the standard of care with renin-angiotensin system (RAS) blockers [92,93,94,95]. The protective effect of the SGLT2is has been widely attributed to normalization of glycemia, natriuresis, body weight reduction, and decrease of intraglomerular pressure [63,96,97]. However, the use of SGLT2is has been associated with reduction of oxidative stress and inflammation as well (Table 2). In type 2 diabetic patients, twelve-week treatment with dapagliflozin significantly reduced the urinary excretion of 8-oxo-7,8-dihydro-2′-deoxyguanosine (8-oxo-dG), a marker of DNA oxidation [98]. In addition, empagliflozin treatment increased 2,2¢-azino-bis-(3-ethylbenzthiazoline-6-sulphonic acid) (ABTS) radical scavenging capacity (a measure of antioxidant capacity) in diabetic patients, suggesting a beneficial equilibrium between oxidant and antioxidant mechanisms despite empagliflozin also increasing serum levels of the thiobarbituric acid reactive substances (TBARS) and malondialdehyde (MDA). The authors suggest that the increased levels of TBARS and MDA (both indicators of lipid peroxidation) can be explained by the ketogenesis induced by SGLT2is that may contribute to lipid peroxidation [99]. Contrarily, Nabrdalik-Leśniak et al. have described that therapy of at least one month with either canagliflozin or empagliflozin decreased total antioxidant capacity (estimated by ABTS urine levels) in diabetic patients as compared to that of diabetic patients not treated with SGLT2is and healthy controls. However, the urinary activity of the antioxidant enzymes SOD and MnSOD was increased in diabetic patients treated with SGLT2is, suggesting that these drugs can also promote the activation of antioxidant mechanisms [100]. In this line, Iannantuoni et al. have described that 24-week long treatment with empagliflozin reduces superoxide production, increases glutathione content (an antioxidant), and enhances the expression of glutathione s-reductase and catalase in leukocytes of diabetic patients. In this study, the authors found that empagliflozin increased the serum levels of the anti-inflammatory interleukin 10 (IL-10) and decreased C reactive protein and myeloperoxidase serum levels suggesting, an antioxidant and anti-inflammatory role of SGLT2is [101]. Similarly, CD34+ endothelial progenitors (markers of endothelial function) isolated from peripheral blood of diabetic patients treated with canagliflozin showed increased SOD2, catalase, and glutathione peroxidase gene expression, which suggests that SGLT2is induce a beneficial antioxidant profile on these cells [102].

Table 2.
Summary of the antioxidant effects of SGLT2is observed in clinical and preclinical studies.
In diabetic experimental models, similar results have been found (Table 2). The administration of SGLT2is in rat and mice models decreases glycemia [106], and in experimental models of diabetic nephropathy, these drugs have demonstrated cardiorenal protection [103,107,108,109,110]. In a high-fat diet (HFD)-induced obesity mouse model, 8 weeks of empagliflozin administration improved cardiac dysfunction via Sestrin2-mediated AMPK-mammalian target of rapamycin (mTOR) pathways by maintaining the redox stability [104]. Similarly, canagliflozin administration for 12 weeks improved atherosclerosis lesions and endothelial dysfunction in streptozotocin-induced diabetic apolipoprotein E-deficient (ApoE−/−) mice. These beneficial effects could be in part ascribed to the reduction of oxidative stress, as canagliflozin decreased the expression of NOX2 and p22phox (both NADPH oxidase subunits) and the urinary excretion of 8-OHdG [105]. Regarding the kidney, Kamezaki et al. have demonstrated that ipragliflozin reduced renal NOX4 expression and oxidative stress in both the tubular epithelium and glomerular podocytes using a model of early diabetic nephropathy (db/db mice) [103]. In obese prediabetic rats, dapagliflozin suppressed renal gluconeogenesis and oxidative stress [111]. Thus, in a diabetic milieu, SGLT2is seem to promote antioxidant effects that could participate in the cardiorenal protective mechanisms of these drugs. Interestingly, blockade of SGLT2 (with SGLT2is or by silencing with siRNAs) ameliorates oxidative stress in endothelial [112,113], tubular [114,115,116], and mesangial cells [117] in culture. This suggests that these drugs have direct antioxidant effects on kidney cells and possibly on other organs (even off-target [113]).
The beneficial antioxidant effects of SGLT2is observed in DKD can in part be attributed to improvement of glycemia and blood pressure control, as other hypoglycemic drugs also reduce oxidative stress [98]. However, other protective mechanisms cannot be ruled out. Recent data obtained from EMPEROR-Reduced and DAPA-CKD studies have confirmed the efficacy of SGLT2is in patients with heart failure or CKD without a history of diabetes [118]. In the EMPEROR-Reduced double-blind trial, patients receiving empagliflozin had a lower risk of cardiovascular death or hospitalization and lesser GFR decline over time, regardless of the presence or absence of diabetes [119]. In addition, among CKD patients participating in the DAPA-CKD clinical trial, those treated with dapagliflozin had better stabilization of GFR and a lower risk of progression to end-stage renal disease or death, again, independent of the presence of diabetes [120]. Although the renal antioxidant effect of the SGLT2is in nondiabetic human CKD needs to be assessed in future research, studies in nondiabetic experimental models of kidney damage have already shown that these drugs protect the kidney by decreasing oxidative stress [121,122]. Furthermore, SGLT2is also have antioxidant properties in organs other than the kidney. Olgar et al. demonstrated that the increase of SGLT2 activity in cardiomyocytes of old Wistar male rats (24-month age) triggers ROS accumulation, alters mitochondrial dynamics, and promotes the loss of mitochondrial membrane potential. Interestingly, these cardiac events were mitigated by dapagliflozin treatment [123].
It has also been demonstrated that targeting SGLT2 with canagliflozin reduces cancer cell proliferation by inhibiting mitochondrial complex I-mediated respiration [124]. In the same line, dapagliflozin and canagliflozin produced cell growth arrest in breast cancer cells both in vitro and in vivo via the AMPK-mTOR pathway. The activation of the AMPK-mTOR pathway basically inhibits energy-consuming processes such as the mitochondrial phosphorylation and increases catabolic processes to restore cellular energy homeostasis [125]. Thus, as SGLT2is have antioxidant effects in nondiabetic CKD and other pathologies not related to the kidney, it is possible that the antioxidant properties of SGLT2is observed in DKD patients happen due to a combination of several factors: (1) glycemia and blood pressure control, (2) reduction of glucose concentration in the target cells, and (3) activation of pathways that improve glucose utilization. Further research is needed to ascertain the exact mechanism by which SGLT2is decrease ROS, but it is a fact that these drugs promote the restoration of the oxidant/antioxidant balance in the diabetic kidney as well as in other scenarios.
5. Conclusions
In conclusion, oxidative stress has an important role in DKD onset. High levels of glucose together with increased ROS production overactivate the SGLT2 transporter in tubular cells, which, in turn, exacerbates oxidative stress. The use of SGLT2is has demonstrated clear cardiovascular benefits which may be in part ascribed by a beneficial balance between oxidant and antioxidant pathways.
Author Contributions
Writing—original draft preparation, C.L.-C., M.M.-V.d.B. and A.V.; writing—review and editing, C.J.-C. and M.J.S.; figures, C.L.-C. and M.M.-V.d.B. All authors have read and agreed to the published version of the manuscript.
Funding
The authors are current recipients of grants from FONDO DE INVESTIGACIÓN SANITARIA-FEDER, ISCIII (PI17/00257 and RICORS RD21/0005/0016), and Fundació la Marató de TV3 (421/C/2020, 759/U/2020 and 215/C/2021).
Conflicts of Interest
A.V. reports personal fees from MUNDIPHARMA, and nonfinancial support from MUNDIPHARMA, SANOFI, and NOVONORDISK outside this. C.J.C. declares travel support and a research grant from TRAVERE THERAPEUTICS outside this work. M.J.S. reports grants from BOEHRINGER, personal fees from NOVONORDISK, JANSSEN, BOHERINGER ASTRAZENECA, FRESENIUS, MUNDIPHARMA, PFIZER, ICU, GE Healthcare BAYER, TRAVERE THERAPEUTICS, and VIFOR, and nonfinancial support from ELI LILLY and ESTEVE, outside this work.
References
- Sies, H. Oxidative stress: A concept in redox biology and medicine. Redox Biol. 2015, 4, 180–183. [Google Scholar] [CrossRef]
- Davies, M.J. The oxidative environment and protein damage. Biochim. Biophys. Acta -Proteins Proteomics 2005, 1703, 93–109. [Google Scholar] [CrossRef]
- Lander, H.M. An essential role for free radicals and derived species in signal transduction. FASEB J. 1997, 11, 118–124. [Google Scholar] [CrossRef]
- Mittal, M.; Siddiqui, M.R.; Tran, K.; Reddy, S.P.; Malik, A.B. Reactive oxygen species in inflammation and tissue injury. Antioxid. Redox Signal. 2014, 20, 1126–1167. [Google Scholar] [CrossRef] [PubMed]
- Houten, S.M.; Wanders, R.J.A. A general introduction to the biochemistry of mitochondrial fatty acid β-oxidation. J. Inherit. Metab. Dis. 2010, 33, 469–477. [Google Scholar] [CrossRef] [PubMed]
- Stahl, A. A current review of fatty acid transport proteins (SLC27). Pflug. Arch. Eur. J. Physiol. 2004, 447, 722–727. [Google Scholar] [CrossRef] [PubMed]
- Romano, A.H.; Conway, T. Evolution of carbohydrate metabolic pathways. Res. Microbiol. 1996, 147, 448–455. [Google Scholar] [CrossRef]
- Napolitano, G.; Fasciolo, G.; Venditti, P. Mitochondrial Management of Reactive Oxygen Species. Antioxidants 2021, 10, 1824. [Google Scholar] [CrossRef] [PubMed]
- Schägger, H.; De Coo, R.; Bauer, M.F.; Hofmann, S.; Godino, C.; Brandt, U. Significance of respirasomes for the assembly/stability of human respiratory chain complex I. J. Biol. Chem. 2004, 279, 36349–36353. [Google Scholar] [CrossRef]
- Peoples, J.N.; Saraf, A.; Ghazal, N.; Pham, T.T.; Kwong, J.Q. Mitochondrial dysfunction and oxidative stress in heart disease. Exp. Mol. Med. 2019, 51, 1–13. [Google Scholar] [CrossRef]
- Boveris, A.; Chance, B. The mitochondrial generation of hydrogen peroxide. General properties and effect of hyperbaric oxygen. Biochem. J. 1973, 134, 707–716. [Google Scholar] [CrossRef] [PubMed]
- Starkov, A.A.; Fiskum, G.; Chinopoulos, C.; Lorenzo, B.J.; Browne, S.E.; Patel, M.S.; Beal, M.F. Mitochondrial α-ketoglutarate dehydrogenase complex generates reactive oxygen species. J. Neurosci. 2004, 24, 7779–7788. [Google Scholar] [CrossRef]
- Pellegrini, M.; Pacini, S.; Baldari, C.T. p66SHC: The apoptotic side of Sch proteins. Apoptosis 2005, 10, 13–18. [Google Scholar] [CrossRef] [PubMed]
- Galimov, E.R. The Role of p66shc in Oxidative Stress and Apoptosis. Acta Nat. 2010, 2, 44–51. [Google Scholar] [CrossRef]
- Giorgio, M.; Migliaccio, E.; Orsini, F.; Paolucci, D.; Moroni, M.; Contursi, C.; Pelliccia, G.; Luzi, L.; Minucci, S.; Marcaccio, M.; et al. Electron transfer between cytochrome c and p66Shc generates reactive oxygen species that trigger mitochondrial apoptosis. Cell 2005, 122, 221–233. [Google Scholar] [CrossRef] [PubMed]
- Lambeth, J.D. NOX enzymes and the biology of reactive oxygen. Nat. Rev. Immunol. 2004, 4, 181–189. [Google Scholar] [CrossRef] [PubMed]
- Taylor, J.P.; Tse, H.M. The role of NADPH oxidases in infectious and inflammatory diseases. Redox Biol. 2020, 48, 102159. [Google Scholar] [CrossRef]
- Borutaite, V.; Budriunaite, A.; Brown, G.C. Reversal of nitric oxide-, peroxynitrite-and S-nitrosothiol-induced inhibition of mitochondrial respiration or complex I activity by light and thiols. Biochim. Biophys. Acta (BBA)-Bioenerg. 2000, 1459, 405–412. [Google Scholar] [CrossRef]
- Patel, R.P.; McAndrew, J.; Sellak, H.; White, C.R.; Jo, H.; Freeman, B.A.; Darley-Usmar, V.M. Biological aspects of reactive nitrogen species. Biochim. Biophys. Acta -Bioenerg. 1999, 1411, 385–400. [Google Scholar] [CrossRef]
- Wu, G.; Morris, S.M. Arginine metabolism: Nitric oxide and beyond. Biochem. J. 1998, 336, 1–17. [Google Scholar] [CrossRef]
- Paolocci, N.; Ekelund, U.E.G.; Isoda, T.; Ozaki, M.; Vandegaer, K.; Georgakopoulos, D.; Harrison, R.W.; Kass, D.A.; Hare, J.M. cGMP-independent inotropic effects of nitric oxide and peroxynitrite donors: Potential role for nitrosylation. Am. J. Physiol. -Heart Circ. Physiol. 2000, 279, H1982–H1988. [Google Scholar] [CrossRef]
- Di Meo, S.; Reed, T.T.; Venditti, P.; Victor, V.M. Role of ROS and RNS Sources in Physiological and Pathological Conditions. Oxid. Med. Cell. Longev. 2016, 2016, 1245049. [Google Scholar] [CrossRef]
- Poderoso, J.J.; Peralta, J.G.; Lisdero, C.L.; Carreras, M.C.; Radisic, M.; Schöpfer, F.; Scho¨pfer, S.; Cadenas, E.; Boveris, A.; Schö, F.-C. Nitric oxide regulates oxygen uptake and hydrogen peroxide release by the isolated beating rat heart. Am. J. Physiol. -Cell Physiol. 1998, 274, C112–C119. [Google Scholar] [CrossRef]
- Simon, D.I.; Mullinst, M.E.; Jiat, L.; Gaston, B.; Singeli, D.J.; Stamlerti, J.S.; Reilly, T. Polynitrosylated proteins: Characterization, bioactivity, and functional consequences. Proc. Natl. Acad. Sci. USA 1996, 93, 4736–4741. [Google Scholar] [CrossRef]
- Van Houten, B.; Woshner, V.; Santos, J.H. Role of mitochondrial DNA in toxic responses to oxidative stress. DNA Repair 2006, 5, 145–152. [Google Scholar] [CrossRef]
- Verkaart, S.; Koopman, W.J.H.; van Emst-de Vries, S.E.; Nijtmans, L.G.J.; van den Heuvel, L.W.P.J.; Smeitink, J.A.M.; Willems, P.H.G.M. Superoxide production is inversely related to complex I activity in inherited complex I deficiency. Biochim. Biophys. Acta -Mol. Basis Dis. 2007, 1772, 373–381. [Google Scholar] [CrossRef] [PubMed]
- Castro, M.d.R.; Suarez, E.; Kraiselburd, E.; Isidro, A.; Paz, J.; Ferder, L.; Ayala-Torres, S. Aging increases mitochondrial DNA damage and oxidative stress in liver of rhesus monkeys. Exp. Gerontol. 2012, 47, 29–37. [Google Scholar] [CrossRef]
- Voets, A.M.; Huigsloot, M.; Lindsey, P.J.; Leenders, A.M.; Koopman, W.J.H.; Willems, P.H.G.M.; Rodenburg, R.J.; Smeitink, J.A.M.; Smeets, H.J.M. Transcriptional changes in OXPHOS complex I deficiency are related to anti-oxidant pathways and could explain the disturbed calcium homeostasis. Biochim. Biophys. Acta -Mol. Basis Dis. 2012, 1822, 1161–1168. [Google Scholar] [CrossRef] [PubMed]
- Sarsour, E.H.; Kumar, M.G.; Chaudhuri, L.; Kalen, A.L.; Goswami, P.C. Redox Control of the Cell Cycle in Health and Disease. Antioxid. Redox Signal. 2009, 11, 2985–3011. [Google Scholar] [CrossRef] [PubMed]
- Sauer, H.; Wartenberg, M.; Hescheler, J. Reactive Oxygen Species as Intracellular Messengers During Cell Growth and Differentiation. Cell. Physiol. Biochem. 2001, 11, 173–186. [Google Scholar] [CrossRef] [PubMed]
- Fukai, T.; Ushio-Fukai, M. Superoxide dismutases: Role in redox signaling, vascular function, and diseases. Antioxid. Redox Signal. 2011, 15, 1583–1606. [Google Scholar] [CrossRef] [PubMed]
- Kawamata, H.; Manfredi, G. Import, maturation, and function of SOD1 and its copper chaperone CCS in the mitochondrial intermembrane space. Antioxid. Redox Signal. 2010, 13, 1375–1384. [Google Scholar] [CrossRef]
- Suzuki, Y.; Ali, M.; Fischer, M.; Riemer, J. Human copper chaperone for superoxide dismutase 1 mediates its own oxidation-dependent import into mitochondria. Nat. Commun. 2013, 4, 2430. [Google Scholar] [CrossRef]
- Gaetani, G.F.; Galiano, S.; Canepa, L.; Ferraris, A.M.; Kirkman, H.N. Catalase and Glutathione Peroxidase Are Equally Active in Detoxification of Hydrogen Peroxide in Human Erythrocytes. Blood 1989, 73, 334–339. [Google Scholar] [CrossRef]
- Radi, R.; Turrens, J.F.; Chang, L.Y.; Bush, K.M.; Crapo, J.D.; Freeman, B.A. Detection of catalase in rat heart mitochondria. J. Biol. Chem. 1991, 266, 22028–22034. [Google Scholar] [CrossRef]
- Irshad, M.; Chaudhuri, P.S. Oxidant-antioxidant system: Role and significance in human body. Indian J. Exp. Biol. 2002, 40, 1233–1239. [Google Scholar]
- Lushchak, V.I. Glutathione Homeostasis and Functions: Potential Targets for Medical Interventions. J. Amino Acids 2012, 2012, 736837. [Google Scholar] [CrossRef]
- Forman, H.J.; Davies, K.J.A.; Ursini, F. How Do Nutritional Antioxidants Really Work: Nucleophilic Tone and Para-Hormesis Versus Free Radical Scavenging in vivo Henry. Free Radic. Biol. Med. 2014, 66, 24–35. [Google Scholar] [CrossRef] [PubMed]
- Mathers, J.; Fraser, J.A.; McMahon, M.; Saunders, R.D.C.; Hayes, J.D.; McLellan, L.I. Antioxidant and cytoprotective responses to redox stress. Biochem. Soc. Symp. 2004, 71, 157–176. [Google Scholar] [CrossRef]
- Simon, N.; Hertig, A. Alteration of fatty acid oxidation in tubular epithelial cells: From acute kidney injury to renal fibrogenesis. Front. Med. 2015, 2, 52. [Google Scholar] [CrossRef] [PubMed]
- Schaub, J.A.; Venkatachalam, M.A.; Weinberg, J.M. Proximal Tubular Oxidative Metabolism in Acute Kidney Injury and the Transition to CKD. Kidney360 2021, 2, 355–364. [Google Scholar] [CrossRef]
- Khan, M.A.; Wang, X.; Giuliani, K.T.K.; Nag, P.; Grivei, A.; Ungerer, J.; Hoy, W.; Healy, H.; Gobe, G.; Kassianos, A.J. Underlying histopathology determines response to oxidative stress in cultured human primary proximal tubular epithelial cells. Int. J. Mol. Sci. 2020, 21, 560. [Google Scholar] [CrossRef]
- Ratliff, B.B.; Abdulmahdi, W.; Pawar, R.; Wolin, M.S. Oxidant Mechanisms in Renal Injury and Disease. Antioxid. Redox Signal. 2016, 25, 119. [Google Scholar] [CrossRef] [PubMed]
- Faivre, A.; Verissimo, T.; Auwerx, H.; Legouis, D.; de Seigneux, S. Tubular Cell Glucose Metabolism Shift During Acute and Chronic Injuries. Front. Med. 2021, 8, 742072. [Google Scholar] [CrossRef] [PubMed]
- Zager, R.A.; Johnson, A.C.M.; Becker, K. Renal cortical pyruvate depletion during AKI. J. Am. Soc. Nephrol. 2014, 25, 998–1012. [Google Scholar] [CrossRef] [PubMed]
- Aranda-Rivera, A.K.; Cruz-Gregorio, A.; Aparicio-Trejo, O.E.; Pedraza-Chaverri, J. Mitochondrial redox signaling and oxidative stress in kidney diseases. Biomolecules 2021, 11, 1144. [Google Scholar] [CrossRef]
- Small, D.M.; Bennett, N.C.; Roy, S.; Gabrielli, B.G.; Johnson, D.W.; Gobe, G.C. Oxidative stress and cell senescence combine to cause maximal renal tubular epithelial cell dysfunction and loss in an in vitro model of kidney disease. Nephron. Exp. Nephrol. 2012, 122, 123–130. [Google Scholar] [CrossRef]
- Aragno, M.; Cutrin, J.C.; Mastrocola, R.; Perrelli, M.G.; Restivo, F.; Poli, G.; Danni, O.; Boccuzzi, G. Oxidative stress and kidney dysfunction due to ischemia/reperfusion in rat: Attenuation by dehydroepiandrosterone. Kidney Int. 2003, 64, 836–843. [Google Scholar] [CrossRef]
- Irazabal, M.V.; Torres, V.E. ROS and Redox signaling in Chronic kidney disease. Cells 2020, 9, 1342. [Google Scholar] [CrossRef]
- Molina-Jijón, E.; Aparicio-Trejo, O.E.; Rodríguez-Muñoz, R.; León-Contreras, J.C.; del Carmen Cárdenas-Aguayo, M.; Medina-Campos, O.N.; Tapia, E.; Sánchez-Lozada, L.G.; Hernández-Pando, R.; Reyes, J.L.; et al. The nephroprotection exerted by curcumin in maleate-induced renal damage is associated with decreased mitochondrial fission and autophagy. BioFactors 2016, 42, 686–702. [Google Scholar] [CrossRef]
- Sedeek, M.; Nasrallah, R.; Touyz, R.M.; Hébert, R.L. NADPH oxidases, reactive oxygen species, and the kidney: Friend and foe. J. Am. Soc. Nephrol. 2013, 24, 1512–1518. [Google Scholar] [CrossRef] [PubMed]
- Thallas-Bonke, V.; Jandeleit-Dahm, K.A.M.; Cooper, M.E. Nox-4 and progressive kidney disease. Curr. Opin. Nephrol. Hypertens. 2015, 24, 74–80. [Google Scholar] [CrossRef] [PubMed]
- You, Y.H.; Quach, T.; Saito, R.; Pham, J.; Sharma, K. Metabolomics reveals a key role for fumarate in mediating the effects of NADPH oxidase 4 in diabetic kidney disease. J. Am. Soc. Nephrol. 2016, 27, 466–481. [Google Scholar] [CrossRef] [PubMed]
- Cowley, A.W., Jr.; Yang, C.; Zheleznova, N.N.; Staruschenko, A.; Kurth, T.; Rein, L.; Kumar, V.; Sadovnikov, K.; Dayton, A.; Hoffman, M.; et al. Evidence of the importance of nox4 in production of hypertension in dahl salt-sensitive rats. Hypertension 2016, 67, 440–450. [Google Scholar] [CrossRef]
- Kahveci, A.S.; Barnatan, T.T.; Kahveci, A.; Adrian, A.E.; Arroyo, J.; Eirin, A.; Harris, P.C.; Lerman, A.; Lerman, L.O.; Torres, V.E.; et al. Oxidative stress and mitochondrial abnormalities contribute to decreased endothelial nitric oxide synthase expression and renal disease progression in early experimental polycystic kidney disease. Int. J. Mol. Sci. 2020, 21, 1994. [Google Scholar] [CrossRef] [PubMed]
- Dikalov, S. Crosstalk between mitochondria and NADPH oxidases Sergey. Free Radic. Biol. Med. 2011, 51, 1289–1301. [Google Scholar] [CrossRef] [PubMed]
- Wenzel, P.; Mollnau, H.; Oelze, M.; Schulz, E.; Wickramanayake, J.M.D.; Müller, J.; Schuhmacher, S.; Hortmann, M.; Baldus, S.; Gori, T.; et al. First evidence for a crosstalk between mitochondrial and NADPH oxidase-derived reactive oxygen species in nitroglycerin-triggered vascular dysfunction. Antioxid. Redox Signal. 2008, 10, 1435–1447. [Google Scholar] [CrossRef]
- Khodo, S.N.; Dizin, E.; Sossauer, G.; Szanto, I.; Martin, P.Y.; Feraille, E.; Krause, K.H.; De Seigneux, S. NADPH-oxidase 4 protects against kidney fibrosis during chronic renal injury. J. Am. Soc. Nephrol. 2012, 23, 1967–1976. [Google Scholar] [CrossRef]
- Brewer, A.C.; Murray, T.V.A.; Arno, M.; Zhang, M.; Anilkumar, N.P.; Mann, G.E.; Shah, A.M. Nox4 regulates Nrf2 and glutathione redox in cardiomyocytes in vivo. Free Radic. Biol. Med. 2011, 51, 205–215. [Google Scholar] [CrossRef]
- Yang, Y.; Liu, H.; Liu, F.; Dong, Z. Mitochondrial Dysregulation and Protection in Cisplatin Nephrotoxicity. Arch Toxicol. 2014, 88, 1249–1256. [Google Scholar] [CrossRef]
- Brooks, C.; Wei, Q.; Cho, S.G.; Dong, Z. Regulation of mitochondrial dynamics in acute kidney injury in cell culture and rodent models. J. Clin. Investig. 2009, 119, 1275–1285. [Google Scholar] [CrossRef]
- Uchida, S.; Endou, H. Substrate specificity to maintain cellular ATP along the mouse nephron. Am. J. Physiol. 1988, 255, F977–F983. [Google Scholar] [CrossRef]
- García-Carro, C.; Vergara, A.; Agraz, I.; Jacobs-Cachá, C.; Espinel, E.; Seron, D.; Soler, M.J. The New Era for Reno-Cardiovascular Treatment in Type 2 Diabetes. J. Clin. Med. 2019, 8, 864. [Google Scholar] [CrossRef] [PubMed]
- Brady, J.A.; Hallow, K.M. Model-Based Evaluation of Proximal Sodium Reabsorption Through SGLT2 in Health and Diabetes and the Effect of Inhibition With Canagliflozin. J. Clin. Pharmacol. 2018, 58, 377–385. [Google Scholar] [CrossRef]
- Onishi, A.; Fu, Y.; Patel, R.; Darshi, M.; Crespo-Masip, M.; Huang, W.; Song, P.; Freeman, B.; Kim, Y.C.; Soleimani, M.; et al. A role for tubular Na+/H+ exchanger NHE3 in the natriuretic effect of the SGLT2 inhibitor empagliflozin. Am. J. Physiol. -Ren. Physiol. 2020, 319, F712–F728. [Google Scholar] [CrossRef] [PubMed]
- Ghezzi, C.; Loo, D.D.F.; Wright, E.M. Physiology of renal glucose handling via SGLT1, SGLT2 and GLUT2. Diabetologia 2018, 61, 2087–2097. [Google Scholar] [CrossRef]
- Gnudi, L.; Coward, R.J.M.; Long, D.A. Diabetic Nephropathy: Perspective on Novel Molecular Mechanisms. Trends Endocrinol. Metab. 2016, 27, 820–830. [Google Scholar] [CrossRef]
- Lastra, G.; Syed, S.; Kurukulasuriya, L.R.; Manrique, C.; Sowers, J.R. Type 2 diabetes mellitus and hypertension: An update. Endocrinol. Metab. Clin. N. Am. 2014, 43, 103–122. [Google Scholar] [CrossRef]
- Packer, M. Activation and Inhibition of Sodium-Hydrogen Exchanger Is a Mechanism That Links the Pathophysiology and Treatment of Diabetes Mellitus With That of Heart Failure. Circulation 2017, 136, 1548–1559. [Google Scholar] [CrossRef] [PubMed]
- Kogot-Levin, A.; Hinden, L.; Riahi, Y.; Israeli, T.; Tirosh, B.; Cerasi, E.; Mizrachi, E.B.; Tam, J.; Mosenzon, O.; Leibowitz, G. Proximal Tubule mTORC1 Is a Central Player in the Pathophysiology of Diabetic Nephropathy and Its Correction by SGLT2 Inhibitors. Cell Rep. 2020, 32, 107954. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.J.; Heo, J.S.; Suh, H.N.; Lee, M.Y.; Han, H.J. Interleukin-6 stimulates α-MG uptake in renal proximal tubule cells: Involvement of STAT3, PI3K/Akt, MAPKs, and NF-κB. Am. J. Physiol. -Ren. Physiol. 2007, 293, 1036–1046. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Tervaert, T.W.C.; Mooyaart, A.L.; Amann, K.; Cohen, A.H.; TerenceCook, H.; Drachenberg, C.B.; Ferrario, F.; Fogo, A.B.; Haas, M.; De Heer, E.; et al. Pathologic classification of diabetic nephropathy. J. Am. Soc. Nephrol. 2010, 21, 556–563. [Google Scholar] [CrossRef]
- Coughlan, M.T.; Nguyen, T.V.; Penfold, S.A.; Higgins, G.C.; Thallas-Bonke, V.; Tan, S.M.; Van Bergen, N.J.; Sourris, K.C.; Harcourt, B.E.; Thorburn, D.R.; et al. Mapping time-course mitochondrial adaptations in the kidney in experimental diabetes. Clin. Sci. 2016, 130, 711–720. [Google Scholar] [CrossRef] [PubMed]
- Lee, W.C.; Chau, Y.Y.; Ng, H.Y.; Chen, C.H.; Wang, P.W.; Liou, C.W.; Lin, T.K.; Chen, J.B. Empagliflozin Protects HK-2 Cells from High Glucose-Mediated Injuries via a Mitochondrial Mechanism. Cells 2019, 8, 1085. [Google Scholar] [CrossRef]
- Takagi, S.; Li, J.; Takagaki, Y.; Kitada, M.; Nitta, K.; Takasu, T.; Kanasaki, K.; Koya, D. Ipragliflozin improves mitochondrial abnormalities in renal tubules induced by a high-fat diet. J. Diabetes Investig. 2018, 9, 1025–1032. [Google Scholar] [CrossRef]
- Szabo, C. Role of nitrosative stress in the pathogenesis of diabetic vascular dysfunction. Br. J. Pharmacol. 2009, 156, 713–727. [Google Scholar] [CrossRef] [PubMed]
- Coughlan, M.T.; Sharma, K. Challenging the dogma of mitochondrial reactive oxygen species overproduction in diabetic kidney disease. Kidney Int. 2016, 90, 272–279. [Google Scholar] [CrossRef]
- Cui, F.Q.; Tang, L.; Gao, Y.B.; Wang, Y.F.; Meng, Y.; Shen, C.; Shen, Z.L.; Liu, Z.Q.; Zhao, W.J.; Liu, W.J. Effect of Baoshenfang Formula on Podocyte Injury via Inhibiting the NOX-4/ROS/p38 Pathway in Diabetic Nephropathy. J. Diabetes Res. 2019, 2019, 2981705. [Google Scholar] [CrossRef] [PubMed]
- Ilatovskaya, D.V.; Blass, G.; Palygin, O.; Levchenko, V.; Pavlov, T.S.; Grzybowski, M.N.; Winsor, K.; Shuyskiy, L.S.; Geurts, A.M.; Cowley, A.W.; et al. A NOX4/TRPC6 Pathway in Podocyte Calcium Regulation and Renal Damage in Diabetic Kidney Disease. J. Am. Soc. Nephrol. 2018, 29, 1917–1927. [Google Scholar] [CrossRef]
- Liu, J.J.; Liu, S.; Gurung, R.L.; Ching, J.; Kovalik, J.P.; Tan, T.Y.; Lim, S.C. Urine Tricarboxylic Acid Cycle Metabolites Predict Progressive Chronic Kidney Disease in Type 2 Diabetes. J. Clin. Endocrinol. Metab. 2018, 103, 4357–4364. [Google Scholar] [CrossRef]
- Jiang, H.; Shao, X.; Jia, S.; Qu, L.; Weng, C.; Shen, X.; Wang, Y.; Huang, H.; Wang, C.; Wang, Y.; et al. The mitochondria-targeted metabolic tubular injury in diabetic kidney disease. Cell. Physiol. Biochem. 2019, 52, 156–171. [Google Scholar] [CrossRef]
- Cao, H.; Wu, J.; Luo, J.; Chen, X.; Yang, J.; Fang, L. Urinary mitochondrial DNA: A potential early biomarker of diabetic nephropathy. Diabetes. Metab. Res. Rev. 2019, 35. [Google Scholar] [CrossRef]
- Al-Kafaji, G.; Aljadaan, A.; Kamal, A.; Bakhiet, M. Peripheral blood mitochondrial DNA copy number as a novel potential biomarker for diabetic nephropathy in type 2 diabetes patients. Exp. Ther. Med. 2018, 16, 1483–1492. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Chi, J.; Wei, F.; Zhou, Y.; Cao, Y.; Wang, Y. Mitochondrial DNA: A New Predictor of Diabetic Kidney Disease. Int. J. Endocrinol. 2020, 2020, 3650937. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Raoof, M.; Chen, Y.; Sumi, Y.; Sursal, T.; Junger, W.; Brohi, K.; Itagaki, K.; Hauser, C.J. Circulating mitochondrial DAMPs cause inflammatory responses to injury. Nature 2010, 464, 104–107. [Google Scholar] [CrossRef]
- Jin, L.; Yu, B.; Armando, I.; Han, F. Mitochondrial DNA-Mediated Inflammation in Acute Kidney Injury and Chronic Kidney Disease. Oxid. Med. Cell. Longev. 2021, 2021, 9985603. [Google Scholar] [CrossRef]
- Chow, F.; Ozols, E.; Nikolic-Paterson, D.J.; Atkins, R.C.; Tesch, G.H. Macrophages in mouse type 2 diabetic nephropathy: Correlation with diabetic state and progressive renal injury. Kidney Int. 2004, 65, 116–128. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, D.; Ping, F.; Mu, W.; Hill, P.; Atkins, R.C.; Chadban, S.J. Macrophage accumulation in human progressive diabetic nephropathy. Nephrology 2006, 11, 226–231. [Google Scholar] [CrossRef]
- Elmarakby, A.A.; Sullivan, J.C. Relationship between oxidative stress and inflammatory cytokines in diabetic nephropathy. Cardiovasc. Ther. 2012, 30, 49–59. [Google Scholar] [CrossRef]
- Isaji, M. SGLT2 inhibitors: Molecular design and potential differences in effect. Kidney Int. 2011, 79, S14–S19. [Google Scholar] [CrossRef]
- Bashier, A.; Khalifa, A.A.; Rashid, F.; Abdelgadir, E.I.; Al Qaysi, A.A.; Ali, R.; Eltinay, A.; Nafach, J.; Alsayyah, F.; Alawadi, F. Efficacy and Safety of SGLT2 Inhibitors in Reducing Glycated Hemoglobin and Weight in Emirati Patients With Type 2 Diabetes. J. Clin. Med. Res. 2017, 9, 499–507. [Google Scholar] [CrossRef] [PubMed]
- Wanner, C.; Heerspink, H.J.L.; Zinman, B.; Inzucchi, S.E.; Koitka-Weber, A.; Mattheus, M.; Hantel, S.; Woerle, H.J.; Broedl, U.C.; Von Eynatten, M.; et al. Empagliflozin and kidney function decline in patients with type 2 diabetes: A slope analysis from the EMPA-REG OUTCOME trial. J. Am. Soc. Nephrol. 2018, 29, 2755–2769. [Google Scholar] [CrossRef] [PubMed]
- Neal, B.; Perkovic, V.; Mahaffey, K.W.; de Zeeuw, D.; Fulcher, G.; Erondu, N.; Shaw, W.; Law, G.; Desai, M.; Matthews, D.R. Canagliflozin and Cardiovascular and Renal Events in Type 2 Diabetes. N. Engl. J. Med. 2017, 377, 644–657. [Google Scholar] [CrossRef]
- Wiviott, S.D.; Raz, I.; Bonaca, M.P.; Mosenzon, O.; Kato, E.T.; Cahn, A.; Silverman, M.G.; Zelniker, T.A.; Kuder, J.F.; Murphy, S.A.; et al. Dapagliflozin and Cardiovascular Outcomes in Type 2 Diabetes. N. Engl. J. Med. 2018, NEJMoa1812389. [Google Scholar] [CrossRef]
- Perkovic, V.; Jardine, M.J.; Neal, B.; Bompoint, S.; Heerspink, H.J.L.; Charytan, D.M.; Edwards, R.; Agarwal, R.; Bakris, G.; Bull, S.; et al. Canagliflozin and Renal Outcomes in Type 2 Diabetes and Nephropathy. N. Engl. J. Med. 2019, 380, 2295–2306. [Google Scholar] [CrossRef]
- Vergara, A.; Jacobs-Cachá, C.; Soler, M.J. Sodium-glucose cotransporter inhibitors: Beyond glycaemic control. Clin. Kidney J. 2019, 12, 322–325. [Google Scholar] [CrossRef] [PubMed]
- Górriz, J.L.; Navarro-González, J.F.; Ortiz, A.; Vergara, A.; Nuñez, J.; Jacobs-Cachá, C.; Martínez-Castelao, A.; Soler, M.J. Sodium-glucose cotransporter 2 inhibition: Towards an indication to treat diabetic kidney disease. Nephrol. Dial. Transplant. 2020, 35, i13–i23. [Google Scholar] [CrossRef]
- van Bommel, E.J.M.; Muskiet, M.H.A.; van Baar, M.J.B.; Tonneijck, L.; Smits, M.M.; Emanuel, A.L.; Bozovic, A.; Danser, A.H.J.; Geurts, F.; Hoorn, E.J.; et al. The renal hemodynamic effects of the SGLT2 inhibitor dapagliflozin are caused by post-glomerular vasodilatation rather than pre-glomerular vasoconstriction in metformin-treated patients with type 2 diabetes in the randomized, double-blind RED trial. Kidney Int. 2020, 97, 202–212. [Google Scholar] [CrossRef]
- Lambadiari, V.; Thymis, J.; Kouretas, D.; Skaperda, Z.; Tekos, F.; Kousathana, F.; Kountouri, A.; Balampanis, K.; Parissis, J.; Andreadou, I.; et al. Effects of a 12-Month Treatment with Glucagon-like Peptide-1 Receptor Agonists, Sodium-Glucose Cotransporter-2 Inhibitors, and Their Combination on Oxidant and Antioxidant Biomarkers in Patients with Type 2 Diabetes. Antioxidants 2021, 10, 1379. [Google Scholar] [CrossRef]
- Nabrdalik-Leśniak, D.; Nabrdalik, K.; Sedlaczek, K.; Główczyński, P.; Kwiendacz, H.; Sawczyn, T.; Hajzler, W.; Drożdż, K.; Hendel, M.; Irlik, K.; et al. Influence of SGLT2 Inhibitor Treatment on Urine Antioxidant Status in Type 2 Diabetic Patients: A Pilot Study. Oxid. Med. Cell. Longev. 2021, 2021, 5593589. [Google Scholar] [CrossRef]
- Iannantuoni, F.; de Marañon, A.M.; Diaz-Morales, N.; Falcon, R.; Bañuls, C.; Abad-Jimenez, Z.; Victor, V.M.; Hernandez-Mijares, A.; Rovira-Llopis, S. The SGLT2 Inhibitor Empagliflozin Ameliorates the Inflammatory Profile in Type 2 Diabetic Patients and Promotes an Antioxidant Response in Leukocytes. J. Clin. Med. 2019, 8, 1814. [Google Scholar] [CrossRef]
- Nandula, S.R.; Kundu, N.; Awal, H.B.; Brichacek, B.; Fakhri, M.; Aimalla, N.; Elzarki, A.; Amdur, R.L.; Sen, S. Role of Canagliflozin on function of CD34+ve endothelial progenitor cells (EPC) in patients with type 2 diabetes. Cardiovasc. Diabetol. 2021, 20, 44. [Google Scholar] [CrossRef]
- Kamezaki, M.; Kusaba, T.; Komaki, K.; Fushimura, Y.; Watanabe, N.; Ikeda, K.; Kitani, T.; Yamashita, N.; Uehara, M.; Kirita, Y.; et al. Comprehensive renoprotective effects of ipragliflozin on early diabetic nephropathy in mice. Sci. Rep. 2018, 8, 4029. [Google Scholar] [CrossRef]
- Sun, X.; Han, F.; Lu, Q.; Li, X.; Ren, D.; Zhang, J.; Han, Y.; Xiang, Y.K.; Li, J. Empagliflozin Ameliorates Obesity-Related Cardiac Dysfunction by Regulating Sestrin2-Mediated AMPK-mTOR Signaling and Redox Homeostasis in High-Fat Diet–Induced Obese Mice. Diabetes 2020, 69, 1292–1305. [Google Scholar] [CrossRef]
- Rahadian, A.; Fukuda, D.; Salim, H.M.; Yagi, S.; Kusunose, K.; Yamada, H.; Soeki, T.; Sata, M. Canagliflozin Prevents Diabetes-Induced Vascular Dysfunction in ApoE-Deficient Mice. J. Atheroscler. Thromb. 2020, 27, 1141–1151. [Google Scholar] [CrossRef]
- Shin, S.J.; Chung, S.; Kim, S.J.; Lee, E.-M.; Yoo, Y.-H.; Kim, J.-W.; Ahn, Y.-B.; Kim, E.-S.; Moon, S.-D.; Kim, M.-J.; et al. Effect of Sodium-Glucose Co-Transporter 2 Inhibitor, Dapagliflozin, on Renal Renin-Angiotensin System in an Animal Model of Type 2 Diabetes. PLoS ONE 2016, 11, e0165703. [Google Scholar] [CrossRef]
- Ye, Y.; Bajaj, M.; Yang, H.-C.; Perez-Polo, J.R.; Birnbaum, Y. SGLT-2 Inhibition with Dapagliflozin Reduces the Activation of the Nlrp3/ASC Inflammasome and Attenuates the Development of Diabetic Cardiomyopathy in Mice with Type 2 Diabetes. Further Augmentation of the Effects with Saxagliptin, a DPP4 Inhibitor. Cardiovasc. Drugs Ther. 2017, 31, 119–132. [Google Scholar] [CrossRef]
- Liang, Y.; Arakawa, K.; Ueta, K.; Matsushita, Y.; Kuriyama, C.; Martin, T.; Du, F.; Liu, Y.; Xu, J.; Conway, B.; et al. Effect of Canagliflozin on Renal Threshold for Glucose, Glycemia, and Body Weight in Normal and Diabetic Animal Models. PLoS ONE 2012, 7, e30555. [Google Scholar] [CrossRef] [PubMed]
- Tahara, A.; Takasu, T. Prevention of progression of diabetic nephropathy by the SGLT2 inhibitor ipragliflozin in uninephrectomized type 2 diabetic mice. Eur. J. Pharmacol. 2018, 830, 68–75. [Google Scholar] [CrossRef] [PubMed]
- Leng, W.; Ouyang, X.; Lei, X.; Wu, M.; Chen, L.; Wu, Q.; Deng, W.; Liang, Z. The SGLT-2 Inhibitor Dapagliflozin Has a Therapeutic Effect on Atherosclerosis in Diabetic ApoE−/− Mice. Mediators Inflamm. 2016, 2016, 6305735. [Google Scholar] [CrossRef] [PubMed]
- Swe, M.T.; Thongnak, L.; Jaikumkao, K.; Pongchaidecha, A.; Chatsudthipong, V.; Lungkaphin, A. Dapagliflozin attenuates renal gluconeogenic enzyme expression in obese rats. J. Endocrinol. 2020, 245, 193–205. [Google Scholar] [CrossRef]
- Li, X.; Römer, G.; Kerindongo, R.P.; Hermanides, J.; Albrecht, M.; Hollmann, M.W.; Zuurbier, C.J.; Preckel, B.; Weber, N.C. Sodium glucose co-transporter 2 inhibitors ameliorate endothelium barrier dysfunction induced by cyclic stretch through inhibition of reactive oxygen species. Int. J. Mol. Sci. 2021, 22, 6044. [Google Scholar] [CrossRef]
- Uthman, L.; Homayr, A.; Juni, R.P.; Spin, E.L.; Kerindongo, R.; Boomsma, M.; Hollmanna Benedikt Preckel, M.W.; Koolwijk, P.; Van Hinsbergh, V.W.M.; Zuurbier, C.J.; et al. Empagliflozin and dapagliflozin reduce ROS generation and restore no bioavailability in tumor necrosis factor α-stimulated human coronary arterial endothelial cells. Cell. Physiol. Biochem. 2019, 53, 865–886. [Google Scholar] [CrossRef]
- Zaibi, N.; Li, P.; Xu, S.Z. Protective effects of dapagliflozin against oxidative stress-induced cell injury in human proximal tubular cells. PLoS ONE 2021, 16, e0247234. [Google Scholar] [CrossRef] [PubMed]
- Shirakawa, K.; Sano, M. Sodium-Glucose Co-Transporter 2 Inhibitors Correct Metabolic Maladaptation of Proximal Tubular Epithelial Cells in High-Glucose Conditions. Int. J. Mol. Sci. 2020, 21, 7676. [Google Scholar] [CrossRef]
- Maeda, S.; Matsui, T.; Takeuchi, M.; Yamagishi, S.I. Sodium-glucose cotransporter 2-mediated oxidative stress augments advanced glycation end products-induced tubular cell apoptosis. Diabetes. Metab. Res. Rev. 2013, 29, 406–412. [Google Scholar] [CrossRef]
- Maki, T.; Maeno, S.; Maeda, Y.; Yamato, M.; Sonoda, N.; Ogawa, Y.; Wakisaka, M.; Inoguchi, T. Amelioration of diabetic nephropathy by SGLT2 inhibitors independent of its glucose-lowering effect: A possible role of SGLT2 in mesangial cells. Sci. Rep. 2019, 9, 4703. [Google Scholar] [CrossRef] [PubMed]
- Nangaku, M. More reasons to use SGLT2 inhibitors: EMPEROR-reduced and DAPA-CKD. Kidney Int. 2020, 98, 1387–1389. [Google Scholar] [CrossRef] [PubMed]
- Packer, M.; Claggett, B.; Lefkowitz, M.P.; McMurray, J.J.V.; Rouleau, J.L.; Solomon, S.D.; Zile, M.R. Effect of neprilysin inhibition on renal function in patients with type 2 diabetes and chronic heart failure who are receiving target doses of inhibitors of the renin-angiotensin system: A secondary analysis of the PARADIGM-HF trial. Lancet Diabetes Endocrinol. 2018, 6, 547–554. [Google Scholar] [CrossRef]
- Heerspink, H.J.L.; Stefánsson, B.V.; Correa-Rotter, R.; Chertow, G.M.; Greene, T.; Hou, F.-F.; Mann, J.F.E.; McMurray, J.J.V.; Lindberg, M.; Rossing, P.; et al. Dapagliflozin in Patients with Chronic Kidney Disease. N. Engl. J. Med. 2020, 383, 1436–1446. [Google Scholar] [CrossRef]
- Nagoya, T.; Kamimura, K.; Goto, R.; Shinagawa-Kobayashi, Y.; Niwa, Y.; Kimura, A.; Sakai, N.; Ko, M.; Nishina, H.; Terai, S. Inhibition of sodium-glucose cotransporter 2 ameliorates renal injury in a novel medaka model of nonalcoholic steatohepatitis-related kidney disease. FEBS Open Bio 2019, 9, 2016–2024. [Google Scholar] [CrossRef]
- Jin, J.; Jin, L.; Luo, K.; Lim, S.W.; Chung, B.H.; Yang, C.W. Effect of Empagliflozin on Tacrolimus-Induced Pancreas Islet Dysfunction and Renal Injury. Am. J. Transplant. 2017, 17, 2601–2616. [Google Scholar] [CrossRef] [PubMed]
- Olgar, Y.; Tuncay, E.; Degirmenci, S.; Billur, D.; Dhingra, R.; Kirshenbaum, L.; Turan, B. Ageing-associated increase in SGLT2 disrupts mitochondrial/sarcoplasmic reticulum Ca 2+ homeostasis and promotes cardiac dysfunction. J. Cell. Mol. Med. 2020, 24, 8567–8578. [Google Scholar] [CrossRef]
- Villani, L.A.; Smith, B.K.; Marcinko, K.; Ford, R.J.; Broadfield, L.A.; Green, A.E.; Houde, V.P.; Muti, P.; Tsakiridis, T.; Steinberg, G.R. The diabetes medication Canagliflozin reduces cancer cell proliferation by inhibiting mitochondrial complex-I supported respiration. Mol. Metab. 2016, 5, 1048–1056. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Zhu, J.; Yu, S.J.; Ma, H.L.; Chen, J.; Ding, X.F.; Chen, G.; Liang, Y.; Zhang, Q. Sodium-glucose co-transporter-2 (SGLT-2) inhibition reduces glucose uptake to induce breast cancer cell growth arrest through AMPK/mTOR pathway. Biomed. Pharmacother. 2020, 132, 110821. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).