Early Blockade of CB1 Receptors Ameliorates Schizophrenia-like Alterations in the Neurodevelopmental MAM Model of Schizophrenia
Abstract
1. Introduction
2. Materials and Methods
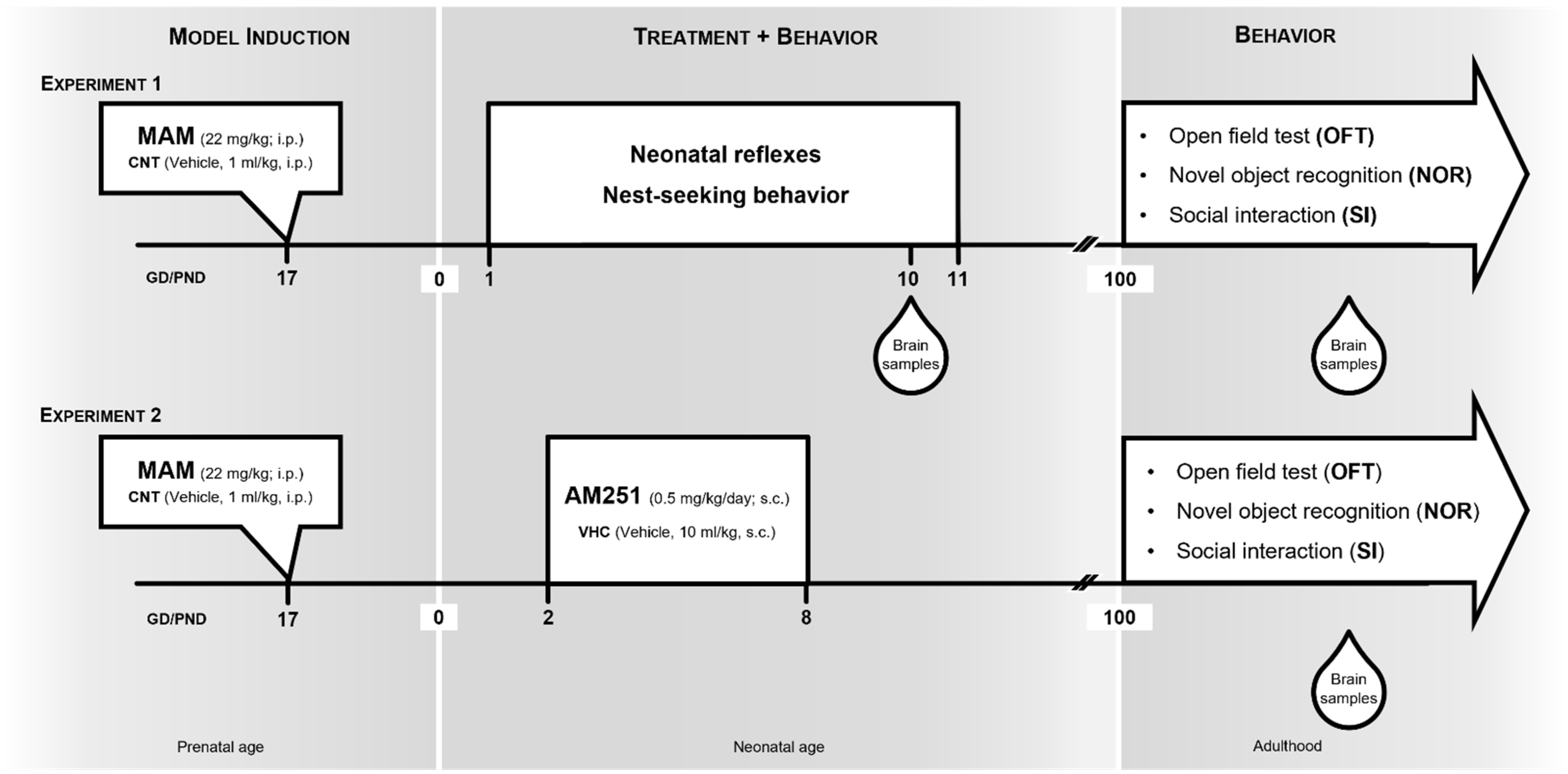
2.1. Animals, MAM Model and Experimental Design
2.2. Behavioral Testing
2.2.1. Neonatal Reflexes
2.2.2. Nest-Seeking Behavior
2.2.3. Spontaneous Locomotor Activity
2.2.4. Social Interaction (SI) Test
2.2.5. Novel Object Recognition (NOR) Test
2.3. Biochemical Methods
2.3.1. Extraction, Purification and Quantification of Endocannabinoids and Endocannabinoids Related Compounds
2.3.2. mRNA Extraction and Quantitative Real-Time Reverse Transcription-Polymerase Chain Reaction (qPCR)
2.3.3. Western Blotting Analysis
2.4. Statistical Analysis
3. Results
3.1. Experiment 1: MAM Rats at Neonatal Age and Adulthood
3.2. Experiment 2: Effects of Early Blockade of CB1 Receptor in MAM Rats at Adulthood
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rapoport, J.; Giedd, J.; Gogtay, N. Neurodevelopmental model of schizophrenia: Update. Mol. Psychiatry 2012, 17, 1228–1238. [Google Scholar] [CrossRef] [PubMed]
- Marenco, S.; Weinberger, D.R. The neurodevelopmental hypothesis of schizophrenia: Following a trail of evidence from cradle to grave. Dev. Psychopathol. 2000, 12, 501–527. [Google Scholar] [CrossRef] [PubMed]
- Phillips, L.J.; Yung, A.R.; Yuen, H.P.; Pantelis, C.; McGorry, P.D. Prediction and prevention of transition to psychosis in young people at incipient risk for schizophrenia. Am. J. Med. Genet. 2002, 114, 929–937. [Google Scholar] [CrossRef] [PubMed]
- Sommer, I.E.; Bearden, C.E.; Van Dellen, E.; Breetvelt, E.J.; Duijff, S.N.; Maijer, K.; Van Amelsvoort, T.; De Haan, L.; Gur, R.E.; Arango, C.; et al. Early interventions in risk groups for schizophrenia: What are we waiting for? NPJ Schizophr. 2016, 2, 16003. [Google Scholar] [CrossRef]
- Loss, C.M.; Teodoro, L.; Rodrigues, G.D.; Moreira, L.R.; Peres, F.F.; Zuardi, A.W.; Crippa, J.A.; Eduardo, J.; Hallak, C.; Abílio, V.C. Is cannabidiol during neurodevelopment a promising therapy for schizophrenia and autism spectrum disorders? Front. Pharmacol. 2021, 11, 635763. [Google Scholar] [CrossRef]
- Salokangas, R.K.; McGlashan, T.H. Early detection and intervention of psychosis. A review. Nord. J. Psychiatry 2008, 62, 92–105. [Google Scholar] [CrossRef]
- Lodge, D.J.; Grace, A.A. Gestational methylazoxymethanol acetate administration: A developmental disruption model of schizophrenia. Behav. Brain Res. 2009, 204, 306–312. [Google Scholar] [CrossRef]
- Micale, V.; Kucerova, J.; Sulcova, A. Leading compounds for the validation of animal models of psychopathology. Cell Tissue Res. 2013, 354, 309–330. [Google Scholar] [CrossRef]
- D’Addario, C.; Micale, V.; Di Bartolomeo, M.; Stark, T.; Pucci, M.; Sulcova, A.; Palazzo, M.; Babinska, Z.; Cremaschi, L.; Drago, F.; et al. A preliminary study of endocannabinoid system regulation in psychosis: Distinct alterations of CNR1 promoter DNA methylation in patients with schizophrenia. Schizophr. Res. 2017, 188, 132–140. [Google Scholar] [CrossRef]
- Večeřa, J.; Bártová, E.; Krejčí, J.; Legartová, S.; Komůrková, D.; Rudá-Kučerová, J.; Štark, T.; Dražanová, E.; Kašpárek, T.; Šulcová, A.; et al. HDAC1 and HDAC3 underlie dynamic H3K9 acetylation during embryonic neurogenesis and in schizo-phrenia-like animals. Cell Physiol. 2018, 233, 530–548. [Google Scholar] [CrossRef]
- Chalkiadaki, K.; Velli, A.; Kyriazidis, E.; Stavroulaki, V.; Vouvoutsis, V.; Chatzaki, E.; Aivaliotis, M.; Sidiropoulou, K. Development of the MAM model of schizophrenia in mice: Sex similarities and differences of hippocampal and prefrontal cortical function. Neuropharmacology 2019, 144, 193–207. [Google Scholar] [CrossRef]
- Micale, V.; Tabiova, K.; Kucerova, J.; Drago, F. Role of endocannabinoid system in depression from preclinical to clinical evidence. In Cannabinoid Modulation of Emotion, Memory, and Motivation; Campolongo, P., Fattore, L., Eds.; Springer: New York, NY, USA, 2015; pp. 97–129. [Google Scholar]
- Micale, V.; Drago, F. Endocannabinoid system, stress and HPA axis. Eur. J. Pharmacol. 2018, 834, 230–239. [Google Scholar] [CrossRef]
- Stark, T.; Di Martino, S.; Drago, F.; Wotjak, C.T.; Micale, V. Phytocannabinoids and schizophrenia: Focus on adolescence as a critical window of enhanced vulnerability and opportunity for treatment. Pharmacol. Res. 2021, 174, 105938. [Google Scholar] [CrossRef] [PubMed]
- Micale, V.; Mazzola, C.; Drago, F. Endocannabinoids and neurodegenerative diseases. Pharmacol. Res. 2007, 56, 382–392. [Google Scholar] [CrossRef] [PubMed]
- Androvicova, R.; Horacek, J.; Stark, T.; Drago, F.; Micale, V. Endocannabinoid system in sexual motivational processes: Is it a novel therapeutic horizon? Pharmacol. Res. 2017, 115, 200–208. [Google Scholar] [CrossRef] [PubMed]
- Viveros, M.P.; Llorente, R.; Suarez, J.; Llorente-Berzal, A.; López-Gallardo, M.; Rodriguez de Fonseca, F. The endocannabinoid system in critical neurodevelopmental periods: Sex differences and neuropsychiatric implications. J. Psychopharmacol. 2012, 26, 164–176. [Google Scholar] [CrossRef] [PubMed]
- Harkany, T.; Cinquina, V. Physiological rules of endocannabinoid action during fetal and neonatal brain development. Cannabis Cannabinoid Res. 2021, 6, 381–388. [Google Scholar] [CrossRef]
- Harkany, T.; Guzmán, M.; Galve-Roperh, I.; Berghuis, P.; Devi, A.L.; Mackie, K. The emerging functions of endocannabinoid signaling during CNS development. Pharmacol. Sci. 2007, 28, 83–92. [Google Scholar] [CrossRef] [PubMed]
- Kucerova, J.; Tabiova, K.; Drago, F.; Micale, V. Therapeutic potential of cannabinoids in schizophrenia. Recent pat. CNS Drug Discov. 2014, 9, 13–25. [Google Scholar]
- Saito, A.; Ballinger, M.D.L.; Pletnikov, M.V.; Wong, D.F.; Kamiya, A. Endocannabinoid system: Potential novel targets for treatment of schizophrenia. Neurobiol. Dis. 2015, 53, 10–17. [Google Scholar] [CrossRef]
- Fox, W.M. Reflex-ontogeny and behavioural development of the mouse. Animal Behav. 1965, 13, 234–241. [Google Scholar] [CrossRef]
- Tonkiss, J.; Harrison, R.H.; Galler, J.R. Differential effects of prenatal protein malnutrition and prenatal cocaine on a test of homing behavior in rat pups. Physiol. Behav. 1996, 60, 1013–1018. [Google Scholar] [CrossRef]
- Baharnoori, M.; Bhardwaj, S.K.; Srivastava, L.K. Neonatal behavioral changes in rats with gestational exposure to lipopoly-saccharide: A prenatal infection model for developmental neuropsychiatric disorders. Schizophr. Bull. 2012, 38, 444–456. [Google Scholar] [CrossRef]
- Ausderau, K.K.; Dammann, C.C.; McManus, K.; Schneider, M.; Emborg, M.E.; Schultz-Darken, N. Cross-species comparison of behavioral neurodevelopmental milestones in the common marmoset monkey and human child. Dev. Psychobiol. 2017, 59, 807–821. [Google Scholar] [CrossRef]
- Ruda-Kucerova, J.; Babinska, Z.; Amchova, P.; Stark, T.; Drago, F.; Sulcova, A.; Micale, V. Reactivity to addictive drugs in the methylazoxymethanol (MAM) model of schizophrenia in male and female rats. World J. Biol. Psychiatry 2017, 18, 129–142. [Google Scholar] [CrossRef]
- Stark, T.; Ruda-Kucerova, S.; Iannotti, F.A.; D’Addario, C.; Di Marco, R.; Pekarik, V.; Drazanova, E.; Piscitelli, F.; Bari, M.; Babinska, Z.; et al. Peripubertal Treatment with cannabidiol reverses behavioral alterations in MAM model of schizophrenia. Neuropharmacology 2019, 146, 212–221. [Google Scholar] [CrossRef]
- Stark, T.; Di Bartolomeo, M.; Di Marco, R.; Drazanova, E.; Platania, C.B.M.; Iannotti, F.A.; Ruda-Kucerova, J.; D’Addario, C.; Kratka, L.; Pekarik, V.; et al. Altered dopamine D3 receptor gene expression in MAM model of schizophrenia is reversed by peripubertal cannabidiol treatment. Biochem. Pharmacol. 2020, 177, 114004. [Google Scholar] [CrossRef]
- Drazanova, E.; Ruda-Kucerova, J.; Kratka, L.; Stark, T.; Kuchar, M.; Maryska, M.; Drago, F.; Starkuk, Z., Jr.; Micale, V. Different effects of prenatal MAM vs. perinatal THC exposure on regional cerebral blood perfusion detected by arterial spin labelling MRI in rats. Sci. Rep. 2019, 9, 6062. [Google Scholar] [CrossRef]
- Horska, K.; Kotolova, H.; Karpisek, M.; Babinska, Z.; Hammer, T.; Prochazka, J.; Stark, T.; Micale, V.; Ruda-Kucerova, J. Metabolic profile of methylazoxymethanol model of schizophrenia in rats and effects of three antipsychotics in long-acting formulation. Toxicol. Appl. Pharmacol. 2020, 406, 115214. [Google Scholar] [CrossRef]
- Kucera, J.; Horska, K.; Hruska, P.; Kuruczova, D.; Micale, V.; Ruda-Kucerova, J.; Bienertova-Vasku, J. Interacting effects of the MAM model of schizophrenia and antipsychotic treatment: Untargeted proteomics approach in adipose tissue. Prog. Neuropsychopharmacol. Biol. Psychiatry 2021, 108, 110165. [Google Scholar] [CrossRef] [PubMed]
- Fride, E.; Ginzburg, Y.; Breuer, A.; Bisogno, T.; Di Marzo, V.; Mechoulam, R. Critical role of the endogenous cannabinoid system in mouse pup suckling and growth. Eur. J. Pharmacol. 2001, 419, 207–214. [Google Scholar] [CrossRef]
- Terzian, A.L.; Drago, F.; Wotjak, C.T.; Micale, V. The dopamine and cannabinoid interaction in the modulation of emotions and cognition: Assessing the role of cannabinoid CB1 receptor in neurons expressing dopamine D1 receptors. Front. Behav. Neurosci. 2011, 5, 49. [Google Scholar] [CrossRef] [PubMed]
- Di Bartolomeo, M.; Stark, T.; Maurel, O.M.; Iannotti, F.A.; Kuchar, M.; Ruda-Kucerova, J.; Piscitelli, F.; Laudani, S.; Pekarik, V.; Salomone, S.; et al. Crosstalk between the transcriptional regulation of dopamine D2 and cannabinoid CB1 receptors in schizophrenia: Analyses in patients and in perinatal Δ9-tetrahydrocannabinol-exposed rats. Pharmacol. Res. 2021, 164, 105357. [Google Scholar] [CrossRef]
- Paxinos, G.; Watson, C. The Rat Brain in Stereotaxic Coordinates, 4th ed.; Acad Press: San Diego, CA, USA, 1998. [Google Scholar]
- Lo Pumo, R.; Bellia, M.; Nicosia, A.; Micale, V.; Drago, F. Long-lasting neurotoxicity of prenatal benzene acute exposure in rats. Toxicology 2006, 223, 227–234. [Google Scholar] [CrossRef] [PubMed]
- Tamburella, A.; Micale, V.; Mazzola, C.; Salomone, S.; Drago, F. The selective norepinephrine reuptake inhibitor atomoxetine counteracts behavioral impairments in trimethyltin-intoxicated rats. Eur. J. Pharmacol. 2012, 683, 148–154. [Google Scholar] [CrossRef]
- Drago, F.; Nicolosi, A.; Micale, V.; Lo Menzo, G. Placebo affects the performance of rats in models of depression: Is it a good control for behavioral experiments? Eur. Neuropsychopharmacol. 2001, 11, 209–213. [Google Scholar] [CrossRef]
- Tamburella, A.; Micale, V.; Navarria, A.; Drago, F. Antidepressant properties of the 5-HT4 receptor partial agonist, SL65.0155: Behavioral and neurochemical studies in rats. Prog. Neuropsychopharmacol. Biol. Psychiatry 2009, 33, 1205–1210. [Google Scholar] [CrossRef]
- Pamplona, F.A.; Henes, K.; Micale, V.; Mauch, C.P.; Takahashi, R.N.; Wotjak, C.T. Prolonged fear incubation leads to gener-alized avoidance behavior in mice. J. Psychiatr. Res. 2011, 45, 354–360. [Google Scholar] [CrossRef] [PubMed]
- Direnberger, S.; Mues, M.; Micale, V.; Wotjak, C.T.; Dietzel, S.; Schubert, M.; Scharr, A.; Hassan, S.; Wahl-Schott, C.; Biel, M.; et al. Biocompatibility of a genetically encoded calcium indicator in a transgenic mouse model. Nat. Commun. 2012, 3, 1031. [Google Scholar] [CrossRef]
- Ruda-Kucerova, J.; Amchova, P.; Babinska, Z.; Dusek, L.; Micale, V.; Sulcova, A. Sex differences in the reinstatement of methamphetamine seeking after forced abstinence in sprague-dawley rats. Front. Psychiatry 2015, 6, 91. [Google Scholar] [CrossRef]
- Uttl, L.; Szczurowska, E.; Hajkova, K.; Horsley, R.R.; Štefková, K.; Hložek, T.; Šíchová, K.; Balíková, M.; Kuchař, M.; Micale, V.; et al. Behavioral and pharmacokinetic profile of indole-derived synthetic cannabinoids JWH-073 and JWH-210 as compared to the phytocannabinoid Delta (9)-THC in rats. Front. Neurosci. 2018, 12, 703. [Google Scholar] [CrossRef] [PubMed]
- Raffaele, M.; Kovacovicova, K.; Biagini, T.; Lo Re, O.; Frohlich, J.; Giallongo, S.; Nhan, J.D.; Giannone, A.G.; Cabibi, D.; Ivanov, M.; et al. Nociceptin/orphanin FQ opioid receptor (NOP) selective ligand MCOPPB links anxiolytic and senolytic effects. GeroScience 2021. [Google Scholar] [CrossRef]
- Terzian, A.L.B.; Micale, V.; Wotjak, C.T. Cannabinoid receptor type 1 receptors on GABAergic vs. glutamatergic neurons dif-ferentially gate sex-dependent social interest in mice. Eur. J. Neurosci. 2014, 40, 2293–2298. [Google Scholar] [CrossRef]
- Chiodi, V.; Domenici, M.R.; Biagini, T.; De Simone, R.; Tartaglione, A.M.; Di Rosa, M.; Lo Re, O.; Mazza, T.; Micale, V.; Vin-ciguerra, M. Systemic depletion of histone macroH2A1.1 boosts hippocampal synaptic plasticity and social behavior in mice. FASEB J. 2021, 3, e21793. [Google Scholar] [CrossRef] [PubMed]
- Brancato, A.; Castelli, V.; Lavanco, G.; Tringali, G.; Micale, V.; Kuchar, M.; Pizzolanti, G.; Feo, S.; Cannizzaro, C. Binge-like alcohol exposure in adolescence: Behavioural, neuroendocrine and molecular evidence of abnormal neuroplasticity…and return. Biomedicines 2021, 9, 1161. [Google Scholar] [CrossRef] [PubMed]
- Iannotti, F.A.; Di Marzo, V.; Petrosino, S. Endocannabinoids and endocannabinoid-related mediators: Targets, metabolism and role in neurological disorders. Prog. Lipid Res. 2016, 62, 107–128. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, A.T.; Armstrong, E.A.; Yager, J.Y. Neurodevelopmental reflex testing in neonatal rat pups. J. Vis. Exp. 2017, 122, 55261. [Google Scholar] [CrossRef]
- Antonelli, T.; Tomasini, M.C.; Tattoli, M.; Cassano, T.; Tanganelli, S.; Finetti, S.; Mazzoni, E.; Trabace, L.; Steardo, L.; Cuomo, V.; et al. Prenatal exposure to the CB1 receptor agonist WIN 55,212-2 causes learning disruption associated with impaired cortical NMDA receptor function and emotional reactivity changes in rat offspring. Cereb. Cortex 2005, 15, 2013–2020. [Google Scholar] [CrossRef] [PubMed]
- Gregory, E.H.; Pfaff, D.W. Development of olfactory-guided behavior in infant rats. Physiol. Behav. 1971, 6, 573–576. [Google Scholar] [CrossRef]
- Compton, M.T.; Walker, E.F. Physical manifestations of neurodevelopmental disruption: Are minor physical anomalies part of the syndrome of schizophrenia? Schizophr. Bull. 2009, 35, 425–436. [Google Scholar] [CrossRef]
- Bramon, E.; Murray, R.M. A plausible model of schizophrenia must incorporate psychological and social, as well as neuro developmental, risk factors. Dialogues Clin. Neurosci. 2001, 3, 243–256. [Google Scholar] [PubMed]
- Llorente, R.; Llorente-Berzal, A.; Petrosino, S.; Marco, E.M.; Guaza, C.; Prada, C.; López-Gallardo, M.; Di Marzo, V.; Viveros, M.P. Gender-dependent cellular and biochemical effects of maternal deprivation on the hippocampus of neonatal rats: A pos-sible role for the endocannabinoid system. Dev. Neurobiol. 2008, 68, 1334–1347. [Google Scholar] [CrossRef]
- Seillier, A.; Advani, T.; Cassano, T.; Hensler, J.G.; Giuffrida, A. Inhibition of fatty-acid amide hydrolase and CB1 receptor antagonism differentially affect behavioural responses in normal and PCP-treated rats. Int. J. Neuropsychopharmacol. 2010, 13, 373–386. [Google Scholar] [CrossRef] [PubMed]
- Bisogno, T.; Howell, F.; Williams, G.; Minassi, A.; Cascio, M.G.; Ligresti, A.; Matias, I.; Schiano-Moriello, A.; Paul, P.; Williams, E.J.; et al. Cloning of the first sn1-DAG lipases points to the spatial and temporal regulation of endocannabinoid signaling in the brain. J. Cell Biol. 2003, 163, 463–468. [Google Scholar] [CrossRef] [PubMed]
- Katona, I.; Urbán, G.M.; Wallace, M.; Ledent, C.; Jung, K.M.; Piomelli, D.; Mackie, K.; Freund, T.F. Molecular composition of the endocannabinoid system at glutamatergic synapses. J. Neurosci. 2006, 26, 5628–5637. [Google Scholar] [CrossRef] [PubMed]
- Melis, M.; Pistis, M.; Perra, S.; Muntoni, A.L.; Pillolla, G.; Gessa, G.L. Endocannabinoids mediate presynaptic inhibition of glutamatergic transmission in rat ventral tegmental area dopamine neurons through activation of CB1 receptors. J. Neurosci. 2014, 24, 53–62. [Google Scholar] [CrossRef]
- Poels, E.M.P.; Kegeles, L.S.; Kantrowitz, J.T.; Slifstein, M.; Javitt, D.C.; Lieberman, J.A.; Abi-Dargham, A.; Girgis, R.R. Imaging glutamate in schizophrenia: Review of findings and implications for drug discovery. Mol. Psychiatry 2014, 19, 20–29. [Google Scholar] [CrossRef]
- Gastambide, F.; Cotel, M.C.; Gilmour, G.; O’Neill, M.J.; Robbins, T.W.; Tricklebank, M.D. Selective remediation of reversal learning deficits in the neurodevelopmental MAM model of schizophrenia by a novel mGlu5 positive allosteric modulator. Neuropsychopharmacology 2012, 37, 1057–1066. [Google Scholar] [CrossRef]
- Hradetzky, E.; Sanderson, T.M.; Tsang, T.M.; Sherwood, J.L.; Fitzjohn, S.M.; Lakics, V.; Malik, N.; Schoeffmann, S.; O’Neill, M.J.; Cheng, T.M.K. The methylazoxymethanol acetate (MAM-E17) rat model: Molecular and functional effects in the hippocampus. Neuropsychopharmacology 2012, 37, 364–377. [Google Scholar] [CrossRef] [PubMed]
- Gulchina, Y.; Xu, S.J.; Snyder, M.A.; Elefant, F.; Gao, W.J. Epigenetic mechanisms underlying NMDA receptor hypofunction in the prefrontal cortex of juvenile animals in the MAM model for schizophrenia. J. Neurochem. 2017, 143, 320–333. [Google Scholar] [CrossRef] [PubMed]
- Snyder, M.A.; Adelman, A.E.; Gao, W.J. Gestational methylazoxymethanol exposure leads to NMDAR dysfunction in hippocampus during early development and lasting deficits in learning. Neuropsychopharmacology 2013, 38, 328–340. [Google Scholar] [CrossRef] [PubMed]
- Unger, E.L.; Paul, T.; Murray-Kolb, L.E.; Felt, B.; Jones, B.C.; Beard, J.L. Early iron deficiency alters sensorimotor development and brain monoamines in rats. J. Nutr. 2007, 137, 118–124. [Google Scholar] [CrossRef] [PubMed]
- Weinstock, M. Alterations induced by gestational stress in brain morphology and behaviour of the offspring. Prog. Neurobiol. 2001, 65, 427–451. [Google Scholar] [CrossRef]
- Igonina, T.N.; Ragaeva, D.N.; Tikhonova, M.A.; Petrova, O.M.; Herbeck, Y.E.; Rozhkova, I.N.; Amstislavskaya, T.G.; Amstislavsky, S.Y. Neurodevelopment and behavior in neonatal OXYS rats with genetically determined accelerated senescence. Brain Res. 2018, 1681, 75–84. [Google Scholar] [CrossRef]
- Sarnat, H.B. Immunocytochemical markers of neuronal maturation in human diagnostic neuropathology. Cell Tiss. Res. 2015, 359, 279–294. [Google Scholar] [CrossRef] [PubMed]
- Young, J.W.; Zhou, X.; Geyer, M.A. Animal models of schizophrenia. Curr. Top Behav. Neurosci. 2010, 4, 391–433. [Google Scholar]
- Guidali, C.; Viganò, D.; Petrosino, S.; Zamberletti, E.; Realini, N.; Binelli, G.; Rubino, T.; Di Marzo, V.; Parolaro, D. Cannabinoid CB1 receptor antagonism prevents neurochemical and behavioural deficits induced by chronic phencyclidine. Int. J. Neuro-psychopharmacol. 2011, 14, 17–28. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kruk-Slomka, M.; Budzynska, B.; Slomka, T.; Banaszkiewicz, I.; Biala, G. The influence of the CB1 receptor ligands on the schizophrenia-like effects in mice induced by MK-801. Neurotox. Res. 2016, 30, 658–676. [Google Scholar] [CrossRef]
- Marsicano, G.; Lutz, B. Expression of the cannabinoid receptor CB1 in distinct neuronal subpopulations in the adult mouse forebrain. Eur. J. Neurosci. 1999, 11, 4213–4225. [Google Scholar] [CrossRef]
- Micale, V.; Stepan, J.; Jurik, A.; Pamplona, F.A.; Marsch, R.; Drago, F.; Eder, M.; Wotjak, C.T. Extinction of avoidance behavior by safety learning depends on endocannabinoid signaling in the hippocampus. J. Psychiatr. Res. 2017, 90, 46–59. [Google Scholar] [CrossRef]
- Gessa, G.L.; Melis, M.; Muntoni, A.L.; Diana, M. Cannabinoids activate mesolimbic dopamine neurons by an action on can-nabinoid CB1 receptors. Eur. J. Pharmacol. 1998, 341, 39–44. [Google Scholar] [CrossRef]
- Tzavara, E.T.; Davis, R.J.; Perry, K.W.; Li, X.; Salhoff, C.; Bymaster, F.P.; Witkin, J.M.; Nomikos, G.G. The CB1 receptor an-tagonist SR141716A selectively increases monoaminergic neurotransmission in the medial prefrontal cortex: Implications for therapeutic actions. Br. J. Pharmacol. 2003, 138, 544–553. [Google Scholar] [CrossRef] [PubMed]
- Zamberletti, E.; Piscitelli, F.; Cadeddu, F.; Rubino, T.; Fratta, W.; Fadda, P.; Di Marzo, V.; Parolaro, D. Chronic blockade of CB (1) receptors reverses startle gating deficits and associated neurochemical alterations in rats reared in isolation. Br. J. Pharmacol. 2011, 167, 1652–1664. [Google Scholar] [CrossRef]
- Pratt, J.A.; Winchester, C.; Egeterton, A.; Cochrane, S.M.; Morris, B.J. Modelling prefrontal cortex deficits in schizophrenia: Implications for treatment. Br. J. Pharmacol. 2008, 153, S465–S470. [Google Scholar] [CrossRef] [PubMed]
- Young, J.W.; Powell, S.B.; Risbrough, V.; Marston, H.M.; Geyer, M.A. Using the MATRICS to guide development of a preclinical cognitive test battery for research in schizophrenia. Pharmacol. Ther. 2009, 122, 150–202. [Google Scholar] [CrossRef]
- Rentzsch, J.; Buntebart, E.; Stadelmeier, A.; Gallinat, J.; Jockers-Scherubl, M.C. Differential effects of chronic cannabis use on preattentional cognitive functioning in abstinent schizophrenic patients and healthy subjects. Schizoph. Res. 2011, 130, 222–227. [Google Scholar] [CrossRef]
- Mas, S.; Gassó, P.; Fernández de Bobadilla, R.; Arnaiz, J.A.; Bernardo, M.; Lafuente, A. Secondary nonmotor negative symp-toms in healthy volunteers after single doses of haloperidol and risperidone: A double-blind, crossover, placebo-controlled trial. Hum. Psychopharmacol. Clin. Exp. 2013, 28, 586–593. [Google Scholar] [CrossRef] [PubMed]
- Seillier, A.; Martinez, A.; Giuffrida, A. Phencyclidine-induced social withdrawal results from from deficient stimulation of cannabinoid CB1 receptors: Implications for schizophrenia. Neuropsychopharmacology 2013, 38, 1816–1824. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Gogos, A.; Langmead, C.; Sullivan, J.C.; Lawrence, A.J. The importance of sex differences in pharmacology research. Br. J. Pharmacol. 2019, 176, 4087–4089. [Google Scholar] [CrossRef]
- Rubino, T.; Parolaro, D. Sexually dimorphic effects of cannabinoid compounds on emotion and cognition. Front. Behav. Neurosci. 2011, 5, 64. [Google Scholar] [CrossRef]
- Fattore, L.; Fratta, W. How important are sex differences in cannabinoid action? Br. J. Pharmacol. 2010, 160, 544–548. [Google Scholar] [CrossRef] [PubMed]
- Micale, V.; Drago, F.; Noerregaard, P.K.; Elling, C.E.; Wotjak, C.T. The cannabinoid CB1 antagonist TM38837 with limited penetrance to the brain shows reduced fear-promoting effects in mice. Front. Pharmacol. 2019, 10, 207. [Google Scholar] [CrossRef] [PubMed]
- Murphy, T.; Le Foll, B. Targeting the endocannabinoid CB1 Receptor to treat body weight disorders: A preclinical and clinical review of the therapeutic potential of past and present CB1 drugs. Biomolecules 2020, 10, 855. [Google Scholar] [CrossRef] [PubMed]
- Hsu, K.L.; Tsuboi, K.; Adibekian, A.; Pugh, H.; Masuda, K.; Cravatt, B.F. DAGLβ inhibition perturbs a lipid network involved in macrophage inflammatory responses. Nat. Chem. Biol. 2012, 8, 999–1007. [Google Scholar] [CrossRef] [PubMed]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stark, T.; Iannotti, F.A.; Di Martino, S.; Di Bartolomeo, M.; Ruda-Kucerova, J.; Piscitelli, F.; Wotjak, C.T.; D’Addario, C.; Drago, F.; Di Marzo, V.; et al. Early Blockade of CB1 Receptors Ameliorates Schizophrenia-like Alterations in the Neurodevelopmental MAM Model of Schizophrenia. Biomolecules 2022, 12, 108. https://doi.org/10.3390/biom12010108
Stark T, Iannotti FA, Di Martino S, Di Bartolomeo M, Ruda-Kucerova J, Piscitelli F, Wotjak CT, D’Addario C, Drago F, Di Marzo V, et al. Early Blockade of CB1 Receptors Ameliorates Schizophrenia-like Alterations in the Neurodevelopmental MAM Model of Schizophrenia. Biomolecules. 2022; 12(1):108. https://doi.org/10.3390/biom12010108
Chicago/Turabian StyleStark, Tibor, Fabio Arturo Iannotti, Serena Di Martino, Martina Di Bartolomeo, Jana Ruda-Kucerova, Fabiana Piscitelli, Carsten T. Wotjak, Claudio D’Addario, Filippo Drago, Vincenzo Di Marzo, and et al. 2022. "Early Blockade of CB1 Receptors Ameliorates Schizophrenia-like Alterations in the Neurodevelopmental MAM Model of Schizophrenia" Biomolecules 12, no. 1: 108. https://doi.org/10.3390/biom12010108
APA StyleStark, T., Iannotti, F. A., Di Martino, S., Di Bartolomeo, M., Ruda-Kucerova, J., Piscitelli, F., Wotjak, C. T., D’Addario, C., Drago, F., Di Marzo, V., & Micale, V. (2022). Early Blockade of CB1 Receptors Ameliorates Schizophrenia-like Alterations in the Neurodevelopmental MAM Model of Schizophrenia. Biomolecules, 12(1), 108. https://doi.org/10.3390/biom12010108











