Liver Stiffness, Albuminuria and Chronic Kidney Disease in Patients with NAFLD: A Systematic Review and Meta-Analysis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Data Sources and Search Strategy
2.2. Study Selection
2.3. Data Extraction and Quality Assessment
2.4. Data Synthesis and Statistical Analysis
3. Results
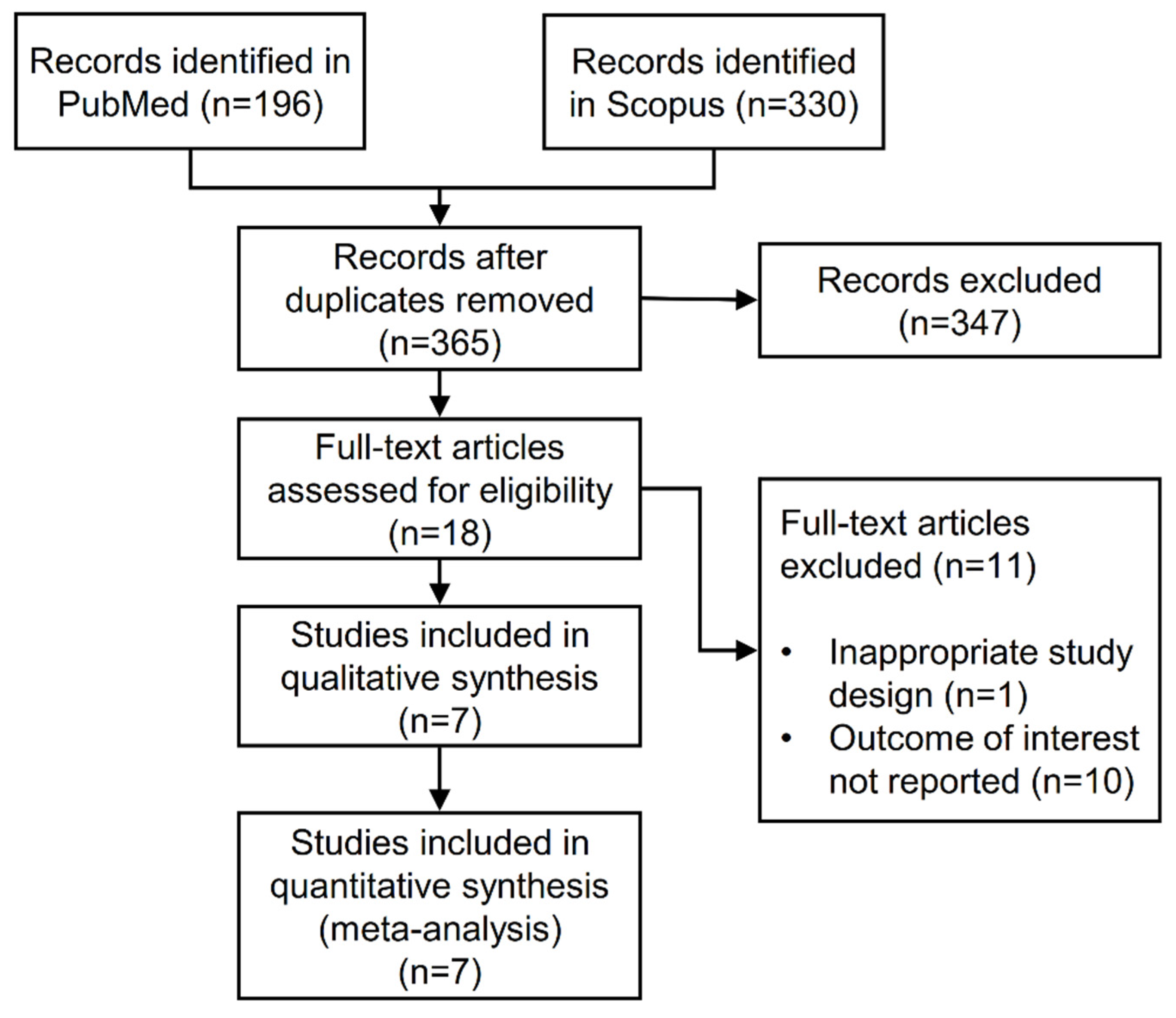
3.1. Search Results
3.2. Features of the Included Articles
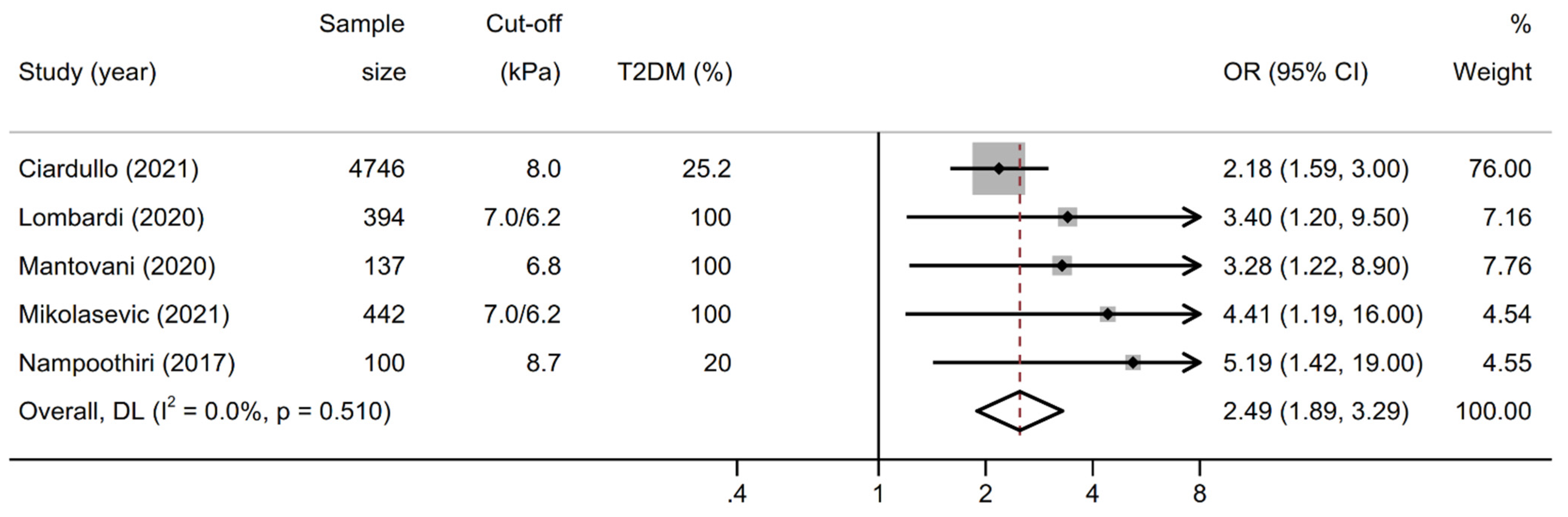
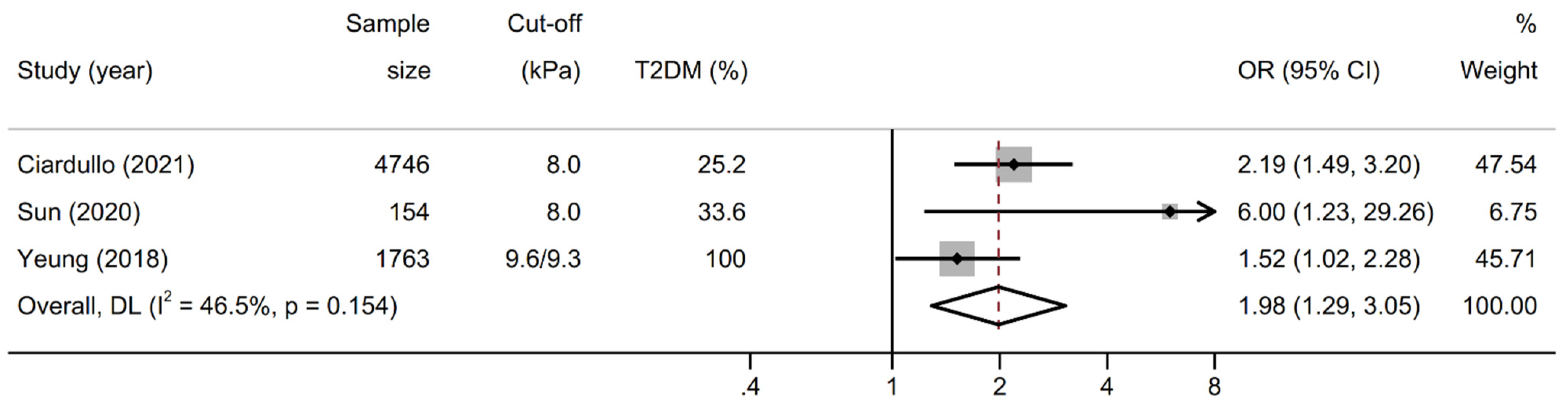
3.3. Association between Liver Stiffness and Kidney Outcomes
3.4. Sensitivity Analyses and Risk of Publication Bias
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Younossi, Z.M.; Koenig, A.B.; Abdelatif, D.; Fazel, Y.; Henry, L.; Wymer, M. Global epidemiology of nonalcoholic fatty liver disease-Meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology 2016, 64, 73–84. [Google Scholar] [CrossRef] [Green Version]
- Ciardullo, S.; Perseghin, G. Prevalence of NAFLD, MAFLD and associated advanced fibrosis in the contemporary United States population. Liver Int. 2021, 41, 1290–1293. [Google Scholar] [CrossRef]
- Ciardullo, S.; Cannistraci, R.; Mazzetti, S.; Mortara, A.; Perseghin, G. Nonalcoholic Fatty Liver Disease, Liver Fibrosis and Cardiovascular Disease in the Adult US Population. Front. Endocrinol. 2021, 12. [Google Scholar] [CrossRef]
- Targher, G.; Day, C.P.; Bonora, E. Risk of Cardiovascular Disease in Patients with Nonalcoholic Fatty Liver Disease. N. Engl. J. Med. 2010, 363, 1341–1350. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Younossi, Z.M.; Stepanova, M.; Ong, J.; Trimble, G.; Alqahtani, S.; Younossi, I.; Ahmed, A.; Racila, A.; Henry, L. Nonalcoholic Steatohepatitis Is the Most Rapidly Increasing Indication for Liver Transplantation in the United States. Clin. Gastroenterol. Hepatol. 2020, 19, 580–589.e5. [Google Scholar] [CrossRef] [PubMed]
- Taylor, R.S.; Taylor, R.J.; Bayliss, S.; Hagström, H.; Nasr, P.; Schattenberg, J.M.; Ishigami, M.; Toyoda, H.; Wong, V.W.-S.; Peleg, N.; et al. Association Between Fibrosis Stage and Outcomes of Patients With Nonalcoholic Fatty Liver Disease: A Systematic Review and Meta-Analysis. Gastroenterology 2020, 158, 1611–1625.e12. [Google Scholar] [CrossRef] [Green Version]
- Dulai, P.S.; Singh, S.; Patel, J.; Soni, M.; Prokop, L.J.; Younossi, Z.; Sebastiani, G.; Ekstedt, M.; Hagstrom, H.; Nasr, P. Increased risk of mortality by fibrosis stage in nonalcoholic fatty liver disease: Systematic review and meta-analysis. Hepatology 2017, 65, 1557–1565. [Google Scholar] [CrossRef]
- Targher, G.; Byrne, C.D.; Lonardo, A.; Zoppini, G.; Barbui, C. Non-alcoholic fatty liver disease and risk of incident cardiovascular disease: A meta-analysis. J. Hepatol. 2016, 65, 589–600. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mantovani, A.; Zaza, G.; Byrne, C.D.; Lonardo, A.; Zoppini, G.; Bonora, E.; Targher, G. Nonalcoholic fatty liver disease increases risk of incident chronic kidney disease: A systematic review and meta-analysis. Metabolism 2018, 79, 64–76. [Google Scholar] [CrossRef]
- Mantovani, A.; Petracca, G.; Beatrice, G.; Csermely, A.; Tilg, H.; Byrne, C.D.; Targher, G. Non-alcoholic fatty liver disease and increased risk of incident extrahepatic cancers: A meta-analysis of observational cohort studies. Gut 2021. [Google Scholar] [CrossRef]
- Mantovani, A.; Petracca, G.; Beatrice, G.; Csermely, A.; Lonardo, A.; Schattenberg, J.M.; Tilg, H.; Byrne, C.D.; Targher, G. Non-alcoholic fatty liver disease and risk of incident chronic kidney disease: An updated meta-analysis. Gut 2020. [Google Scholar] [CrossRef]
- Rockey, D.C.; Caldwell, S.H.; Goodman, Z.D.; Nelson, R.C.; Smith, A.D. Liver biopsy. Hepatology 2008, 49, 1017–1044. [Google Scholar] [CrossRef]
- Castera, L.; Friedrich-Rust, M.; Loomba, R. Noninvasive Assessment of Liver Disease in Patients with Nonalcoholic Fatty Liver Disease. Gastroenterology 2019, 156, 1264–1281.e4. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ciardullo, S.; Ballabeni, C.; Trevisan, R.; Perseghin, G. Liver fibrosis assessed by transient elastography is independently associated with albuminuria in the general United States population. Dig. Liver. Dis. 2021, 53, 866–872. [Google Scholar] [CrossRef] [PubMed]
- Ciardullo, S.; Monti, T.; Perseghin, G. High Prevalence of Advanced Liver Fibrosis Assessed by Transient Elastography Among U.S. Adults With Type 2 Diabetes. Diabetes Care 2020, 44, 519–525. [Google Scholar] [CrossRef] [PubMed]
- Mikolasevic, I.; Rahelic, D.; Turk-Wensween, T.; Ruzic, A.; Domislovic, V.; Hauser, G.; Matic, T.; Radic-Kristo, D.; Krznaric, Z.; Radic, M.; et al. Significant liver fibrosis, as assessed by fibroscan, is independently associated with chronic vascular complications of type 2 diabetes: A multicenter study. Diabetes Res. Clin. Pract. 2021, 177, 108884. [Google Scholar] [CrossRef] [PubMed]
- Lombardi, R.; Airaghi, L.; Targher, G.; Serviddio, G.; Maffi, G.; Mantovani, A.; Maffeis, C.; Colecchia, A.; Villani, R.; Rinaldi, L.; et al. Liver fibrosis by FibroScan(®) independently of established cardio-vascular risk parameters associates with macrovascular and microvascular complications in patients with type 2 diabetes. Liver Int. Off. J. Int. Assoc. Study Liver 2020, 40, 347–354. [Google Scholar]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G.; Group, P. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. PLoS Med. 2009, 6, e1000097. [Google Scholar] [CrossRef] [Green Version]
- Stroup, D.F.; Berlin, J.A.; Morton, S.C.; Olkin, I.; Williamson, G.D.; Rennie, D.; Moher, D.; Becker, B.J.; Sipe, T.A.; Thacker, S.B.; et al. Meta-analysis of observational studies in epidemiology: A proposal for reporting. JAMA 2000, 283, 2008–2012. [Google Scholar] [CrossRef]
- Higgins, J.P.; Thomas, J.; Chandler, J.; Cumpston, M.; Li, T.; Page, M.J.; Welch, V.A. Cochrane Handbook for Systematic Reviews of Interventions; John Wiley & Sons: Hoboken, NJ, USA, 2019. [Google Scholar]
- Higgins, J.P.; Thompson, S.G.; Deeks, J.J.; Altman, D.G. Measuring inconsistency in meta-analyses. BMJ 2003, 327, 557–560. [Google Scholar] [CrossRef] [Green Version]
- Begg, C.B.; Mazumdar, M. Operating characteristics of a rank correlation test for publication bias. Biometrics 1994, 50, 1088–1101. [Google Scholar] [CrossRef] [PubMed]
- Berzigotti, A.; Tsochatzis, E.; Boursier, J.; Castera, L.; Cazzagon, N.; Friedrich-Rust, M.; Petta, S.; Thiele, M. EASL Clinical Practice Guidelines on non-invasive tests for evaluation of liver disease severity and prognosis—2021 update. J. Hepatol. 2021, 75, 659–689. [Google Scholar] [CrossRef] [PubMed]
- Byrne, C.D.; Targher, G. NAFLD as a driver of chronic kidney disease. J. Hepatol. 2020, 72, 785–801. [Google Scholar] [CrossRef] [Green Version]
- Romeo, S.; Kozlitina, J.; Xing, C.; Pertsemlidis, A.; Cox, D.; Pennacchio, L.A.; Boerwinkle, E.; Cohen, J.C.; Hobbs, H.H. Genetic variation in PNPLA3 confers susceptibility to nonalcoholic fatty liver disease. Nat. Genet. 2008, 40, 1461–1465. [Google Scholar] [CrossRef] [Green Version]
- Oniki, K.; Saruwatari, J.; Izuka, T.; Kajiwara, A.; Morita, K.; Sakata, M.; Otake, K.; Ogata, Y.; Nakagawa, K. Influence of the PNPLA3 rs738409 polymorphism on non-alcoholic fatty liver disease and renal function among normal weight subjects. PLoS ONE 2015, 10, e0132640. [Google Scholar] [CrossRef] [Green Version]
- Yilmaz, Y.; Alahdab, Y.O.; Yonal, O.; Kurt, R.; Kedrah, A.E.; Celikel, C.A.; Ozdogan, O.; Duman, D.; Imeryuz, N.; Avsar, E. Micro-albuminuria in nondiabetic patients with nonalcoholic fatty liver disease: Association with liver fibrosis. Metabolism 2010, 59, 1327–1330. [Google Scholar] [CrossRef]
- Mantovani, A.; Turino, T.; Lando, M.G.; Gjini, K.; Byrne, C.D.; Zusi, C.; Ravaioli, F.; Colecchia, A.; Maffeis, C.; Salvagno, G.; et al. Screening for non-alcoholic fatty liver disease using liver stiffness measurement and its association with chronic kidney disease and cardiovascular complications in patients with type 2 diabetes. Diabetes Metab. 2020, 46, 296–303. [Google Scholar] [CrossRef] [PubMed]
- Nampoothiri, R.V.; Duseja, A.; Rathi, M.; Agrawal, S.; Sachdeva, N.; Mehta, M.; Dhaliwal, H.S.; Dhiman, R.K.; Chawla, Y. Renal Dysfunction in Patients With Nonalcoholic Fatty Liver Disease is Related to the Presence of Diabetes Mellitus and Severity of Liver Disease. J. Clin. Exp. Hepatol. 2019, 9, 22–28. [Google Scholar] [CrossRef]
- Sun, D.Q.; Ye, F.Z.; Kani, H.T.; Yang, J.R.; Zheng, K.I.; Zhang, H.Y.; Targher, G.; Byrne, C.D.; Chen, Y.P.; Yuan, W.J.; et al. Higher liver stiffness scores are associated with early kidney dysfunction in patients with histologically proven non-cirrhotic NAFLD. Diabetes Metab. 2020, 46, 288–295. [Google Scholar] [CrossRef]
- Yeung, M.W.; Wong, G.L.H.; Choi, K.C.; Luk, A.O.Y.; Kwok, R.; Shu, S.S.T.; Chan, A.W.H.; Lau, E.S.H.; Ma, R.C.W.; Chan, H.L.Y.; et al. Advanced liver fibrosis but not steatosis is independently associated with albuminuria in Chinese patients with type 2 diabetes. J. Hepatol. 2018, 68, 147–156. [Google Scholar] [CrossRef]
| Author | Year | Country | Study Design | Setting | Sample | Male (%) | Diabetes (%) | Mean Age (years) | Outcome Assessed | Adjustment | NOS Score |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Ciardullo [14] | 2021 | USA | cross-sectional | general us population | 4746 | 57.4 | 25.2 | 51.0 | CKD, increased UACR | age, sex, race-ethnicity, BMI, diabetes, blood pressure, HbA1c, ACE-ARB therapy, CAP | 8 |
| Lombardi [17] | 2020 | Italy | cross-sectional | five diabetes centers | 394 | 52.0 | 100.0 | 68.0 | CKD | age, sex, smoking, diabetes duration, HbA1c, waist circumference, blood pressure, ACE-ARB therapy, statins, uric acid, LDL, HDL, insulin therapy, steatosis grade | 8 |
| Mantovani [23] | 2020 | Italy | cross-sectional | single diabetes center | 137 | 48.2 | 100.0 | 69.9 | CKD | age, sex, diabetes duration, HbA1c, smoking, blood pressure, dyslipidemia, BMI, HOMA-IR, hs-CRP | 8 |
| Mikolasevic [16] | 2021 | Croatia | cross-sectional | two diabetes centers | 442 | 47.2 | 100.0 | 62.0 | CKD | age, sex, BMI, diabetes duration, blood pressure, dyslipidemia, ACE-ARB therapy, statins, HbA1c, uric acid, hs-CRP | 8 |
| Nampoothiri [24] | 2017 | India | cross-sectional | single medical hospital | 100 | 56.0 | 20.0 | 42.0 | CKD | age, BMI, metabolic syndrome, HOMA-IR, transaminases, steatosis on ultrasound | 7 |
| Sun [25] | 2020 | China | cross-sectional | two medical centers | 154 | 66.4 | 33.6 | 43.1 | increased UACR | age, sex, ethnicity, waist circumference, uric acid, dyslipidemia, blood pressure, diabetes, HOMA-IR | 8 |
| Yeung [26] | 2018 | China | cross-sectional | single medical center | 1763 | 56.0 | 100.0 | 60.7 | increased UACR | age, sex, education, smoking, diabetes medications, statins, diabetes duration, dyslipidemia, HbA1c, retinopathy, blood pressure, ACE-ARB therapy, BMI | 8 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ciardullo, S.; Ballabeni, C.; Trevisan, R.; Perseghin, G. Liver Stiffness, Albuminuria and Chronic Kidney Disease in Patients with NAFLD: A Systematic Review and Meta-Analysis. Biomolecules 2022, 12, 105. https://doi.org/10.3390/biom12010105
Ciardullo S, Ballabeni C, Trevisan R, Perseghin G. Liver Stiffness, Albuminuria and Chronic Kidney Disease in Patients with NAFLD: A Systematic Review and Meta-Analysis. Biomolecules. 2022; 12(1):105. https://doi.org/10.3390/biom12010105
Chicago/Turabian StyleCiardullo, Stefano, Cinzia Ballabeni, Roberto Trevisan, and Gianluca Perseghin. 2022. "Liver Stiffness, Albuminuria and Chronic Kidney Disease in Patients with NAFLD: A Systematic Review and Meta-Analysis" Biomolecules 12, no. 1: 105. https://doi.org/10.3390/biom12010105
APA StyleCiardullo, S., Ballabeni, C., Trevisan, R., & Perseghin, G. (2022). Liver Stiffness, Albuminuria and Chronic Kidney Disease in Patients with NAFLD: A Systematic Review and Meta-Analysis. Biomolecules, 12(1), 105. https://doi.org/10.3390/biom12010105






