TRP Channels as Molecular Targets to Relieve Cancer Pain
Abstract
:1. Introduction
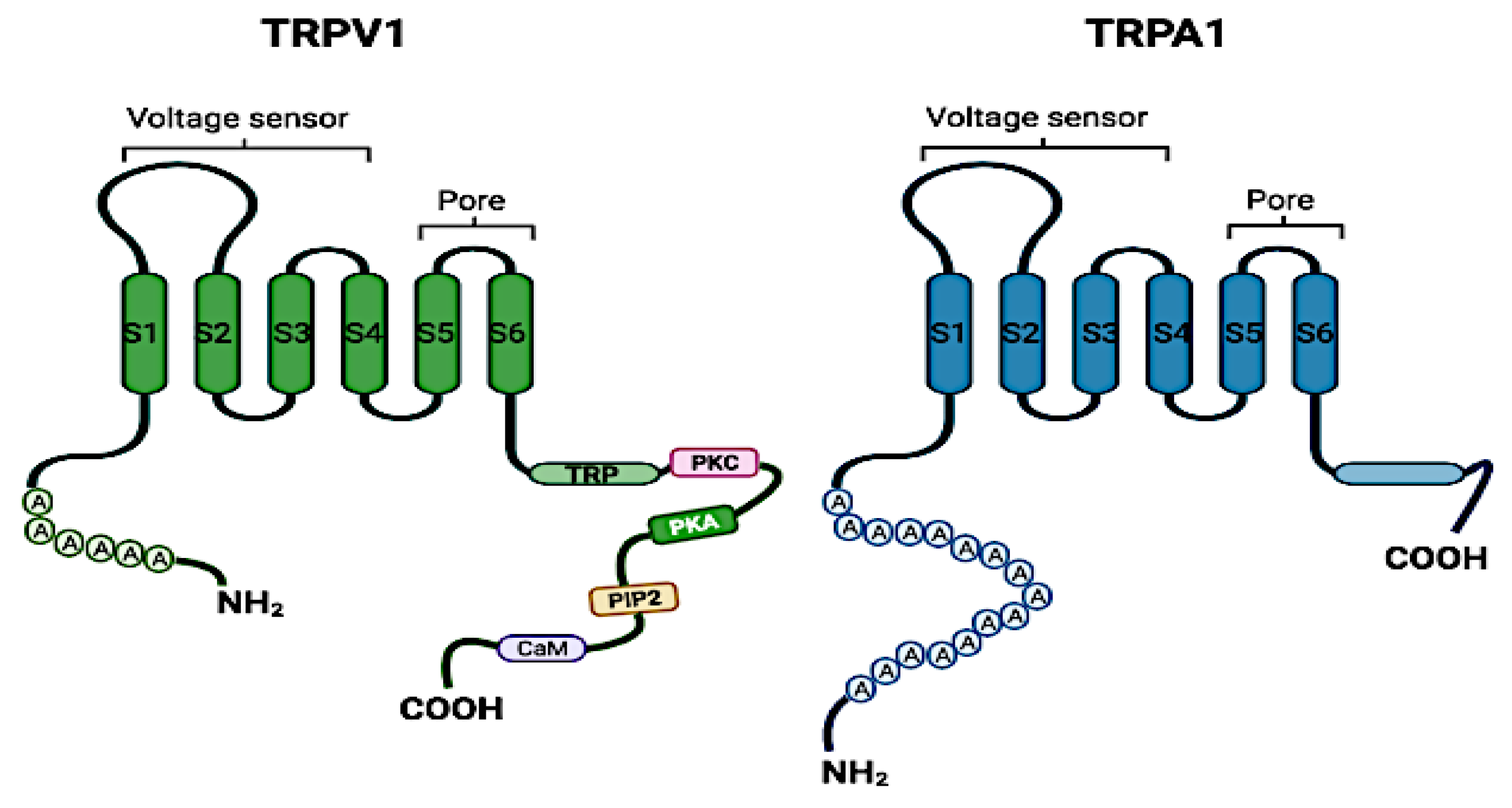
2. The Structure and Physiological Functions of TRP Channels
3. Activation Mechanisms of TRPV1 and TRPA1
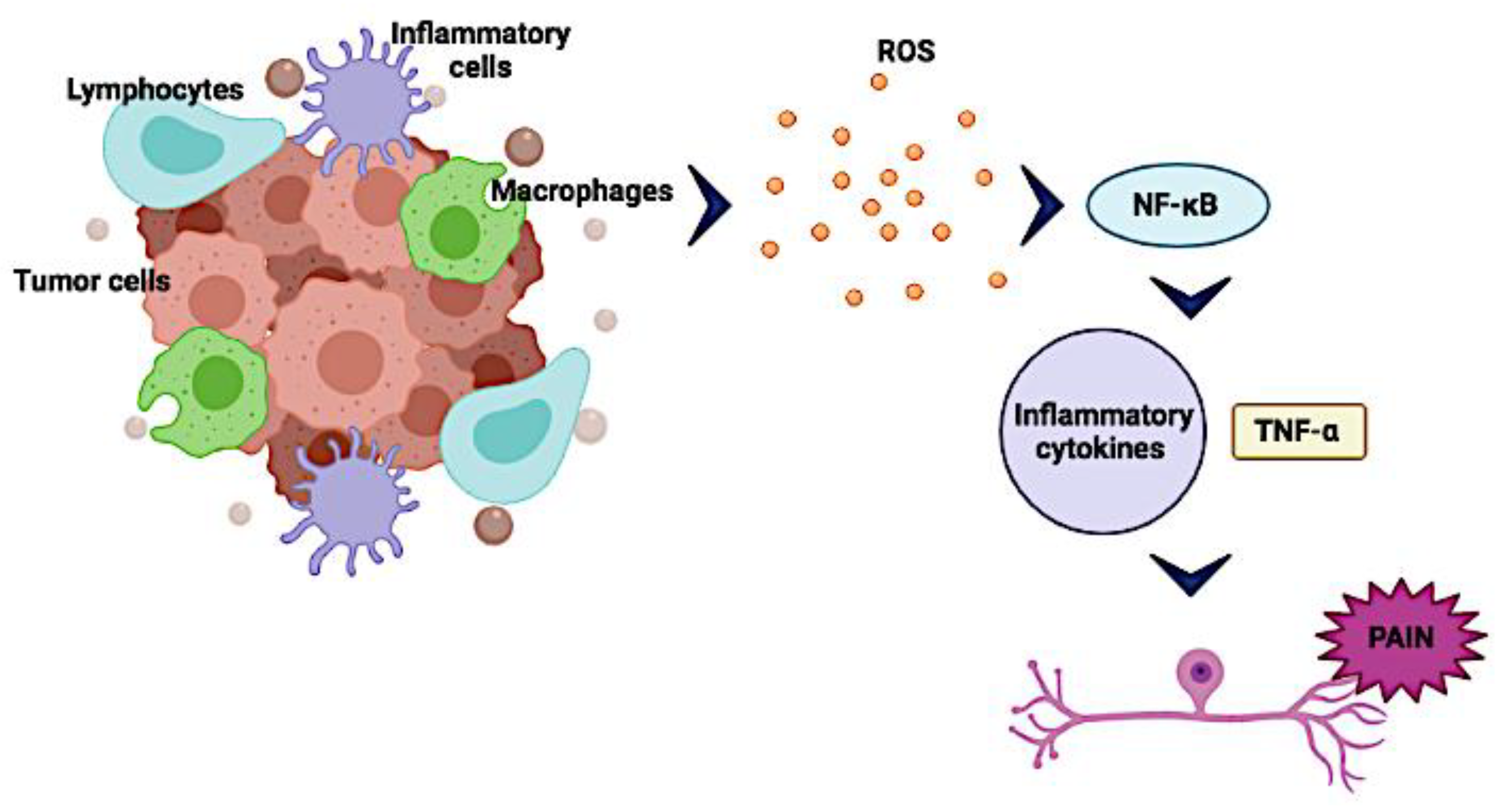
4. Tumor Microenvironment (TM) and Cancer Pain
4.1. Pro-Inflammatory Mediators Associated with Nociceptive Processes
4.2. Acidification of the Tumor Microenvironment
5. The Role of TRP Channels in Cancer Pain
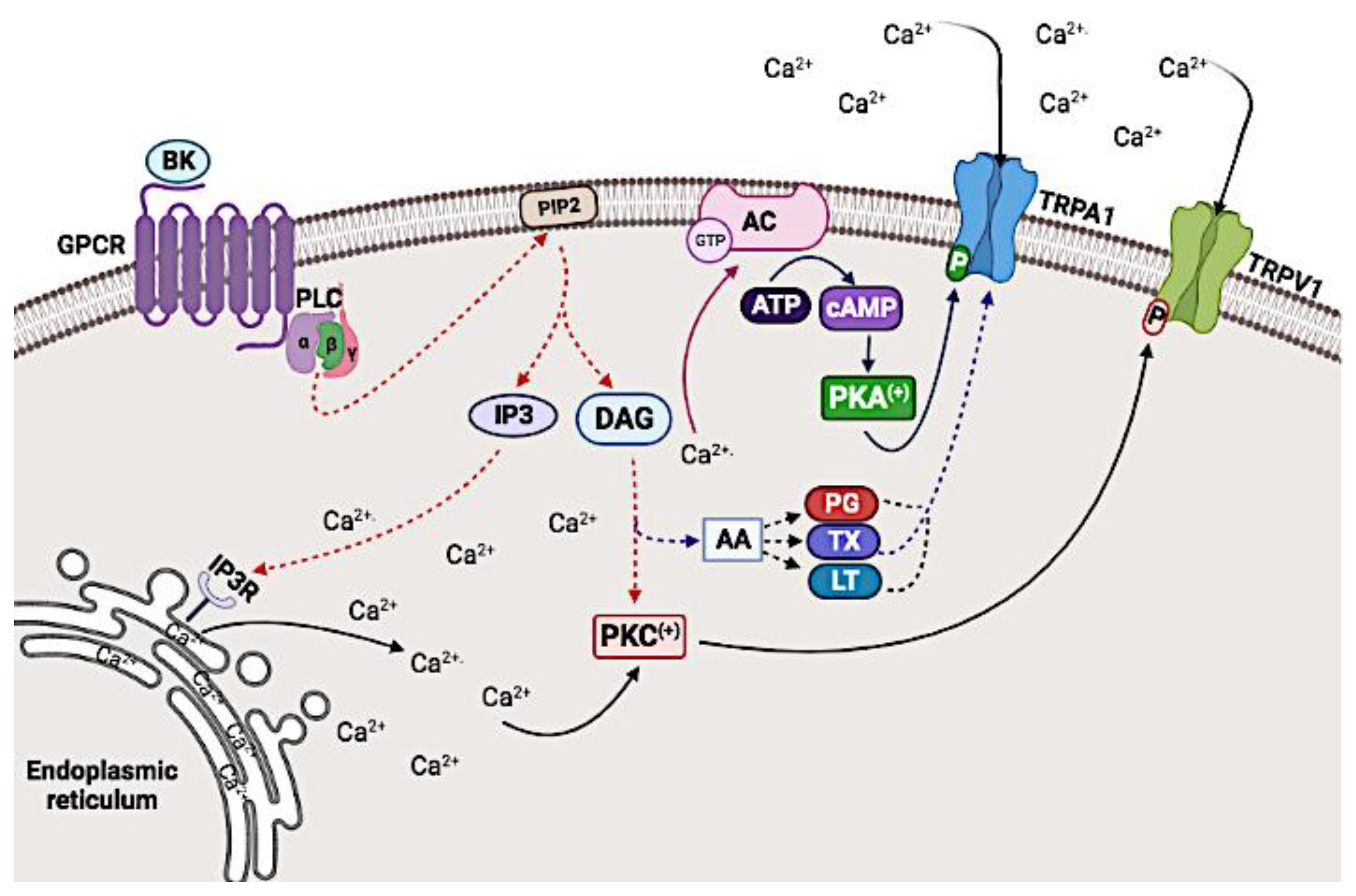
5.1. G protein Coupled Receptor (GPCR)-Mediated Modulation of TRPV1 and TRPA1 Channel Activity
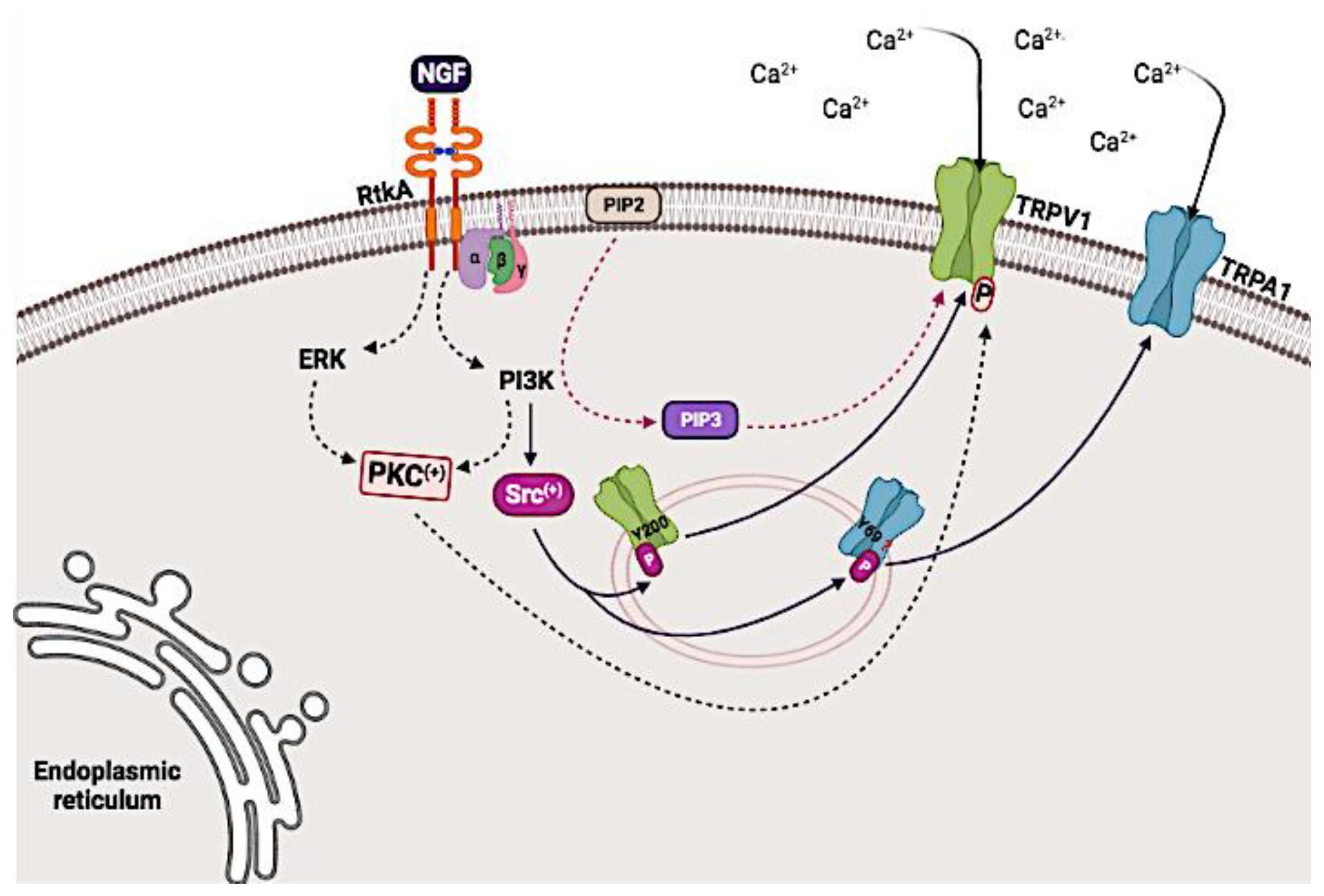
5.2. Modulation of the Activity of the TRPV1 and TRPA1 Channels Mediated through Receptor Tyrosine Kinase A (TrkA)
5.3. Other Mechanisms
6. The Role of TRP Channel Agonists and Antagonists in Cancer Pain
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Catterall, W.A.; Swanson, T.M. Structural basis for pharmacology of voltage-gated sodium and calcium channels. Mol. Pharmacol. 2015, 88, 141–150. [Google Scholar] [CrossRef] [PubMed]
- Harteneck, C.; Plant, T.D.; Schultz, G. From worm to man: Three subfamilies of TRP channels. Trends Neurosci. 2000, 23, 159–166. [Google Scholar] [CrossRef]
- Gaudet, R. TRP channels entering the structural era. J. Physiol. 2008, 586, 3565–3575. [Google Scholar] [CrossRef] [PubMed]
- Nilius, B.; Owsianik, G. The transient receptor potential family of ion channels. Genome Biol. 2011, 12, 218. [Google Scholar] [CrossRef] [Green Version]
- Smith, G.D.; Gunthorpe, M.J.; Kelsell, R.E.; Hayes, P.D.; Reilly, P.; Facer, P.; Wright, J.E.; Jerman, J.C.; Walhin, J.-P.; Ooi, L.; et al. TRPV3 is a temperature-sensitive vanilloid receptor-like protein. Nature 2002, 418, 186–190. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Ramsey, I.S.; Kotecha, S.A.; Moran, M.M.; Chong, J.A.; Lawson, D.; Ge, P.; Lilly, J.; Silos-Santiago, I.; Xie, Y.; et al. TRPV3 is a calcium-permeable temperature-sensitive cation channel. Nature 2002, 418, 181–186. [Google Scholar] [CrossRef]
- Gees, M.; Colsoul, B.; Nilius, B. The role of transient receptor potential cation channels in Ca2+ signaling. Cold Spring Harb. Perspect. Biol. 2010, 2, a003962. [Google Scholar] [CrossRef] [Green Version]
- Samanta, A.; Hughes, T.E.T.; Moiseenkova-Bell, V.Y. Transient receptor potential (TRP) channels. In Subcellular Biochemistry; Springer: New York, NY, USA, 2018; pp. 141–165. [Google Scholar]
- Caterina, M.J.; Rosen, T.A.; Tominaga, M.; Brake, A.J.; Julius, D. A capsaicin-receptor homologue with a high threshold for noxious heat. Nature 1999, 398, 436–441. [Google Scholar] [CrossRef]
- Mandadi, S.; Sokabe, T.; Shibasaki, K.; Katanosaka, K.; Mizuno, A.; Moqrich, A.; Patapoutian, A.; Fukumi-Tominaga, T.; Mizumura, K.; Tominaga, M. TRPV3 in keratinocytes transmits temperature information to sensory neurons via ATP. Pflügers Archiv. 2009, 458, 1093–1102. [Google Scholar] [CrossRef] [Green Version]
- Vennekens, R.; Owsianik, G. Vanilloid transient receptor potential cation channels: An overview. Curr. Pharm. Des. 2008, 14, 18–31. [Google Scholar]
- Legrand, C.; Merlini, J.M.; De Senarclens-Bezençon, C.; Michlig, S. New natural agonists of the transient receptor potential Ankyrin 1 (TRPA1) channel. Sci. Rep. 2020, 10, 11238. [Google Scholar] [CrossRef]
- Laursen, W.J.; Bagriantsev, S.N.; Gracheva, E.O. TRPA1 channels: Chemical and temperature sensitivity. In Current Topics in Membranes; Elsevier: Amsterdam, The Netherlands, 2014; Volume 74, pp. 89–112. [Google Scholar] [CrossRef]
- Meents, J.E.; Ciotu, C.I.; Fischer, M.J.M. Trpa1: A molecular view. J. Neurophysiol. 2019, 121, 427–443. [Google Scholar] [CrossRef]
- Meents, J.E.; Fischer, M.J.; McNaughton, P.A. Sensitization of TRPA1 by protein kinase A. PLoS ONE 2017, 12, e0170097. [Google Scholar] [CrossRef] [PubMed]
- Kheradpezhouh, E.; Choy, J.M.C.; Daria, V.R.; Arabzadeh, E. TRPA1 expression and its functional activation in rodent cortex. Open Biol. 2017, 7, 160314. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Andrei, S.R.; Sinharoy, P.; Bratz, I.N.; Damron, D.S. TRPA1 is functionally co-expressed with TRPV1 in cardiac muscle: Co-localization at z-discs, costameres and intercalated discs. Channels 2016, 10, 395–409. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Wang, S.; Kobayashi, K.; Hao, Y.; Kanda, H.; Kondo, T.; Kogure, Y.; Yamanaka, H.; Yamamoto, S.; Li, J.; et al. TRPA1-expressing lamina propria mesenchymal cells regulate colonic motility. JCI Insight 2019, 9, e122402. [Google Scholar] [CrossRef]
- Büch, T.R.H.; Schäfer, E.A.M.; Demmel, M.-T.; Boekhoff, I.; Thiermann, H.; Gudermann, T.; Steinritz, D.; Schmidt, A. Functional expression of the transient receptor potential channel TRPA1, a sensor for toxic lung inhalants, in pulmonary epithelial cells. Chem. Biol. Interact. 2013, 206, 462–471. [Google Scholar] [CrossRef]
- Du, S.; Araki, I.; Kobayashi, H.; Zakoji, H.; Sawada, N.; Takeda, M. Differential expression profile of cold (TRPA1) and cool (TRPM8) receptors in human urogenital organs. Urology 2008, 72, 450–455. [Google Scholar] [CrossRef]
- Kochukov, M.; McNearney, T.A.; Fu, Y.; Westlund, K.N. Thermosensitive TRP ion channels mediate cytosolic calcium response in human synoviocytes. Am. J. Physiol. Cell Physiol. 2006, 291, C424–C432. [Google Scholar] [CrossRef]
- Osterloh, M.; Böhm, M.; Kalbe, B.; Osterloh, S.; Hatt, H. Identification and functional characterization of TRPA1 in human myoblasts. Pflügers Archiv. 2015, 468, 321–333. [Google Scholar] [CrossRef]
- Takahashi, N.; Mizuno, Y.; Kozai, D.; Yamamoto, S.; Kiyonaka, S.; Shibata, T.; Uchida, K.; Mori, Y. Molecular characterization of TRPA1 channel activation by cysteine-reactive inflammatory mediators. Channels 2008, 2, 287–298. [Google Scholar] [CrossRef] [Green Version]
- Corey, D.P.; Garcia-Añoveros, J.; Holt, J.R.; Kwan, K.Y.; Lin, S.Y.; Vollrath, M.A.; Zhang, D.S. TRPA1 is a candidate for the mechanosensitive transduction channel of vertebrate hair cells. Nature 2004, 432, 723–730. [Google Scholar] [CrossRef] [PubMed]
- Nagata, K.; Duggan, A.; Kumar, G.; García-Añoveros, J. Nociceptor and hair cell transducer properties of TRPA1, a channel for pain and hearing. J. Neurosci. 2005, 25, 4052–4061. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nilius, B. Transient Receptor Potential (TRP) channels in the Brain: The good and the ugly. Eur. Rev. 2012, 20, 343–355. [Google Scholar] [CrossRef]
- Katz, B.; Payne, R.; Minke, B. TRP channels in vision. In Neurobiology of TRP Channels; CRC Press: Boca Raton, FL, USA, 2017; pp. 27–63. [Google Scholar]
- Cortright, D.W.; Szallasi, A. Biochemical pharmacology of the vanilloid receptor TRPV1: An update. Eur. J. Biochem. 2004, 271, 1814–1819. [Google Scholar] [CrossRef]
- Jung, J.; Shin, J.S.; Lee, S.Y.; Hwang, S.W.; Koo, J.; Cho, H.; Oh, U. Phosphorylation of vanilloid receptor 1 by Ca2+/Calmodulin-dependent kinase II regulates Its vanilloid binding. J. Biol. Chem. 2004, 279, 7048–7054. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Caterina, M.J.; Schumacher, M.A.; Tominaga, M.; Rosen, T.A.; Levine, J.D.; Julius, D. The capsaicin receptor: A heat-activated ion channel in the pain pathway. Nature 1997, 389, 816–824. [Google Scholar] [CrossRef]
- Tominaga, M.; Caterina, M.J.; Malmberg, A.B.; Rosen, T.A.; Gilbert, H.; Skinner, K.; Julius, D. The cloned capsaicin receptor integrates multiple pain-producing stimuli. Neuron 1998, 21, 531–543. [Google Scholar] [CrossRef] [Green Version]
- Cao, E. Structural mechanisms of transient receptor potential ion channels. J. Gen. Physiol. 2020, 152, e201811998. [Google Scholar] [CrossRef]
- Hwang, I.; Harper, J.F.; Liang, F.; Sze, H. Calmodulin activation of an endoplasmic reticulum-located calcium pump involves an interaction with the N-terminal autoinhibitory domain. Plant Physiol. 2000, 122, 157–168. [Google Scholar] [CrossRef] [Green Version]
- Bhave, G.; Zhu, W.; Wang, H.; Brasier, D.J.; Oxford, G.S.; Gereau, R.W., IV. cAMP-dependent protein kinase regulates desensitization of the capsaicin receptor (VR1) by direct phosphorylation. Neuron 2002, 35, 721–731. [Google Scholar] [CrossRef] [Green Version]
- Burgess, G.; Mullaney, I.; McNeill, M.; Dunn, P.; Rang, H. Second messengers involved in the mechanism of action of bradykinin in sensory neurons in culture. J. Neurosci. 1989, 9, 3314–3325. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vlachová, V.; Teisinger, J.; Sušánková, K.; Lyfenko, A.; Ettrich, R.; Vyklický, L. Functional role of C-terminal cytoplasmic tail of rat vanilloid receptor 1. J. Neurosci. 2003, 23, 1340–1350. [Google Scholar] [CrossRef] [PubMed]
- Varga, V.; Helenius, J.; Tanaka, K.; Hyman, A.; Tanaka, T.; Howard, J. Yeast kinesin-8 depolymerizes microtubules in a length-dependent manner. Nature 2006, 8, 957–962. [Google Scholar] [CrossRef] [PubMed]
- Rosenbaum, T.; Gordon-Shaag, A.; Munari, M.; Gordon, S.E. Ca2+/Calmodulin modulates TRPV1 activation by capsaicin. J. Gen. Physiol. 2004, 123, 53–62. [Google Scholar] [CrossRef] [Green Version]
- Jin, X.; Morsy, N.; Winston, J.; Pasricha, P.J.; Garrett, K.; Akbarali, H.I. Modulation of TRPV1 by nonreceptor tyrosine kinase, c-Src kinase. Am. J. Physiol. Cell Physiol. 2004, 287, 558–563. [Google Scholar] [CrossRef] [Green Version]
- Shin, J.; Cho, H.; Hwang, S.W.; Jung, J.; Shin, C.Y.; Lee, S.-Y.; Kim, S.H.; Lee, M.G.; Choi, Y.H.; Kim, J.; et al. Bradykinin-12-lipoxygenase-VR1 signaling pathway for inflammatory hyperalgesia. Proc. Natl. Acad. Sci. USA 2002, 99, 10150–10155. [Google Scholar] [CrossRef] [Green Version]
- Zarpelon, A.C.; Cunha, T.M.; Alves-Filho, J.C.; Pinto, L.G.; Ferreira, S.H.; McInnes, I.B.; Verri, W.A., Jr. IL-33/ST2 signalling contributes to carrageenin-induced innate inflammation and inflammatory pain: Role of cytokines, endothelin-1 and prostaglandin E 2. Br. J. Pharmacol. 2013, 169, 90–101. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, L.-J.; Wang, X.-L.; Cui, L.-W.; Liu, Z.; Gao, Y.-M.; Wang, S.; Li, H.; Liu, H.-X. Effects of TRPA1 activation and inhibition on TRPA1 and CGRP expression in dorsal root ganglion neurons. Neural Regen. Res. 2019, 14, 140. [Google Scholar] [CrossRef]
- Viana, F. TRPA1 channels: Molecular sentinels of cellular stress and tissue damage. J. Physiol. 2016, 594, 4151–4169. [Google Scholar] [CrossRef] [Green Version]
- Moparthi, L.; Kichko, T.I.; Eberhardt, M.; Högestätt, E.D.; Kjellbom, P.; Johanson, U.; Zygmunt, P.M. Human TRPA1 is a heat sensor displaying intrinsic U-shaped thermosensitivity. Sci. Rep. 2016, 6, 28763. [Google Scholar] [CrossRef] [PubMed]
- De La Roche, J.; Eberhardt, M.J.; Klinger, A.B.; Stanslowsky, N.; Wegner, F.; Koppert, W.; Leffler, A. The molecular basis for species-specific activation of human TRPA1 protein by protons involves poorly conserved residues within transmembrane domains 5 and 6. J. Biol. Chem. 2013, 288, 20280–20292. [Google Scholar] [CrossRef] [Green Version]
- Zimova, M.; Hackländer, K.; Good, J.M.; Melo-Ferreira, J.; Alves, P.C.; Mills, L.S. Function and underlying mechanisms of seasonal colour moulting in mammals and birds: What keeps them changing in a warming world? Biol. Rev. 2018, 93, 1478–1498. [Google Scholar] [CrossRef] [Green Version]
- Kimura, H. Signaling molecules: Hydrogen sulfide and polysulfide. Antioxid. Redox Signal. 2015, 22, 362–376. [Google Scholar] [CrossRef] [Green Version]
- Chen, J.; Hackos, D.H. TRPA1 as a drug target—Promise and challenges. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2015, 388, 451–463. [Google Scholar] [CrossRef] [Green Version]
- Trevisan, G.; Hoffmeister, C.; Rossato, M.F.; Oliveira, S.M.; Silva, M.A.; Silva, C.R.; Fusi, C.; Tonello, R.; Minocci, D.; Guerra, G.P.; et al. TRPA1 receptor stimulation by hydrogen peroxide is critical to trigger hyperalgesia and inflammation in a model of acute gout. Free Radic. Biol. Med. 2014, 72, 200–209. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fujita, F.; Uchida, K.; Moriyama, T.; Shima, A.; Shibasaki, K.; Inada, H.; Sokabe, T.; Tominaga, M. Intracellular alkalization causes pain sensation through activation of TRPA1 in mice. J. Clin. Investig. 2008, 118, 4049–4057. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yuan, Y.; Jiang, Y.-C.; Sun, C.-K.; Chen, Q.-M. Role of the tumor microenvironment in tumor progression and the clinical applications (Review). Oncol. Rep. 2016, 35, 2499–2515. [Google Scholar] [CrossRef] [Green Version]
- Baghban, R.; Roshangar, L.; Jahanban-Esfahlan, R.; Seidi, K.; Ebrahimi-Kalan, A.; Jaymand, M.; Kolahian, S.; Javaheri, T.; Zare, P. Tumor microenvironment complexity and therapeutic implications at a glance. Cell Commun. Signal. 2020, 18, 59. [Google Scholar] [CrossRef] [Green Version]
- Whiteside, T.L. The tumor microenvironment and its role in promoting tumor growth. Oncogene 2008, 27, 5904–5912. [Google Scholar] [CrossRef] [Green Version]
- Aller, M.; Arias, J.L.; Nava, M.P. Posttraumatic inflammation is a complex response based on the pathological expression of the nervous, immune, and endocrine functional systems. Exp. Biol. Med. 2004, 229, 170–181. [Google Scholar] [CrossRef]
- Lluis, J.M.; Buricchi, F.; Chiarugi, P.; Morales, A.; Fernandez-Checa, J.C. Dual role of mitochondrial reactive oxygen species in hypoxia signaling: Activation of nuclear factor-KB via c-SRC- and oxidant-dependent cell death. Cancer Res. 2007, 67, 7368–7377. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jahanban-Esfahlan, R.; Seidi, K.; Shotorbani, B.B.; Jahanban-Esfahlan, A.; Yousefi, B. Combination of nanotechnology with vascular targeting agents for effective cancer therapy. J. Cell. Physiol. 2017, 233, 2982–2992. [Google Scholar] [CrossRef]
- Jahanban-Esfahlan, R.; Seidi, K.; Zarghami, N. Tumor vascular infarction: Prospects and challenges. Int. J. Hematol. 2017, 105, 244–256. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.-M.; An, J. Cytokines, inflammation and pain. Int. Anesth. Clin. 2009, 69, 482–489. [Google Scholar]
- Schmidt, B.L. The neurobiology of cancer pain. Neuroscience 2014, 20, 546–562. [Google Scholar]
- Ronga, I.; Gallucci, F.; Riccardi, F.; Uomo, G. Anorexia–cachexia syndrome in pancreatic cancer: Recent advances and new pharmacological approach. Adv. Med. Sci. 2014, 59, 1–6. [Google Scholar] [CrossRef]
- Meng, J.; Wang, J.; Steinhoff, M.; Dolly, J.O. TNFα induces co-trafficking of TRPV1/TRPA1 in VAMP1-containing vesicles to the plasmalemma via Munc18-1/syntaxin1/SNAP-25 mediated fusion. Sci. Rep. 2016, 6, 21226. [Google Scholar] [CrossRef]
- Fang, D.; Kong, L.Y.; Cai, J.; Li, S.; Liu, X.D.; Han, J.S.; Xing, G.G. Interleukin-6-mediated functional upregulation of TRPV1 receptors in dorsal root ganglion neurons through the activation of JAK/PI3K signaling pathway: Roles in the development of bone cancer pain in a rat model. Pain 2015, 156, 1124–1144. [Google Scholar] [CrossRef]
- Couture, R.; Harrisson, M.; Vianna, R.M.J.; Cloutier, F. Kinin receptors in pain and inflammation. Eur. J. Pharmacol. 2001, 429, 161–176. [Google Scholar] [CrossRef]
- Fox, A.; Wotherspoon, G.; McNair, K.; Hudson, L.; Patel, S.; Gentry, C.; Winter, J. Regulation and function of spinal and peripheral neuronal B1 bradykinin receptors in inflammatory mechanical hyperalgesia. Pain 2003, 104, 683–691. [Google Scholar] [CrossRef]
- Sevcik, M.A.; Ghilardi, J.R.; Halvorson, K.G.; Lindsay, T.H.; Kubota, K.; Mantyh, P.W. Analgesic efficacy of bradykinin B1 antagonists in a murine bone cancer pain model. J. Pain 2005, 6, 771–775. [Google Scholar] [CrossRef] [PubMed]
- Weisberg, S.P.; McCann, D.; Desai, M.; Rosenbaum, M.; Leibel, R.L.; Ferrante, A.W. Obesity is associated with macrophage accumulation in adipose tissue. J. Clin. Investig. 2003, 112, 1796–1808. [Google Scholar] [CrossRef]
- Schecterson, L.C.; Bothwell, M. Neurotrophin receptors: Old friends with new partners. Dev. Neurobiol. 2010, 70, 332–338. [Google Scholar] [CrossRef] [PubMed]
- Watson, J.J.; Allen, S.J.; Dawbarn, D. Targeting nerve growth factor in pain. BioDrugs 2008, 22, 349–359. [Google Scholar] [CrossRef]
- Jimenez-Andrade, J.M.; Bloom, A.P.; Stake, J.I.; Mantyh, W.; Taylor, R.N.; Freeman, K.T.; Ghilardi, J.R.; Kuskowski, M.A.; Mantyh, P.W. Pathological sprouting of adult nociceptors in chronic prostate cancer-induced bone pain. J. Neurosci. 2010, 30, 14649–14656. [Google Scholar] [CrossRef] [PubMed]
- Ye, Y.; Dang, D.; Zhang, J.; Viet, C.T.; Lam, D.K.; Dolan, J.C.; Gibbs, J.L.; Schmidt, B. Nerve growth factor links oral cancer progression, pain, and cachexia. Mol. Cancer Ther. 2011, 10, 1667–1676. [Google Scholar] [CrossRef] [Green Version]
- Kolokythas, A.; Cox, D.P.; Dekker, N.; Schmidt, B.L. Nerve growth factor and tyrosine kinase a receptor in oral squamous cell carcinoma: Is there an association with perineural invasion? J. Oral Maxillofac. Surg. 2010, 68, 1290–1295. [Google Scholar] [CrossRef]
- Kato, Y.; Ozawa, S.; Miyamoto, C.; Maehata, Y.; Suzuki, A.; Maeda, T.; Baba, Y. Acidic extracellular microenvironment and cancer. Cancer Cell Int. 2013, 13, 89. [Google Scholar] [CrossRef] [Green Version]
- Sennino, B.; McDonald, D.M. Controlling escape from angiogenesis inhibitors. Nat. Rev. Cancer 2012, 12, 699–709. [Google Scholar] [CrossRef]
- Corbet, C.; Feron, O. Cancer cell metabolism and mitochondria: Nutrient plasticity for TCA cycle fueling. Biochim. Et Biophys. Acta Rev. Cancer 2017, 1868, 7–15. [Google Scholar] [CrossRef]
- Corbet, C.; Feron, O. Tumour acidosis: From the passenger to the driver’s seat. Nat. Rev. Cancer 2017, 17, 577–593. [Google Scholar] [CrossRef] [PubMed]
- DeLeo, J.A.; Yezierski, R.P. The role of neuroinflammation and neuroimmune activation in persistent pain. Pain 2001, 90, 1–6. [Google Scholar] [CrossRef]
- Li, B.; Cai, S.; Zhao, Y.; He, Q.; Yu, X.; Cheng, L.; Zhang, Y.; Hu, X.; Ke, M.; Chen, S.; et al. Nerve growth factor modulates the tumor cells migration in ovarian cancer through the WNT/β-catenin pathway. Oncotarget 2016, 7, 81026–81048. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Davar, G. Endothelin-1 and Metastatic Cancer Pain. Pain Med. 2001, 2, 24–27. [Google Scholar] [CrossRef] [Green Version]
- Grace, M.; Birrell, M.A.; Dubuis, E.; Maher, S.A.; Belvisi, M.G. Transient receptor potential channels mediate the tussive response to prostaglandin E2 and bradykinin. Thorax 2012, 67, 891–900. [Google Scholar] [CrossRef] [Green Version]
- Mantyh, P.W. Cancer pain and its impact on diagnosis, survival and quality of life. Nat. Rev. Neurosci. 2006, 7, 797–809. [Google Scholar] [CrossRef]
- Berridge, M.J.; Lipp, P.; Bootman, M.D. The versatility and universality of calcium signalling. Nat. Rev. Mol. Cell Biol. 2000, 1, 11–21. [Google Scholar] [CrossRef] [PubMed]
- Nilius, B.; Szallasi, A. Transient receptor potential channels as drug targets: From the science of basic research to the art of medicine. Pharmacol. Rev. 2014, 66, 676–814. [Google Scholar] [CrossRef]
- Oprée, A.; Kress, M. Involvement of the proinflammatory cytokines tumor necrosis factor-α, IL-1β, and IL-6 but Not IL-8 in the development of heat hyperalgesia: Effects on heat-evoked calcitonin gene-related peptide release from rat skin. J. Neurosci. 2000, 20, 6289–6293. [Google Scholar] [CrossRef] [PubMed]
- Nadler, R.B.; Koch, A.E.; Calhoun, E.A.; Campbell, P.L.; Pruden, D.L.; Bennett, C.L.; Yarnold, P.R.; Schaeffer, A.J. IL-1β and TNF-α IN prostatic secretions are indicators in the evaluation of men with chronic prostatitis. J. Urol. 2000, 164, 214–218. [Google Scholar] [CrossRef]
- Watkins, L.R.; Goehler, L.E.; Relton, J.; Brewer, M.T.; Maier, S.F. Mechanisms of tumor necrosis factor-α (TNF-α) hyperalgesia. Brain Res. 1995, 692, 244–250. [Google Scholar] [CrossRef]
- Poon, R.T.-P.; Fan, S.-T.; Wong, J. Clinical implications of circulating angiogenic factors in cancer patients. J. Clin. Oncol. 2001, 19, 1207–1225. [Google Scholar] [CrossRef] [PubMed]
- Clapham, D.E.; Runnels, L.W.; Strübing, C. The TRP ion channel family_Review. Nat. Rev. Neurosci. 2001, 2, 387–396. [Google Scholar] [CrossRef] [PubMed]
- Cao, E.; Morales, J.C.; Liu, B.; Qin, F.; Julius, D. TRPV1 channels are intrinsically heat sensitive and negatively regulated by phosphoinositide lipids. Neuron 2013, 77, 667–679. [Google Scholar] [CrossRef] [Green Version]
- Mrozkova, P.; Spicarova, D.; Palecek, J. Hypersensitivity induced by activation of spinal cord PAR2 receptors is partially mediated by TRPV1 receptors. PLoS ONE 2016, 11, e0163991. [Google Scholar] [CrossRef] [Green Version]
- Numazaki, M.; Tominaga, T.; Takeuchi, K.; Murayama, N.; Toyooka, H.; Tominaga, M. Structural determinant of TRPV1 desensitization interacts with calmodulin. Proc. Natl. Acad. Sci. USA 2003, 100, 8002–8006. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, S.; Joseph, J.; Ro, J.Y.; Chung, M.K. Modality-specific mechanisms of protein kinase C-induced hypersensitivity of TRPV1: S800 is a polymodal sensitization site. Pain 2015, 156, 931–941. [Google Scholar] [CrossRef] [Green Version]
- Montell, C. The TRP superfamily of cation channels. Sci. STKE 2005, 2005, re3. [Google Scholar] [CrossRef] [Green Version]
- Diogenes, A.; Akopian, A.; Hargreaves, K. NGF up-regulates TRPA1: Implications for orofacial pain. J. Dent. Res. 2007, 86, 550–555. [Google Scholar] [CrossRef]
- Dai, Y.; Wang, S.; Tominaga, M.; Yamamoto, S.; Fukuoka, T.; Higashi, T.; Noguchi, K. Sensitization of TRPA1 by PAR2 contributes to the sensation of inflammatory pain. J. Clin. Investig. 2007, 117, 1979–1987. [Google Scholar] [CrossRef] [Green Version]
- Lukacs, V.; Rives, J.M.; Sun, X.; Zakharian, E.; Rohacs, T. Promiscuous activation of transient receptor potential vanilloid 1 (TRPV1) channels by negatively charged intracellular lipids: The key role of endogenous phosphoinositides in maintaining channel activity. J. Biol. Chem. 2013, 288, 35003–35013. [Google Scholar] [CrossRef] [Green Version]
- Lishko, P.V.; Procko, E.; Jin, X.; Phelps, C.B.; Gaudet, R. The ankyrin repeats of TRPV1 bind multiple ligands and modulate channel sensitivity. Neuron 2007, 54, 905–918. [Google Scholar] [CrossRef] [Green Version]
- Lau, S.-Y.; Procko, E.; Gaudet, R. Distinct properties of Ca2+–calmodulin binding to N- and C-terminal regulatory regions of the TRPV1 channel. J. Gen. Physiol. 2012, 140, 541–555. [Google Scholar] [CrossRef] [Green Version]
- Malin, S.; Molliver, D.; Christianson, J.A.; Schwartz, E.S.; Cornuet, P.; Albers, K.M.; Davis, B.M. TRPV1 and TRPA1 Function and Modulation Are Target Tissue Dependent. J. Neurosci. 2011, 31, 10516–10528. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tominaga, M.; Wada, M.; Masu, M. Potentiation of capsaicin receptor activity by metabotropic ATP receptors as a possible mechanism for ATP-evoked pain and hyperalgesia. Proc. Natl. Acad. Sci. USA 2001, 98, 6951–6956. [Google Scholar] [CrossRef] [Green Version]
- Numazaki, M.; Tominaga, T.; Toyooka, H.; Tominaga, M. Direct phosphorylation of capsaicin receptor VR1 by protein kinase Cε and identification of two target serine residues. J. Biol. Chem. 2002, 277, 13375–13378. [Google Scholar] [CrossRef] [Green Version]
- Sugiura, T.; Kobayashi, Y.; Oka, S.; Waku, K. Biosynthesis and degradation of anandamide and 2-arachidonoylglycerol and their possible physiological significance. Prostaglandins Leukot. Essent. Fat. Acids 2002, 66, 173–192. [Google Scholar] [CrossRef] [PubMed]
- Stein, A.T.; Ufret-Vincenty, C.A.; Hua, L.; Santana, L.; Gordon, S.E. Phosphoinositide 3-kinase binds to TRPV1 and mediates NGF-stimulated TRPV1 trafficking to the plasma membrane. J. Gen. Physiol. 2006, 128, 509–522. [Google Scholar] [CrossRef] [Green Version]
- Por, E.D.; Samelson, B.K.; Belugin, S.; Akopian, A.N.; Scott, J.D.; Jeske, N.A. PP2B/calcineurin-mediated desensitization of TRPV1 does not require AKAP150. Biochem. J. 2010, 432, 549–556. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Southall, M.; Vasko, M. Prostaglandin receptor subtypes, EP3C and EP4, mediate the prostaglandin E2-induced cAMP production and sensitization of sensory neurons. J. Biol. Chem. 2001, 276, 16083–16091. [Google Scholar] [CrossRef] [Green Version]
- Malmberg, A.B.; Brandon, E.P.; Idzerda, R.L.; Liu, H.; McKnight, G.S.; Basbaum, A.I. Diminished inflammation and nociceptive pain with preservation of neuropathic pain in mice with a targeted mutation of the type I regulatory subunit of cAMP-dependent protein kinase. J. Neurosci. 1997, 17, 7462–7470. [Google Scholar] [CrossRef]
- Mohapatra, D.P.; Nau, C. Regulation of Ca2+-dependent desensitization in the vanilloid receptor TRPV1 by calcineurin and cAMP-dependent protein kinase. J. Biol. Chem. 2005, 280, 13424–13432. [Google Scholar] [CrossRef] [Green Version]
- Taylor-Clark, T.E.; Undem, B.J.; MacGlashan, D.W.; Ghatta, S.; Carr, M.J.; McAlexander, M.A. Prostaglandin-induced activation of nociceptive neurons via direct interaction with transient receptor potential A1 (TRPA1). Mol. Pharmacol. 2007, 73, 274–281. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.Y.; Chang, R.B.; Waters, H.N.; McKemy, D.D.; Liman, E.R. The nociceptor ion channel TRPA1 is potentiated and inactivated by permeating calcium ions. J. Biol. Chem. 2008, 283, 32691–32703. [Google Scholar] [CrossRef] [Green Version]
- Ferguson, G.D.; Storm, D.R. Why calcium-stimulated adenylyl cyclases? Physiology 2004, 19, 271–276. [Google Scholar] [CrossRef] [Green Version]
- Jeske, N.A.; Diogenes, A.; Ruparel, N.B.; Fehrenbacher, J.C.; Henry, M.; Akopian, A.N.; Hargreaves, K.M. A-kinase anchoring protein mediates TRPV1 thermal hyperalgesia through PKA phosphorylation of TRPV1. Pain 2008, 138, 604–616. [Google Scholar] [CrossRef] [PubMed]
- Fischer, M.J.; Btesh, J.; McNaughton, P. Disrupting sensitization of transient receptor potential vanilloid subtype 1 inhibits inflammatory hyperalgesia. J. Neurosci. 2013, 33, 7407–7414. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zimova, L.; Barvikova, K.; Macikova, L.; Vyklicka, L.; Sinica, V.; Barvik, I.; Vlachova, V. Proximal C-terminus serves as a signaling hub for TRPA1 channel regulation via its interacting molecules and supramolecular complexes. Front. Physiol. 2020, 11, 189. [Google Scholar] [CrossRef] [PubMed]
- Lukacs, V.; Thyagarajan, B.; Varnai, P.; Balla, A.; Balla, T.; Rohacs, T. Dual regulation of TRPV1 by phosphoinositides. J. Neurosci. 2007, 27, 7070–7080. [Google Scholar] [CrossRef]
- Samad, A.; Sura, L.; Benedikt, J.; Ettrich, R.; Minofar, B.; Teisinger, J.; Vlachova, V. The C-terminal basic residues contribute to the chemical- and voltage-dependent activation of TRPA1. Biochem. J. 2010, 433, 197–204. [Google Scholar] [CrossRef]
- Kádková, A.; Synytsya, V.; Krusek, J.; Zímová, L.; Vlachová, V. Molecular basis of TRPA1 regulation in nociceptive neurons. A review. Physiol. Res. 2017, 66, 425–439. [Google Scholar] [CrossRef] [PubMed]
- Redmond, W.J.; Gu, L.; Camo, M.; McIntyre, P.; Connor, M. Ligand determinants of fatty acid activation of the pronociceptive ion channel TRPA1. PeerJ 2014, 2, e248. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gouin, O.; L’Herondelle, K.; Lebonvallet, N.; Le Gall-Ianotto, C.; Sakka, M.; Buhé, V.; Plée-Gautier, E.; Carré, J.-L.; Lefeuvre, L.; Misery, L.; et al. TRPV1 and TRPA1 in cutaneous neurogenic and chronic inflammation: Pro-inflammatory response induced by their activation and their sensitization. Protein Cell 2017, 8, 644–661. [Google Scholar] [CrossRef] [Green Version]
- Rohacs, T. Regulation of transient receptor potential channels by the phospholipase C pathway. Adv. Biol. Regul. 2013, 53, 341–355. [Google Scholar] [CrossRef] [Green Version]
- Jeske, N.A.; Patwardhan, A.M.; Ruparel, N.B.; Akopian, A.N.; Shapiro, M.S.; Henry, M.A. A-kinase anchoring protein 150 controls protein kinase C-mediated phosphorylation and sensitization of TRPV1. Pain 2009, 146, 301–307. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, X.; Li, L.; McNaughton, P.A. Proinflammatory mediators modulate the heat-activated ion channel TRPV1 via the scaffolding protein AKAP79/150. Neuron 2008, 59, 450–461. [Google Scholar] [CrossRef] [Green Version]
- Docherty, R.J.; Yeats, J.C.; Bevan, S.; Boddeke, H.W.G.M. Inhibition of calcineurin inhibits the desensitization of capsaicin-evoked currents in cultured dorsal root ganglion neurones from adult rats. Pflugers Arch. Eur. J. Physiol. 1996, 431, 828–837. [Google Scholar] [CrossRef]
- Patwardhan, A.M.; Jeske, N.A.; Price, T.; Gamper, N.; Akopian, A.N.; Hargreaves, K.M. The cannabinoid WIN 55,212-2 inhibits transient receptor potential vanilloid 1 (TRPV1) and evokes peripheral antihyperalgesia via calcineurin. Proc. Natl. Acad. Sci. USA 2006, 103, 11393–11398. [Google Scholar] [CrossRef] [Green Version]
- Galoyan, S.M.; Petruska, J.C.; Mendell, L.M. Mechanisms of sensitization of the response of single dorsal root ganglion cells from adult rat to noxious heat. Eur. J. Neurosci. 2003, 18, 535–541. [Google Scholar] [CrossRef]
- Zhu, W.; Oxford, G.S. Phosphoinositide-3-kinase and mitogen activated protein kinase signaling pathways mediate acute NGF sensitization of TRPV1. Mol. Cell. Neurosci. 2007, 34, 689–700. [Google Scholar] [CrossRef] [Green Version]
- Zhuang, Z.-Y.; Xu, H.; Clapham, D.E.; Ji, R.-R. Phosphatidylinositol 3-kinase activates ERK in primary sensory neurons and mediates inflammatory heat hyperalgesia through TRPV1 sensitization. J. Neurosci. 2004, 24, 8300–8309. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bandell, M.; Story, G.M.; Hwang, S.W.; Viswanath, V.; Eid, S.R.; Petrus, M.J.; Earley, T.J.; Patapoutian, A. Noxious cold ion channel TRPA1 is activated by pungent compounds and bradykinin. Neuron 2004, 41, 849–857. [Google Scholar] [CrossRef] [Green Version]
- Chuang, H.-H.; Prescott, E.D.; Kong, H.; Shields, S.; Jordt, S.-E.; Basbaum, A.I.; Chao, M.; Julius, D. Bradykinin and nerve growth factor release the capsaicin receptor from PtdIns(4,5)P2-mediated inhibition. Nature 2001, 411, 957–962. [Google Scholar] [CrossRef]
- Obata, K.; Katsura, H.; Mizushima, T.; Yamanaka, H.; Kobayashi, K.; Dai, Y.; Noguchi, K. TRPA1 induced in sensory neurons contributes to cold hyperalgesia after inflammation and nerve injury (Journal of Clinical Investigation (2005) 115, (2393–2401)). J. Clin. Investig. 2010, 120, 394. [Google Scholar] [CrossRef] [Green Version]
- Andrade, E.; Meotti, F.; Calixto, J. TRPA1 antagonists as potential analgesic drugs. Pharmacol. Ther. 2011, 133, 189–204. [Google Scholar] [CrossRef] [PubMed]
- Doerner, J.F.; Gisselmann, G.; Hatt, H.; Wetzel, C.H. Transient receptor potential channel A1 is directly gated by calcium ions. J. Biol. Chem. 2007, 282, 13180–13189. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morgan, K.; Sadofsky, L.R.; Crow, C.; Morice, A.H. Human TRPM8 and TRPA1 pain channels, including a gene variant with increased sensitivity to agonists (TRPA1 R797T), exhibit differential regulation by SRC-tyrosine kinase inhibitor. Biosci. Rep. 2014, 34, 469–478. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, X.; Huang, J.; McNaughton, P. NGF rapidly increases membrane expression of TRPV1 heat-gated ion channels. EMBO J. 2005, 24, 4211–4223. [Google Scholar] [CrossRef] [Green Version]
- Talavera, K.; Startek, J.B.; Alvarez-Collazo, J.; Boonen, B.; Alpizar, Y.A.; Sanchez, A.; Naert, R.; Nilius, B. Mammalian transient receptor potential TRPA1 channels: From structure to disease. Physiol. Rev. 2020, 100, 725–803. [Google Scholar] [CrossRef]
- Lennertz, R.C.; Kossyreva, E.A.; Smith, A.K.; Stucky, C.L. TRPA1 Mediates Mechanical Sensitization in Nociceptors during Inflammation. PLoS ONE 2012, 7, e43597. [Google Scholar] [CrossRef]
- Bonnington, J.K.; McNaughton, P.A. Signalling pathways involved in the sensitisation of mouse nociceptive neurones by nerve growth factor. J. Physiol. 2003, 551, 433–446. [Google Scholar] [CrossRef]
- Zhu, W.; Galoyan, S.M.; Petruska, J.; Oxford, G.S.; Mendell, L. A developmental switch in acute sensitization of small Dorsal Root Ganglion (DRG) neurons to capsaicin or noxious heating by NGF. J. Neurophysiol. 2004, 92, 3148–3152. [Google Scholar] [CrossRef] [Green Version]
- Neri, D.; Supuran, C.T. Interfering with pH regulation in tumours as a therapeutic strategy. Nat. Rev. Drug Discov. 2011, 10, 767–777. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koplas, P.A.; Rosenberg, R.L.; Oxford, G.S. The role of calcium in the desensitization of capsaicin responses in rat dorsal root ganglion neurons. J. Neurosci. 1997, 17, 3525–3537. [Google Scholar] [CrossRef]
- Martin, T.; Ye, L.; Sanders, A.; Lane, J.; Jiang, W. Cancer invasion and metastasis: Molecular and cellular perspective cancer invasion and metastasis: The role of cell adhesion molecules. Cancer Invasion Metastasis Mol. Cell Perspect 2014, 9, 135–168. [Google Scholar]
- Mantyh, P. Bone cancer pain: Causes, consequences, and therapeutic opportunities. Pain 2013, 154, S54–S62. [Google Scholar] [CrossRef] [PubMed]
- Teitelbaum, S.L. Bone resorption by osteoclasts. Science 2000, 289, 1504–1508. [Google Scholar] [CrossRef] [PubMed]
- Jordt, S.-E.; Tominaga, M.; Julius, D. Acid potentiation of the capsaicin receptor determined by a key extracellular site. Proc. Natl. Acad. Sci. USA 2000, 97, 8134–8139. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hellwig, N.; Plant, T.D.; Janson, W.; Schäfer, M.; Schultz, G. TRPV1 Acts as proton channel to induce acidification in nociceptive neurons. J. Biol. Chem. 2004, 279, 34553–34561. [Google Scholar] [CrossRef] [Green Version]
- Wang, G.J.; Randall, R.D.; Thayer, S.A. Glutamate-induced intracellular acidification of cultured hippocampal neurons demonstrates altered energy metabolism resulting from Ca2+ loads. J. Neurophysiol. 1994, 72, 2563–2569. [Google Scholar] [CrossRef] [PubMed]
- Paalasmaa, P.; Kaila, K. Role of voltage-gated calcium channels in the generation of activity-induced extracellular pH transients in the rat hippocampal slice. J. Neurophysiol. 1996, 75, 2354–2360. [Google Scholar] [CrossRef]
- Wang, Y.Y.; Chang, R.B.; Liman, E.R. TRPA1 is a component of the nociceptive response to CO2. J. Neurosci. 2010, 30, 12958–12963. [Google Scholar] [CrossRef]
- Dai, Y. TRPs and pain. Semin Immunopathol. 2016, 38, 277–291. [Google Scholar] [CrossRef]
- Moparthi, L.; Zygmunt, P.M. Human TRPA1 is an inherently mechanosensitive bilayer-gated ion channel. Cell Calcium 2020, 91, 102255. [Google Scholar] [CrossRef] [PubMed]
- Hayes, J.D.; Dinkova-Kostova, A.T.; Tew, K.D. Oxidative stress in cancer. Cancer Cell 2020, 38, 167–197. [Google Scholar] [CrossRef] [PubMed]
- Lehto, S.G.; Tamir, R.; Deng, H.; Klionsky, L.; Kuang, R.; Le, A.; Lee, D.; Louis, J.-C.; Magal, E.; Manning, B.H.; et al. Antihyperalgesic effects of (R,E)-N-(2-Hydroxy-2,3-dihydro-1H-inden-4-yl)-3-(2-(piperidin-1-yl)-4-(trifluoromethyl)phenyl)-acrylamide (AMG8562), a novel transient receptor potential vanilloid type 1 modulator that does not cause hyperthermia in rats. J. Pharmacol. Exp. Ther. 2008, 326, 218–229. [Google Scholar] [CrossRef] [Green Version]
- Hashim, A.I.; Zhang, X.; Wojtkowiakk, J.W.; Martinez, G.V.; Gillies, R.J. Imaging pH and metastasis. NMR Biomed. 2011, 24, 582–591. [Google Scholar] [CrossRef] [PubMed]
- Stubbs, M.; McSheehy, P.M.; Griffiths, J.R.; Bashford, C. Causes and consequences of tumour acidity and implications for treatment. Mol. Med. Today 2000, 6, 15–19. [Google Scholar] [CrossRef]
- Bode, A.M.; Dong, Z. The Two Faces of Capsaicin: Figure 1. Cancer Res. 2011, 71, 2809–2814. [Google Scholar] [CrossRef] [Green Version]
- Hayman, M.; Kam, P.C. Capsaicin: A review of its pharmacology and clinical applications. Curr. Anaesth. Crit. Care 2008, 19, 338–343. [Google Scholar] [CrossRef]
- Szallasi, A.; Cortright, D.N.; Blum, C.A.; Eid, S.R. The vanilloid receptor TRPV1: 10 years from channel cloning to antagonist proof-of-concept. Nat. Rev. Drug Discov. 2007, 6, 357–372. [Google Scholar] [CrossRef]
- Remadevi, R.; Szallisi, A. Adlea (ALGRX-4975), an injectable capsaicin (TRPV1 receptor agonist) formulation for longlasting pain relief. IDrugs Investig. Drugs J. 2008, 11, 120–132. [Google Scholar]
- Nolano, M.; Simone, D.A.; Wendelschafer-Crabb, G.; Johnson, T.; Hazen, E.; Kennedy, W.R. Topical capsaicin in humans: Parallel loss of epidermal nerve fibers and pain sensation. Pain 1999, 81, 135–145. [Google Scholar] [CrossRef]
- Fattori, V.; Hohmann, M.S.N.; Rossaneis, A.C.; Pinho-Ribeiro, F.A.; Verri, W.A. Capsaicin: Current Understanding of Its Mechanisms and Therapy of Pain and Other Pre-Clinical and Clinical Uses. Molecules 2016, 21, 844. [Google Scholar] [CrossRef] [Green Version]
- Smith, H.; Brooks, J.R. Capsaicin-Based Therapies for Pain Control. Prog. Drug Res. 2014, 68, 129–146. [Google Scholar] [CrossRef]
- Watson, P.N.; Evans, R.J.; Watt, V.R.; Birkett, N. Post-herpetic neuralgia: 208 cases. Pain 1988, 35, 289–297. [Google Scholar] [CrossRef]
- Kiani, J.; Sajedi, F.; Nasrollahi, S.A.; Esna-Ashari, F. A randomized clinical trial of efficacy and safety of the topical clonidine and capsaicin in the treatment of painful diabetic neuropathy. J. Res. Med. Sci. 2015, 20, 359–363. [Google Scholar] [PubMed]
- Zis, P.; Apsokardos, A.; Isaia, C.; Sykioti, P.; Vadalouca, A. Posttraumatic and postsurgical neuropathic pain responsive to treatment with capsaicin 8% topical patch. Pain Physician 2014, 17, E213–E218. [Google Scholar]
- Robbins, W.R.; Staats, P.S.; Levine, J.; Fields, H.L.; Allen, R.W.; Campbell, J.N.; Pappagallo, M. Treatment of intractable pain with topical large-dose capsaicin: Preliminary report. Anesth. Analg. 1998, 86, 579–583. [Google Scholar] [CrossRef]
- Basith, S.; Cui, M.; Hong, S.; Choi, S. Harnessing the Therapeutic Potential of Capsaicin and Its Analogues in Pain and Other Diseases. Molecules 2016, 21, 966. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Derry, S.; Rice, A.S.; Cole, P.; Tan, T.; Moore, R.A. Topical capsaicin (high concentration) for chronic neuropathic pain in adults. Cochrane Database Syst. Rev. 2017, 2021, CD007393. [Google Scholar] [CrossRef] [Green Version]
- Ellison, N.; Loprinzi, C.L.; Kugler, J.; Hatfield, A.K.; Miser, A.; Sloan, J.A.; Wender, D.B.; Rowland, K.M.; Molina, R.; Cascino, T.L.; et al. Phase III placebo-controlled trial of capsaicin cream in the management of surgical neuropathic pain in cancer patients. J. Clin. Oncol. 1997, 15, 2974–2980. [Google Scholar] [CrossRef]
- Berger, A.; Henderson, M.; Nadoolman, W.; Duffy, V.; Cooper, D.; Saberski, L.; Bartoshuck, L. Oral capsaicin provides temporary relief for oral mucositis pain secondary to chemotherapy/radiation therapy. J. Pain Symptom. Manag. 1995, 10, 243–248. [Google Scholar] [CrossRef]
- Bölcskei, K.; Tékus, V.; Dézsi, L.; Szolcsányi, J.; Petho, G. Antinociceptive desensitizing actions of TRPV1 receptor agonists capsaicin, resiniferatoxin and N-oleoyldopamine as measured by determination of the noxious heat and cold thresholds in the rat. Eur. J. Pain 2010, 14, 480–486. [Google Scholar] [CrossRef] [PubMed]
- Ghilardi, J.R.; Röhrich, H.; Lindsay, T.H.; Sevcik, M.A.; Schwei, M.J.; Kubota, K.; Halvorsonj, J.; Poblete, J.; Chaplan, S.; Dublin, A.; et al. Selective blockade of the capsaicin receptor TRPV1 attenuates bone cancer pain. J. Neurosci. 2005, 25, 3126–3131. [Google Scholar] [CrossRef]
- Namazi, H. The effect of epidural resiniferatoxin in the neuropathic pain rat model randomized trial: A complementary mechanism. Pain Physician. 2012, 15, E750. [Google Scholar] [CrossRef]
- Heiss, J.; Iadarola, M.; Cantor, F.; Oughourli, A.; Smith, R.; Mannes, A. (364) A Phase I study of the intrathecal administration of resiniferatoxin for treating severe refractory pain associated with advanced cancer. J. Pain 2014, 15, S67. [Google Scholar] [CrossRef]
- Mannes, A.; Hughes, M.; Quezado, Z.; Berger, A.; Fojo, T.; Smith, R.; Butman, J.; Lonser, R.; Ladarola, M. Resiniferatoxin, a potent TRPV1 agonist: Intrathecal administration to treat severe pain associated with advanced cancer—case report. J. Pain 2010, 11, S43. [Google Scholar] [CrossRef]
- Watson, C.P.; Tyler, K.; Bickers, D.R.; Millikan, L.E.; Smith, S.; Coleman, E. A randomized vehicle-controlled trial of topical capsaicin in the treatment of postherpetic neuralgia. Clin. Ther. 1993, 15, 510–526. [Google Scholar] [CrossRef]
- Gomes Júnior, A.L.; Islam, M.T.; Nicolau, L.A.D.; De Souza, L.K.M.; de Souza Lopes Araújo, T.; Lopes De Oliveira, G.A.; Nogueira, K.; Lopes da Silva, L.; Medieros, J.; Mubarak, M.; et al. Anti-Inflammatory, Antinociceptive, and Antioxidant Properties of Anacardic Acid in Experimental Models. ACS Omega 2020, 5, 19506–19515. [Google Scholar] [CrossRef] [PubMed]
- Bartolome, A.P.; Villaseñor, I.M.; Yang, W.C. Bidens pilosa L. (Asteraceae): Botanical properties, traditional uses, phytochemistry, and pharmacology. Evid.-Based Complementary Altern. Med. 2013, 2013, 340215. [Google Scholar] [CrossRef] [Green Version]
- Xuan, T.D.; Khanh, T.D. Chemistry and pharmacology of Bidens pilosa: An overview. J. Pharm. Investig. 2016, 46, 91–132. [Google Scholar] [CrossRef] [PubMed]
- Guerrini, A.; Sacchetti, G.; Rossi, D.; Paganetto, G.; Muzzoli, M.; Andreotti, E.; Tognolini, M.; Maldonado, M.E.; Bruni, R. Bioactivities of Piper aduncum L. and Piper obliquum Ruiz & Pavon (Piperaceae) essential oils from Eastern Ecuador. Environ. Toxicol. Pharmacol. 2009, 27, 39–48. [Google Scholar] [CrossRef]
- Santos, M.L.; Magalhães, C.F.; da Rosa, M.B.; de Assis Santos, D.; Brasileiro, B.G.; de Carvalho, L.M.; Barreto da Silva, M.; Zani, C.; Pessoa de Siqueira, E.; Peres, R.; et al. Antifungal activity of extracts from Piper aduncum leaves prepared by different solvents and extraction techniques against dermatophytes Trichophyton rubrum and Trichophyton interdigitale. Braz. J. Microbiol. 2013, 44, 1275–1278. [Google Scholar] [CrossRef] [Green Version]
- López, M.A.; Stashenko, E.; Fuentes, J.L. Chemical composition and antigenotoxic properties of Lippia alba essential oils. Genet. Mol. Biol. 2011, 34, 479–488. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ezeonwumelu, J.O.C.; Ntale, M.; Ogbonnia, S.O.; Agwu, E.; Tanayen, J.K.; Adedeji, A.A.; Okonkwo, C.O.; Akunne, A.A.; Ebosie, J.C.; Byarugaba, F. Analgesic Appraisal of Bidens pilosa (Asteraceae) Leaf Extracts Used in Management of Oral Lesion Pain in HIV/AIDS Patients in Rodents. Pharmacol. Pharm. 2018, 9, 175–192. [Google Scholar] [CrossRef] [Green Version]
- Haldar, S.; Kar, B.; Dolai, N.; Kumar, R.B.S.; Behera, B.; Haldar, P.K. In Vivo anti-nociceptive and anti-inflammatory activities of Lippia alba. Asian Pacific J. Trop Dis. 2012, 2, S667–S670. [Google Scholar] [CrossRef]
- McNamara, F.N.; Randall, A.; Gunthorpe, M.J. Effects of piperine, the pungent component of black pepper, at the human vanilloid receptor (TRPV1). Br. J. Pharmacol. 2005, 144, 781–790. [Google Scholar] [CrossRef] [Green Version]
- Pearce, L.V.; Petukhov, P.A.; Szabo, T.; Kedei, N.; Bizik, F.; Kozikowski, A.P.; Blumberg, P. Evodiamine functions as an agonist for the vanilloid receptor TRPV1. Org. Biomol. Chem. 2004, 2, 2281–2286. [Google Scholar] [CrossRef] [Green Version]
- Bisogno, T.; Hanuš, L.; De Petrocellis, L.; Tchilibon, S.; Ponde, D.E.; Brandi, I.; Moriello, A.S.; Davis, J.B.; Mechoulam, R.; Di Marzo, V. Molecular targets for cannabidiol and its synthetic analogues: Effect on vanilloid VR1 receptors and on the cellular uptake and enzymatic hydrolysis of anandamide. Br. J. Pharmacol. 2001, 134, 845–852. [Google Scholar] [CrossRef] [PubMed]
- Andre, E.; Campi, B.; Trevisani, M.; Ferreira, J.; Malheiros, A.; Yunes, R.A.; Calixto, J.B.; Geppetti, P. Pharmacological characterisation of the plant sesquiterpenes polygodial and drimanial as vanilloid receptor agonists. Biochem. Pharmacol. 2006, 71, 1248–1254. [Google Scholar] [CrossRef]
- Szallasi, A.; Jonassohn, M.; Ács, G.; Bíró, T.; Ács, P.; Blumberg, P.; Sterner, O. The stimulation of capsaicin-sensitive neurones in a vanilloid receptor-mediated fashion by pungent terpenoids possessing an unsaturated 1,4-dialdehyde moiety. Br. J. Pharmacol. 1996, 119, 283–290. [Google Scholar] [CrossRef] [Green Version]
- Tóth, A.; Kedei, N.; Szabó, T.; Wang, Y.; Blumberg, P.M. Thapsigargin binds to and inhibits the cloned vanilloid receptor-1. Biochem. Biophys. Res. Commun. 2002, 293, 777–782. [Google Scholar] [CrossRef]
- Dessaint, J.; Yu, W.; Krause, J.E.; Yue, L. Yohimbine inhibits firing activities of rat dorsal root ganglion neurons by blocking Na+ channels and vanilloid VR1 receptors. Eur. J. Pharmacol. 2003, 485, 11–20. [Google Scholar] [CrossRef] [PubMed]
- Jordt, S.E.; Bautista, D.M.; Chuang, H.H.; McKemy, D.D.; Zygmunt, P.M.; Högestätt, E.D.; Högestätt, E.; Meng, I.; Julius, D. Mustard oils and cannabinoids excite sensory nerve fibres through the TRP channel ANKTM1. Nature 2004, 427, 260–265. [Google Scholar] [CrossRef]
- Xu, H.; Delling, M.; Jun, J.C.; Clapham, D.E. Oregano, thyme and clove-derived flavors and skin sensitizers activate specific TRP channels. Nat. Neurosci. 2006, 9, 628–635. [Google Scholar] [CrossRef]
- Macpherson, L.J.; Hwang, S.W.; Miyamoto, T.; Dubin, A.E.; Patapoutian, A.; Story, G.M. More than cool: Promiscuous relationships of menthol and other sensory compounds. Mol. Cell. Neurosci. 2006, 32, 335–343. [Google Scholar] [CrossRef]
- Yang, B.H.; Piao, Z.G.; Kim, Y.-B.; Lee, C.-H.; Lee, J.K.; Park, K.; Kim, J.S.; Oh, S.B. Activation of Vanilloid Receptor 1 (VR1) by Eugenol. J. Dent. Res. 2003, 82, 781–785. [Google Scholar] [CrossRef]
- Iwasaki, Y.; Morita, A.; Iwasawa, T.; Kobata, K.; Sekiwa, Y.; Morimitsu, Y.; Kubota, K.; Watanabe, T. A nonpungent component of steamed ginger-[10]-shogaol-increases adrenaline secretion via the activation of TRPV1. Nutr. Neurosci. 2006, 9, 169–178. [Google Scholar] [PubMed]
- Xu, H.; Blair, N.T.; Clapham, D.E. Camphor Activates and Strongly Desensitizes the Transient Receptor Potential Vanilloid Subtype 1 Channel in a Vanilloid-Independent Mechanism. J. Neurosci. 2005, 25, 8924–8937. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Macpherson, L.; Geierstanger, B.H.; Viswanath, V.; Bandell, M.; Eid, S.R.; Hwang, S.W.; Patapoutian, A. The Pungency of Garlic: Activation of TRPA1 and TRPV1 in Response to Allicin. Curr. Biol. 2005, 15, 929–934. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dolai, N.; Karmakar, I.; Kumar, R.B.S.; Haldar, P.K. CNS depressant activity of Castanopsis indica leaves. Orient. Pharm. Exp. Med. 2012, 12, 135–140. [Google Scholar] [CrossRef]
| Channel | Compound | Source | Activity | Reference | |
|---|---|---|---|---|---|
| Agonist | Antagonist | ||||
| TRPV1 | Capsaicin | Capsicum | † | [30] | |
| Piperine | Piper nigrum | † | [182] | ||
| Resiniferatoxin | Euphorbia resinifera | † | [30] | ||
| Evodiamine | Evodia rutaecarpa | † | [183] | ||
| Cannabidiol | Cannabis sativa | † | [184] | ||
| Polygodial | Polygonum hydropiper | † | [185] | ||
| Isovelleral | Lactarius vellereus | † | [186] | ||
| Tapsigargina | Thapsia garganica | † | [187] | ||
| Yohimbine | Pausinystalia yohimbe | † | [188] | ||
| TRPA1 | Allyl isothiocyanate | Wasabi, mustard oil | † | [189] | |
| Benzyl isothiocyanate | Yellow mustard | † | [189] | ||
| Phenylethyl isothiocyanate | Brussels sprouts | † | [189] | ||
| Isopropyl isothiocyanate | Nasturtium seeds | † | [189] | ||
| Methyl isothiocyanate | Capparis spinosa | † | [189] | ||
| Cinnamaldehyde | Cinnamomum cassia/Cinnamomum zeylanicum | † | [126] | ||
| Methyl salicylate | Wintergreen oil | † | [126] | ||
| Carvacrol | Origanum genus | † | [190] | ||
| Tetrahydrocannabinol | Cannabis genus | † | [189] | ||
| Menthol | Mentha | † | [191] | ||
| Both of them | Eugenol | Clove oil | TRPV1/TRPA1 | [192] | |
| Gingerol | Zingiber officinale | TRPV1/TRPA1 | [126,193] | ||
| Camphor | Cinnamomum camphora | TRPV1 | TRPA1 | [194] | |
| Allicin | Allium sativum | TRPV1/TRPA1 | [195] | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Duitama, M.; Moreno, Y.; Santander, S.P.; Casas, Z.; Sutachan, J.J.; Torres, Y.P.; Albarracín, S.L. TRP Channels as Molecular Targets to Relieve Cancer Pain. Biomolecules 2022, 12, 1. https://doi.org/10.3390/biom12010001
Duitama M, Moreno Y, Santander SP, Casas Z, Sutachan JJ, Torres YP, Albarracín SL. TRP Channels as Molecular Targets to Relieve Cancer Pain. Biomolecules. 2022; 12(1):1. https://doi.org/10.3390/biom12010001
Chicago/Turabian StyleDuitama, Milena, Yurany Moreno, Sandra Paola Santander, Zulma Casas, Jhon Jairo Sutachan, Yolima P. Torres, and Sonia L. Albarracín. 2022. "TRP Channels as Molecular Targets to Relieve Cancer Pain" Biomolecules 12, no. 1: 1. https://doi.org/10.3390/biom12010001
APA StyleDuitama, M., Moreno, Y., Santander, S. P., Casas, Z., Sutachan, J. J., Torres, Y. P., & Albarracín, S. L. (2022). TRP Channels as Molecular Targets to Relieve Cancer Pain. Biomolecules, 12(1), 1. https://doi.org/10.3390/biom12010001





