Tailored Functionalization of Natural Phenols to Improve Biological Activity
Abstract
:1. Introduction
2. Monophenols
2.1. Carvacrol
2.2. Thymol
2.3. Eugenol
3. Diphenols
3.1. Resveratrol
3.2. Hispolon
3.3. Hydroxytyrosol
4. Phenolic Acids
4.1. Caffeic Acid
4.2. Ferulic Acid
4.3. Miscellanea
5. Lipidic Phenols
5.1. Biocatalyzed Syntheses of Lipidic Phenols
5.2. Chemical Syntheses of Lipidic Phenols
6. Polyphenols
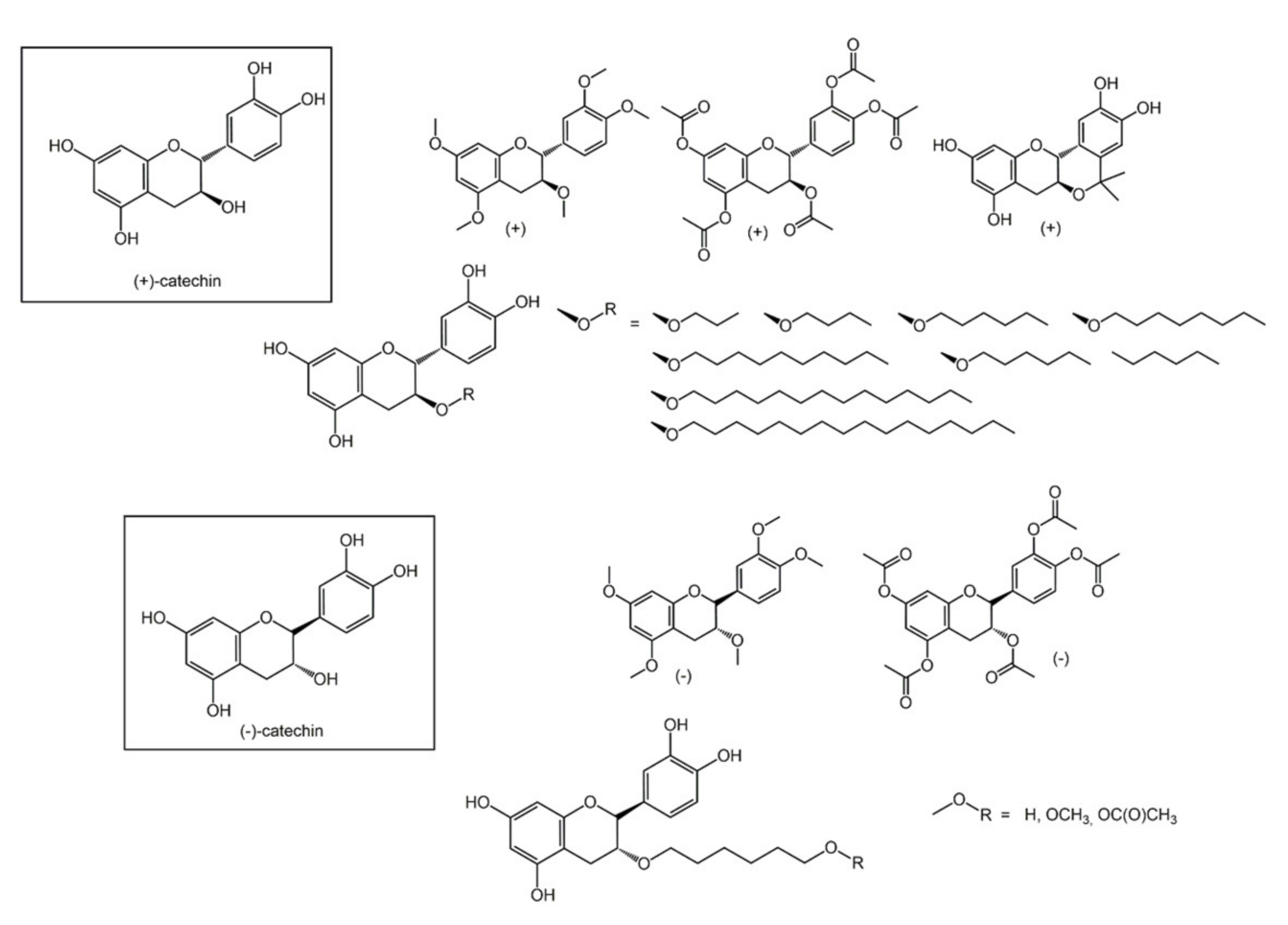
6.1. Phenols from Chroman
6.2. Phenols from Chromen
6.3. Phenols from Chromon
6.4. Phenols from 2,3-dihydrochromon
7. Curcumin and Curcuminoids
7.1. Minor Structural Changes
7.2. Substituents in the Unsaturated Chain
7.3. Modification of the β-dicarbonyl Moiety
7.4. Partial Replacement of the β-dicarbonyl Moiety
7.5. Reducing the Length of Unsaturated Chain
7.6. Derivatives with Only “Half” of the Curcumin Structure
7.7. Photosensityzers
8. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References and Notes
- Staszowska-Karkut, M.; Materska, M. Phenolic composition, mineral content, and beneficial bioactivities of leaf extracts from black Currant (Ribes nigrum L.), Raspberry (Rubus idaeus), and Aronia (Aronia melanocarpa). Nutrients 2020, 12, 463. [Google Scholar] [CrossRef] [Green Version]
- Metsämuuronen, S.; Sirén, H. Bioactive phenolic compounds, metabolism and properties: A review on valuable chemical compounds in Scots pine and Norway spruce. Phytochem. Rev. 2019, 18, 623–664. [Google Scholar] [CrossRef] [Green Version]
- Mouwakeh, A.; Kincses, A.; Nové, M.; Mosolygó, T.; Mohácsi-Farkas, C.; Kiskó, G.; Spengler, G. Nigella sativa essential oil and its bioactive compounds as resistance modifiers against Staphylococcus aureus. Phytother. Res. 2019, 33, 1010–1018. [Google Scholar] [CrossRef]
- Chibane, L.B.; Degraeve, P.; Ferhout, H.; Bouajila, J.; Oulahal, N. Plant Antimicrobial polyphenols as potential natural food preservatives. J. Sci. Food Agric. 2019, 99, 1457–1474. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Muhammad, D.R.A.; Tuenter, E.; Patria, G.D.; Foubert, K.; Pieters, L.; Dewettinck, K. Phytochemical composition and antioxidant activity of Cinnamomum burmannii Blume extracts and their potential application in white chocolate. Food Chem. 2021, 340, 127983. [Google Scholar] [CrossRef]
- Pavlić, B.; Tesli, N.; Zengin, G.; Đurović, S.; Rakić, D.; Cvetanović, A.; Gunes, A.K.; Zeković, Z. Antioxidant and enzyme-inhibitory activity of peppermint extracts and essential oils obtained by conventional and emerging extraction techniques. Food Chem. 2021, 338, 127724. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Gutiérrez, J.A.; Moreno-Lorenzana, D.; Álvarez-Bernal, D.; Rodríguez-Campos, J.; Medina-Medrano, J.R. Phenolic profile, antioxidant and anti-proliferative activities of methanolic extracts from Asclepias linaria Cav. Leaves. Molecules 2020, 25, 54. [Google Scholar] [CrossRef] [Green Version]
- Bodoira, R.; Maestri, D. Phenolic compounds from nuts: Extraction, chemical profiles, and bioactivity. J. Agric. Food Chem. 2020, 68, 927–942. [Google Scholar] [CrossRef] [PubMed]
- Singh, B.; Singh, J.P.; Kaur, A.; Singh, N. Phenolic compounds as beneficial phytochemicals in pomegranate (Punica granatum L.) peel: A review. Food Chem. 2018, 26, 75–86. [Google Scholar] [CrossRef]
- Servili, M.; Sordini, B.; Esposto, S.; Urbani, S.; Veneziani, G.; Di Maio, I.; Selvaggini, R.; Taticchi, A. Biological activities of phenolic compounds of extra virgin olive oil. Antioxidants 2014, 3, 1–23. [Google Scholar] [CrossRef] [Green Version]
- Bouyahya, A.; Chamkhi, I.; Benali, T.; Guaouguaou, F.-E.; Balahbibe, A.; El Omari, N.; Taha, D.; Belmehdi, O.; Ghokhan, Z.; El Menyiy, N. Traditional use, phytochemistry, toxicology, and pharmacology of Origanum majorana L. J. Ethnoph. 2021, 265, 113318. [Google Scholar] [CrossRef]
- Mamede, L.; Ledoux, A.; Jansen, O.; Frédérich, M. Natural phenolic compounds and derivatives as potential antimalarial agents. Planta Med. 2020, 86, 585–618. [Google Scholar] [CrossRef] [Green Version]
- Mazumder, K.; Biswas, B.; Raja, I.M.; Fukase, K. A review of cytotoxic plants of the indian subcontinent and a broad-spectrum analysis of their bioactive compounds. Molecules 2020, 25, 1904. [Google Scholar] [CrossRef] [PubMed]
- Mansoori, B.; Mohammadi, A.; Doustvandi, M.A.; Mohammadnejad, F.; Kamari, F.; Gjerstorff, M.F.; Baradaran, B.; Hamblin, M.R. Photodynamic therapy for cancer: Role of natural products. Photodiag. Photodyn. Ther. 2019, 26, 395–404. [Google Scholar] [CrossRef]
- Da Fonsêca, D.V.; da Silva Maia Bezerra, C., Jr.; Cardoso Lima, T.; Nóbrega de Almeida, R.; Pergentino de Sousa, D. Anticonvulsant essential oils and their relationship with oxidative stress in epilepsy. Biomolecules 2019, 9, 835. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tabassum, H.; Ahmad, A.; Ahmad, I.Z. Nigella sativa L. and its bioactive constituents as hepatoprotectant: A review. Curr. Pharm. Biotechnol. 2018, 19, 43–67. [Google Scholar] [CrossRef]
- Tepe, B.; Cakir, A.; Tepe, A.S. Medicinal uses, phytochemistry, and pharmacology of Origanum onites (L.): A review. Chem. Biodiv. 2016, 13, 504–520. [Google Scholar] [CrossRef] [PubMed]
- Yamada, M.; Ono, K.; Hamaguchi, T.; Noguchi-Shinohara, M. Natural phenolic compounds as therapeutic and preventive agents for cerebral amyloidosis. In Natural Compounds as Therapeutic Agents for Amyloidogenic Diseases; Advances in Experimental Medicine and Biology; Vassallo, N., Ed.; Springer International Publishing: Cham, Switzerland, 2015; Volume 863, pp. 79–94. [Google Scholar]
- Gutiérrez-Grijalva, E.P.; Ambriz-Pére, D.L.; Leyva-López, N.; Castillo-López, R.I.; Heredia, J.B. Review: Dietary phenolic compounds, health benefits and bioaccessibility. Archiv. Latinoam. Nutr. 2016, 66, 87–100. [Google Scholar]
- Kimura, H.; Ohtsuka, K.; Matsumoto, A.; Ougi, T.; Ishibashi, Y.; Yamano, K. High performance phenolic resin based on untreated natural herbaceous lignin. Polym. Polym. Compos. 2015, 23, 525–534. [Google Scholar] [CrossRef]
- Campos, D.; Betalleluz, I.; Tauquino, R.; Chirinos, R.; Pedreschi, R. Nutritional and functional characterisation of Andean chicuru (Stangea rhizanta). Food Chem. 2009, 112, 63–70. [Google Scholar] [CrossRef]
- Lombardo, L.; Grasso, F.; Lanciano, F.; Loria, S.; Monetti, E. Broad-Spectrum health protection of extra virgin olive oil compounds. In Studies in Natural Products Chemistry; Atta-ur-Rahman, Ed.; Elsevier: Amsterdam, The Netherlands, 2018; Volume 57, pp. 41–77. [Google Scholar]
- Kaushik, P.; Andújar, I.; Vilanova, S.; Plazas, M.; Gramazio, P.; Herraiz, F.J.; Brar, N.S.; Prohens, J. Breeding vegetables with increased content in bioactive phenolic acids. Molecules 2015, 20, 18464–18481. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dall’Acqua, S.; Ak, G.; Sut, S.; Zengin, G.; Yıldıztugay, E.; Mahomoodally, M.F.; Sinan, K.I.; Lobine, D. Comprehensive bioactivity and chemical characterization of the endemic plant Scorzonera hieraciifolia Hayek extracts: A promising source of bioactive compounds. Food Res. Int. 2020, 137, 109371. [Google Scholar] [CrossRef]
- Zengin, G.; Mahomoodally, M.F.; Rocchetti, G.; Lucini, L.; Sieniawska, E.; Świątek, Ł.; Rajtar, B.; Polz-Dacewicz, M.; Senkardes, I.; Aktumsek, A.; et al. Chemical characterization and bioactive properties of different extracts from Fibigia clypeata, an unexplored plant food. Foods 2020, 9, 705. [Google Scholar] [CrossRef]
- Quílez, M.; Ferreres, F.; López-Miranda, S.; Salazar, E.; Jordán, M.J. Seed oil from mediterranean aromatic and medicinal plants of the lamiaceae family as a source of bioactive components with nutritional. Antioxidants 2020, 9, 510. [Google Scholar] [CrossRef]
- Silva, V.; Falco, V.; Dias, M.I.; Barros, L.; Silva, A.; Capita, R.; Alonso-Calleja, C.; Amaral, J.S.; Igrejas, G.; Ferreira, I.C.F.R.; et al. Evaluation of the phenolic profile of Castanea sativa mill. by-products and their antioxidant and antimicrobial activity against multiresistant bacteria. Antioxidants 2020, 9, 87. [Google Scholar] [CrossRef] [Green Version]
- Golkar, P.; Moattar, F. Essential oil composition, bioactive compounds, and antioxidant activities in Iberis amara L. Nat. Prod. Commun. 2019, 14, 1–8. [Google Scholar] [CrossRef]
- Sut, S.; Dall’Acqua, S.; Zengin, G.; Senkardes, I.; Bulut, G.; Cvetanović, A.; Stupar, A.; Mandić, A.; Picot-Allain, C.; Dogan, A.; et al. Influence of different extraction techniques on the chemical profile and biological properties of Anthemis cotula L.: Multifunctional aspects for potential pharmaceutical applications. J. Pharm. Biomed. Anal. 2019, 173, 75–85. [Google Scholar] [CrossRef]
- Petrović, M.; Pastor, F.; Ðurović, S.; Veljović, S.; Gorjanović, S.; Sredojević, M.; Vukosavljević, P. Evaluation of novel green walnut liqueur as a source of antioxidants: Multi-method approach. J. Food. Sci. Technol. 2021, 58, 2160–2169. [Google Scholar] [CrossRef]
- Walsh, D.J.; Livinghouse, T.; Goeres, D.M.; Mettler, M.; Stewart, P.S. Antimicrobial activity of naturally occurring phenols and derivatives against biofilm and planktonic bacteria. Front. Chem. 2019, 7, 653. [Google Scholar] [CrossRef] [PubMed]
- Caleja, C.; Finimundy, T.C.; Pereira, C.; Barros, L.; Calhelha, R.C.; Sokovic, M.; Ivanov, M.; Carvalho, A.M.; Rosa, E.; Ferreira, I.C.F.R. Challenges of traditional herbal teas: Plant infusions and their mixtures with bioactive properties. Food Funct. 2019, 10, 5939–5951. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Andrade, C.; Ferreres, F.; Gomes, N.G.M.; Duangsrisai, S.; Srisombat, N.; Vajrodaya, S.; Pereira, D.M.; Gil-Izquierdo, A.; Andrade, P.B.; Valentão, P. Phenolic profiling and biological potential of ficus curtipes corner leaves and stem bark: 5-lipoxygenase inhibition and interference with NO levels in LPS-stimulated RAW264.7 macrophages. Biomolecules 2019, 9, 400. [Google Scholar] [CrossRef] [Green Version]
- Garzon, A.G.; Drago, S.R. Free a-amino acids, g-Aminobutyric acid (GABA), phenolic compounds and their relationships with antioxidant properties of sorghum malted in different conditions. J. Food Sci. Technol. 2018, 55, 3188–3198. [Google Scholar] [CrossRef] [PubMed]
- Quintero-Flórez, A.; Pereira-Caro, G.; Sánchez-Quezada, C.; Moreno-Rojas, J.M.; Gaforio, J.J.; Jimenez, A.; Beltran, G. Effect of olive cultivar on bioaccessibility and antioxidant activity of phenolic fraction of virgin olive oil. Eur. J. Nutr. 2018, 57, 1925–1946. [Google Scholar] [CrossRef]
- Uzun, Y.; Dalar, A.; Konczak, I. Sempervivum davisii: Phytochemical composition, antioxidant and lipase-inhibitory activities. Pharm. Biol. 2017, 55, 532–540. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Atiya, A.; Sinha, B.N.; Lal, U.R. Bioactive phenylpropanoid analogues from Piper betle L. var. birkoli leaves. Nat. Prod. Res. 2017, 31, 2604–2611. [Google Scholar] [CrossRef]
- Freitas dos Santos, H.; Ferreira Campos, J.; Miranda dos Santos, C.; Perrella Balestieri, J.B.; Brentan Silva, D.; Carollo, C.A.; de Picoli Souza, K.; Estevinho, L.M.; dos Santos, E.L. Chemical profile and antioxidant, anti-inflammatory, antimutagenic and antimicrobial activities of geopropolis from the stingless bee Melipona orbignyi. Int. J. Mol. Sci. 2017, 18, 953. [Google Scholar] [CrossRef]
- Martini, S.; Conte, A.; Tagliazucchi, D. Phenolic compounds profile and antioxidant properties of six sweet cherry (Prunus avium) cultivars. Food Res. Int. 2017, 97, 15–26. [Google Scholar] [CrossRef] [PubMed]
- Guenes, M.E.; Şahin, S.; Demir, C.; Borum, E.; Tosunoğlu, A. Determination of phenolic compounds profile in chestnut and floral honeys and their antioxidant and antimicrobial activities. J. Food Biochem. 2016, 41, e12345. [Google Scholar]
- Herraiz, F.J.; Villano, D.; Plazas, M.; Vilanova, S.; Ferreres, F.; Prohens, J.; Moreno, D.A. Phenolic profile and biological activities of the pepino (Solanum muricatum) fruit and its wild relative S. caripense. Int. J. Mol. Sci. 2016, 17, 394. [Google Scholar] [CrossRef] [Green Version]
- Mathew, S.; Abraham, T.E.; Zakaria, Z.A. Reactivity of phenolic compounds towards free radicals under in vitro conditions. J. Food Sci. Tech. 2015, 52, 5790–5798. [Google Scholar] [CrossRef] [Green Version]
- Detsi, A.; Kavetsou, E.; Kostopoulou, I.; Pitterou, I.; Pontillo, A.R.N.; Tzani, A.; Christodoulou, P.; Siliachli, A.; Zoumpoulakis, P. Nanosystems for the encapsulation of natural products: The case of chitosan biopolymer as a matrix. Pharmaceutics 2020, 12, 669. [Google Scholar] [CrossRef]
- Delogu, G.; Juliano, C.C.A.; Usai, M. Thymus catharinae camarda essential oil: β-cyclodextrin inclusion complexes, evaluation of antimicrobial activity. Nat. Prod. Res. 2015, 30, 2049–2057. [Google Scholar] [CrossRef] [PubMed]
- Dall’Acqua, S.; Kumar, G.; Sinan, K.I. An insight into Cochlospermum planchonii extracts obtained by traditional and green extraction methods: Relation between chemical compositions and biological properties by multivariate analysis. Ind. Crops Prod. 2020, 147, 112226. [Google Scholar] [CrossRef]
- Nazeam, J.A.; AL-Shareef, W.A.; Helmy, M.W.; El-Haddad, A.E. Bioassay-guided isolation of potential bioactive constituents from pomegranate agrifood by-product. Food Chem. 2020, 326, 126993. [Google Scholar] [CrossRef] [PubMed]
- Borrás Linares, I.; Arráez-Romána, D.; Herrero, M.; Ibáñez, E.; Segura-Carretero, A.; Fernández-Gutiérrez, A. Comparison of different extraction procedures for the comprehensive characterization of bioactive phenolic compounds in Rosmarinus officinalis by reversed-phase high-performance liquid chromatography with diode array detection coupled to electrospray time-of-flight mass spectrometry. J. Chrom. A 2011, 1218, 7682–7690. [Google Scholar]
- Alves-Silva, J.M.; Guerra, I.; Gonçalves, M.J.; Cavaleiro, C.; Cruz, M.T.; Figueirinha, A.; Salgueiro, L. Chemical composition of Crithmum maritimum L. essential oil and hydrodistillation residual water by GC-MS and HPLC-DAD-MS/MS, and their biological activities. Ind. Crops Prod. 2020, 149, 112329. [Google Scholar] [CrossRef]
- Mahomoodally, M.F.; Dall’Acqua, S.; Sinan, K.I.; Sut, S.; Ferrarese, I.; Etienne, O.K.; Sadeer, N.B.; Ak, G.; Zengin, G. Phenolic compounds analysis of three Euphorbia species by LC-DAD-MSn and their biological properties. J. Pharm. Biomed. Anal. 2020, 189, 113477. [Google Scholar] [CrossRef]
- Ma, W.; Tang, B.; Row, K.H. Exploration of a ternary deep eutectic solvent of methyltriphenylphosphonium bromide/chalcone/formic acid for the selective recognition of rutin and quercetin in Herba Artemisiae scopariae. J. Sep. Sci. 2017, 40, 3248–3256. [Google Scholar] [CrossRef] [PubMed]
- Arun, K.P.; Brindha, P. Investigations into phenolic and alkaloid constituents of Jatropha tanjorensis by LC-MS/MS and evaluating its bioactive property. Asian J. Chem. 2015, 27, 3249–3253. [Google Scholar] [CrossRef]
- Oldoni, T.L.C.; Melo, P.S.; Massarioli, A.P.; Moreno, I.A.M.; Bezerra, R.M.N.; Rosalen, P.L.; da Silva, G.V.J.; Nascimento, A.M.; Alencar, S.M. Bioassay-guided isolation of proanthocyanidins with antioxidant activity from peanut (Arachis hypogaea) skin by combination of chromatography techniques. Food Chem. 2016, 192, 306–312. [Google Scholar] [CrossRef] [Green Version]
- Alagawany, M.; Farag, M.R.; Salah, A.A.; Mahmoud, M.A. The role of oregano herb and its derivatives as immunomodulators in fish. Rev. Aquacult. 2020, 12, 2481–2492. [Google Scholar] [CrossRef]
- Sorrenti, V.; Fortinguerra, S.; Caudullo, G.; Buriani, A. Deciphering the role of polyphenols in sports performance: From nutritional genomics to the gut microbiota toward phytonutritional epigenomics. Nutrients 2020, 12, 1265. [Google Scholar] [CrossRef] [PubMed]
- Araghi, M.; Moslehi, Z.; Nafchi, A.A.; Mostahsan, A.; Salamat, N.; Garmakhany, A.D. Cold water fish gelatin modification by a natural phenolic cross-linker (ferulic acid and caffeic acid). Food Sci. Nutr. 2015, 3, 370–375. [Google Scholar] [CrossRef]
- Zhang, X.; Do, M.D.; Casey, P.; Sulistio, A.; Qiao, G.G.; Lundin, L.; Lillford, P.; Kosaraju, S. Chemical modification of gelatin by a natural phenolic cross-linker, tannic acid. J. Agric. Food Chem. 2010, 58, 6809–6815. [Google Scholar] [CrossRef] [PubMed]
- Alirezalua, K.; Pateiro, M.; Yaghoubi, M.; Alirezalu, A.; Peighambardoust, S.H.; Lorenzo, J.M. Phytochemical constituents, advanced extraction technologies and technofunctional properties of selected Mediterranean plants for use in meat products. A comprehensive review. Trends Food Sci. Technol. 2020, 100, 292–306. [Google Scholar] [CrossRef]
- Gullón, P.; Astray, G.; Gullón, B.; Tomasevic, I.; Lorenzo, J.M. Pomegranate peel as suitable source of high-added value bioactives: Tailored functionalized meat products. Molecules 2020, 25, 2859. [Google Scholar] [CrossRef] [PubMed]
- Mainente, F.; Menin, A.; Alberton, A.; Zoccatelli, G.; Rizzi, C. Evaluation of the sensory and physical properties of meat and fish derivatives containing grape pomace powders International. J. Food Sci. Tech. 2018, 54, 952–958. [Google Scholar] [CrossRef]
- Gutiérrez-del-Río, I.; Fernández, J.; Lombó, F. Plant nutraceuticals as antimicrobial agents in food preservation: Terpenoids, polyphenols and thiols. Int. J. Antimicr. Agents 2018, 52, 309–315. [Google Scholar] [CrossRef] [PubMed]
- Akl, E.M.; Dacrory, S.; Abdel-Aziz, M.S.; Kamel, S.; Fahim, A.M. Preparation and characterization of novel antibacterial blended films based on modified carboxymethyl cellulose/phenolic compounds. Polym. Bull. 2021, 78, 1061–1085. [Google Scholar] [CrossRef]
- Ibrahim, S.A.; Soliman, O.R. Nano-encapsulation of bioactive oils through emulsion polymerization as antimicrobials and its adhesion to packaging films. Der Pharm. Lett. 2016, 8, 367–373. [Google Scholar]
- Jing, S.; Li, T.; Li, X.; Xu, Q.; Hu, J.; Li, R. Phenolic foams modified by cardanol through bisphenol modification. J. Appl. Polym. Sci. 2014, 131, 39942. [Google Scholar] [CrossRef]
- Mahendran, A.R.; Wuzella, G.; Aust, N.; Mueller, U.; Kandelbauer, A. Processing and characterization of natural fibre reinforced composites using lignin phenolic binder. Polym. Polym. Comp. 2013, 21, 199–205. [Google Scholar] [CrossRef]
- Rahim, M.A.; Kristufek, S.L.; Pan, S.; Richardson, J.J.; Caruso, F. Phenolic building blocks for the assembly of functional materials. Angew. Chem. Int. Ed. 2019, 58, 1904–1927. [Google Scholar] [CrossRef]
- Lomonaco, D.; Maia, F.J.N.; Clemente, C.S.; Mota, J.P.F.; Costa, A.E., Jr.; Mazzetto, S.E. Thermal studies of new biodiesel antioxidants synthesized from a natural occurring phenolic lipid. Fuel 2012, 97, 552–559. [Google Scholar] [CrossRef] [Green Version]
- Morales, J.C.; Lucas, R. Structure-activity relationship of phenolic antioxidants and olive components. In Olives and Olive Oil in Health and Disease Prevention; Preedy, V.R., Watson, R.R., Eds.; Elsevier: Amsterdam, The Netherlands, 2010; pp. 905–914. [Google Scholar]
- Chen, Y.; Xiao, H.; Zheng, J.; Liang, G. Structure-thermodynamics-antioxidant activity relationships of selected natural phenolic acids and derivatives: An experimental and theoretical evaluation. PLoS ONE 2015, 10, e0121276. [Google Scholar] [CrossRef]
- Zeljković, S.C.; Topčagic, A.; Požgan, F.; Štefane, B.; Tarkowski, P.; Maksimović, M. Antioxidant activity of natural and modified phenolic extracts from Satureja montana L. Ind. Crops Prod. 2015, 76, 1094–1099. [Google Scholar] [CrossRef]
- Sun, Q.; Heilmann, J.; König, B. Natural phenolic metabolites with anti-angiogenic properties—A review from the chemical point of view. Beilstein J. Org. Chem. 2015, 11, 249–264. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Groussin, A.-L.; Antoniotti, S. Valuable chemicals by the enzymatic modification of molecules of natural origin: Terpenoids, steroids, phenolics and related compounds. Biores. Technol. 2012, 115, 237–243. [Google Scholar] [CrossRef] [PubMed]
- Cravens, A.; Payne, J.; Smolke, C.D. Synthetic biology strategies for microbial biosynthesis of plant natural products. Nat. Commun. 2019, 10, 2142. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zielińska-Błajet, M.; Feder-Kubis, J. Monoterpenes and their derivatives—Recent development in biological and medical applications. Int. J. Mol. Sci. 2020, 21, 7078. [Google Scholar] [CrossRef]
- Pawełczyk, A.; Olender, D.; Sowa-Kasprzak, K.; Zaprutko, L. Linked drug-drug conjugates based on triterpene and phenol structures. Rational synthesis, molecular properties, toxicity and bioactivity prediction. Arab. J. Chem. 2020, 13, 8793–8806. [Google Scholar] [CrossRef]
- Nesterkina, M.; Kravchenko, I. Synthesis and pharmacological properties of novel esters based on monoterpenoids and glycine. Pharmaceuticals 2017, 10, 47. [Google Scholar] [CrossRef] [PubMed]
- Rajput, J.D.; Bagul, S.D.; Pete, U.D.; Zade, C.M.; Padhye, S.B.; Bendre, R.S. Perspectives on medicinal properties of natural phenolic monoterpenoids and their hybrids. Mol. Divers. 2018, 22, 225–245. [Google Scholar] [CrossRef]
- Mastelić, J.; Jerković, I.; Blažević, I.; Poljak-Blaži, M.; Borović, S.; Ivanćić-Baće, I.; Smrećki, V.; Žarković, N.; Brćić-Kostic, K.; Vikić-Topić, D. Comparative study on the antioxidant and biological activities of carvacrol, thymol, and eugenol derivatives. J. Agric. Food Chem. 2008, 56, 3989–3996. [Google Scholar] [CrossRef] [PubMed]
- Schreiner, L.; Bauer, J.; Ortner, E.; Buettner, A. Structure−Odor activity studies on derivatives of aromatic and oxygenated monoterpenoids synthesized by modifying p-cymene. J. Nat. Prod. 2020, 83, 834–842. [Google Scholar] [CrossRef] [PubMed]
- Marković, A.K.; Torić, J.; Barbarić, M.; Brala, C.J. Hydroxytyrosol, tyrosol and derivatives and their potential effects on human health. Molecules 2019, 24, 2001. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zang, H.; Shen, P.; Xu, Q.; Zhang, L.; Xia, G.; Sun, J.; Zhu, J.; Yang, X. Synthesis and biological activities of tyrosol phenolic acid ester derivatives. Chem. Nat. Compd. 2019, 55, 1043–1049. [Google Scholar] [CrossRef]
- Barontini, M.; Bernini, R.; Carastro, I.; Gentili, P.; Romani, A. Synthesis and DPPH radical scavenging activity of novel compounds obtained from tyrosol and cinnamic acid derivatives. New J. Chem. 2014, 38, 809–816. [Google Scholar] [CrossRef]
- Lombrea, A.; Antal, D.; Ardelean, F.; Avram, S.; Pavel, I.Z.; Vlaia, L.; Mut, A.-M.; Diaconeasa, Z.; Dehelean, C.A.; Soica, C.; et al. A recent insight regarding the phytochemistry and bioactivity of Origanum vulgare L. essential oil. Int. J. Mol. Sci. 2020, 21, 9653. [Google Scholar] [CrossRef]
- Aljaafari, M.N.; AlAli, A.O.; Baqais, L.; Alqubaisy, M.; AlAli, M.; Molouki, A.; Ong-Abdullah, J.; Abushelaibi, A.; Lai, K.-S.; Erin Lim, S.-H. An overview of the potential therapeutic applications of essential oils. Molecules 2021, 26, 628. [Google Scholar] [CrossRef]
- Nabavi, S.; Marchese, A.; Izadi, M.; Curti, V.; Daglia, M.; Nabavi, S.F. Plants belonging to the genus Thymus as antibacterial agents: From farm to pharmacy. Food Chem. 2015, 173, 339–347. [Google Scholar] [CrossRef]
- Marinelli, L.; Di Stefano, A.; Cacciatore, I. Carvacrol and its derivatives as antibacterial agents. Phytochem. Rev. 2018, 17, 903–921. [Google Scholar] [CrossRef]
- De Mesquita, B.M.; do Nascimento, P.G.G.; Souza, L.G.S.; de Farias, I.F.; da Silva, R.A.C.; de Lemos, T.L.G.; Monte, F.J.Q.; Oliveira, I.R.; Trevisan, M.T.S.; da Silva, H.C.; et al. Synthesis, larvicidal and acetylcholinesterase ihibitory activities of carvacrol/thymol and derivatives. Quim. Nova 2018, 41, 412–416. [Google Scholar]
- Damasceno, S.R.B.; Oliveira, F.R.A.M.; Carvalho, N.S.; Brito, C.F.C.; Silva, I.S.; Sousa, F.B.M.; Silva, R.O.; Sousa, D.P.; Barbosa, A.L.R.; Freitas, R.M.; et al. Carvacryl acetate, a derivative of carvacrol, reduces nociceptive and inflammatory response in mice. Life Sci. 2014, 94, 58–66. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pires, L.F.; Muratori Costa, L.; Cardoso de Almeida, A.A.; Almeida Silva, O.; Santos Cerqueira, G.; de Sousa, D.P.; de Freitas, R.M. Is there a correlation between in vitro antioxidant potential and in vivo effect of carvacryl acetate against oxidative stress in mice hippocampus? Neurochem. Res. 2014, 39, 758–769. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Jiang, S.; Pu, T.; Fan, L.; Su, F.; Ye, M. Antifungal activity of phenolic monoterpenes and structure-related compounds against plant pathogenic fungi. Nat. Prod. Res. 2019, 33, 1423–1430. [Google Scholar] [CrossRef]
- Pires, L.F.; Muratori Costa, L.; Almeida Silva, O.; Cardoso de Almeida, A.A.; Cerqueira, G.S.; de Sousa, D.P.; de Freitas, R.M. Anxiolytic-like effects of carvacryl acetate, a derivative of carvacrol in mice. Pharmacol. Biochem. Behav. 2013, 112, 42–48. [Google Scholar] [CrossRef] [Green Version]
- Novato, T.; Gomes, G.A.; Zeringóta, V.; Franco, C.T.; de Oliveira, D.R.; Melo, D.; de Carvalho, M.G.; Daemond, E.; de Oliveira Monteiroc, C.M. In vitro assessment of the acaricidal activity of carvacrol, thymol, eugenol and their acetylated derivatives on Rhipicephalus microplus (Acari: Ixodidae). Vet. Parasitol. 2018, 260, 1–4. [Google Scholar] [CrossRef]
- Konig, I.F.M.; Gonçalves, R.R.P.; Oliveira, M.V.S.; Silva, C.M.; Thomasi, S.S.; Peconick, A.P.; Remedio, R.N. Sublethal concentrations of acetylcarvacrol strongly impact oocyte development of engorged female cattle ticks Rhipicephalus microplus (Canestrini, 1888) (Acari: Ixodidae). Ticks Tick Borne Dis. 2019, 10, 766–774. [Google Scholar] [CrossRef]
- De Santana, M.T.; Barros Silva, V.; de Brito, R.G.; dos Santos, P.L.; de Holanda Cavalcanti, S.C.; Oliveira Barreto, E.; de Souza Ferro, J.N.; Viana dos Santos, M.R.; de Sousa Araújo, A.A.; Quintans-Júnior, L.J. Synthesis and pharmacological evaluation of carvacrol propionate. Inflammation 2014, 37, 1575–1587. [Google Scholar] [CrossRef]
- Nesterkina, M.; Kravchenko, I. Synthesis and pharmacological properties of novel esters based on monocyclic terpenes and GABA. Pharmaceuticals 2016, 9, 32. [Google Scholar] [CrossRef] [Green Version]
- Nesterkina, M.; Kravchenko, I. Analgesic activity of novel GABA esters after transdermal delivery. Nat. Prod. Commun. 2016, 11, 1419–1420. [Google Scholar] [CrossRef] [Green Version]
- Ashraf, Z.; Rafiq, M.; Nadeem, H.; Hassan, M.; Afzal, S.; Waseem, M.; Afzal, K.; Latip, J. Carvacrol derivatives as mushroom tyrosinase inhibitors; synthesis, kinetics mechanism and molecular docking studies. PLoS ONE 2017, 12, e178069. [Google Scholar] [CrossRef] [Green Version]
- Mathela, C.S.; Singh, K.K.; Gupta, V.K. Synthesis and in vitro antibacterial activity of thymol and carvacrol derivatives. Acta Pol. Pharm. 2010, 67, 375–380. [Google Scholar]
- Alokam, R.; Jeankumar, V.U.; Sridevi, J.P.; Matikonda, S.S.; Peddi, S.; Alvala, M.; Yogeeswari, P.; Sriram, D. Identification and structure–activity relationship study of carvacrol derivatives as Mycobacterium tuberculosis chorismate mutase inhibitors. J. Enzyme Inhib. Med. Chem. 2014, 29, 547–554. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Jiang, S.; Yang, Y.; Fan, L.; Su, F.; Ye, M. Synthesis and antifungal activity of carvacrol and thymol esters with heteroaromatic carboxylic acids. Nat. Prod. Res. 2019, 33, 1924–1930. [Google Scholar] [CrossRef]
- Walsh, D.J.; Livinghouse, T.; Durling, G.M.; Chase-Bayless, Y.; Arnold, A.D.; Stewart, P.S. Sulfenate esters of simple phenols exhibit enhanced activity against biofilms. ACS Omega 2020, 5, 6010–6020. [Google Scholar] [CrossRef] [Green Version]
- Pinheiro, P.F.; Parreira Menini, L.A.; Campos Bernardes, P.; Henriques Saraiva, S.; Mesquita Carneiro, J.W.; Vidal Costa, A.; Rodrigues Arruda, T.; Ribeiro Lage, M.; Martins Gonçalves, P.; de Oliveira Bernardes, C.; et al. Semisynthetic phenol derivatives obtained from natural phenols: Antimicrobial activity and molecular properties. J. Agric. Food Chem. 2018, 66, 323–330. [Google Scholar] [CrossRef] [PubMed]
- Marinelli, L.; Fornasari, E.; Eusepi, P.; Ciulla, M.; Genovese, S.; Epifano, F.; Fiorito, S.; Turkez, H.; Ortücü, S.; Mingoia, M.; et al. Carvacrol prodrugs as novel antimicrobial agents. Eur. J. Med. Chem. 2019, 178, 515–529. [Google Scholar] [CrossRef] [PubMed]
- Cacciatore, I.; Di Giulio, M.; Fornasari, E.; Di Stefano, A.; Cerasa, L.S.; Marinelli, L.; Turkez, H.; Di Campli, E.; Di Bartolomeo, S.; Robuffo, I.; et al. Carvacrol codrugs: A new approach in the antimicrobial plan. PLoS ONE 2015, 10, e0120937. [Google Scholar] [CrossRef] [Green Version]
- Gharbi, A.; Legigan, T.; Humblot, V.; Papot, S.; Berjeaud, J.-M. Surface functionalization by covalent immobilization of an innovative carvacrol derivative to void fungal biofilm formation. AMB Express 2015, 5, 9. [Google Scholar] [CrossRef] [Green Version]
- Bonfim, R.R.; Paiva-Souza, I.O.; Moraes, J.P.; Pereira, D.S.; Santos, C.A.; Santana, D.G.; Thomazzi, S.M.; Ferro, J.N.S.; Barreto, E.O.; Sousa, D.P.; et al. Isopropoxy-carvacrol, a derivative obtained from carvacrol, reduces acute inflammation and nociception in rodents. Basic Clin. Pharmacol. 2014, 115, 237–243. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Patil, J.U.; Suryawanshi, K.C.; Patil, P.B.; Chaudhary, S.R.; Pawar, N.S. Synthesis and antibacterial activity of carvacryl ethers. J. Asian Nat. Prod. Res. 2010, 12, 129–133. [Google Scholar] [CrossRef] [PubMed]
- Sisto, F.; Carradori, S.; Guglielmi, P.; Traversi, C.B.; Spano, M.; Sobolev, A.P.; Secci, D.; Di Marcantonio, M.C.; Haloci, E.; Grande, R.; et al. Synthesis and biological evaluation of carvacrol-based derivatives as dual inhibitors of H. pylori strains and ags cell proliferation. Pharmaceuticals 2020, 13, 405. [Google Scholar] [CrossRef]
- Bkhaitan, M.M.; Alarjah, M.; Mirza, A.Z.; Abdalla, A.N.; El-Said, H.M.; Faidah, H.S. Preparation and biological evaluation of metronidazole derivatives with monoterpenes and eugenol. Chem. Biol. Drug Des. 2018, 92, 1954–1962. [Google Scholar] [CrossRef] [PubMed]
- Nesterkina, M.; Bilokon, S.; Alieksieieva, T.; Chebotar, S.; Kravchenko, I. Toxic effect and genotoxicity of carvacrol ethers in Drosophila melanogaster. Mutat. Res. Fund. Mol. Mech. Mutagen. 2020, 821, 111713. [Google Scholar] [CrossRef]
- Brotzman, N.; Xu, Y.; Graybill, A.; Cocolas, A.; Ressler, A.; Seeram, N.P.; Ma, H.; Henry, G.E. Synthesis and tyrosinase inhibitory activities of 4-oxobutanoate derivatives of carvacrol and thymol. Bioorg. Med. Chem. Lett. 2019, 27, 3805–3812. [Google Scholar] [CrossRef]
- Uddin, A.; Singh, V.; Irfan, I.; Mohammad, T.; Singh Hada, R.; Hassan, M.I.; Abid, M.; Singh, S. Identification and structure–activity relationship (SAR) studies of carvacrol derivatives as potential anti-malarial against Plasmodium falciparum falcipain-2 protease. Bioorg. Chem. 2020, 103, 104142. [Google Scholar] [CrossRef]
- Aneja, B.; Azam, M.; Alam, S.; Perwez, A.; Maguire, R.; Yadava, U.; Kavanagh, K.; Daniliuc, C.G.; Rizvi, M.M.A.; Haq, Q.M.R.; et al. Natural product-based 1,2,3-triazole/sulfonate analogues as potential chemotherapeutic agents for bacterial infections. ACS Omega 2018, 3, 6912–6930. [Google Scholar] [CrossRef] [Green Version]
- Bytyqi-Damoni, A.; Kestane, A.; Taslimi, P.; Tuzun, B.; Zengin, M.; Bilgicli, H.G.; Gulcin, İ. Novel carvacrol based new oxypropanolamine derivatives: Design, synthesis, characterization, biological evaluation, and molecular docking studies. J. Mol. Struct. 2020, 23, 878–893. [Google Scholar] [CrossRef]
- Kurt, B.Z.; Gazioglu, I.; Dag, A.; Salmas, R.E.; Kayık, G.; Durdagi, S.; Sonmez, F. Synthesis, anticholinesterase activity and molecular modeling study of novel carbamate-substituted thymol/carvacrol derivative. Bioorg. Med. Chem. 2017, 25, 1352–1363. [Google Scholar] [CrossRef]
- Zengin Kurt, B.; Durdagi, S.; Celebi, G.; Ekhteiari Salmas, R.; Sonmez, F. Synthesis, anticholinesterase activity and molecular modeling studies of novel carvacrol substituted amide derivatives. J. Biomol. Struct. Dyn. 2020, 38, 841–859. [Google Scholar] [CrossRef] [PubMed]
- Bassanetti, I.; Carcelli, M.; Buschini, A.; Montalbano, S.; Leonardi, G.; Pelagatti, P.; Tosi, G.; Massi, P.; Fiorentini, L.; Rogolino, D. Investigation of antibacterial activity of new classes of essential oils derivatives. Food Control 2017, 73, 606–612. [Google Scholar] [CrossRef]
- Chen, G.-Q.; Sun, D.; Yang, J.-M.; Zhang, S.; Tian, Y.-E.; Che, Z.-P.; Liu, S.-M.; Jiang, J.; Lin, X.-M. Synthesis of sulfonate derivatives of carvacrol and thymol as anti-oomycetes agents. J. Asian Nat. Prod. Res. 2021, 23, 692–702. [Google Scholar] [CrossRef]
- Rajput, J.D.; Bagul, S.D.; Hosamani, A.A.; Patil, M.A.; Bendre, R.S. Synthesis, characterizations, biological activities and docking studies of novel dihydroxy derivatives of natural phenolic monoterpenoids containing azomethine linkage. Res. Chem. Intermed. 2017, 43, 5377–5393. [Google Scholar] [CrossRef]
- Rajput, J.D.; Bagul, S.D.; Bendre, R.S. Design, synthesis, biological screenings and docking simulations of novel carvacrol and thymol derivatives containing acetohydrazone linkage. Res. Chem. Intermed. 2017, 43, 4893–4906. [Google Scholar] [CrossRef]
- Rajput, J.D.; Bagul, S.D.; Bendre, R.S. Synthesis, biological activities and molecular docking simulation of hydrazone scaffolds of carvacrol, thymol and eugenol. Res. Chem. Intermed. 2017, 43, 6601–6616. [Google Scholar] [CrossRef]
- Bagul, S.D.; Rajput, J.D.; Tadavi, S.K.; Bendre, R.S. Design, synthesis and biological activities of novel 5-isopropyl-2-methylphenolhydrazide-based sulfonamide derivatives. Res. Chem. Intermed. 2017, 43, 2241–2252. [Google Scholar] [CrossRef]
- Sobotta, L.; Lijewski, S.; Dlugaszewska, J.; Nowicka, J.; Mielcarek, J.; Goslinski, T. Photodynamic inactivation of Enterococcus faecalis by conjugates of zinc(II) phthalocyanines with thymol and carvacrol loaded into lipid vesicles. Inorg. Chim. Acta 2019, 489, 180–190. [Google Scholar] [CrossRef]
- Marchese, A.; Orhan, I.E.; Daglia, M.; Barbieri, R.; Di Lorenzo, A.; Nabavi, S.F.; Gortzi, O.; Izadi, M.; Nabavi, S.M. Antibacterial and antifungal activities of thymol: A brief review of the literature. Food Chem. 2016, 210, 402–414. [Google Scholar] [CrossRef]
- Kumar, A.; Singh, S.P.; Chhokar, S.S. Thymol and its derivatives as antimicrobial agents. Nat. Prod. Commun. 2008, 3, 823–828. [Google Scholar] [CrossRef] [Green Version]
- Talavera-Alemán, A.; Rodríguez-García, G.; López, Y.; García-Gutiérrez, H.A.; Torres-Valencia, J.M.; del Río, R.E.; Cerda-García-Rojas, C.M.; Joseph-Nathan, P.; Gómez-Hurtado, M.A. Systematic evaluation of thymol derivatives possessing stereogenic or prostereogenic centers. Phytochem. Rev. 2016, 15, 251–277. [Google Scholar] [CrossRef]
- De Silvestro, I.; Drew, S.L.; Nichol, G.S.; Duarte, F.; Lawrence, A.L. Total synthesis of a dimeric thymol derivative isolated from Arnica sachalinensis. Angew. Chem. Int. Ed. 2017, 56, 6813–6817. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- El-Miligy, M.M.M.; Hazzaa, A.A.; El-Zemity, S.R.; Al-Kubeisi, A.K. Synthesis of thymol derivatives as potential non-irritant antimicrobial and insecticidal agents. Curr. Bioact. Comp. 2019, 15, 125–137. [Google Scholar] [CrossRef]
- Cherkasov, R.A.; Nizamov, I.S.; Gabdullina, G.T.; Almetkina, L.A.; Shamilov, R.R.; Sofronov, A.V. Dithiophosphoric and dithiophosphonic acids and their derivatives on the basis of thymol: Synthesis and antimicrobial activity. Phosphorus Sulfur 2013, 188, 33–35. [Google Scholar] [CrossRef]
- Matela, G.; Aman, R.; Sharma, C.; Chaudhray, S. Reactions of tin and triorganotin(IV) isopropoxides with thymol derivative: Synthesis, characterization and in vitro antimicrobial screening. J. Serb. Chem. Soc. 2013, 78, 1323–1333. [Google Scholar] [CrossRef]
- Robledo, S.; Osorio, E.; Munõz, D.; Jaramillo, L.M.; Restrepo, A.; Arango, G.; Vélez, I. In vitro and in vivo cytotoxicities and antileishmanial activities of thymol and hemisynthetic derivatives. Antimicrob. Agents Chemother. 2005, 49, 1652–1655. [Google Scholar] [CrossRef] [Green Version]
- More, D.H.; Pawar, N.S.; Dewang, P.M.; Patil, S.L.; Mahulikar, P.P. Microwave-assisted Sinthesis of thymyl ethers and esters in aqueous medium. Russ. J. Gen. Chem. 2004, 74, 217–218. [Google Scholar] [CrossRef]
- Sabour, A.; El Asbahani, A.; Bentahar, S.; Ait taleb, M.; Lacherai, A.; Jilale, A. Synthesis of some thymol derivatives for enhanced antibacterial activity. Mor. J. Chem. 2019, 7, 748–757. [Google Scholar]
- Barros Silva, V.; Lima Travassos, D.; Nepel, A.; Barison, A.; Vilaça Costa, E.; Scotti, L.; Scotti, M.T.; Bezerra Mendonça-Junior, F.J.; La Corte dos Santos, R.; Cabral de Holanda Cavalcanti, S. Synthesis and chemometrics of thymol and carvacrol derivatives as larvicides against Aedes aegypti. J. Arthropod-Borne Dis. 2017, 11, 315–330. [Google Scholar]
- De Morais, S.M.; Vila-Nova, N.S.; Bevilaqua, C.M.L.; Rondon, F.C.; Lobo, C.H.; Noronha Moura, A.d.A.A.; Sales, A.D.; Ribeiro Rodrigues, A.P.; de Figuereido, J.R.; Campello, C.C.; et al. Thymol and eugenol derivatives as potential antileishmanial agents. Bioorg. Med. Chem. 2014, 22, 6250–6255. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- André, W.P.P.; Cavalcante, G.S.; Correia Ribeiro, W.L.; dos Santos, J.M.L.; Freitas Macedo, I.T.; de Paula, H.C.B.; de Morais, S.M.; de Melo, J.V.; Leal Bevilaqua, C.M. Anthelmintic effect of thymol and thymol acetate on sheep gastrointestinal nematodes and their toxicity in mice. Braz. J. Vet. Parasitol. 2017, 26, 323–330. [Google Scholar] [CrossRef]
- Xavier, F.J.S.; Rodrigues, K.A.d.F.; De Oliveira, R.G.; Lima Junior, C.G.; Da Câmara Rocha, J.; Keesen, T.S.L.; De Oliveira, M.R.; Silva, F.P.L.; Vasconcellos, M.L.A.d.A. Synthesis and in vitro anti Leishmania amazonensis biological screening of morita-baylis-hillman adducts prepared from eugenol, thymol and carvacrol. Molecules 2016, 21, 1483. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hassib, S.T.; Hassan, G.S.; El-Zaher, A.A.; Fouad, M.A.; Abd El-Ghafar, O.A.; Taha, E.A. Synthesis and biological evaluation of new prodrugs of etodolac and tolfenamic acid with reduced ulcerogenic potential. Eur. J. Pharm. Sci. 2019, 140, 105101. [Google Scholar] [CrossRef] [PubMed]
- Sawraj, S.; Bhardawaj, T.R.; Sharma, P.D. Design, synthesis, and evaluation of novel indomethacin-antioxidant codrugs as gastrosparing NSAIDs. Med. Chem. Res. 2012, 21, 834–843. [Google Scholar] [CrossRef]
- Sehajpal, S.; Prasad, D.N.; Singh, R.K. Synthesis and evaluation of prodrugs of ketoprofen with antioxidants as gastroprotective NSAIDs. Asian J. Chem. 2018, 30, 2145–2150. [Google Scholar] [CrossRef]
- Dhaneshwar, S.; Patel, V.; Patil, D.; Meena, G. Studies on synthesis, stability, release and pharmacodynamic profile of a novel diacerein-thymol prodrug. Bioorg. Med. Chem. Lett. 2013, 23, 55–61. [Google Scholar] [CrossRef]
- Ashraf, Z.; Rafiq, M.; Seo, S.-Y.; Kwon, K.S.; Babar, M.M.; Sadaf Zaidi, N.U.S. Kinetic and in silico studies of novel hydroxy-based thymol analogues as inhibitors of mushroom tyrosinase. Eur. J. Med. Chem. 2015, 98, 203–211. [Google Scholar] [CrossRef]
- Kang, H.H.; Rho, H.S.; Hwang, J.S.; Oh, S.-G. Depigmenting activity and low cytotoxicity of alkoxy benzoates or alkoxy cinnamate in cultured melanocytes. Chem. Pharm. Bull. 2003, 51, 1085–1088. [Google Scholar] [CrossRef] [Green Version]
- Hwan Lee, J.; Lee, E.-S.; Bae, H.; Hwang, J.-A.; Kim, S.-H.; Kim, D.-Y.; Park, N.-H.; Rho, H.S.; Kim, Y.J.; Oh, S.-G.; et al. Antimelanogenic efficacy of melasolv (3,4,5-Trimethoxycinnamate Thymol Ester) in melanocytes and three-dimensional human skin equivalent. Ski. Pharmacol. Physiol. 2017, 30, 190–196. [Google Scholar]
- Sheng, Z.; Ge, S.; Xu, X.; Zhang, Y.; Wu, P.; Zhang, K.; Xu, X.; Li, C.; Zhao, D.; Tang, X. Design, synthesis and evaluation of cinnamic acid ester derivatives as mushroom tyrosinase inhibitors. Med. Chem. Commun. 2018, 9, 853–861. [Google Scholar] [CrossRef]
- Zengin, M.; Genca, H.; Taslimi, P.; Kestane, A.; Guclu, E.; Ogutlu, A.; Karabay, O.; Gulçin, I. Novel thymol bearing oxypropanolamine derivatives as potent some metabolic enzyme inhibitors—Their antidiabetic, anticholinergic and antibacterial potentials. Bioorg. Chem. 2018, 81, 119–126. [Google Scholar] [CrossRef] [PubMed]
- Kumar, T.V.S.; Sankar, K.-U.; Divakar, S. Synthesis of thymol glycosides under SCCO2 conditions using amyloglucosidase from Rhizopus mold. J. Food Sci. Technol. 2013, 50, 803–808. [Google Scholar] [CrossRef] [PubMed]
- Epps, S.V.R.; Harvey, R.B.; Byrd, J.A.; Petrujkić, B.T.; Sedej, I.; Beier, R.C.; Phillips, T.D.; Hume, M.E.; Anderson, R.C.; Nisbet, D.J. Comparative effect of thymol or its glucose conjugate, thymol-β-D-glucopyranoside, on Campylobacter in avian gut contents. J. Environ. Sci. Health B 2015, 50, 55–61. [Google Scholar] [CrossRef]
- James Bound, D.; Bettadaiah, B.K.; Srinivas, P. ZnBr2-Catalyzed and Microwave-Assisted Synthesis of 2,3-Unsaturated Glucosides of Hindered Phenols and Alcohols. Synth. Commun. 2014, 44, 2565–2576. [Google Scholar] [CrossRef]
- Bound, J.S.; Murthy, P.S.; Srinivas, P. 2,3-Dideoxyglucosides of selected terpene phenols and alcohols as potent antifungal compounds. Food Chem. 2016, 210, 371–380. [Google Scholar] [CrossRef]
- Havasi, M.H.; Ressler, A.J.; Parks, E.L.; Cocolas, A.H.; Weaver, A.; Seeram, N.P.; Henry, G.E. Antioxidant and tyrosinase docking studies of heterocyclic sulfide derivatives containing a thymol moiety. Inorg. Chim. Acta 2020, 505, 119495. [Google Scholar] [CrossRef]
- Piombino, C.; Lange, H.; Sabuzi, F.; Galloni, P.; Conte, V.; Crestini, C. lignosulfonate microcapsules for delivery and controlled release of thymol and derivatives. Molecules 2020, 25, 866. [Google Scholar] [CrossRef] [Green Version]
- Swain, S.S.; Paidesetty, S.K.; Padhy, R.N. Antibacterial activity, computational analysis and host toxicity study of thymol-sulfonamide conjugates. Biomed. Pharmacother. 2017, 88, 181–193. [Google Scholar] [CrossRef]
- Sathe, P.S.; Rajput, J.D.; Gunaga, S.S.; Patel, H.M.; Bendre, R.S. Synthesis, characterization, and antioxidant activity of thymol-based paracetamol analogues. Res. Chem. Intermed. 2019, 45, 5487–5498. [Google Scholar] [CrossRef]
- Swain, S.S.; Paidesetty, S.K.; Padhy, R.N. Synthesis of novel thymol derivatives against MRSA and ESBL producing pathogenic bacteria. Nat. Prod. Res. 2019, 33, 3181–3189. [Google Scholar] [CrossRef] [PubMed]
- Radwan, M.A.; El-Zemity, S.R.; Mohamed, S.A.; Sherby, S.M. Potential of some monoterpenoids and their new N-methylcarbamate derivatives against Schistosomiasis snail vector, Biomphalaria alexandrina. Ecotoxicol. Environ. Saf. 2008, 71, 889–894. [Google Scholar] [CrossRef] [PubMed]
- El-Zemity, S.R.; Radwan, M.A.; El-Monam Mohamed, S.A.; Sherby, S.M. Antibacterial screening of some essential oils, monoterpenoids and novel N-methyl carbamates based on monoterpenoids against Agrobacterium tumefaciens and Erwinia carotovora. Arch. Phytopathol. Plant Prot. 2008, 41, 451–461. [Google Scholar] [CrossRef]
- Latacz, G.; Lubelska, A.; Jastrzębska-Więsek, M.; Partyka, A.; Marć, M.A.; Satała, G.; Wilczyńska, D.; Kotańska, M.; Więcek, M.; Kamińska, K.; et al. The 1,3,5-triazine derivatives as innovative chemical family of 5-ht6 serotonin receptor agents with therapeutic perspectives for cognitive impairment. Int. J. Mol. Sci. 2019, 20, 3420. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nagle, P.; Pawar, Y.; Sonawane, A.; Bhosale, S.; More, D. Docking simulation, synthesis and biological evaluation of novel pyridazinone containing thymol as potential antimicrobial agents. Med. Chem. Res. 2014, 23, 918–926. [Google Scholar] [CrossRef]
- Nagle, P.S.; Pawar, Y.A.; Sonawane, A.E.; Bhosale, S.M.; More, D.H. Synthesis and evaluation of antioxidant and antimicrobial properties of thymol containing pyridone moieties. Med. Chem. Res. 2012, 21, 1395–1402. [Google Scholar] [CrossRef]
- Kulabaş, N.; Tatar, E.; Özakpınar, Ö.B.; Özsavcı, D.; Pannecouque, C.; De Clercq, E.; Küçükgüzel, I. Synthesis and antiproliferative evaluation of novel 2-(4H-1,2,4-triazole-3-ylthio)acetamide derivatives as inducers of apoptosis in cancer cells. Eur. J. Med. Chem. 2016, 121, 58–70. [Google Scholar] [CrossRef] [PubMed]
- Inci Gul, H.; Yamali, C.; Tugce Yasa, A.; Unluer, E.; Sakagami, H.; Tanc, M.; Supuran, C.T. Carbonic anhydrase inhibition and cytotoxicity studies of Mannich base derivatives of thymol. J. Enzym. Inhib. Med. Chem. 2016, 31, 1375–1380. [Google Scholar] [CrossRef] [Green Version]
- Raghuvanshi, D.S.; Verma, N.; Singh, S.V.; Khare, S.; Pal, A.; Negi, A.S. Synthesis of thymol-based pyrazolines: An effort to perceive novel potent antimalarials. Bioorg. Chem. 2019, 88, 102933. [Google Scholar] [CrossRef]
- Kaur, R.; Darokar, M.P.; Chattopadhyay, S.K.; Krishna, V.; Ahmad, A. Synthesis of halogenated derivatives of thymol and their antimicrobial activities. Med. Chem. Res. 2014, 23, 2212–2217. [Google Scholar] [CrossRef]
- Getrey, L.; Krieg, T.; Hollmann, F.; Schrader, J.; Holtmann, D. Enzymatic halogenation of the phenolic monoterpenes thymol and carvacrol with chloroperoxidase. Green Chem. 2014, 16, 1104–1108. [Google Scholar] [CrossRef]
- Sabuzi, F.; Churakova, E.; Galloni, P.; Wever, R.; Hollmann, F.; Floris, B.; Conte, V. Thymol bromination—A comparison between enzymatic and chemical catalysis. Eur. J. Inorg. Chem. 2015, 2015, 3519–3525. [Google Scholar] [CrossRef] [Green Version]
- Floris, B.; Sabuzi, F.; Coletti, A.; Conte, V. Sustainable vanadium-catalyzed oxidation of organic substrates with H2O2. Cat. Today 2017, 285, 49–56. [Google Scholar] [CrossRef]
- Sabuzi, F.; Pomarico, G.; Floris, B.; Valentini, F.; Galloni, P.; Conte, V. Sustainable bromination of organic compounds: A critical review. Coord. Chem. Rev. 2019, 385, 100–136. [Google Scholar] [CrossRef]
- Galloni, P.; Conte, V.; Sabuzi, F.; Migliore, L.; Thaller, M.C.; Matteucci, G. Sustainable process for the preparation of highly pure 4-bromothymol and its application as antimicrobial agent. World Patent WO 2018/046584 A1, 15 March 2018. [Google Scholar]
- Ulanowska, M.; Olas, B. Biological properties and prospects for the application of eugenol-A review. Int. J. Mol. Sci. 2021, 22, 3671. [Google Scholar] [CrossRef] [PubMed]
- Modjinou, T.; Versace, D.-L.; Abbad-Andallousi, S.; Bousserrhine, N.; Dubot, P.; Langlois, V.; Renard, E. Antibacterial and antioxidant bio-based networks derived from eugenol using photo-activated thiol-ene reaction. React. Funct. Polym. 2016, 101, 47–53. [Google Scholar] [CrossRef]
- Da Silva, F.F.M.; Monte, F.J.Q.; de Lemos, T.L.G.; do Nascimento, P.G.G.; de Medeiros Costa, A.K.; de Paiva, L.M.M. Eugenol derivatives: Synthesis, characterization, and evaluation of antibacterial and antioxidant activities. Chem. Cent. J. 2018, 12, 34. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Charan Raja, M.R.; Velappan, A.B.; Chellappan, D.; Debnath, J.; Mahapatra, S.K. Eugenol derived immunomodulatory molecules against visceral leishmaniasis. Eur. J. Med. Chem. 2017, 139, 503–518. [Google Scholar] [CrossRef]
- Rahim, N.H.C.A.; Asari, A.; Ismail, N.; Osman, H. Synthesis and antibacterial study of eugenol derivatives. Asian J. Chem. 2017, 29, 22–26. [Google Scholar] [CrossRef]
- Behrouz, S.; Rad, M.N.S.; Taghavi Shahraki, B.; Fathalipour, M.; Behrouz, M.; Mirkhani, H. Design, synthesis, and in silico studies of novel eugenyloxy propanol azole derivatives having potent antinociceptive activity and evaluation of their β-adrenoceptor blocking property. Mol. Divers. 2019, 23, 147–164. [Google Scholar] [CrossRef]
- Fernandes, M.J.G.; Pereira, R.B.; Pereira, D.M.; Fortes, A.G.; Castanheira, E.M.S.; Gonçalves, M.S.T. New eugenol derivatives with enhanced insecticidal activity. Int. J. Mol. Sci. 2020, 21, 9257. [Google Scholar] [CrossRef] [PubMed]
- Lenardão, E.J.; Jacob, R.G.; Mesquita, K.D.; Lara, R.G.; Webber, R.; Martinez, D.M.; Savegnago, L.; Mendes, S.R.; Alvesa, D.; Perin, G. Glycerol as a promoting and recyclable medium for catalyst-free synthesis of linear thioethers: New antioxidants from eugenol. Green Chem. Lett. Rev. 2012, 6, 269–276. [Google Scholar] [CrossRef] [Green Version]
- Carrasco, H.; Raimondi, M.; Svetaz, L.; Di Liberto, M.; Rodriguez, M.V.; Espinoza, L.; Madrid, A.; Zacchino, S. Antifungal activity of eugenol analogues. influence of different substituents and studies on mechanism of action. Molecules 2012, 17, 1002–1024. [Google Scholar] [CrossRef] [Green Version]
- Rudyanto, M.; Ekowati, J.; Widiandani, T.; Honda, T. Synthesis and brine shrimp lethality test of some benzoxazine and aminomethyl derivatives of eugenol. Int. J. Pharm. Pharm. Sci. 2014, 6, 96–98. [Google Scholar]
- De Carvalho, L.I.S.; Alvarenga, D.J.; do Carmo, L.C.F.; de Oliveira, L.G.; Silva, N.C.; Dias, A.L.T.; Coelho, L.F.L.; de Souza, T.B.; Dias, D.F.; Carvalho, D.T. Antifungal activity of new eugenol-benzoxazole hybrids against Candida spp. J. Chem. 2017, 2017, 5207439. [Google Scholar] [CrossRef] [Green Version]
- Olea, A.; Bravo, A.; Martínez, R.; Thomas, M.; Sedan, C.; Espinoza, L.; Zambrano, E.; Carvajal, D.; Silva-Moreno, E.; Carrasco, H. Antifungal activity of eugenol derivatives against Botrytis cinerea. Molecules 2019, 24, 1239. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gul, F.; Mohammed Khan, K.; Adhikari, A.; Zafar, S.; Akram, M.; Khan, H.; Saeed, M. Antimicrobial and antioxidant activities of a new metabolite from Quercus incana. Nat. Prod. Res. 2016, 31, 1901–1909. [Google Scholar] [CrossRef]
- Anjum, N.F.; Purohit, M.N.; Yogish Kumar, H.; Ramya, K.; Javid, S.; Salahuddin, M.D.; Prashantha Kumar, B.R. Semisynthetic derivatives of eugenol and their biological properties: A fleeting look at the promising molecules. J. Biol. Act. Prod. Nat. 2020, 10, 379–404. [Google Scholar]
- Kaufman, T.S. The multiple faces of eugenol. A versatile starting material and building block for organic and bio-organic synthesis and a convenient precursor toward bio-based fine chemicals. J. Braz. Chem. Soc. 2015, 26, 1055–1085. [Google Scholar] [CrossRef]
- Espinoza-Hicks, J.C.; Zaragoza-Galán, G.; Chávez-Flores, D.; Ramos-Sánchez, V.H.; Tamariz, J.; Camacho-Dávila, A.A. A convergent total synthesis of the biologically active benzofurans ailanthoidol, egonol and homoegonol from biomass-derived eugenol. Synthesis 2018, 50, 3493–3498. [Google Scholar]
- Kamiloglu, A.A.; Direkel, S.; Yalazan, H.; Kantekin, H.; Acar, I. Octa- and tetra-substituted phthalocyanines with methoxyeugenol group: Synthesis, characterization and in vitro antimicrobial activity. J. Coord. Chem. 2020, 73, 1177–1190. [Google Scholar] [CrossRef]
- Nguyen Thi Thanh, C.; Truong Thi Cam, M.; Pham Van, T.; Nguyen, L.; Nguyen Ha, M.; Van Meervelt, L. Synthesis, structure and in vitro cytotoxicity of platinum(II) complexes containing eugenol and a quinolin-8-ol-derived chelator. Acta Cryst. 2017, C73, 1030–1037. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Naddeo, M.; Vigliotta, G.; Pellecchia, C.; Pappalardo, D. Synthesis of biobased polymacrolactones with pendant eugenol moieties as novel antimicrobial thermoplastic materials. React. Funct. Polym. 2020, 155, 104714. [Google Scholar] [CrossRef]
- Maurya, A.K.; Agarwal, K.; Gupta, A.C.; Saxena, A.; Nooreen, Z.; Tandon, S.; Ahmad, A.; Bawankule, D.U. Synthesis of eugenol derivatives and its antiinflammatory activity against skin inflammation. Nat. Prod. Res. 2020, 34, 251–260. [Google Scholar] [CrossRef] [PubMed]
- D’ Avila Farias, M.; Oliveira, P.S.; Pereira Dutra, F.S.; Jacobsen Fernandes, T.; de Pereira, C.M.P.; Quintana de Oliveira, S.; Moro Stefanello, F.; Leonetti Lencina, C.; Gatto Barschak, A. Eugenol derivatives as potential anti-oxidants: Is phenolic hydroxyl necessary to obtain an effect? J. Pharm. Pharmacol. 2014, 66, 733–746. [Google Scholar] [CrossRef]
- Mastelari Martins, R.; D’Avila Farias, M.; Nedel, F.; de Pereira, C.M.P.; Lencina, C.; Guerra Lund, R. Antimicrobial and cytotoxic evaluation of eugenol derivatives. Med. Chem. Res. 2016, 25, 2360–2367. [Google Scholar] [CrossRef]
- Makuch, E.; Nowak, A.; Günther, A.; Pełech, R.; Kucharski, L.; Duchnik, W.; Klimowicz, A. Enhancement of the antioxidant and skin permeation properties of eugenol by the esterifcation of eugenol to new derivatives. AMB Expr. 2020, 10, 187. [Google Scholar] [CrossRef]
- Lone, S.A.; Wani, M.Y.; Fru, P.; Ahmad, A. Cellular apoptosis and necrosis as therapeutic targets for novel eugenol tosylate congeners against Candida albicans. Sci. Rep. 2020, 10, 1191. [Google Scholar] [CrossRef] [Green Version]
- Lone, S.H.; Ahmad, A. Inhibitory effect of novel eugenol tosylate congeners on pathogenicity of Candida albicans. BMC Complement. Med. Ther. 2020, 20, 131. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, A.; Wani, M.Y.; Khan, A.; Manzoor, N.; Molepo, J. Synergistic interactions of eugenol-tosylate and its congeners with fluconazole against Candida albicans. PLoS ONE 2015, 10, e0145053. [Google Scholar] [CrossRef]
- Li, J.-Y.; Yu, Y.-G.; Wang, Q.-W.; Zhang, J.-Y.; Yang, Y.-J.; Li, B.; Zhou, X.-Z.; Niu, J.-R.; Wei, X.-J.; Liu, X.-W.; et al. Synthesis of aspirin eugenol ester and its biological activity. Med. Chem. Res. 2012, 21, 995–999. [Google Scholar] [CrossRef]
- Ma, N.; Liu, X.-W.; Kong, X.-J.; Li, S.-H.; Jiao, Z.-H.; Qin, Z.; Yang, Y.-J.; Li, J.-Y. Aspirin eugenol ester regulates cecal contents metabolomic profile and microbiota in an animal model of hyperlipidemia. BMC Vet. Res. 2018, 14, 405. [Google Scholar] [CrossRef]
- Shen, D.S.; Yang, Y.-J.; Kong, X.-J.; Ma, N.; Liu, X.-W.; Li, S.-H.; Jiao, Z.H.; Qin, Z.; Huang, M.-Z.; Li, J.-Y. Eugenol ester inhibits agonist-induced platelet aggregation in vitro by regulating PI3K/Akt, MAPK and Sirt 1/CD40L pathways. Eur. J. Pharmacol. 2019, 852, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.-D.; Yang, Y.-J.; Liu, X.-W.; Qin, Z.; Li, S.-H.; Li, J.-Y. The protective effect of aspirin eugenol ester on paraquat-induced acute liver injury rats. Front. Med. 2020, 7, 589011. [Google Scholar] [CrossRef] [PubMed]
- Redasani, V.K.; Bari, S.B. Synthesis and evaluation of mutual prodrugs of ibuprofen with menthol, thymol, and eugenol. Eur. J. Med. Chem. 2012, 56, 134–138. [Google Scholar] [CrossRef]
- Topal, F.; Gulcin, I.; Dastan, A.; Guney, M. Novel eugenol derivatives: Potent acetylcholinesterase and carbonic anhydrase inhibitors. Int. J. Biol. Macromol. 2017, 94, 845–851. [Google Scholar] [CrossRef]
- Bilgiçli, H.G.; Ergön, D.; Taslimi, P.; Tüzün, B.; Kuru, I.A.; Zengin, M.; Gülçin, I. Novel propanolamine derivatives attached to 2-metoxifenol moiety: Synthesis, characterization, biological properties, and molecular docking studies. Bioorg. Chem. 2020, 101, 103969. [Google Scholar] [CrossRef] [PubMed]
- Bilgiçli, H.G.; Kestane, A.; Taslimi, P.; Karabay, O.; Bytyqi-Damoni, A.; Zengin, M.; Gulçin, I. Novel eugenol bearing oxypropanolamines: Synthesis, characterization, antibacterial, antidiabetic, and anticholinergic potentials. Bioorg. Chem. 2019, 88, 102931. [Google Scholar] [CrossRef] [PubMed]
- Taia, A.; Essaber, M.; Oubella, A.; Aatif, A.; Bodiguel, J.; Jamart-Gregoire, B.; Itto, M.Y.A.; Morjani, H. Synthesis, characterization, and biological evaluation of new heterocyclic systems 1, 2, 3-triazole-isoxazoline from eugenol by the mixed condensation reactions. Synth. Commun. 2020, 50, 2052–2065. [Google Scholar] [CrossRef]
- De Oliveira, A.S.; Gazolla, P.A.R.; Oliveira, A.F.C.d.S.; Pereira, W.L.; Viol, L.C.d.S.; Maia, A.F.d.S.; Santos, E.G.; da Silva, I.E.P.; de Oliveira Mendes, T.A.; da Silva, A.M.; et al. Discovery of novel West Nile Virus protease inhibitor based on isobenzonafuranone and triazolic derivatives of eugenol and indan-1,3-dione scaffolds. PLoS ONE 2019, 14, e0223017. [Google Scholar]
- Teixeira, R.R.; Rodrigues Gazolla, P.A.; da Silva, A.M.; Gonçalves Borsodi, M.P.; Rossi Bergmann, B.; Salgado Ferreira, R.; Vaz, B.G.; Vasconcelos, G.A.; Lima, W.P. Synthesis and leishmanicidal activity of eugenol derivatives bearing 1,2,3-triazole functionalities. Eur. J. Med. Chem. 2018, 146, 274–286. [Google Scholar] [CrossRef] [PubMed]
- De Souza, T.B.; Caldas, I.S.; Paula, F.R.; Rodrigues, C.C.; Carvalho, D.T.; Dias, D.F. Synthesis, activity, and molecular modeling studies of 1,2,3-triazole derivatives from natural phenylpropanoids as new trypanocidal agents. Chem. Biol. Drug Des. 2020, 95, 124–129. [Google Scholar] [CrossRef] [PubMed]
- Dos Santos, T.; Coelho, C.M.; Elias, T.C.; Siqueira, F.S.; Nora, E.S.S.D.; de Campos, M.M.A.; de Souza, G.A.P.; Coelho, L.F.L.; Carvalho, D.T. Synthesis and biological evaluation of new eugenol-derived 1,2,3-triazoles as antimycobacterial agents. J. Braz. Chem. Soc. 2019, 30, 1425–1436. [Google Scholar] [CrossRef]
- De Souza, T.B.; Raimundo, P.O.B.; Andrade, D.F.; Hipólito, T.M.M.; Silva, N.C.; Dias, A.L.T.; Ikegaki, M.; Rocha, R.P.; Coelho, L.F.L.; Veloso, M.P.; et al. Synthesis and antimicrobial activity of 6-triazolo-6-deoxy eugenol glucosides. Carbohyd. Res. 2015, 410, 1–8. [Google Scholar] [CrossRef]
- Rohane, S.H.; Chauhan, A.J.; Fuloria, N.K.; Fuloria, S. Synthesis and in vitro antimycobacterial potential of novel hydrazones of eugenol. Arab. J. Chem. 2020, 13, 4495–4504. [Google Scholar] [CrossRef]
- Hipólito, T.M.M.; Bastos, G.T.L.; Barbosa, T.W.L.; de Souza, T.B.; Coelho, L.F.L.; Dias, A.L.T.; Rodríguez, I.C.; dos Santos, M.H.; Ferreira Dias, D.; Franco, L.L.; et al. Synthesis, activity and docking studies of eugenol-based glucosides as new agents against Candida sp. Chem. Biol. Drug Des. 2018, 92, 1514–1524. [Google Scholar] [CrossRef]
- Braga Resende, D.; de Abreu Martins, H.H.; de Souza, T.B.; Carvalho, D.T.; Hilsdorf Piccoli, R.; Schwan, R.F.; Dias, D.R. Synthesis and sp. evaluation of peracetyl and deacetyl glycosides of eugenol, isoeugenol and dihydroeugenol acting against food contaminating bacteria. Food Chem. 2017, 237, 1025–1029. [Google Scholar] [CrossRef]
- De Souza, T.B.; Orlandi, M.; Coelho, L.F.L.; Malaquias, L.C.C.; Dias, A.L.T.; de Carvalho, R.R.; Silva, N.C.; Carvalho, D.T. Synthesis and in vitro evaluation of antifungal and cytotoxic activities of eugenol glycosides. Med. Chem. Res. 2014, 23, 496–502. [Google Scholar] [CrossRef]
- De Souza, T.B.; de Oliveira Brito, K.M.; Silva, N.C.; Rocha, R.P.; de Sousa, G.F.; Duarte, L.P.; Coelho, L.F.L.; Dias, A.L.T.; Veloso, M.P.; Carvalho, D.T.; et al. New eugenol glucoside-based derivative shows fungistatic and fungicidal activity against opportunistic Candida glabrata. Chem. Biol. Drug Des. 2016, 87, 83–90. [Google Scholar] [CrossRef]
- Fernández-Mar, M.I.; Mateos, R.; García-Parrilla, M.C.; Puertas, B.; Cantos-Villar, E. Bioactive compounds in wine: Resveratrol, hydroxytyrosol and melatonin: A review. Food Chem. 2012, 130, 797–813. [Google Scholar] [CrossRef]
- Mattio, L.M.; Catinella, G.; Dallavalle, S.; Pinto, A. Stilbenoids: A natural arsenal against bacterial pathogens. Antibiotics 2020, 9, 336. [Google Scholar] [CrossRef] and references cited therein.
- Mattio, L.M.; Catinella, G.; Pinto, A.; Dallavalle, S. Natural and nature-inspired stilbenoids as antiviral agents. Eur. J. Med. Chem. 2020, 202, 112541. [Google Scholar] [CrossRef] and references cited therein.
- Deng, L.-J.; Qi, M.; Li, N.; Lei, Y.-H.; Zhang, D.-M.; Chen, J.-X. Natural products and their derivatives: Promising modulators of tumor immunotherapy. J. Leukoc. Biol. 2020, 108, 493–508. [Google Scholar] [CrossRef]
- Ashrafizadeh, M.; Rafiei, H.; Mohammadinejad, R.; Farkhondeh, T.; Samarghandian, S. Anti-tumor activity of resveratrol against gastric cancer: A review of recent advances with an emphasis on molecular pathways. Cancer Cell Int. 2021, 21, 66. [Google Scholar] [CrossRef]
- Ferraz da Costa, D.C.; Rangel, L.P.; da Cunha Martins-Dinis, M.M.D.; da Silva Ferretti, G.D.; Ferreira, V.F.; Silva, J.L. Anticancer potential of resveratrol, b-lapachone and their analogues. Molecules 2020, 25, 893. [Google Scholar] [CrossRef] and references cited therein.[Green Version]
- Li, K.-X.; Ji, M.-J.; Sun, H.-J. An updated pharmacological insight of resveratrol in the treatment of diabetic nephropathy. Gene 2021, 780, 145532. [Google Scholar] [CrossRef] [PubMed]
- Lin, M.-H.; Hung, C.-F.; Sung, H.-C.; Yang, S.-C.; Yu, H.-P.; Fang, J.-Y. The bioactivities of resveratrol and its naturally occurring derivatives on skin. Food Drug Anal. 2021, 29, 15–38. [Google Scholar] [CrossRef]
- Shaito, A.; Posadino, A.M.; Younes, N.; Hasan, H.; Halabi, S.; Alhababi, D.; Al-Mohannadi, A.; Abdel-Rahman, W.M.; Eid, A.H.; Nasrallah, G.K.; et al. Potential adverse effects of resveratrol: A literature review. J. Mol. Sci. 2020, 21, 2084. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Intagliata, S.; Modica, M.N.; Santagati, L.M.; Montenegro, L. Strategies to improve resveratrol systemic and topical bioavailability: An update. Antioxidants 2019, 8, 244. [Google Scholar] [CrossRef] and references cited therein.[Green Version]
- Vervandier-Fasseur, D.; Vang, O.; Latruffe, N. Special Issue: Improvements for resveratrol efficacy. Molecules 2017, 22, 1737. [Google Scholar] [CrossRef] and references cited therein.[Green Version]
- Nawaz, W.; Zhou, Z.; Deng, S.; Ma, X.; Ma, X.; Li, C.; Shu, X. Therapeutic versatility of resveratrol derivatives. Nutrients 2017, 9, 1188. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Singh, D.; Mendonsa, R.; Koli, M.; Subramanian, M.; Nayak, S.K. Antibacterial activity of resveratrol structural analogues: A mechanistic evaluation of the structure-activity relationship. Toxicol. Appl. Pharmacol. 2019, 367, 23–32. [Google Scholar] [CrossRef] [PubMed]
- Pecyna, P.; Wargula, J.; Murias, M.; Kucinska, M. More than resveratrol: New insights into stilbene-based compounds. Biomolecules 2020, 10, 1111. [Google Scholar] [CrossRef] and references cited therein.
- Biasutto, L.; Mattarei, A.; Azzolini, M.; La Spina, M.; Sassi, N.; Romio, M.; Paradisi, C.; Zoratti, M. Resveratrol derivatives as a pharmacological tool. Ann. N. Y. Acad. Sci 2017, 1403, 27–37. [Google Scholar] [CrossRef] and references cited therein.
- Latruffe, N.; Vervandier-Fasseur, D. Strategic syntheses of vine and wine resveratrol derivatives to explore their effects on cell functions and dysfunctions. Diseases 2018, 6, 110. [Google Scholar] [CrossRef] [Green Version]
- Peñalver, P.; Belmonte-Reche, E.; Adán, N.; Caro, M.; Mateos-Martín, M.L.; Delgado, M.; González-Rey, E.; Morales, J.C. Alkylated resveratrol prodrugs and metabolites as potential therapeutics for neurodegenerative diseases. Eur. J. Med. Chem. 2018, 146, 123–138. [Google Scholar] [CrossRef]
- Yang, M.-F.; Yao, X.; Chen, L.-M.; Gu, J.-Y.; Yang, Z.-H.; Chen, H.-F.; Zheng, X.; Zheng, Z.-T. Synthesis and biological evaluation of resveratrol derivatives with anti-breast cancer activity. Arch. Pharmazie 2020, 353, e2000044. [Google Scholar] [CrossRef]
- Ahmadi, R.; Ebrahimzadeh, M.A. Resveratrol—A comprehensive review of recent advances in anticancer drug design and development. Eur. J. Med. Chem. 2020, 200, 112356. [Google Scholar] [CrossRef] [PubMed] and references cited therein.
- Venkateswarlu, S.; Ramachandra, M.S.; Sethuramu, K.; Subbaraju, V. Synthesis and antioxidant activity of hispolon, a yellow pigment from Inonotus hispidius. Ind. J. Chem. 2002, 41B, 875–877. [Google Scholar]
- Khan, M.F.; Aktar, S.; Bin Rashid, R.; Rashid, M.A. In silico investigation of physicochemical, pharmacokinetic and toxicological properties of hispolon. Pharma Chem. 2017, 9, 9–13. [Google Scholar]
- Sarfraz, A.; Rasul, A.; Sarfraz, I.; Shah, M.A.; Hussain, G.; Shafiq, N.; Masood, M.; Adem, S.; Sarker, S.D.; Li, X. Hispolon: A natural polyphenol and emerging cancer killer by multiple cellular signaling pathways. Environ. Res. 2020, 190, 110017. [Google Scholar] [CrossRef] [PubMed]
- Paul, M.; Panda, M.K.; Thatoi, H. Developing Hispolon based novel anticancer therapeutics against human (NF-κβ) using in silico approach of modelling, docking and protein dynamics. J. Biomol. Struct. Dyn. 2019, 37, 3947–3967. [Google Scholar] [CrossRef]
- Rossi, M.; Caruso, F.; Costanzini, I.; Kloer, C.; Sulovari, A.; Monti, E.; Gariboldi, M.; Marras, E.; Balaji, N.V.; Ramani, M.V.; et al. X-ray crystal structures, density functional theory and docking on deacetylase enzyme for antiproliferative activity of hispolon derivatives on HCT116 colon cancer. Bioorg. Med. Chem. 2019, 29, 56–58. [Google Scholar] [CrossRef] [PubMed]
- Fan, H.-C.; Hsieh, Y.-C.; Li, L.-H.; Chang, C.-C.; Janoušková, K.; Ramani, M.V.; Subbaraju, G.V.; Cheng, K.-T.; Chang, C.-C. Dehydroxyhispolon methyl ether, a hispolon derivative, inhibits WNT/-catenin signaling to elicit human colorectal carcinoma cell apoptosis. Int. J. Mol. Sci. 2020, 21, 8839. [Google Scholar] [CrossRef]
- Balaji, N.V.; Ramani, M.V.; Viana, A.G.; Sanglard, L.P.; White, J.; Mulabagal, V.; Lee, C.; Gana, T.J.; Egiebor, N.O.; Subbaraju, G.V.; et al. Design, synthesis and in vitro cell-based evaluation of the anti-cancer activities of hispolon analogs. Bioorg. Med. Chem. 2015, 23, 2148–2158. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Balaji, N.V.; Babu, B.H.; Subbaraju, G.V.; Nagasree, K.P.; Kumar, M.M.K. Synthesis, screening and docking analysis of hispolon analogs as potential antitubercular agents. Bioorg. Med. Chem. Lett. 2017, 27, 11–15. [Google Scholar] [CrossRef] [PubMed]
- Chethna, P.; Iyer, S.S.; Gandhi, V.V.; Kunwar, A.; Singh, B.G.; Barik, A.; Balaji, N.V.; Ramani, M.V.; Subbaraju, G.V.; Priyadarsini, K.I. Toxicity and antigenotoxic effect of hispolon derivatives: Role of structure in modulating cellular redox state and thioredoxin reductase. ACS Omega 2018, 3, 5958–5970. [Google Scholar] [CrossRef]
- Shaikh, S.A.M.; Barik, A.; Singh, B.G.; Madukuri, R.V.; Balaji, N.V.; Subbaraju, G.V.; Naik, D.B.; Priyadarsini, K.I. Free radical reactions of isoxazole and pyrazole derivatives of hispolon: Kinetics correlated with molecular descriptors. Free Radical Res. 2016, 50, 1361–1373. [Google Scholar] [CrossRef]
- Wei, X.; Yang, Y.; Ge, J.; Lin, X.; Liu, D.; Wang, S.; Zhang, J.; Zhou, G.; Li, S. Synthesis, characterization, DNA/BSA interactions and in vitro cytotoxicity study of palladium(II) complexes of hispolon derivatives. J. Inorg. Biochem. 2020, 202, 110857. [Google Scholar] [CrossRef]
- Bertelli, M.; Kiani, A.K.; Paolacci, S.; Manara, E.; Baglivo, M.; Kurti, D.; Dhuli, K.; Bushati, V.; Miertus, J.; Pangallo, D.; et al. Hydroxytyrosol: A natural compound with promising pharmacological activities. J. Biotechnol. 2020, 309, 29–33. [Google Scholar] [CrossRef] [PubMed]
- Wei, J.; Wang, S.; Pei, D.; Qu, L.; Li, Y.; Chen, J.; Di, D.; Gao, K. Antibacterial activity of hydroxytyrosol acetate from olive leaves (Olea Europaea L.). Nat. Prod. Res. 2018, 32, 1967–1970. [Google Scholar] [CrossRef] [PubMed]
- Yao, F.; Yang, G.; Xian, Y.; Wang, G.; Zheng, Z.; Jin, Z.; Xie, Y.; Wang, W.; Gue, J.; Lin, R. Protective effect of hydroxytyrosol acetate against inflammation of vascular endothelial cells partly through SIRT6-mediated PKM2 signaling pathway. Food Funct. 2019, 10, 5789–5803. [Google Scholar] [CrossRef]
- Qin, C.; Hu, S.; Zhang, S.; Zhao, D.; Wang, Y.; Li, L.; Peng, Y.; Shi, L.; Xu, X.; Wang, C.; et al. Hydroxytyrosol acetate improves the cognitive function of APP/PS1 transgenic mice in ERβ-dependent manner. Mol. Nutr. Food Res. 2021, 65, 2000797. [Google Scholar] [CrossRef]
- Aparicio-Soto, M.; Sánchez-Fidalgo, S.; González-Benjumea, A.; Maya, I.; Fernández-Bolaños, J.G.; Alarcón-de-la-Lastra, C. Naturally occurring hydroxytyrosol derivatives: Hydroxytyrosyl acetate and 3,4-dihydroxyphenylglycol modulate inflammatory response in murine peritoneal macrophages. Potential utility as new dietary supplements. J. Agric. Food Chem. 2015, 63, 836–846. [Google Scholar] [CrossRef] [PubMed]
- Martin, D.; Garcia-Serrano, A.; Casado, V.; Vázquez, L.; Reglero, G.; Torres, C.F. Antioxidant activity of phosphatidyl derivatives of hydroxytyrosol in edible oils. Eur. J. Lipid Sci. Technol. 2014, 116, 1035–1043. [Google Scholar] [CrossRef]
- Martin, D.; Moran-Valero, M.I.; Casado, V.; Reglero, G.; Torres, C.F. Phosphatidyl derivative of hydroxytyrosol. In Vitro intestinal digestion, bioaccessibility, and its effect on antioxidant activity. J. Agric. Food Chem. 2014, 62, 9751–9759. [Google Scholar] [CrossRef] [PubMed]
- Montoya, T.; Aparicio-Soto, M.; Castejón, M.L.; Rosillo, M.A.; Sánchez-Hidalgo, M.; Begines, P.; Fernández-Bolaños, J.G.; Alarcón-de-la-Lastra, C. Peracetylated hydroxytyrosol, a new hydroxytyrosol derivate, attenuates LPS-induced inflammatory response in murine peritoneal macrophages via regulation of non-canonical inflammasome, Nrf2/HO1 and JAK/STAT signaling pathways. J. Nutr. Biochem. 2018, 57, 110–120. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Gutiérrez, G.; Rubio-Senent, F.; Gómez-Carretero, A.; Maya, I.; Fernández-Bolaños, J.; Duthie, G.G.; de Roos, B. Selenium and sulphur derivatives of hydroxytyrosol: Inhibition of lipid peroxidation in liver microsomes of vitamin E-deficient rats. Eur. J. Nutr. 2019, 58, 1847–1851. [Google Scholar] [CrossRef] [Green Version]
- Majhi, S.; Das, D. Chemical derivatization of natural products: Semisynthesis and pharmacological aspects—A decade update. Tetrahedron 2021, 78, 131801. [Google Scholar] [CrossRef]
- Tofani, D.; Balducci, V.; Gasperi, T.; Incerpi, S.; Gambacorta, A. Fatty acid hydroxytyrosyl esters: Structure/antioxidant activity relationship by ABTS and in cell-culture dcf assays. J. Agric. Food Chem. 2010, 58, 5292–5299. [Google Scholar] [CrossRef]
- Zhou, D.-A.; Sun, Y.-X.; Shahidi, F. Preparation and antioxidant activity of tyrosol and hydroxytyrosol esters. J. Funct. Food. 2017, 37, 66–73. [Google Scholar] [CrossRef]
- Balducci, V.; Incerpi, S.; Stano, P.; Tofani, D. Antioxidant activity of hydroxytyrosyl esters studied in liposome models. BBA Biomembr. 2018, 1860, 600–610. [Google Scholar] [CrossRef] [PubMed]
- Lopes, R.; Costa, M.; Ferreira, M.; Gameiro, P.; Paiva-Martins, F. A new family of hydroxytyrosol phenolipids for the antioxidant protection of liposomal systems. BBA Biomembr. 2021, 1863, 183505. [Google Scholar] [CrossRef]
- Candiracci, M.; Madrona, A.; Espartero, J.L.; Zappia, G.; Piatti, E. Lipophilic hydroxytyrosol esters significantly improve the oxidative state of human red blood cells. J. Funct. Food. 2016, 23, 339–347. [Google Scholar] [CrossRef]
- Belmonte-Reche, E.; Martínez-García, M.; Peñalver, P.; Gómez-Pérez, V.; Lucas, R.; Gamarro, F.; Pérez-Victoria, J.M.; Morales, J.C. Tyrosol and hydroxytyrosol derivatives as antitrypanosomal and antileishmanial agents. Eur. J. Med. Chem. 2016, 119, 132–140. [Google Scholar] [CrossRef]
- Funakohi-Tago, M.; Sakata, T.; Fujiwara, S.; Sakakura, A.; Sugai, T.; Tago, K.; Tamura, H. Hydroxytyrosol butyrate inhibits 6-OHDA-induced apoptosis through activation of the Nrf2/HO-1 axis in SH-SY5Y cells. Eur. J. Pharmacol. 2018, 834, 246–256. [Google Scholar]
- Xie, Y.; Xu, Y.; Chen, Z.; Lu, W.; Li, N.; Wang, Q.; Shao, L.; Li, Y.; Yang, G.; Bian, X. A new multifunctional hydroxytyrosol-fenofibrate with antidiabetic, antihyperlipidemic, antioxidant and antiinflammatory action. Biomed. Pharmacother. 2017, 95, 1749–1758. [Google Scholar] [CrossRef]
- Xie, Y.-D.; Chen, Z.-Z.; Li, N.; Lu, W.-F.; Xu, Y.-H.; Lin, Y.Y.; Shao, L.-H.; Wang, Q.-T.; Guo, L.-Y.; Gao, Y.-Q.; et al. Hydroxytyrosol nicotinate, a new multifunctional hypolipidemic and hypoglycemic agent. Biomed. Pharmacother. 2018, 99, 715–724. [Google Scholar] [CrossRef]
- Crisante, F.; Taresco, V.; Donelli, G.; Vuotto, C.; Martinelli, A.; D’Ilario, L.; Pietrelli, L.; Francolini, I.; Piozzi, A. Antioxidant hydroxytyrosol-based polyacrylate with antimicrobial and antiadhesive activity versus Staphylococcus epidermidis. In Advances in Microbiology, Infectious Diseases and Public Health, 1st ed.; Doninelli, G., Ed.; Springer: Cham, Switzerland, 2016; Volume 2, pp. 25–36. [Google Scholar]
- Romanucci, V.; Giordano, M.; De Tommaso, G.; Iuliano, M.; Bernini, R.; Clemente, M.; Garcia-Viñuales, S.; Milardi, D.; Zarrelli, A.; Di Fabio, G. Synthesis of new tyrosol-based phosphodiester derivatives: Effect on amyloid β aggregation and metal chelation ability. Chem. Med. Chem. 2021, 16, 1172–1183. [Google Scholar] [CrossRef] [PubMed]
- Marrero, A.D.; Castilla, L.; Espartero, J.L.; Madrona, A.; Quesada, A.R.; Medina, M.A.; Martínez-Poveda, B. A comparative study of the antiangiogenic activity of hydroxytyrosyl alkyl ethers. Food Chem. 2020, 333, 127476. [Google Scholar] [CrossRef]
- Muñoz-Marín, J.; De La Cruz, J.P.; Reyes, J.J.; López-Villodres, J.A.; Guerrero, A.; López-Leiva, I.; Espartero, J.L.; Labajos, M.T.; González-Correa, J.A. Hydroxytyrosyl alkyl ether derivatives inhibit platelet activation after oral administration to rats. Food Chem. Toxicol. 2013, 58, 295–300. [Google Scholar] [CrossRef] [PubMed]
- Pereira-Caro, G.; Mateos, R.; Traka, M.H.; Bacon, J.R.; Bongaerts, R.; Sarriá, B.; Bravo, L.; Kroon, P.A. Hydroxytyrosyl ethyl ether exhibits stronger intestinal anticarcinogenic potency and effects on transcript profiles compared to hydroxytyrosol. Food Chem. 2013, 138, 1172–1182. [Google Scholar] [CrossRef]
- Cert, R.; Madrona, A.; Espartero, J.L.; Pérez-Camino, M.C. Antioxidant activity of alkyl hydroxytyrosyl ethers in unsaturated lipids. Food Funct. 2015, 6, 1999–2007. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nieto-Domínguez, M.; de Eugenio, L.I.; Peñalver, P.; Belmonte-Reche, E.; Morales, J.C.; Poveda, A.; Jiménez-Barbero, J.; Prieto, A.; Plou, F.J.; Martínez, M.J. Enzymatic synthesis of a novel neuroprotective hydroxytyrosyl glycoside. J. Agric. Food Chem. 2017, 65, 10526–10533. [Google Scholar] [CrossRef]
- Trujillo, M.; Gallardo, E.; Madrona, A.; Bravo, L.; Sarriá, B.; González-Correa, J.A.; Mateos, R.; Espartero, J.L. Synthesis and antioxidant activity of nitrohydroxytyrosol and its acyl derivatives. J. Agric. Food Chem. 2014, 62, 10297–10303. [Google Scholar] [CrossRef] [Green Version]
- Gallardo, E.; Palma-Valdés, R.; Sarriá, B.; Gallardo, I.; de la Cruz, J.P.; Bravo, L.; Mateos, R.; Espartero, J.L. Synthesis and antioxidant activity of Alkyl nitroderivatives of hydroxytyrosol. Molecules 2016, 21, 656. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fernandez-Pastor, I.; Fernandez-Hernandez, A.; Rivas, F.; Martinez, A.; Garcia-Granados, A.; Parra, A. Synthesis and antioxidant activity of hydroxytyrosol Alkyl-carbonate derivatives. J. Nat. Prod. 2016, 79, 1737–1745. [Google Scholar] [CrossRef]
- Fernandez-Pastor, I.; Martínez-García, M.; Medina-O’Donnell, M.; Rivas, F.; Martinez, A.; Pérez-Victoria, J.M.; Parra, A. Semisynthesis of ω-hydroxyalkylcarbonate derivatives of hydroxytyrosol as antitrypanosome agents. J. Nat. Prod. 2018, 81, 2075–2082. [Google Scholar] [CrossRef] [PubMed]
- Servili, M.; Esposto, S.; Fabiani, R.; Urbani, S.; Taticchi, A.; Mariucci, F.; Selvaggini, R.; Montedoro, G.F. Phenolic compounds in olive oil: Antioxidant, health and organoleptic activities according to their chemical structure. Inflammopharmacology 2009, 17, 76–84. [Google Scholar] [CrossRef]
- Khadem, S.; Marles, R.J. Monocyclic phenolic acids; hydroxy- and polyhydroxybenzoic acids: Occurrence and recent bioactivity studies. Molecules 2010, 15, 7985–8005. [Google Scholar] [CrossRef] [PubMed]
- Heleno, S.A.; Martins, A.; Queiroz, M.J.R.P.; Ferreira, I.C.F.R. Bioactivity of phenolic acids: Metabolites versus parent compounds: A review. Food Chem. 2015, 173, 501–513. [Google Scholar] [CrossRef] [Green Version]
- Muronetz, V.I.; Barinova, K.; Kudryavtseva, S.; Medvedeva, M.; Melnikova, A.; Sevostyanova, I.; Semenyuk, P.; Stroylova, Y.; Sova, M. Natural and synthetic derivatives of hydroxycinnamic acid modulating the pathological transformation of amyloidogenic proteins. Molecules 2020, 25, 4647. [Google Scholar] [CrossRef]
- Kumar, N.; Goel, N. Phenolic acids: Natural versatile molecules with promising therapeutic applications. Biotechnol. Reports 2019, 24, e00370. [Google Scholar] [CrossRef]
- Manuja, R.; Sachdeva, S.; Jain, A.; Chaudhary, J. A comprehensive review on biological activities of p-hydroxy benzoic acid and its derivatives. Int. J. Pharm. Sci. Rev. Res. 2013, 22, 109–115. [Google Scholar]
- Vinayagam, R.; Jayachandran, M.; Xu, B. Antidiabetic effects of simple phenolic acids: A comprehensive review. Phytother. Res. 2016, 30, 184–199. [Google Scholar] [CrossRef]
- Kakkar, S.; Bais, S. A review on protocatechuic acid and its pharmacological potential. ISRN Pharmacol. 2014, 26, 952943. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khan, A.K.; Rashid, R.; Nighat, F.; Sadaf, M.; Mir, S.; Khan, S.; Jabeen, N.; Murtaza, G. Pharmacological activities of protocatechuic acid. Acta Pol. Pharm. Drug Res. 2015, 72, 643–650. [Google Scholar]
- Al Zahrani, N.A.; El-Shishtawy, R.M.; Asiri, A.M. Recent developments of gallic acid derivatives and their hybrids in medicinal chemistry: A review. Eur. J. Med. Chem. 2020, 204, 112609. [Google Scholar] [CrossRef]
- Naira, N.; Asdaq, S.M.B.; Heba, S.; Said, A.H.E.l.A. Gallic acid: A promising lead molecule for drug development. J. App. Pharm. 2016, 8, 213. [Google Scholar]
- Ou, S.; Kwok, K.-C. Ferulic acid: Pharmaceutical functions, preparation and applications in foods. J. Sci. Food Agric. 2004, 84, 1261–1269. [Google Scholar] [CrossRef]
- Zhao, Z.; Moghadasian, M.H. Chemistry, natural sources, dietary intake and pharmacokinetic properties of ferulic acid: A review. Food Chem. 2008, 109, 691–702. [Google Scholar] [CrossRef] [PubMed]
- Brenelli de Paiva, L.; Goldbeck, R.; Dantas dos Santos, W.; Squina, F.M. Ferulic acid and derivatives: Molecules with potential application in the pharmaceutical field. Braz. J. Pharm. Sci. 2013, 49, 395–411. [Google Scholar] [CrossRef] [Green Version]
- Pei, K.; Ou, J.; Huang, C.; Ou, S. Derivatives of ferulic acid: Structure, preparation and biological activities. Ann. Res. Rev. Biol. 2015, 5, 512–528. [Google Scholar] [CrossRef]
- Zduńska, K.; Dana, A.; Kolodziejczak, A.; Rotsztejn, H. Antioxidant properties of ferulic acid and its possible application. Skin Pharmacol. Physiol. 2018, 31, 332–336. [Google Scholar] [CrossRef] [PubMed]
- Jiang, R.-W.; Lau, K.-M.; Hon, P.-M.; Mak, T.C.W.; Woo, K.-S.; Fung, K.-P. Chemistry and biological activities of caffeic acid derivatives from Salvia miltiorrhiza. Curr. Med. Chem. 2005, 12, 237–246. [Google Scholar] [CrossRef]
- Zhang, P.; Tang, Y.; Li, N.-G.; Zhu, Y.; Duan, J.-A. Bioactivity and chemical synthesis of caffeic acid phenethyl ester and its derivatives. Molecules 2014, 19, 16458–16476. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, T.; Zhu, G.; Meng, X.; Zhang, X. Recent developments of small molecules with anti-inflammatory activities for the treatment of acute lung injury. Eur. J. Med. Chem. 2020, 207, 112660. [Google Scholar] [CrossRef]
- Wang, J.; Mahajani, M.; Jackson, S.L.; Yang, Y.; Chen, M.; Ferreira, E.M.; Lin, Y.; Yan, Y. Engineering a bacterial platform for total biosynthesis of caffeic acid derived phenethyl esters and amides. Metab. Eng. 2017, 44, 89–99. [Google Scholar] [CrossRef]
- Widjaja, A.; Yeh, T.-H.; Ju, Y.-H. Enzymatic synthesis of caffeic acid phenethyl ester. J. Chin. Inst. Chem. Eng. 2008, 39, 413–418. [Google Scholar] [CrossRef]
- Chen, H.-C.; Chen, J.-H.; Chang, C.; Shieh, C.-J. Optimization of ultrasound-accelerated synthesis of enzymatic caffeic acid phenethyl ester by response surface methodology. Ultrasonics Sonochem. 2011, 18, 455–459. [Google Scholar] [CrossRef]
- Kurata, A.; Kitamura, Y.; Irie, S.; Takemoto, S.; Akai, Y.; Hirota, Y.; Fujita, T.; Iwai, K.; Furusawa, M.; Kishimoto, N. Enzymatic synthesis of caffeic acid phenethyl ester analogues in ionic liquid. J. Biotechnol. 2010, 148, 133–138. [Google Scholar] [CrossRef]
- De Cássia Orlandi Sardi, J.; Gullo, F.P.; Almeida Freires, I.; de Souza Pitangui, N.; Segalla, M.P.; Fusco-Almeida, A.M.; Rosalen, P.L.; Regasini, L.O.; Soares Mendes-Giannini, M.J. Synthesis, antifungal activity of caffeic acid derivative esters, and their synergism with fluconazole and nystatin against Candida spp. Diagn. Microbiol. Infect. Dis. 2016, 86, 387–391. [Google Scholar] [CrossRef] [Green Version]
- Da Cunha, F.M.; Duma, D.; Assreuy, J.; Buzzi, F.C.; Niero, R.; Campos, M.M.; Calixto, J. Caffeic acid derivatives: In Vitro and In Vivo anti-inflammatory properties. Free Rad. Res. 2004, 38, 1241–1253. [Google Scholar] [CrossRef]
- Shi, H.; Xie, D.; Yang, R.; Cheng, Y. Synthesis of caffeic acid phenethyl ester derivatives, and their cytoprotective and neuritogenic activities in PC12 cells. J. Agric. Food Chem. 2014, 62, 5046–5053. [Google Scholar] [CrossRef]
- Chiang, E.-P.I.; Tsai, S.-Y.; Kuo, Y.-H.; Pai, M.-H.; Chiu, H.-L.; Rodriguez, R.L.; Tang, F.-Y. Caffeic acid derivatives inhibit the growth of colon cancer: Involvement of the PI3-k/akt and ampk signaling pathways. PLoS ONE 2014, 9, e99631. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, J.; Xu, L.-X.; Xu, X.-S.; Li, B.-W.; Wang, R.; Fu, J.-J. Synthesis and effects of new caffeic acid derivatives on nitric oxide production in lipopolysaccharide-induced RAW 264.7 macrophages. Int. J. Clin. Exp. Med. 2014, 7, 1022–1027. [Google Scholar]
- De Lucia, D.; Lucio, O.M.; Musio, B.; Bender, A.; Listing, M.; Dennhardt, S.; Koeberle, A.; Garscha, U.; Rizzo, R.; Manfredini, S.; et al. Design, synthesis and evaluation of semi-synthetic triazole-containing caffeic acid analogues as 5-lipoxygenase inhibitors. Eur. J. Med. Chem. 2015, 101, 573–583. [Google Scholar] [CrossRef]
- Rajan, P.; Vedernikova, I.; Cos, P.; Vanden Berghe, D.; Augustyns, K.; Haemers, A. Synthesis and evaluation of caffeic acid amides as antioxidants. Bioorg. Med. Chem. Lett. 2001, 11, 215–217. [Google Scholar] [CrossRef]
- Xie, Y.; Huang, B.; Yu, K.; Shi, F.; Liu, T.; Xu, W. Caffeic acid derivatives: A new type of influenza neuraminidase inhibitors. Bioorg. Med. Chem. Lett. 2013, 23, 3556–3560. [Google Scholar] [CrossRef] [PubMed]
- Chao, X.; He, X.; Yang, Y.; Zhou, X.; Jin, M.; Liu, S.; Cheng, Z.; Liu, P.; Wang, Y.; Yu, J.; et al. Design, synthesis and pharmacological evaluation of novel tacrine-caffeic acid hybrids as multi-targeted compounds against Alzheimer’s disease. Bioorg. Med. Chem. Lett. 2012, 22, 6498–6502. [Google Scholar] [CrossRef] [PubMed]
- Hezam Al-Ostoot, F.; Zabiulla, S.; Grisha, S.; Hussein Eissa Mohammed, Y.; Vivek, H.K.; Ara Khanum, S. Molecular docking and synthesis of caffeic acid analogous and its anti-inflammatory, analgesic and ulcerogenic studies. Bioorg. Med. Chem. Lett. 2021, 33, 127743. [Google Scholar] [CrossRef]
- Zhou, D.; Weng, X. A novel butylated caffeic acid derivative protects hacat keratinocytes from squalene peroxidation-induced stress. Skin Pharmacol. Physiol. 2019, 32, 307–317. [Google Scholar] [CrossRef]
- Chigorimbo-Murefu, N.T.L.; Riva, S.; Burton, S.G. Lipase-catalysed synthesis of esters of ferulic acid with natural compounds and evaluation of their antioxidant properties. J. Mol. Catal. B Enzym. 2009, 56, 277–282. [Google Scholar] [CrossRef]
- Lee, G.-S.; Widjaja, A.; Ju, Y.-H. Enzymatic synthesis of cinnamic acid derivatives. Biotechnol. Lett. 2006, 28, 581–585. [Google Scholar] [CrossRef] [PubMed]
- Sandoval, G.; Quintana, P.G.; Baldessari, A.; Ballesteros, A.O.; Plou, F.J. Lipase-catalyzed preparation of mono- and diesters of ferulic acid. Biocat. Biotransf. 2015, 33, 89–97. [Google Scholar] [CrossRef]
- Adeyemi, O.S.; Atolani, O.; Banerjee, P.; Arolasafe, G.; Preissner, R.; Etukudoh, P.; Ibraheem, O. Computational and experimental validation of antioxidant properties of synthesized bioactive ferulic acid derivatives. Int. J. Food Prop. 2018, 21, 101–113. [Google Scholar] [CrossRef]
- Ravi Kiran, T.N.; Sri Alekhya, C.; Lokesh, B.V.S.; Madhu Latha, A.V.S.; Rajendra Prasad, Y.; Naga Mounika, T. Synthesis, characterization and biological screening of ferulic acid derivatives. J. Cancer Ther. 2015, 6, 917–931. [Google Scholar] [CrossRef] [Green Version]
- Nomura, E.; Kashiwada, A.; Hosoda, A.; Nakamura, K.; Morishita, H.; Tsuno, T.; Taniguchi, H. Synthesis of amide compounds of ferulic acid, and their stimulatory effects on insulin secretion in vitro. Bioorg. Med. Chem. 2003, 11, 3807–3813. [Google Scholar] [CrossRef]
- Lee, Y.-T.; Hsieh, Y.-L.; Yeh, Y.-H.; Huang, C.-Y. Synthesis of phenolic amides and evaluation of their antioxidant and anti-inflammatory activity in vitro and in vivo. RSC Adv. 2015, 5, 85806–85815. [Google Scholar] [CrossRef]
- Huang, G.-Y.; Cui, C.; Wang, Z.-P.; Li, Y.-Q.; Xiong, L.-X.; Wang, L.-Z.; Yu, S.-J.; Li, Z.-M.; Zhao, W.-G. Synthesis and characteristics of (Hydrogenated) ferulic acid derivatives as potential antiviral agents with insecticidal activity. Chem. Cent. J. 2013, 7, 33. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cui, C.; Wang, Z.-P.; Du, X.; Wang, L.-Z.; Yu, S.-J.; Liu, X.-H.; Li, Z.-M.; Zhao, W.-G. Synthesis and antiviral activity of hydrogenated ferulic acid derivatives. J. Chem. 2013, 2013, 269434. [Google Scholar] [CrossRef]
- Firdaus, P.; Soekamto, N.H.; Seniwati, P.; Islam, M.F.; Sultan, P. Phenethyl ester and amide of ferulic acids: Synthesis and bioactivity against P388 leukemia murine cells. IOP Conf. Ser. J. Phys. Conf. Ser. 2018, 979, 012016. [Google Scholar] [CrossRef] [Green Version]
- Firdaus, H.D.R.; Naid, T.; Seniwati, K.; Soekamto, N.H.; Sumarna, S.; Islam, M.F. Synthesis of piperidine and morpholine amides of ferulic acid and their bioactivity against P-388 leukemia cells. Int. J. Chemtech. Res. 2017, 10, 27. [Google Scholar]
- Kumar, N.; Kumar, S.; Abbat, S.; Nikhil, K.; Sondhi, S.M.; Bharatam, P.V.; Roy, P.; Pruthi, V. Ferulic acid amide derivatives as anticancer and antioxidant agents: Synthesis, thermal, biological and computational studies. Med. Chem. Res. 2015, 25, 1175–1192. [Google Scholar] [CrossRef]
- Serafim, T.L.; Carvalho, F.S.; Marques, M.P.M.; Calheiros, R.; Silva, T.; Garrido, J.; Milhazes, N.; Borges, F.; Roleira, F.; Silva, E.T.; et al. Lipophilic caffeic and ferulic acid derivatives presenting cytotoxicity against human breast cancer cells. Chem. Res. Toxicol. 2011, 24, 763–774. [Google Scholar] [CrossRef] [Green Version]
- Wang, F.; Lu, W.; Zhang, T.; Dong, J.; Gao, H.; Li, P.; Wang, S.; Zhang, J. Development of novel ferulic acid derivatives as potent histone deacetylase inhibitors. Bioorg. Med. Chem. 2013, 21, 6973–6980. [Google Scholar] [CrossRef]
- Han, B.S.; Park, C.B.; Takasuka, N.; Naito, A.; Sekine, K.; Nomura, E.; Taniguchi, H.; Tsuno, T.; Tsuda, H. A ferulic acid derivative, ethyl 3-(4′-geranyloxy-3-methoxyphenyl)-2-propenoate, as a new candidate chemopreventive agent for colon carcinogenesis in the rat. Jpn. J. Cancer Res. 2001, 92, 404–409. [Google Scholar] [CrossRef]
- Gaspar, A.; Martins, M.; Silva, P.; Garrido, E.M.; Garrido, J.; Firuzi, O.; Miri, R.; Saso, L.; Borge, F. Dietary phenolic acids and derivatives. Evaluation of the antioxidant activity of sinapic acid and its alkyl esters. J. Agric. Food Chem. 2010, 58, 11273–11280. [Google Scholar] [CrossRef] [PubMed]
- Flausino, O.A., Jr.; Dufau, L.; Regasini, L.O.; Petrônio, M.S.; Silva, D.H.S.; Rose, T.; Bolzani, V.S.; Reboud-Ravaux, M. Alkyl hydroxybenzoic acid derivatives that inhibit HIV-1 protease dimerization. Curr. Med. Chem. 2012, 19, 4534–4540. [Google Scholar] [CrossRef] [PubMed]
- Daréa, R.G.; Oliveira, M.M.; Truiti, M.C.T.; Nakamura, C.V.; Ximenes, V.F.; Lautenschlager, S.O.S. Abilities of protocatechuic acid and its alkyl esters, ethyl and heptyl protocatechuates, to counteract UVB-induced oxidative injuries and photoaging in fibroblasts L929 cell line. J. Photochem. Photobiol. B Biol. 2020, 203, 111771. [Google Scholar] [CrossRef] [PubMed]
- Savi, L.A.; Leal, P.C.; Vieira, T.O.; Rosso, R.; Nunes, R.J.; Yunes, R.A.; Creczynski-Pasa, T.B.; Barardic, C.R.M.; Simões, C.M.O. Evaluation of anti-herpetic and antioxidant activities, and cytotoxic and genotoxic effects of synthetic Alkyl-esters of gallic acid. Arzneim. Forsch. Drug Res. 2005, 55, 66–75. [Google Scholar] [CrossRef] [PubMed]
- Locatelli, C.; Filippin-Monteiro, F.B.; Creczynski-Pasa, T.B. Alkyl esters of gallic acid as anticancer agents: A review. Eur. J. Med. Chem. 2013, 60, 233–239. [Google Scholar] [CrossRef] [PubMed]
- Arunkumar, S.; Ilango, K.; Mandikandan, R.S.; Ramalakshmi, N. Synthesis and anti-inflammatory activity of some novel pyrazole derivatives of gallic acid. J. Chem. 2009, 6, S123–S128. [Google Scholar] [CrossRef] [Green Version]
- Belkheiri, N.; Bouguerne, B.; Bedos-Belval, F.; Duran, H.; Bernis, C.; Salvayre, R.; Nègre-Salvayre, A.; Baltas, M. Synthesis and antioxidant activity evaluation of a syringic hydrazones family. Eur. J. Med. Chem. 2010, 45, 3019–3026. [Google Scholar] [CrossRef]
- See, as an example: Koufaki, M. Vitamin E derivatives: A patent review (2010–2015). Expert Opin. Ther. Pat. 2016, 26, 35–47. [Google Scholar] [CrossRef]
- Kozubek, A.; Tyman, J.H.P. Resorcinolic lipids, the natural non-isoprenoid phenolic amphiphiles and their biological activity. Chem. Rev. 1999, 99, 1–25. [Google Scholar] [CrossRef] [PubMed]
- Stasiuk, M.; Kozubek, A. Biological activity of phenolic lipids. Cell. Mol. Life Sci. 2010, 67, 841–860. [Google Scholar] [CrossRef]
- Meshginfar, N.; Tavakoli, H.; Dornan, K.; Hosseinian, F.; Hosseinian, F. Phenolic lipids as unique bioactive compounds: A comprehensive review on their multifunctional activity toward the prevention of Alzheimer’s disease. Crit. Rev. Food Sci. Nutr. 2021, 61, 1394–1403. [Google Scholar] [CrossRef]
- Teixeira dos Santos, A.; Coelho Bernardo Guerra, G.; Marques, J.I.; Torres-Rêgo, M.; Ferreira Alves, J.S.; Carvalho Vasconcelos, R.; Fernandes de Souza Araújo, D.; Silva Abreu, L.; Gomes de Carvalho, T.; Cavalcante de Araújo, D.R.; et al. Potentialities of cashew nut (Anacardium occidentale) by-product for pharmaceutical applications: Extraction and purification technologies, safety, and anti-inflammatory and anti-arthritis activities. Rev. Bras. Farm. 2020, 30, 652–666. [Google Scholar] [CrossRef]
- Hamdy Roby, M.H. Synthesis and characterization of phenolic lipids. In Phenolic Compounds—Natural Sources, Importance and Applications; Soto-Hernandex, M., Palma-Tenango, M., Garcia-Mateos, M.d.R., Eds.; IntechOpen: London, UK, 2017. [Google Scholar] and references cited therein.
- Reddy Jala, R.C.; Hu, P.; Yang, T.; Jiang, Y.; Zheng, Y.; Xu, X. Lipases as biocatalysts for the synthesis of structured lipids. Methods Mol. Biol. 2012, 861, 403–433. [Google Scholar]
- Torres de Pinedo, A.; Penalver, P.; Rondon, D.; Morales, J.C. Efficient lipase-catalyzed synthesis of new lipid antioxidants based on a catechol structure. Tetrahedron 2005, 61, 7654–7660. [Google Scholar] [CrossRef]
- Sabally, K.; Karboune, S.; St-Louis, R.; Kermasha, S. Lipase-catalyzed transesterification of dihydrocaffeic acid with flaxseed oil for the synthesis of phenolic lipids. J. Biotechnol. 2006, 127, 167–176. [Google Scholar] [CrossRef] [PubMed]
- Karboune, S.; St-Louis, R.; Kermasha, S. Enzymatic synthesis of structured phenolic lipids by acidolysis of flaxseed oil with selected phenolic acids. J. Mol. Catal. B Enzym. 2008, 52-53, 96–105. [Google Scholar] [CrossRef]
- Safari, M.; Karboune, S.; St-Louis, R.; Kermasha, S. Enzymatic synthesis of structured phenolic lipids by incorporation of selected phenolic acids into triolein. Biocatal. Biotransf. 2006, 24, 272–279. [Google Scholar] [CrossRef]
- Sabally, K.; Karboune, S.; St-Louis, R.; Kermasha, S. Lipase-catalyzed synthesis of phenolic lipids from fish liver oil and dihydrocaffeic acid. Biocat. Biotransf. 2007, 25, 211–218. [Google Scholar] [CrossRef]
- Aziz, S.; St-Louis, R.; Yaylayan, V.; Kermasha, S. Chromatographic separation of synthesized phenolic lipids from krill oil and dihydroxyphenyl acetic acid. J. Am. Oil. Chem. Soc. 2012, 89, 597–608. [Google Scholar] [CrossRef]
- Sorour, N.; Karboune, S.; Saint-Louis, R.; Kermasha, S. Lipase-catalyzed synthesis of structured phenolic lipids in solvent-free system using flaxseed oil and selected phenolic acids as substrates. J. Biotechnol. 2012, 158, 128–136. [Google Scholar] [CrossRef]
- Sorour, N.; Karboune, S.; Saint-Louis, R.; Kermasha, S. Enzymatic synthesis of phenolic lipids in solvent-free medium using flaxseed oil and 3,4-dihydroxyphenyl acetic acid. Proc. Biochem. 2012, 47, 1813–1819. [Google Scholar] [CrossRef]
- Ciftci, D.; Saldaña, M.D.A. Enzymatic synthesis of phenolic lipids using flaxseed oil and ferulic acid in supercritical carbon dioxide media. J. Supercrit. Fluids 2012, 72, 255–262. [Google Scholar] [CrossRef]
- Reddy, K.K.; Shanker, K.S.; Ravinder, T.; Prasad, R.B.N.; Kanjilal, S. Chemo-enzymatic synthesis and evaluation of novel structured phenolic lipids as potential lipophilic antioxidants. Eur. J. Lip. Sci. Techn. 2010, 112, 600–608. [Google Scholar] [CrossRef]
- Balakrishna, M.; Shanker Kaki, S.; Karuna, M.S.L.; Sarada, S.C.; Kumar, C.G.; Prasad, R.B.N. Synthesis and in vitro antioxidant and antimicrobial studies of novel structured phosphatidylcholines with phenolic acids. Food Chem. 2016, 221, 664–672. [Google Scholar] [CrossRef] [PubMed]
- Pande, G.; Akoh, C.C. Enzymatic synthesis of tyrosol-based phenolipids: Characterization and effect of Alkyl chain unsaturation on the antioxidant activities in bulk oil and oil-in-water emulsion. J. Am. Oil Chem. Soc. 2016, 93, 329–337. [Google Scholar] [CrossRef]
- Kaki, S.S.; Grey, C.; Adlercreutz, P. Bioorganic synthesis, characterization and antioxidant activity of esters of natural phenolics and a-lipoic acid. J. Biotechnol. 2012, 157, 344–349. [Google Scholar] [CrossRef]
- Micillo, R.; Sirés-Campos, J.; García-Borrón, J.C.; Panzella, L.; Napolitano, A.; Olivares, C. Conjugation with dihydrolipoic acid imparts caffeic acid ester potent inhibitory effect on dopa oxidase activity of human tyrosinase. Int. J. Mol. Sci. 2018, 19, 2156. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Johny, J.; Kontham, V.; Kaki Shiva, S.; Veeragoni, D.; Misra, S. Bioorganic synthesis, characterization and evaluation of a natural phenolic lipid. Biotechnol. Rep. 2019, 24, e00375. [Google Scholar] [CrossRef]
- Sari, M.; Chung, Y.; Agatha, F.; Kim, H.K. Evaluation of antioxidant and antimicrobial activity of phenolic lipids produced by the transesterification of 4-hydroxyphenylacetic acid and triglycerides. Appl. Biol. Chem. 2019, 62, 5. [Google Scholar] [CrossRef]
- Dynczuki Navarro, S.; Adilson, B.; Meza, A.; Pesarini, J.R.; da Silva Gomes, R.; Bilhar Karaziack, C.; Cunha-Laura, A.L.; Duenhas Monreal, A.C.; Wanderson, R.; Lacerda, V., Jr.; et al. A new synthetic resorcinolic lipid 3-Heptyl-3,4,6-trimethoxy-3H-isobenzofuran-1-one: Evaluation of toxicology and ability to potentiate the mutagenic and apoptotic effects of cyclophosphamide. Eur. J. Med. Chem. 2014, 75, 132–142. [Google Scholar] [CrossRef]
- Kaki, S.S.; Kunduru, K.R.; Kanjilal, S.; Prasad, R.B.N. Synthesis and characterization of a novel phenolic lipid for use as potential lipophilic antioxidant and as a prodrug of butyric acid. J. Oleo Sci. 2015, 64, 845–852. [Google Scholar] [CrossRef] [Green Version]
- Buonanno, F.; Catalani, E.; Cervia, D.; Proietti Serafini, F.; Picchietti, S.; Fausto, A.M.; Giorgi, S.; Lupidi, G.; Rossi, F.V.; Marcantoni, E.; et al. Bioactivity and structural properties of novel synthetic analogues of the protozoan toxin climacostol. Toxins 2019, 11, 42. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yarra, M.; Kaki, S.S.; Prasad, R.B.N.; Mallampalli, K.S.L.; Yedla, P.; Ganesh, K.C. Synthesis of novel (Z)-methyl-12-aminooctadec-9-enoate-based phenolipids as potential antioxidants and chemotherapeutic agents. Eur. J. Lip. Sci. Technol. 2016, 118, 622–630. [Google Scholar] [CrossRef]
- Xie, Y.; Yang, Y.; Li, S.; Xu, Y.; Lu, W.; Chen, Z.; Yang, G.; Li, Y.; Cao, Y.; Bian, X. Phenylsulfonylfuroxan NO-donor phenols: Synthesis and multifunctional activities evaluation. Bioorg. Med. Chem. 2017, 25, 4407–4413. [Google Scholar] [CrossRef] [PubMed]
- Roleira, F.M.F.; Siquet, C.; Orrù, E.; Garrido, E.M.; Garrido, J.; Milhazes, N.; Podda, G.; Paiva-Martins, F.; Reis, S.; Carvalho, R.A.; et al. Borges, Lipophilic phenolic antioxidants: Correlation between antioxidant profile, partition coefficients and redox properties. Bioorg. Med. Chem. 2010, 18, 5816–5825. [Google Scholar] [CrossRef]
- Anankanbil, S.; Perez, B.; Widzisz, K.M.; Guo, Z.; Fernandes, I.; Mateus, N.; Wang, Z. A new group of synthetic phenolic-containing amphiphilic molecules for multipurpose applications: Physico-chemical characterization and cell-toxicity study. Sci. Rep. 2018, 8, 832. [Google Scholar] [CrossRef] [Green Version]
- Logrado, L.P.L.; Santos, C.O.; Romeiro, L.A.S.; Costa, A.M.; Ferreira, J.R.O.; Cavalcanti, B.C.; de Moraes, M.O.; Costa-Lotufo, L.V.; Pessoa, C.; dos Santos, M.L. Synthesis and cytotoxicity screening of substituted isobenzofuranones designed from anacardic acids. Eur. J. Med. Chem. 2010, 45, 3480–3489. [Google Scholar] [CrossRef] [PubMed]
- Torres de Pinedo, A.; Penalver, P.; Perez-Victoria, I.; Rondon, D.; Morales, J.C. Synthesis of new phenolic fatty acid esters and their evaluation as lipophilic antioxidants in an oil matrix. Food Chem. 2007, 105, 657–665. [Google Scholar] [CrossRef]
- Jin, W.; Zjawiony, J.K. 5-Alkylresorcinols from Merulius incarnates. J. Nat. Prod. 2006, 69, 704–706. [Google Scholar] [CrossRef]
- Parikka, K.; Wahala, K. An expedient synthesis of 5-n-alkylresorcinols and novel 5-n-alkylresorcinol haptens. Beilst. J. Org. Chem. 2009, 5, 22. [Google Scholar] [CrossRef] [Green Version]
- Menezes, J.C.J.M.D.S.; Kamat, S.P.; Cavaleiro, J.A.S.; Gaspar, A.; Garrido, J.; Borges, F. Synthesis and antioxidant activity of long chain alkyl hydroxycinnamates. Eur. J. Med. Chem. 2011, 46, 773–777. [Google Scholar] [CrossRef] [Green Version]
- Kashyap, D.; Kumar Garg, V.; Singh Tuli, H.; Yerer, M.B.; Sak, K.; Kumar Sharma, A.; Kumar, M.; Aggarwal, V.; Singh Sandhu, S. Fisetin and quercetin: Promising flavonoids with chemopreventive potential. Biomolecules 2019, 9, 174. [Google Scholar] [CrossRef] [Green Version]
- Anand David, A.V.; Arulmoli, R.; Parasuraman, S. Overviews of biological importance of quercetin: A bioactive flavonoid. Pharmacogn. Rev. 2016, 10, 84–89. [Google Scholar] [PubMed] [Green Version]
- Abbas, M.; Saeed, F.; Anjum, F.M.; Afzaal, M.; Tufail, T.; Bashir, M.S.; Ishtiaq, A.; Hussain, S.; Suleria, H.A.R. Natural polyphenols: An overview. Int. J. Food Prop. 2017, 20, 1689–1699. [Google Scholar] [CrossRef] [Green Version]
- Hirpara, K.V.; Aggarwal, P.; Mukherjee, A.J.; Joshi, N.; Burman, A.C. Quercetin and its derivatives: Synthesis, pharmacological uses with special emphasis on anti-tumor properties and prodrug with enhanced bio-availability. Anti-Canc. Ag. Med. Chem. 2009, 9, 138–161. [Google Scholar] [CrossRef] [PubMed]
- Saladino, R.; Gualandi, G.; Farina, A.; Crestini, C.; Nencioni, L.; Palamara, A.T. Advances and challenges in the synthesis of highly oxidised natural phenols with antiviral, antioxidant and cytotoxic activities. Curr. Med. Chem. 2008, 15, 1500–1519. [Google Scholar] [CrossRef]
- Dudnik, A.; Gaspar, P.; Neves, A.R.; Forster, J. Engineering of microbial cell factories for the production of plant polyphenols with health-beneficial properties. Curr. Pharm. Des. 2018, 24, 2208–2225. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Veluri, R.; Weir, T.L.; Pal Bais, H.; Stermitz, F.R.; Vivanco, J.M. Phytotoxic and antimicrobial activities of catechin derivatives. J. Agric. Food Chem. 2004, 52, 1077–1082. [Google Scholar] [CrossRef]
- Park, K.D.; Cho, S.J. Synthesis and antimicrobial activities of 3-O-alkyl analogues of (+)-catechin: Improvement of stability and proposed action mechanism. Eur. J. Med. Chem. 2010, 45, 1028–1033. [Google Scholar] [CrossRef]
- Kumar, D.; Poornima, M.; Kushwaha, R.N.; Won, T.-J.; Ahn, C.; Ganesh Kumar, C.; Jang, K.; Shin, D.-S. Antimicrobial and docking studies of (-)-catechin derivatives. J. Kor. Soc. Appl. Biol. Chem. 2015, 58, 581–585. [Google Scholar] [CrossRef]
- Fukuhara, K.; Ohno, A.; Nakanishi, I.; Imai, K.; Nakamura, A.; Anzai, K.; Miyata, N.; Okuda, H. Novel ninhydrin adduct of catechin with potent antioxidative activity. Tetrahedron Lett. 2009, 50, 6989–6992. [Google Scholar] [CrossRef]
- Yen, C.-T.; Nakagawa-Goto, K.; Hwang, T.-L.; Wu, P.-C.; Morris-Natschke, S.L.; Lai, W.-C.; Bastow, K.F.; Chang, F.-R.; Wub, Y.-C.; Lee, K.-H. Antitumor agents. 271: Total synthesis and evaluation of brazilein and analogs as anti-inflammatory and cytotoxic agents. Bioorg. Med. Chem. Lett. 2010, 20, 1037–1039. [Google Scholar] [CrossRef] [Green Version]
- Pan, C.; Zeng, X.; Guan, Y.; Jiang, X.; Li, L.; Zhang, H. Design and synthesis of brazilin-like compounds. Synlett 2011, 2011, 425–429. [Google Scholar]
- Kostova, I. Synthetic and natural coumarins as cytotoxic agents. Curr. Med. Chem. Anti Cancer Agents 2005, 5, 29–46. [Google Scholar] [CrossRef]
- Stefanachi, A.; Leonetti, F.; Pisani, L.; Catto, M.; Carotti, A. Coumarin: A natural, privileged and versatile scaffold for bioactive compounds. Molecules 2018, 23, 250. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khoobi, M.; Foroumadi, A.; Emami, S.; Safavi, M.; Dehghan, G.; Alizadeh, B.H.; Ramazani, A.; Ardestani, S.K.; Shafiee, A. Coumarin-based bioactive compounds: Facile synthesis and biological evaluation of coumarin-fused 1,4-thiazepines. Chem. Biol. Drug Des. 2011, 78, 580–586. [Google Scholar] [CrossRef] [PubMed]
- Symeonidis, T.; Chamilos, M.; Hadjipavlou-Litina, D.J.; Kallitsakis, M.; Litinas, K.E. Synthesis of hydroxycoumarins and hydroxybenzo[f]- or [h]coumarins as lipid peroxidation inhibitors. Bioorg. Med. Chem. Lett. 2009, 19, 1139–1142. [Google Scholar] [CrossRef]
- Perez-Vizcaino, F.; Duarte, J.; Jimenez, R.; Santos-Buelga, C.; Osuna, A. Antihypertensive effects of the flavonoid quercetin. Pharm. Rep. 2009, 61, 67–75. [Google Scholar] [CrossRef]
- Massi, A.; Bortolini, O.; Ragno, D.; Bernardi, T.; Sacchetti, G.; Tacchini, M.; De Risi, C. Research progress in the modification of quercetin leading to anticancer agents. Molecules 2017, 22, 1270. [Google Scholar] [CrossRef]
- Halevasa, E.; Pekou, A.; Papi, R.; Mavroidi, B.; Hatzidimitriou, A.G.; Zahariou, G.; Litsardakis, G.; Sagnou, M.; Pelecanou, M.; Pantazaki, A.A. Synthesis, physicochemical characterization and biological properties of two novel Cu(II) complexes based on natural products curcumin and quercetin. J. Inorg. Biochem. 2020, 208, 111083. [Google Scholar] [CrossRef]
- Sharma, A.; Kashyap, D.; Sak, K.; Singh Tuli, H.; Sharma, A.K. Therapeutic charm of quercetin and its derivatives: A review of research and patents. Pharm. Pat. Anal. 2018, 7, 15–32. [Google Scholar] [CrossRef] [PubMed]
- Teles, Y.C.F.; Souza, M.S.R.; de Souza, M.F.V. Sulphated flavonoids: Biosynthesis, structures, and biological activities. Molecules 2018, 23, 480. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koirala, N.; Pandey, R.P.; Parajuli, P.; Jung, H.J.; Sohng, J.K. Methylation and subsequent glycosylation of 7,8-dihydroxyflavone. J. Biotechnol. 2014, 184, 128–137. [Google Scholar] [CrossRef]
- Antonopoulou, I.; Varriale, S.; Topakas, E.; Rova, U.; Christakopoulos, P.; Faraco, V. Enzymatic synthesis of bioactive compounds with high potential for cosmeceutical application. Appl. Microbiol. Biotechnol. 2016, 100, 6519–6543. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mecenas, A.S.; Malafaia, C.R.A.; Sangenito, L.S.; Simas, D.L.R.; de Barros Machado, T.; Amaral, A.C.F.; Santos, A.L.S.D.; Freire, D.M.G.; Leal, I.C.R. Rutin derivatives obtained by transesterification reactions catalyzed by Novozym 435: Antioxidant properties and absence of toxicity in mammalian cells. PLoS ONE 2018, 13, e0203159. [Google Scholar] [CrossRef] [PubMed] and references cited therein.
- Li, X.; Mai, W.; Chen, D. Chemical study on protective effect against hydroxyl-induced dna damage and antioxidant mechanism of myricitrin. J. Chin. Chem. Soc. 2014, 61, 383–390. [Google Scholar] [CrossRef]
- Deng, X.; Wang, Z.; Liu, J.; Xiong, S.; Xiong, R.; Cao, X.; Chen, Y.; Zheng, X.; Tang, G. Design, synthesis and biological evaluation of flavonoid salicylate derivatives as potential anti-tumor agents. RSC Adv. 2017, 7, 38171–38178. [Google Scholar] [CrossRef] [Green Version]
- Poerwono, H.; Sasaki, S.; Hattori, Y.; Higashiyama, K. Efficient microwave-assisted prenylation of pinostrobin and biological evaluation of its derivatives as antitumor agents. Bioorg. Med. Chem. Lett. 2010, 20, 2086–2089. [Google Scholar] [CrossRef]
- Marliyana, S.D.; Mujahidin, D.; Syah, Y.M. pinostrobin derivatives from prenylation reaction and their antibacterial activity against clinical bacteria. IOP Conf. Ser. Mater. Sci. Eng. 2018, 349, 012057. [Google Scholar] [CrossRef]
- Tian, J.-L.; Liu, T.-L.; Xue, J.-J.; Hong, W.; Zhang, Y.; Zhang, D.-X.; Cui, C.-C.; Liu, M.-C.; Niu, S.-L. Flavanoids derivatives from the root bark of Broussonetia papyrifera as a tyrosinase inhibitor. Ind. Crop. Prod. 2019, 138, 111445. [Google Scholar] [CrossRef]
- Thuan, N.H.; Malla, S.; Trung, N.T.; Dhakal, D.; Pokhrel, A.R.; Chu, L.L.; Sohng, J.K. Microbial production of astilbin, a bioactive rhamnosylated flavanonol, from taxifolin. World J. Microbiol. Biotechnol. 2017, 33, 36. [Google Scholar] [CrossRef]
- Thapa, S.B.; Pandey, R.P.; Bashyal, P.; Tokutaro, Y.; Sohng, J.Y. Cascade biocatalysis systems for bioactive naringenin glucosides and quercetin rhamnoside production from sucrose. Appl. Microbiol. Biotechnol. 2019, 103, 7953–7969. [Google Scholar] [CrossRef] [PubMed]
- Ben Kaab, S.; Lins, L.; Hanafi, M.; Bettaieb Rebey, I.; Deleu, M.; Fauconnier, M.-L.; Ksouri, R.; Jijakli, M.H.; De Clerck, C. Cynara cardunculus crude extract as a powerful natural herbicide and insight into the mode of action of its bioactive molecules. Biomolecules 2020, 10, 209. [Google Scholar] [CrossRef] [Green Version]
- Preetha, A.; Sherin, G.T.; Ajaikumar, B.K.; Chitra, S.; Kuzhuvelil, B.H.; Bokyung, S.; Sheeja, T.T.; Krishna, M.; Priyadarsini, I.K.; Rajasekharan, K.N.; et al. Biological activities of curcumin and its analogues (Congeners) made by man and Mother Nature. Biochem. Pharm. 2008, 76, 1590–1611. [Google Scholar]
- Bukhari, S.N.A.; Bin Jantan, I.; Jasamai, M.; Ahmad, W.; Bin Amjad, W. Synthesis and biological evaluation of curcumin analogues. J. Med. Sci. 2013, 13, 501–513. [Google Scholar] [CrossRef] [Green Version]
- Amalraj, A.; Pius, A.; Gopi, S.; Gopi, S. Biological activities of curcuminoids, other biomolecules from turmeric and their derivatives—A review. J. Trad. Compl. Med. 2017, 7, 205–233. [Google Scholar] [CrossRef] [Green Version]
- Oglah, M.K.; Mustafa, Y.F.; Bashir, M.K.; Jasim, M.H. Curcumin and its derivatives: A review of their biological activities. Sys. Rev. Pharm. 2020, 11, 472–481. [Google Scholar]
- Rodrigues, F.C.; Anil Kumar, N.V.; Thakur, G. Developments in the anticancer activity of structurally modified curcumin: An up-to-date review. Eur. J. Med. Chem. 2019, 177, 76–104. [Google Scholar] [CrossRef] [PubMed]
- Noureddin, S.S.; El-Shishtawy, R.M.; Al-Footy, K.O. Curcumin analogues and their hybrid molecules as multifunctional drugs. Eur. J. Med. Chem. 2019, 182, 111631. [Google Scholar] [CrossRef]
- Mbese, Z.; Khwaza, V.; Aderibigbe, B.A. Curcumin and its derivatives as potential therapeutic agents in prostate, colon and breast cancers. Molecules 2019, 24, 4386. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zangui, M.; Atkin, S.L.; Majeed, M.; Sahebkar, A. Current evidence and future perspectives for curcumin and its analogues as promising adjuncts to oxaliplatin: State-of-the-art. Pharmacol. Res. 2019, 141, 343–356. [Google Scholar] [CrossRef]
- Selvam, C.; Prabu, S.L.; Jordan, B.C.; Purushothaman, Y.; Umamaheswari, A.; Hosseini Zare, M.S.; Thilagavathi, R. Molecular mechanisms of curcumin and its analogs in colon cancer prevention and treatment. Life Sci. 2019, 239, 117032. [Google Scholar] [CrossRef] [PubMed]
- Lo Cascio, F.; Marzullo, P.; Kayed, R.; Palumbo Piccionello, A. Curcumin as scaffold for drug discovery against neurodegenerative diseases. Biomedicines 2021, 9, 173. [Google Scholar] [CrossRef]
- Chainoglou, E.; Hadjipavlou-Litina, D. Curcumin in health and diseases: Alzheimer’s disease and curcumin analogues, derivatives, and hybrids. Int. J. Mol. Sci. 2020, 21, 1975. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mansouri, K.; Rasoulpoor, S.; Daneshkhah, A.; Abolfathi, S.; Salari, N.; Mohammadi, M.; Rasoulpoor, S.; Shabani, S. Clinical effects of curcumin in enhancing cancer therapy: A systematic review. BMC Cancer 2020, 20, 791. [Google Scholar] [CrossRef] [PubMed]
- Abdellah, A.M.; Sliem, M.A.; Bakr, M.; Amin, R.M. Green synthesis and biological activity of silver–curcumin nanoconjugates. Future Med. Chem. 2018, 10, 2577–2588. [Google Scholar] [CrossRef] [PubMed]
- Koo, H.J.; Shin, S.; Choi, J.Y.; Lee, K.H.; Kim, B.T.; Choe, Y.S. Introduction of methyl groups at C2 and C6 positions enhances the antiangiogenesis activity of curcumin. Sci. Rep. 2015, 5, 14205. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Edwards, R.L.; Luis, P.B.; Varuzza, P.V.; Joseph, A.I.; Presley, S.H.; Chaturvedi, R.; Schneider, C. The anti-inflammatory activity of curcumin is mediated by its oxidative metabolites. J. Biol. Chem. 2017, 292, 21243–21252. [Google Scholar] [CrossRef] [Green Version]
- Joseph, A.I.; Edwards, R.L.; Luis, P.B.; Presley, S.H.; Porter, N.A.; Schneider, C. Stability and anti-inflammatory activity of the reduction-resistant curcumin analog, 2,6-dimethyl-curcumin. Org. Biomol. Chem. 2018, 16, 3273–3281. [Google Scholar] [CrossRef]
- De Cássia Orlandi Sardi, J.; Polaquini, C.R.; Almeida Freires, I.; Câmara de Carvalho Galvão, L.; Goldoni Lazarini, J.; Silva Torrezan, G.; Regasini, L.O.; Rosalen, P.L. Antibacterial activity of diacetylcurcumin against Staphylococcus aureus results in decreased biofilm and cellular adhesion. J. Med. Microbiol. 2017, 66, 816–824. [Google Scholar] [CrossRef]
- Escobedo-Martínez, C.; Guzmán-Gutiérrez, S.L.; Carrillo-López, M.I.; Deveze-Álvarez, M.A.; Trujillo-Valdivia, A.; Meza-Morales, W.; Enríquez, R.G. Diacetylcurcumin: Its potential antiarthritic effect on a freund’s complete adjuvant-induced murine model. Molecules 2019, 24, 2643. [Google Scholar] [CrossRef] and references cited therein.[Green Version]
- Somparn, P.; Phisalaphong, C.; Nakornchai, S.; Unchern, S.; Morales, N.P. Comparative antioxidant activities of curcumin and its demethoxy and hydrogenated derivatives. Biol. Pharm. Bull. 2007, 30, 74–78. [Google Scholar] [CrossRef] [Green Version]
- Rosa, A.; Atzeri, A.; Deiana, M.; Melis, M.P.; Incani, A.; Minassi, A.; Cabboi, B.; Appendino, G. Prenylation preserves antioxidant properties and effect on cell viability of the natural dietary phenol curcumin. Food Res. Int. 2014, 57, 225–233. [Google Scholar] [CrossRef]
- Abonia, R.; Laali, K.K.; Raja Somu, D.; Bunge, S.D.; Wang, E.C. A flexible strategy for modular synthesis of curcuminoid-BF2/curcuminoid pairs and their comparative anti-proliferative activity in human cancer cell lines. Chem. Med. Chem. 2020, 15, 354–362. [Google Scholar] [CrossRef] [PubMed]
- Buyandelger, U.; Walker, D.G.; Taguchi, H.; Yanagisawa, D.; Tooyama, I. Novel fluorinated derivative of curcumin negatively regulates thioredoxin-interacting protein expression in retinal pigment epithelial and macrophage cells. Biochem. Biophys. Res. Commun. 2020, 532, 668–674. [Google Scholar] [CrossRef] [PubMed]
- Mishra, S.; Karmodiya, K.; Surolia, N.; Surolia, A. Synthesis and exploration of novel curcumin analogues as anti-malarial agents. Bioorg. Med. Chem. 2008, 16, 2894–2902. [Google Scholar] [CrossRef]
- Radha, A.; Rukhmini, S.D.; Vilasini, S.; Sakunthala, P.R.; Sreedharan, B.; Velayudhan, M.P.; Abraham, A. Bioactive derivatives of curcumin attenuate cataract formation in vitro. Chem. Biol. Drug. Des. 2012, 80, 887–892. [Google Scholar] [CrossRef] [PubMed]
- Wolosewicz, K.; Podgorska, K.; Rutkowska, E.; Lazny, R. Synthesis of dicarbonyl curcumin analogues containing the tropane scaffold. Eur. J. Org. Chem. 2019, 2019, 4662–4674. [Google Scholar] [CrossRef] and references cited therein.
- Liu, Y.; Dargusch, R.; Maher, P.; Schubert, D. A broadly neuroprotective derivative of curcumin. J. Neurochem. 2008, 105, 1336–1345. [Google Scholar] [CrossRef] [PubMed]
- Mahera, P.; Akaishi, T.; Schubert, D.; Abe, K. A pyrazole derivative of curcumin enhances memory. Neurobiol. Aging 2010, 3, 706–709. [Google Scholar] [CrossRef]
- Akaishi, T.; Yamamoto, S.; Abe, K. The synthetic curcumin derivative CNB-001 attenuates thrombin-stimulated microglial inflammation by inhibiting the ERK and p38 MAPK pathways. Biol. Pharm. Bull. 2020, 43, 138–144. [Google Scholar] [CrossRef] [Green Version]
- Khaldi-Khellafi, N.; Makhloufi-Chebli, M.; Oukacha-Hikem, D.; Bouaziz, S.T.; Lamara, K.O.; Idir, T.; Benazzouz-Touami, A.; Dumas, F. Green synthesis, antioxidant and antibacterial activities of 4-aryl-3,4-dihydropyrimidinones/thiones derivatives of curcumin. Theoretical calculations and mechanism study. J. Mol. Struct. 2019, 1181, 261–269. [Google Scholar] [CrossRef]
- Huang, J.; Fu, J.; Liu, B.; Wang, R.; You, T. A Synthetic curcuminoid analog, (2E,6E)-2,6-bis(2-(trifluoromethyl)benzylidene)cyclohexanone, ameliorates impaired wound healing in streptozotocin-induced diabetic mice by increasing miR-146a. Molecules 2020, 25, 920. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pignanelli, C.; Ma, D.; Noel, M.; Ropat, J.; Mansour, F.; Curran, C.; Pupulin, S.; Larocque, K.; Wu, J.; Liang, G.; et al. Selective targeting of cancer cells by oxidative vulnerabilities with novel curcumin analogs. Sci. Rep. 2017, 7, 1603. [Google Scholar]
- Parashar, K.; Sood, S.; Mehaidli, A.; Curran, C.; Vegh, C.; Nguyen, C.; Pignanelli, C.; Wu, J.; Liang, G.; Wang, Y. Evaluating the anti-cancer efficacy of a synthetic curcumin analog on human melanoma cells and its interaction with standard chemotherapeutics. Molecules 2019, 24, 2483. [Google Scholar] [CrossRef] [Green Version]
- Hisamuddin, N.; Shaik Mossadeq, W.M.; Sulaiman, M.R.; Abas, F.; Leong, S.W.; Kamarudin, N.; Ong, H.M.; Ahmad Azmi, A.F.; Ayumi, R.R.; Talib, M. Anti-edematogenic and anti-granuloma activity of a synthetic curcuminoid analog, 5-(3,4-dihydroxyphenyl)-3-hydroxy-1-(2-hydroxyphenyl)penta-2,4-dien-1-one, in mouse models of inflammation. Molecules 2019, 24, 2614. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, E.S.; Kwon, M.-H.; Kim, H.M.; Woo, H.B.; Ahn, C.M.; Chung, C.H. Curcumin analog CUR5–8 ameliorates nonalcoholic fatty liver disease in mice with high-fat diet-induced obesity. Metab. Clin. Exp. 2020, 103, 154015. [Google Scholar] [CrossRef] [Green Version]
- Kazantzis, K.T.; Koutsonikoli, K.; Mavroidi, B.; Zachariadis, M.; Alexiou, P.; Pelecanou, M.; Politopoulos, K.; Alexandratou, E.; Sagnou, M. Curcumin derivatives as photosensitizers in photodynamic therapy: Photophysical properties and in vitro studies with prostate cancer cells. Photochem. Photobiol. Sci. 2020, 19, 193–206. [Google Scholar] [CrossRef] [PubMed]
- Yanagisawa, D.; Shirai, N.; Amatsubo, T.; Taguchi, H.; Hirao, K.; Urushitani, M.; Morikawa, S.; Inubushi, T.; Kato, M.; Kato, F.; et al. Relationship between the tautomeric structures of curcumin derivatives and their Aβ-binding activities in the context of therapies for Alzheimer’s disease. Biomaterials 2010, 31, 4179–4185. [Google Scholar] [CrossRef]
- Taguchi, H.; Yanagisawa, D.; Morikawa, S.; Hirao, K.; Shirai, N.; Tooyama, I. Synthesis and tautomerism of curcumin derivatives and related compounds. Aust. J. Chem. 2015, 68, 224–229. [Google Scholar] [CrossRef]
- Yanagisawa, D.; Taguchi, H.; Morikawa, S.; Kato, T.; Hirao, K.; Shirai, N.; Tooyama, I. Novel curcumin derivatives as potent inhibitors of amyloid β aggregation. Biochem. Biophys. Rep. 2015, 4, 357–368. [Google Scholar] [CrossRef] [Green Version]
- Yanagisawa, D.; Kato, T.; Taguchi, H.; Shirai, N.; Hirao, K.; Sogabe, T.; Tomiyama, T.; Gamo, K.; Hirahara, Y.; Kitada, M.; et al. Keto form of curcumin derivatives strongly binds to Aβ oligomers but not fibrils. Biomaterials 2021, 270, 120686. [Google Scholar] [CrossRef] [PubMed]
- Ferrari, E.; Pignedoli, F.; Imbriano, C.; Marverti, G.; Basile, V.; Venturi, E.; Saladini, M. Newly synthesized curcumin derivatives: Crosstalk between chemico-physical properties and biological activity. J. Med. Chem. 2011, 54, 8066–8077. [Google Scholar] [CrossRef]
- Ahmed, M.; Qadir, M.A.; Shafiq, M.I.; Muddassar, M.; Samra, Z.Q.; Hameed, A. Synthesis, characterization, biological activities and molecular modeling of Schiff bases of benzene sulfonamides bearing curcumin scaffold. Arab. J. Chem. 2019, 12, 41–53. [Google Scholar] [CrossRef]
- Liang, G.; Li, X.; Chen, L.; Yang, S.; Wu, X.; Studer, E.; Gurley, E.; Hylemon, P.B.; Ye, F.; Li, Y.; et al. Synthesis and anti-inflammatory activities of mono-carbonyl analogues of curcumin. Bioorg. Med. Chem. Lett. 2008, 18, 1525–1529. [Google Scholar] [CrossRef] [Green Version]
- Liang, G.; Shao, L.; Wang, Y.; Zhao, C.; Chu, Y.; Xiao, J.; Zhao, Y.; Li, X.; Yang, S. Exploration and synthesis of curcumin analogues with improved structural stability both in vitro and in vivo as cytotoxic agents. Bioorg. Med. Chem. 2009, 17, 2623–2631. [Google Scholar] [CrossRef]
- Vanchinathan, K.; Bhagavannarayana, G.; Muthu, K.; Meenakshisundaram, S.P. Synthesis, crystal growth and characterization of 1,5-diphenylpenta-1,4- dien-3-one: An organic crystal. Phys. B Condens. Matter. 2011, 406, 4195–4199. [Google Scholar] [CrossRef]
- Patel, H.; Mothia, B.; Patel, J.; Fasanya, O.; Sooda, K.; Javid, F.; Wyatt, P.B. Cytotoxicity of some synthetic bis(arylidene) derivatives of cyclic ketones towards cisplatin-resistant human ovarian carcinoma cells. Med. Chem. Res. 2020, 29, 935–941. [Google Scholar] [CrossRef]
- Da Silva, C.C.; Silveira Pacheco, B.; das Neves, R.N.; Dié Alves, M.S.; Sena-Lopes, Ǻ.; Moura, S.; Borsuk, S.; de Pereira, C.M.P. Antiparasitic activity of synthetic curcumin monocarbonyl analogues against Trichomonas vaginalis. Biomed. Pharmacother. 2019, 111, 367–377. [Google Scholar] [CrossRef]
- Tavaf, Z.; Dangolani, S.K.; Yousefi, R.; Panahi, F.; Shahsavani, M.B.; Khalafi-Nezhad, A. Synthesis of new curcumin derivatives as influential antidiabetic α-glucosidase and α-amylase inhibitors with anti-oxidant activity. Carbohydr. Res. 2020, 494, 108069. [Google Scholar] [CrossRef]
- Nakamae, I.; Morimoto, T.; Shima, H.; Shionyu, M.; Fujiki, H.; Yoneda-Kato, N.; Yokoyama, T.; Kanaya, S.; Kakiuchi, K.; Shirai, T.; et al. Curcumin derivatives verify the essentiality of ROS upregulation in tumor suppression. Molecules 2019, 24, 4067. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ali, N.; Yeap, S.K.; Abu, N.; Lim, K.L.; Ky, H.; Pauzi, A.Z.M.; Ho, W.Y.; Tan, S.W.; Alan-Ong, H.K.; Zareen, S.; et al. Synthetic curcumin derivative DK1 possessed G2/M arrest and induced apoptosis through accumulation of intracellular ROS in MCF-7 breast cancer cells. Cancer Cell Signal 2017, 23, 1816–1823. [Google Scholar] [CrossRef] [Green Version]
- Yazmin, H.; Muhammad Nazirul Mubin, A.; Nurul Fattin Che, R.; Swee Keong, Y.; Nurul Elyani, M.; Mas Jaffri, M.; Noraini, N.; Afizan-Nik Abd Rahman, N.M.; Yong, C.Y.; Nadeem Akhtar, M.; et al. DK1 induces apoptosis via mitochondria-dependent signaling pathway in human colon carcinoma cell lines in vitro. Int. J. Mol. Sci. 2018, 19, 1151. [Google Scholar] [CrossRef] [Green Version]
- Aziz, M.N.M.; Hussin, Y.; Rahim, N.F.C.; Nordin, N.; Mohamad, N.E.; Yeap, S.K.; Yong, C.Y.; Masarudin, M.J.; Cheah, Y.K.; Abu, N.; et al. Curcumin analog DK1 induces apoptosis in human osteosarcoma cells in vitro through mitochondria-dependent signaling pathway. Molecules 2018, 23, 75. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Obregón-Mendoza, M.A.; Estévez-Carmona, M.M.; Hernández-Ortega, S.; Soriano-García, M.; Ramírez-Apan, M.T.; Orea, L.; Pilotzi, H.; Gnecco, D.; Cassani, J.; Enríquez, R.G. Retro-curcuminoids as mimics of dehydrozingerone and curcumin: Synthesis, nmr, X-ray, and cytotoxic activity. Molecules 2017, 22, 33. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Freitas Silva, M.; Ferreira Coelho, L.; Mitestainer Guirelli, I.; Machado Pereira, R.; Ferreira da Silva, G.Á.; Graravelli, G.Y.; de Oliveira Horvath, R.; Caixeta Nogueira, E.S.; Ionta, M.; Viegas, C. Synthetic resveratrol-curcumin hybrid derivative inhibits mitosis progression in estrogen positive mcf-7 breast cancer cells. Toxicol. Vitro 2018, 50, 75–85. [Google Scholar] [CrossRef]
- Spaeth, A.; Graeler, A.; Maisch, T.; Plaetzer, K. CureCuma–cationic curcuminoids with improved properties and enhanced antimicrobial photodynamic activity. Eur. J. Med. Chem. 2018, 159, 423–440. [Google Scholar] [CrossRef] [PubMed]
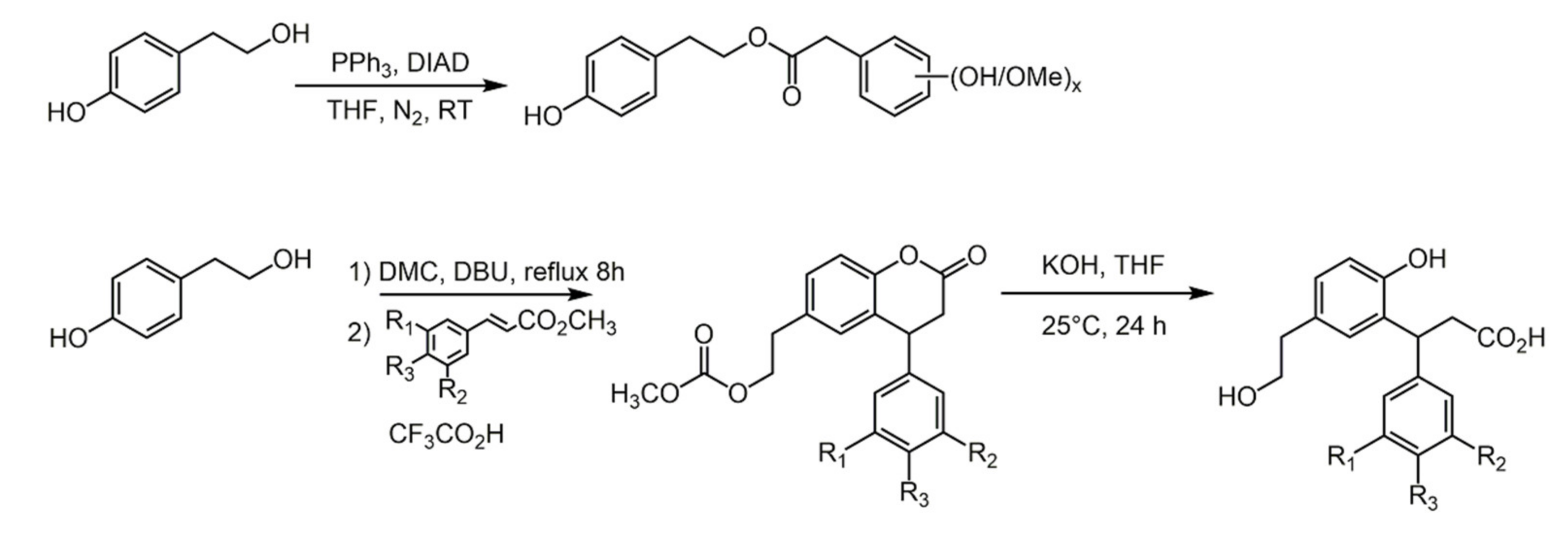

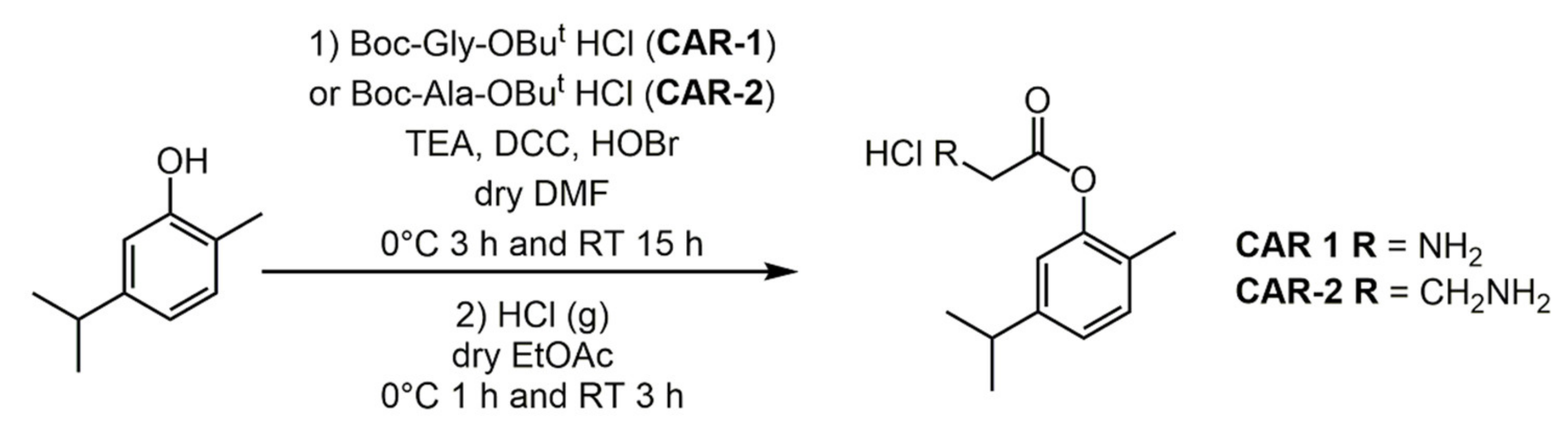
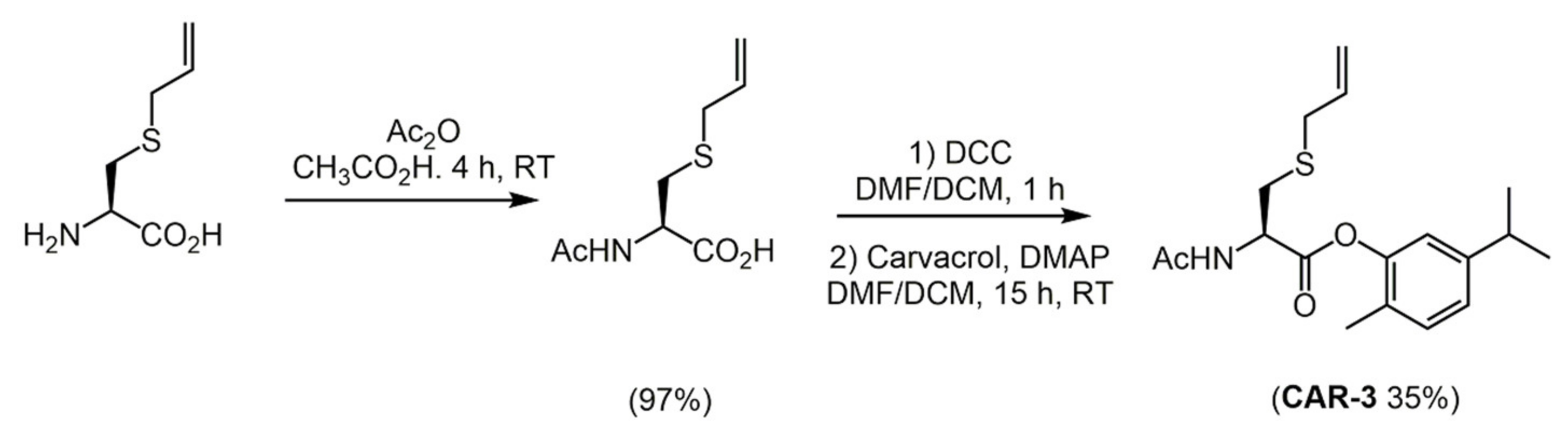
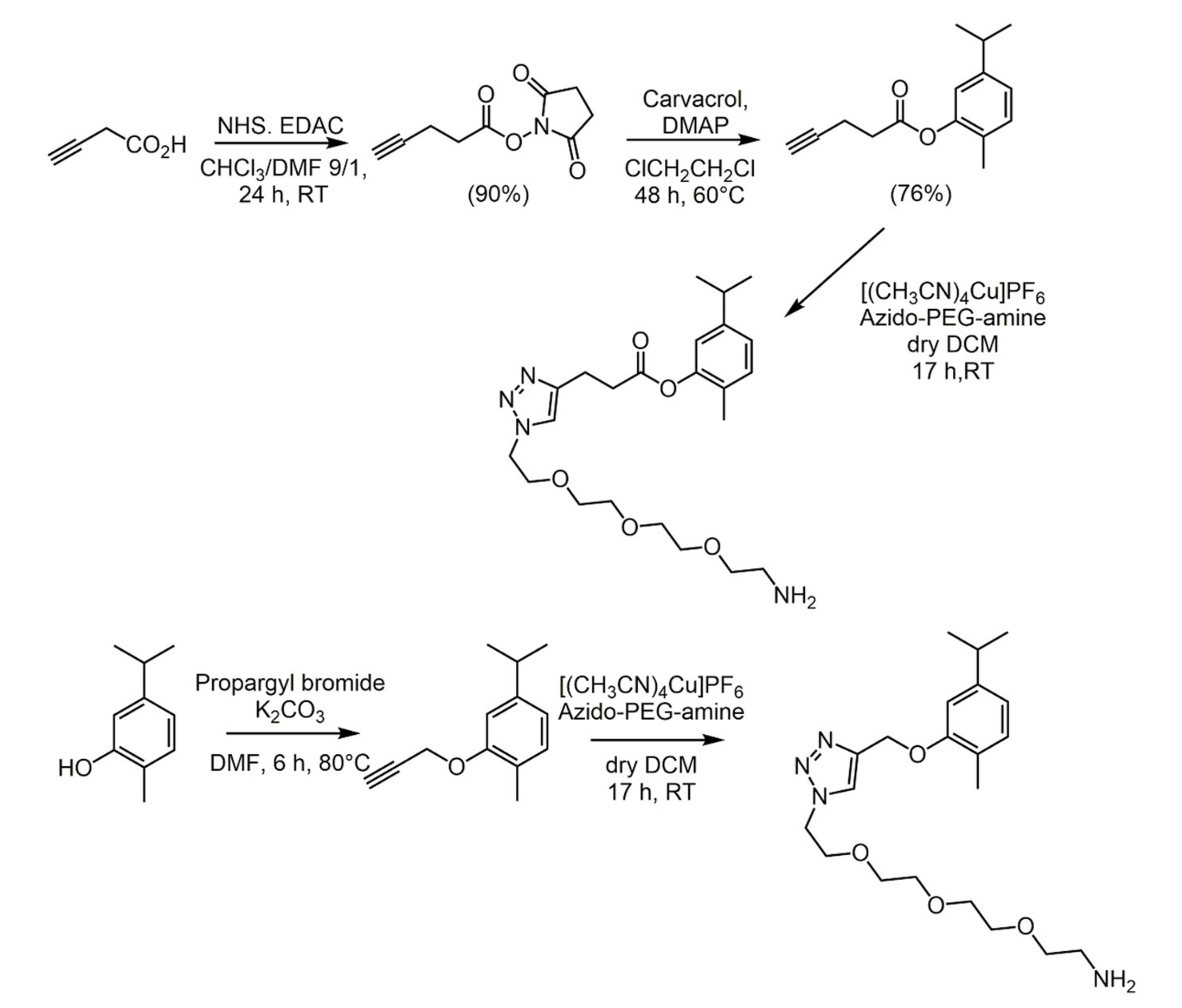
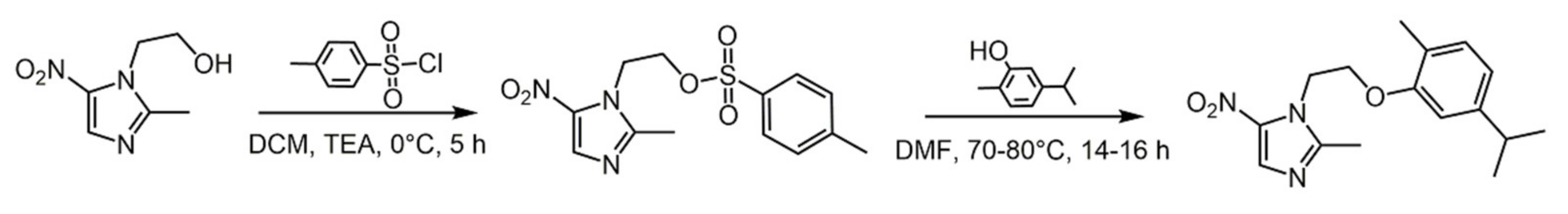
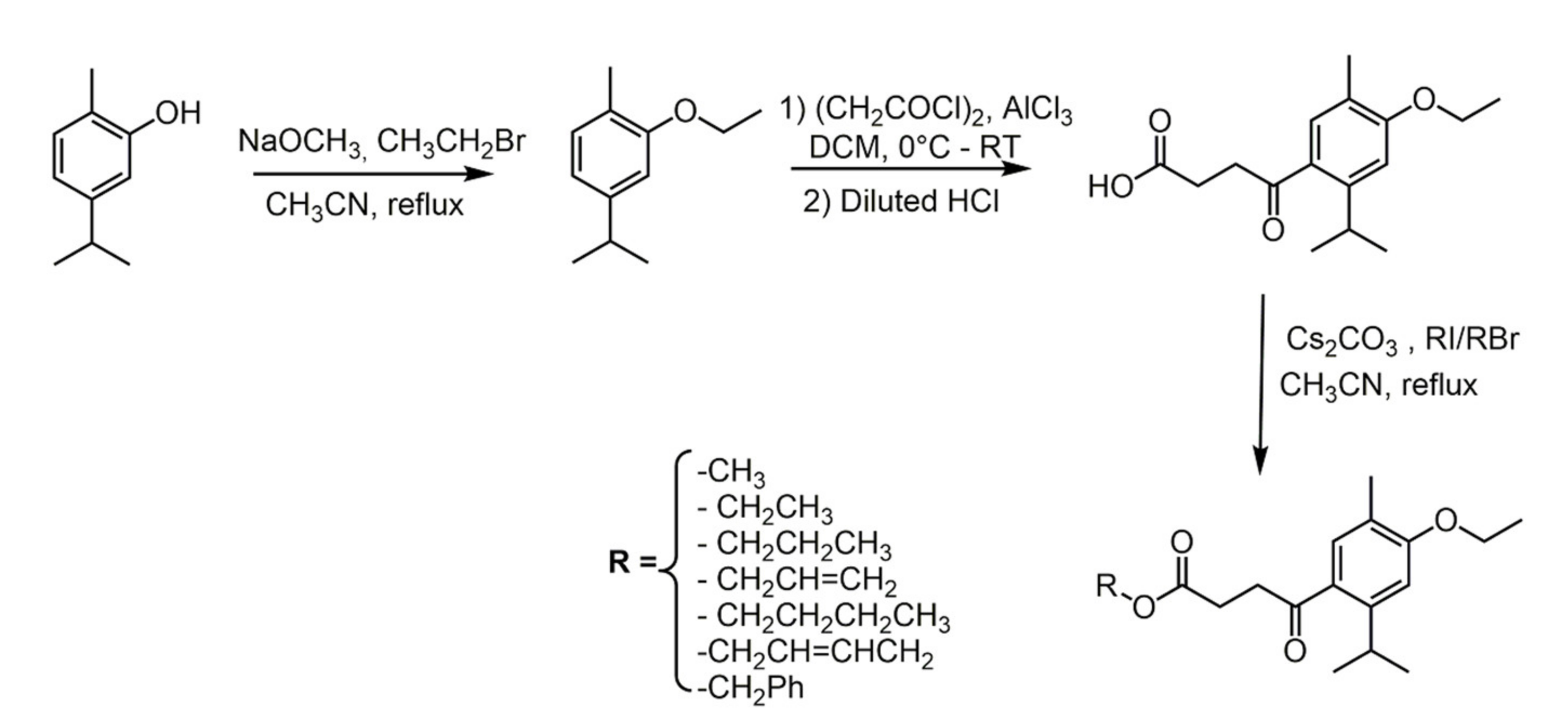
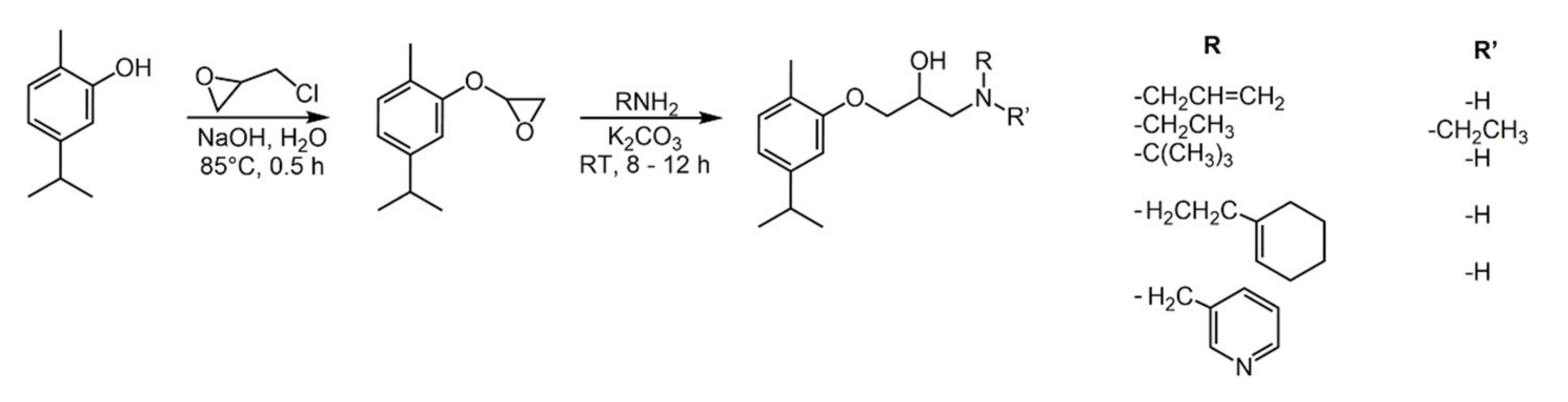
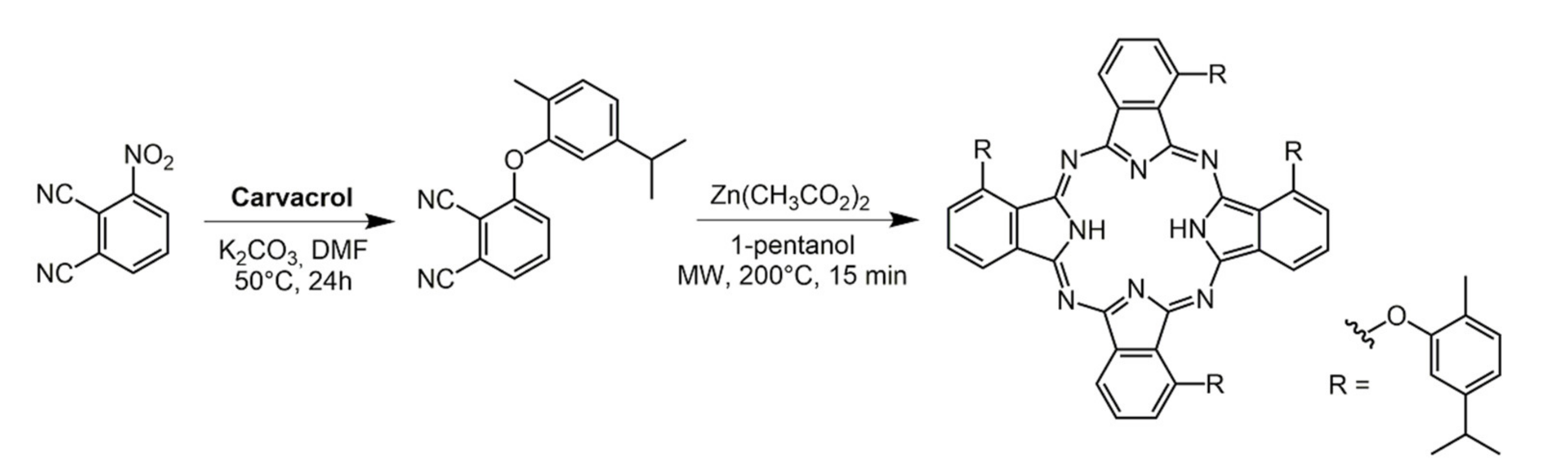
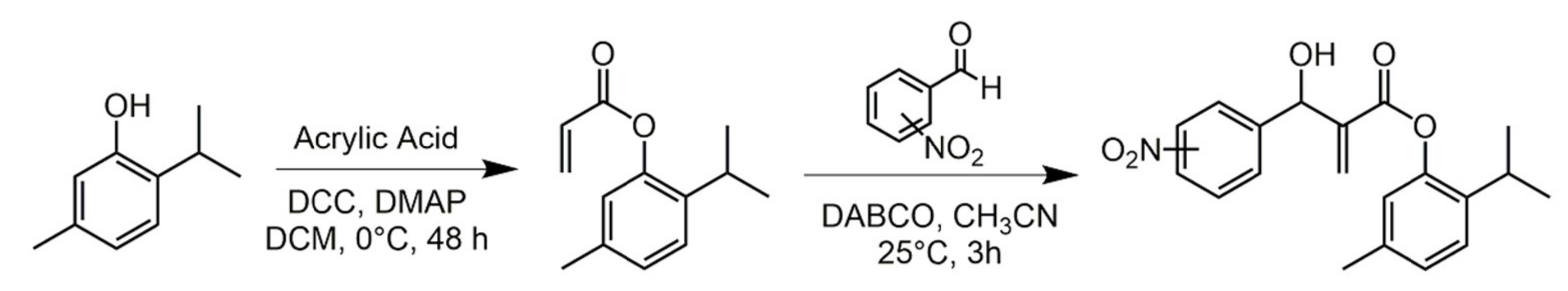
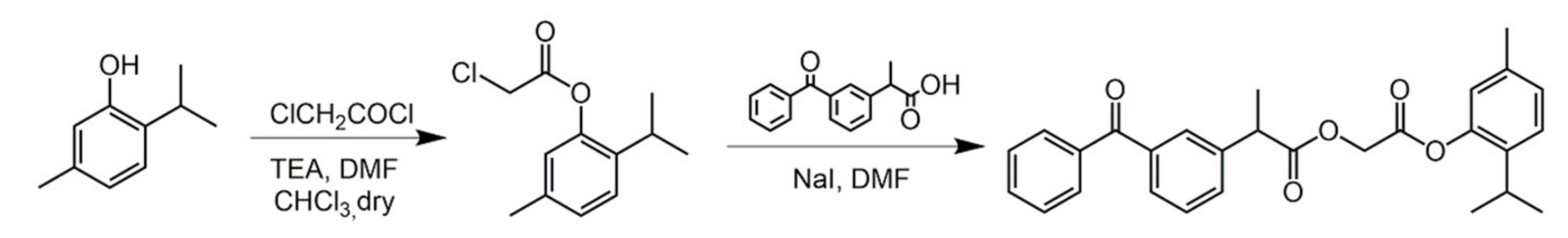
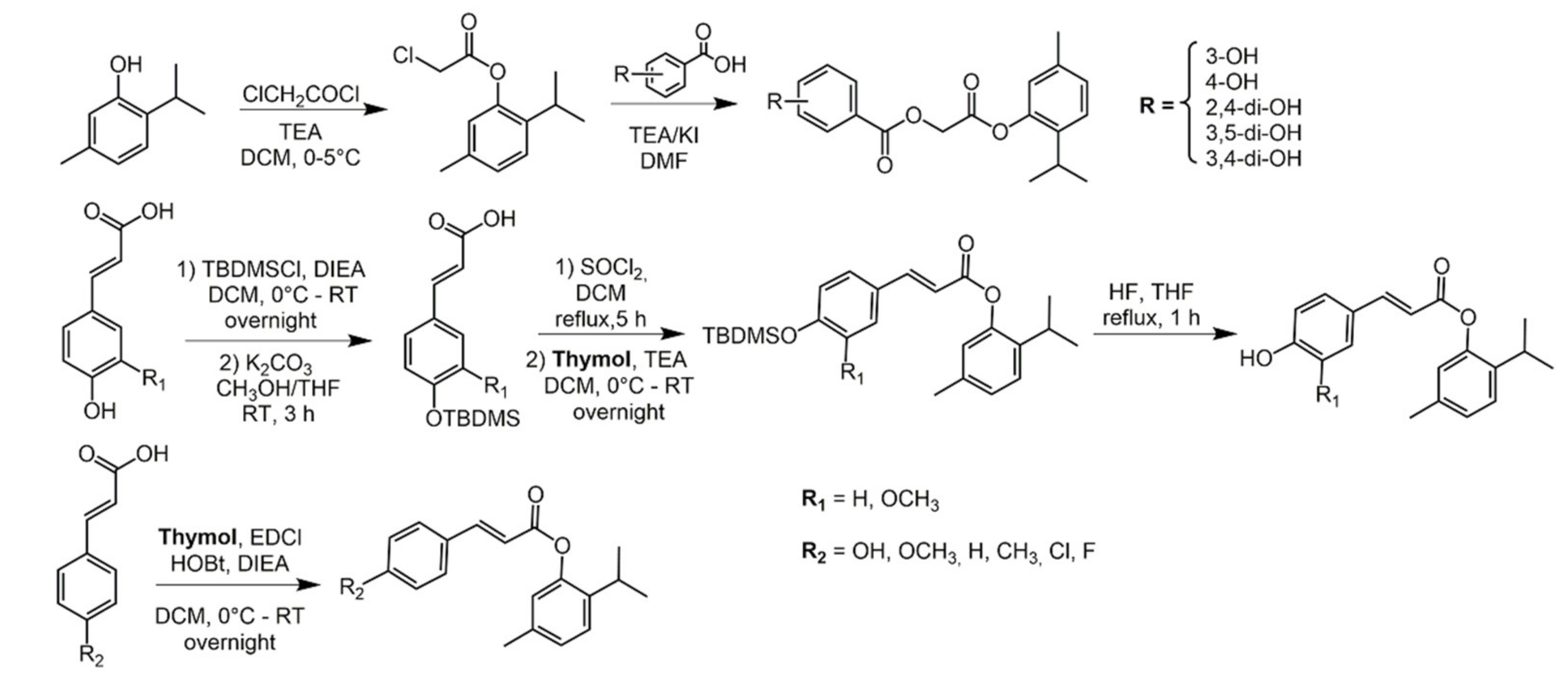
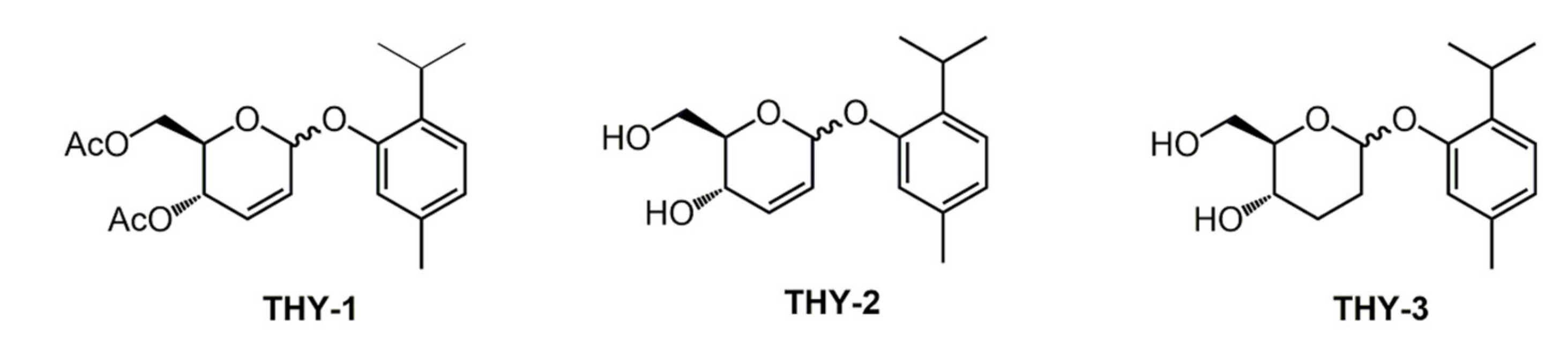
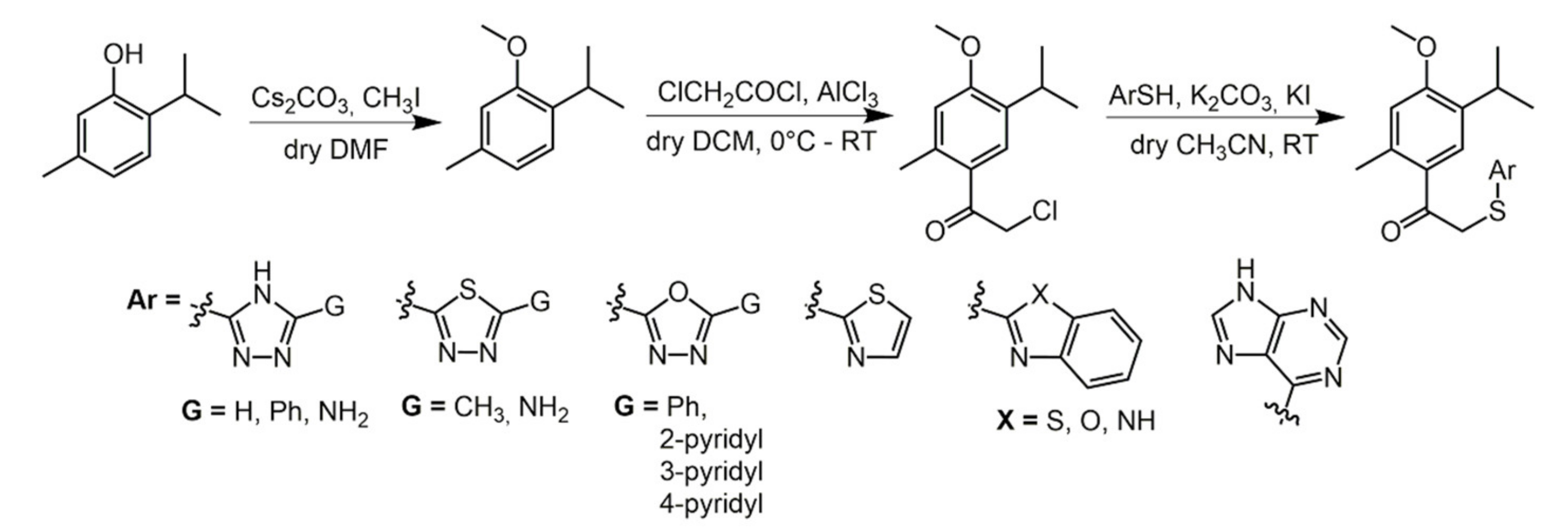
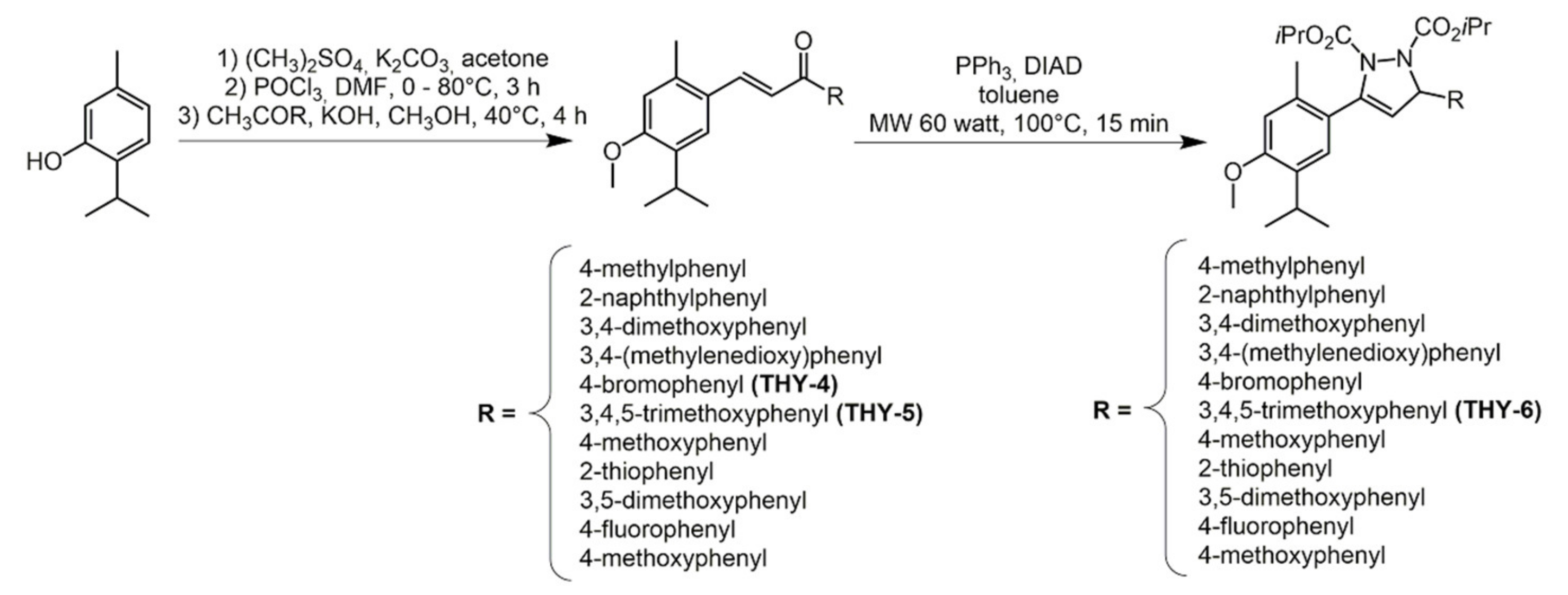
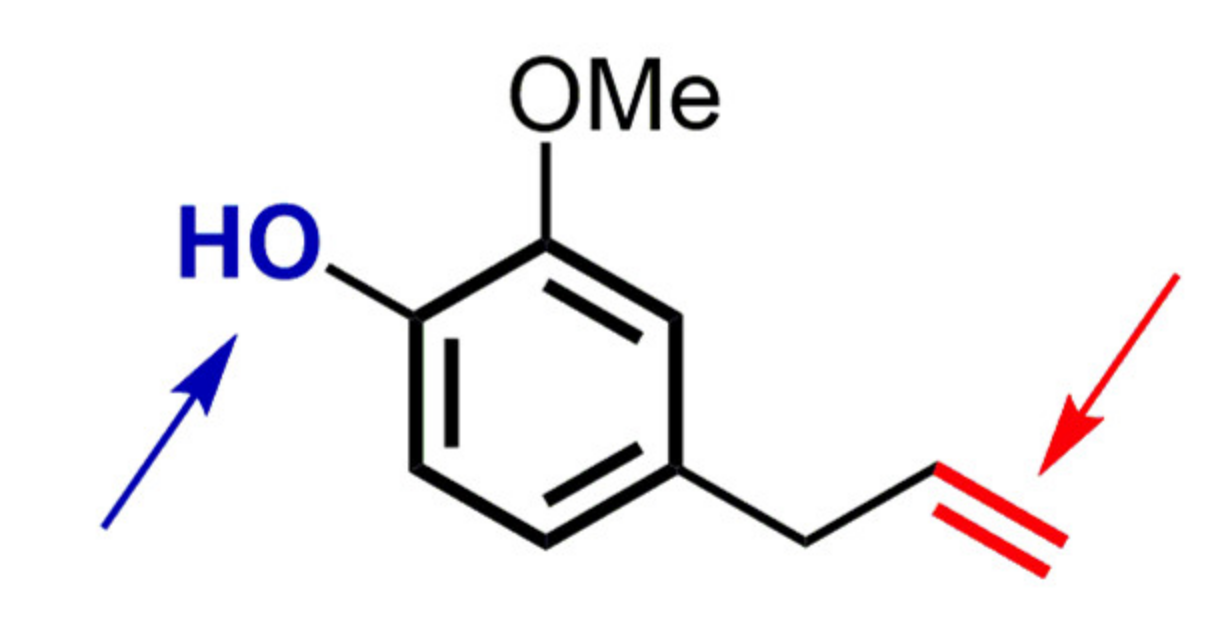

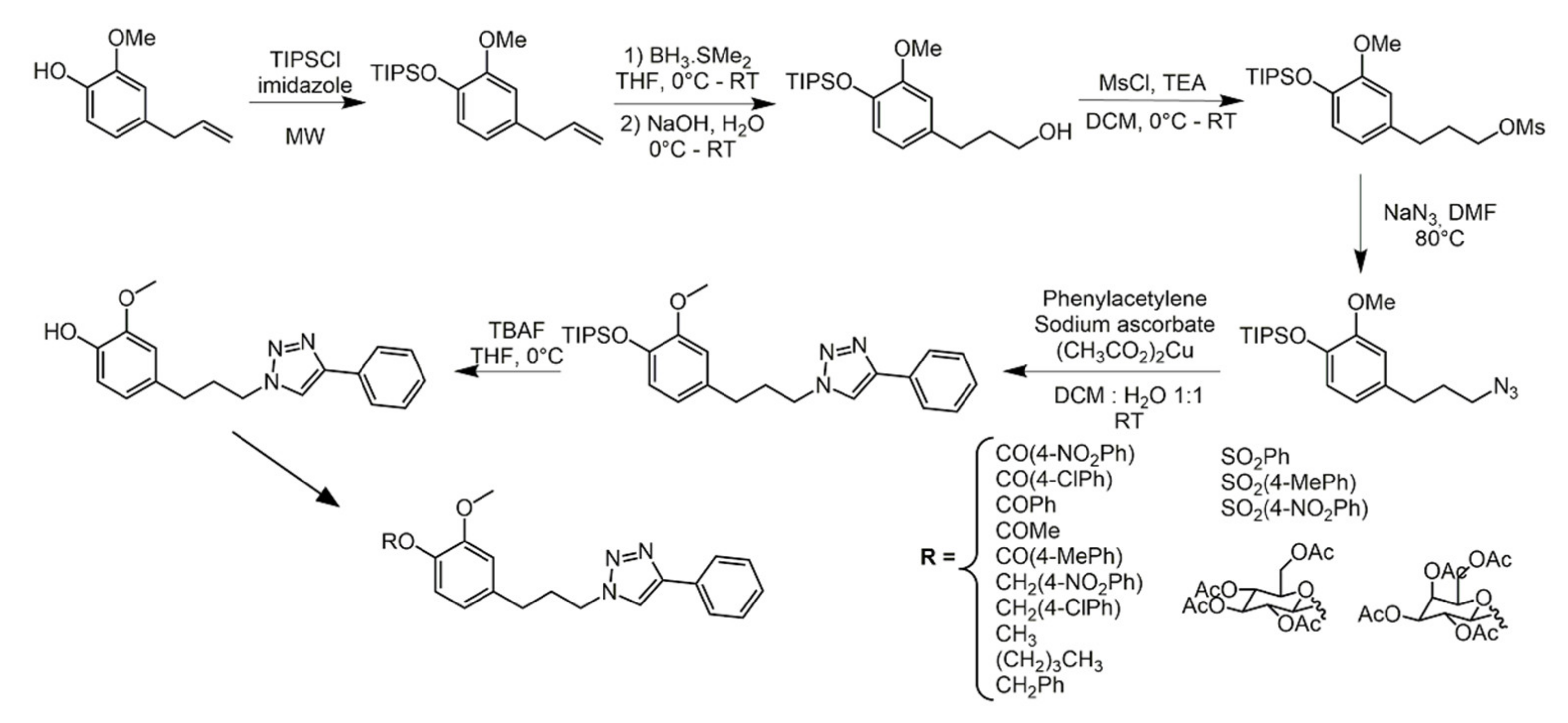
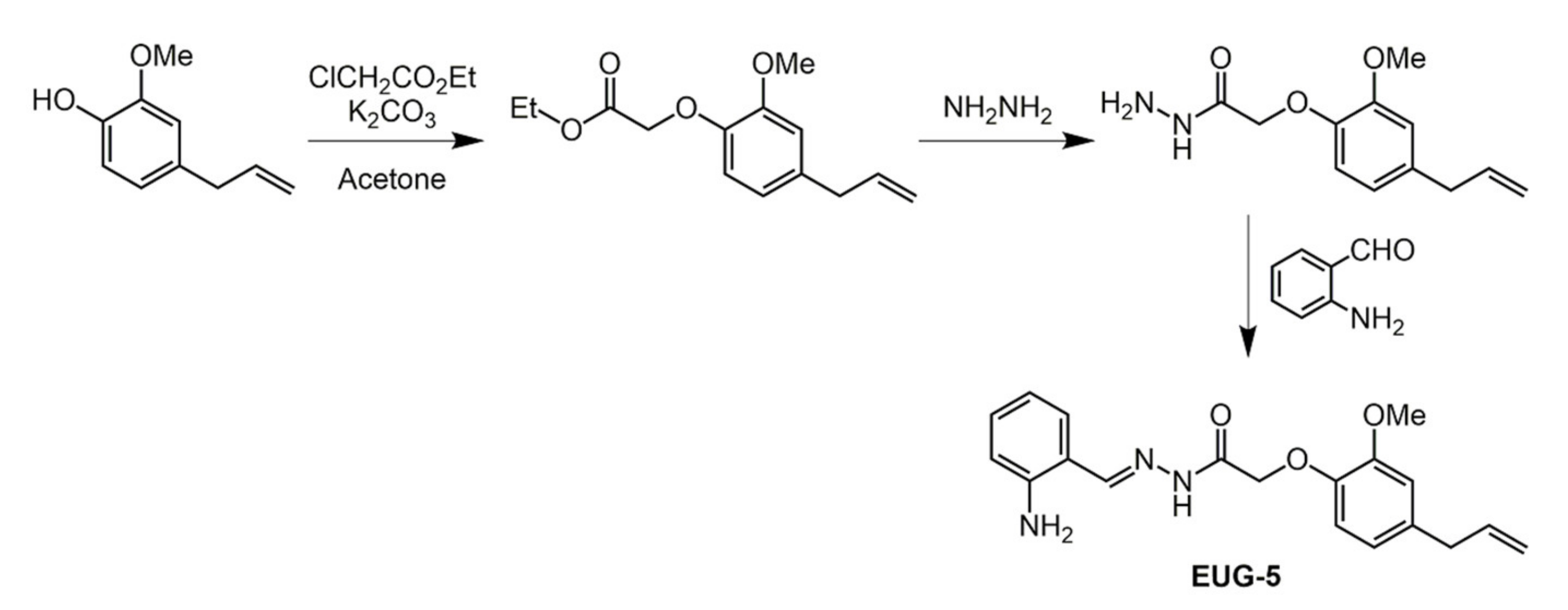
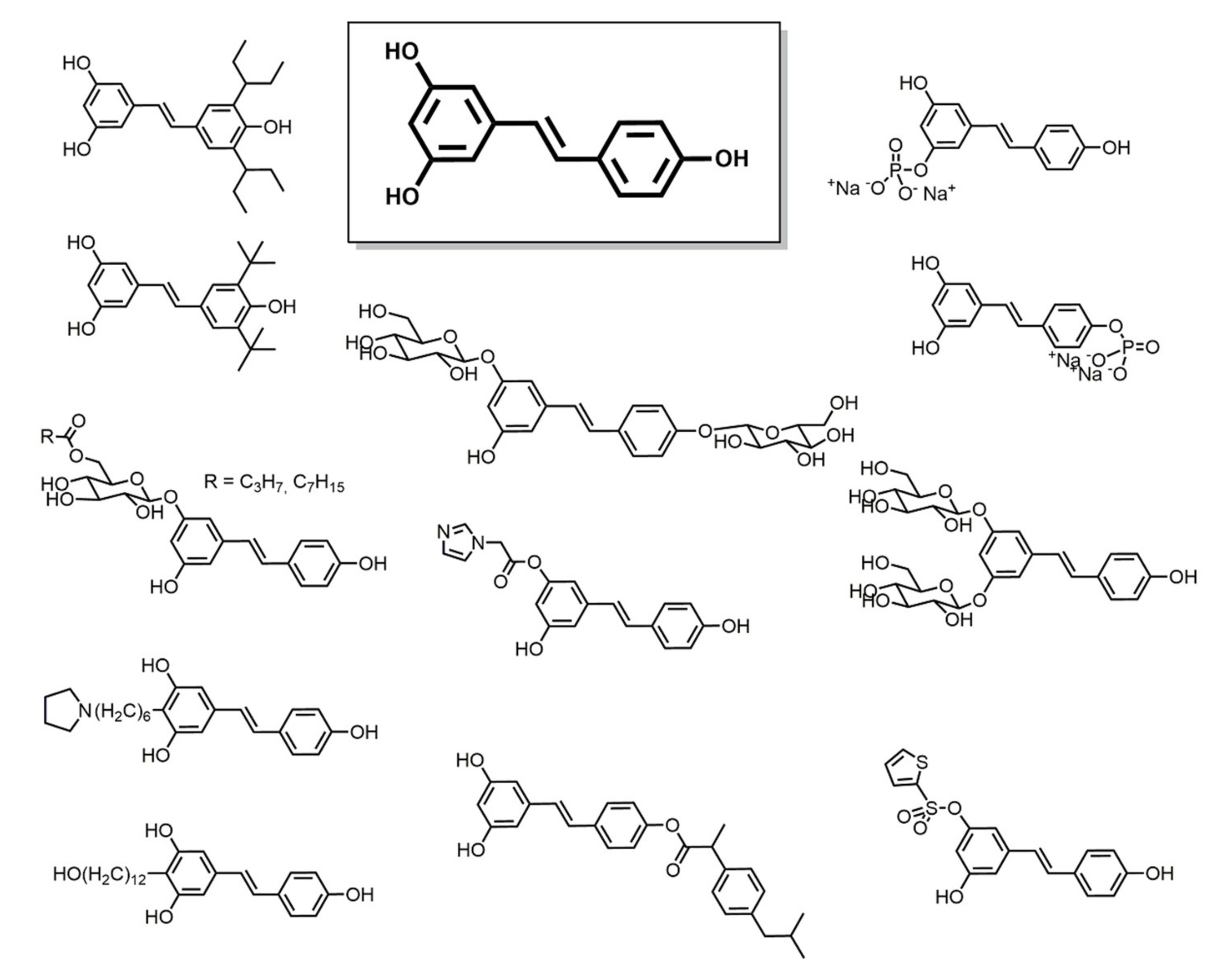
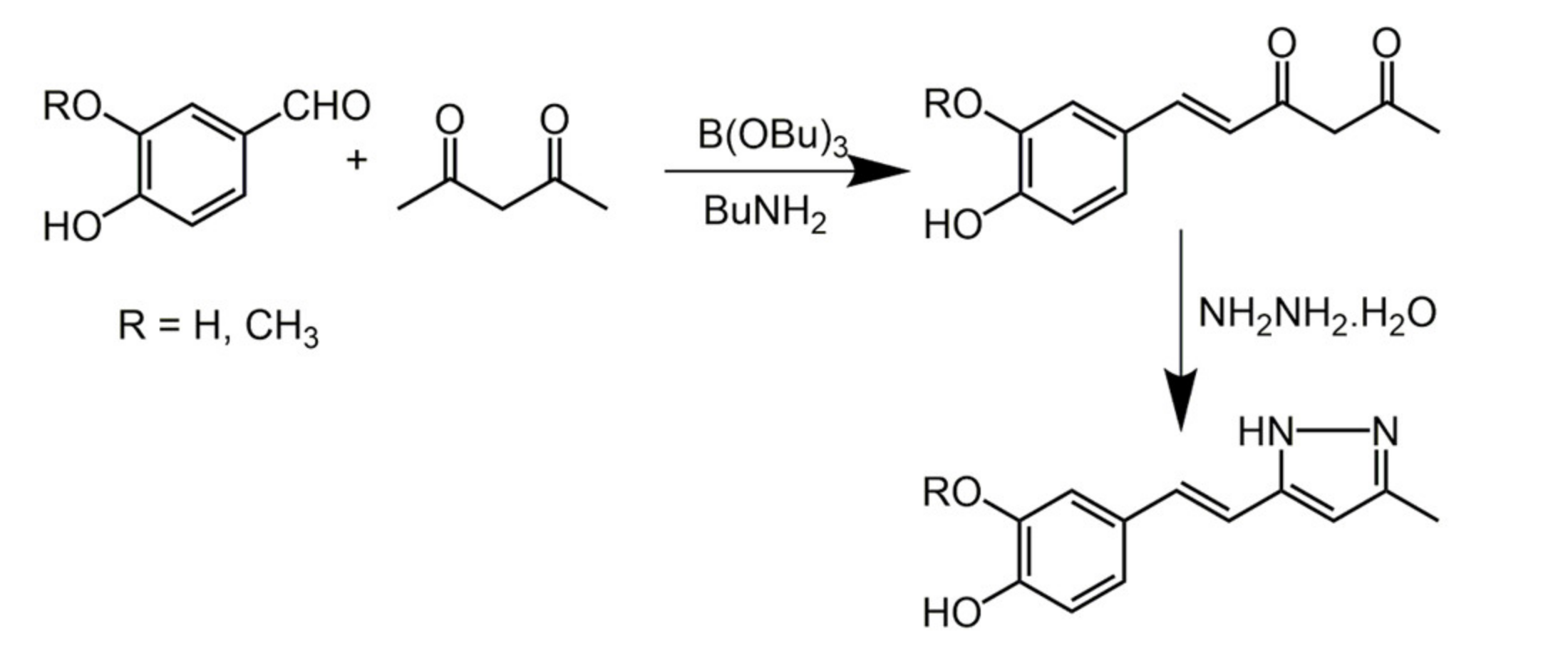
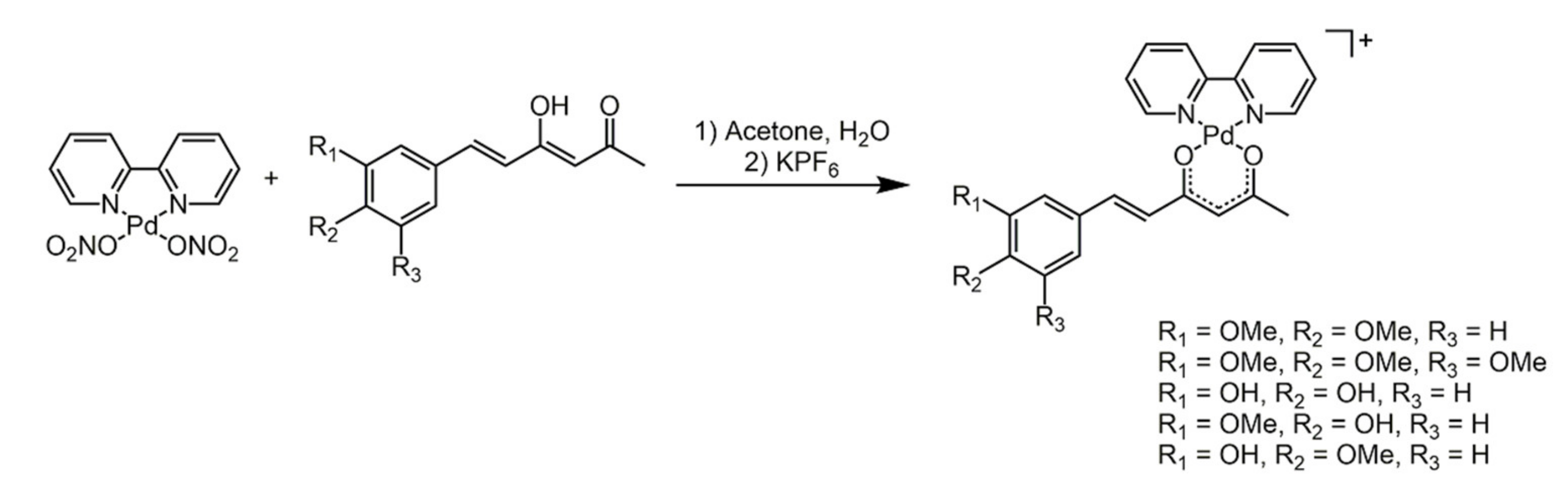
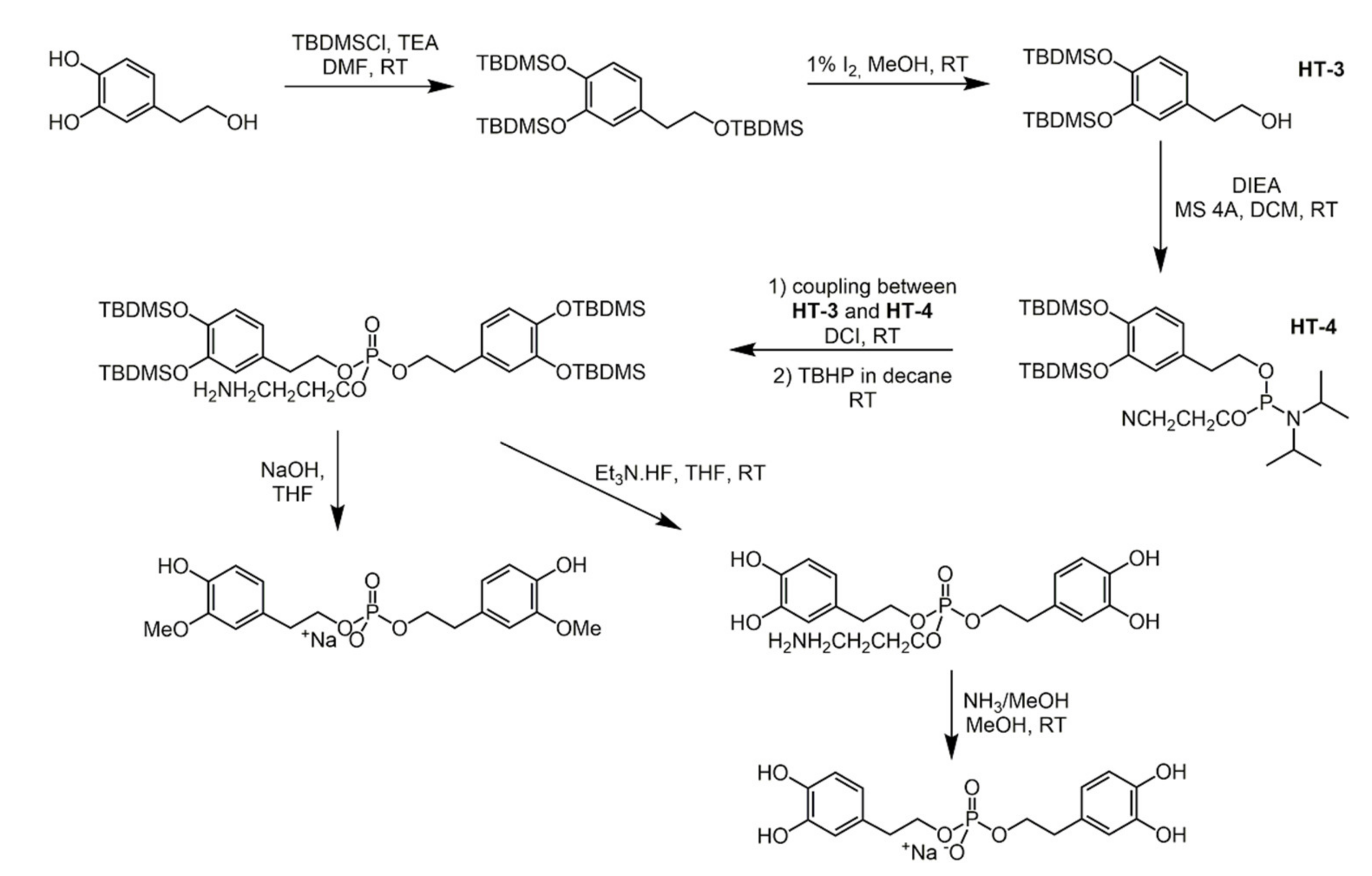
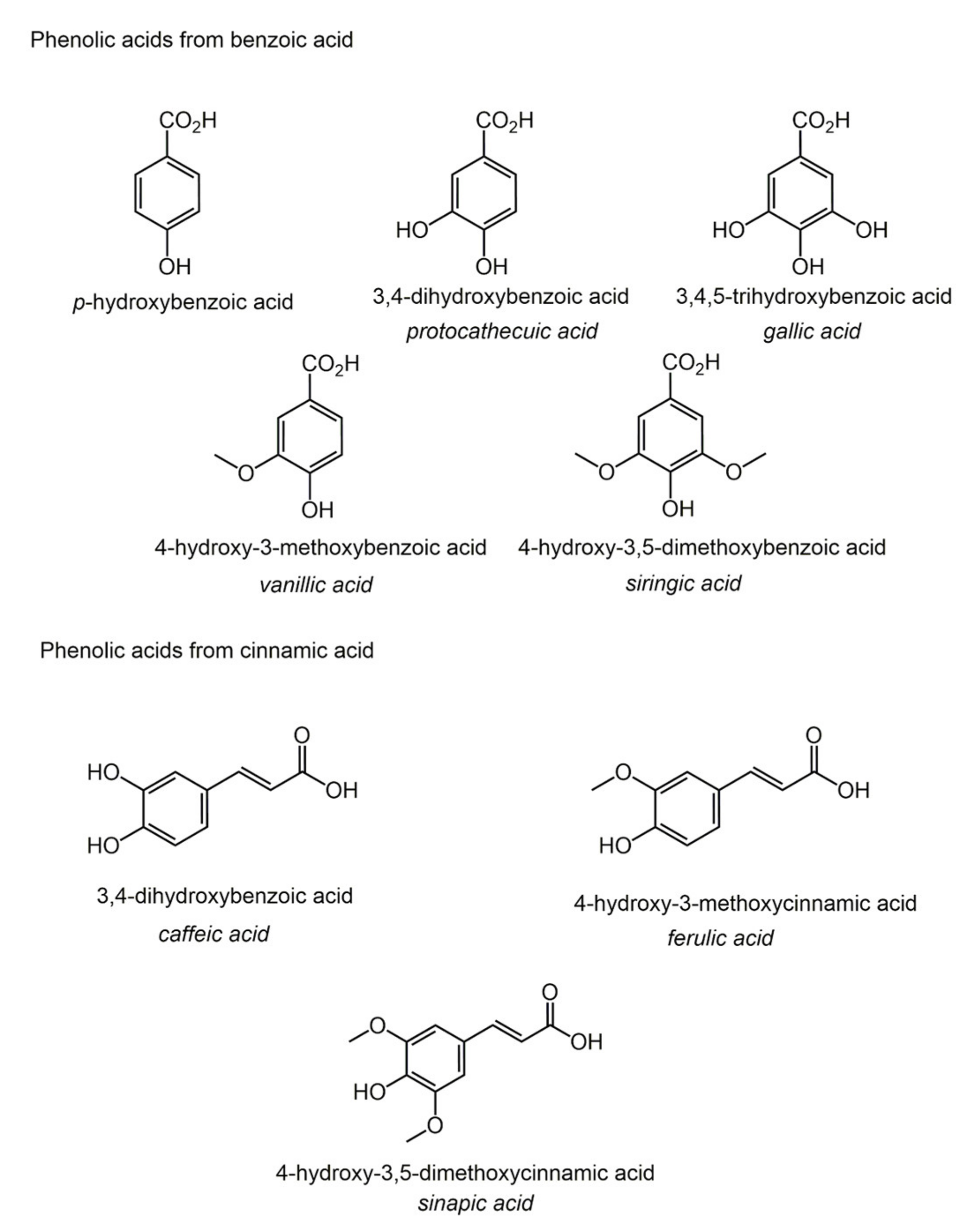


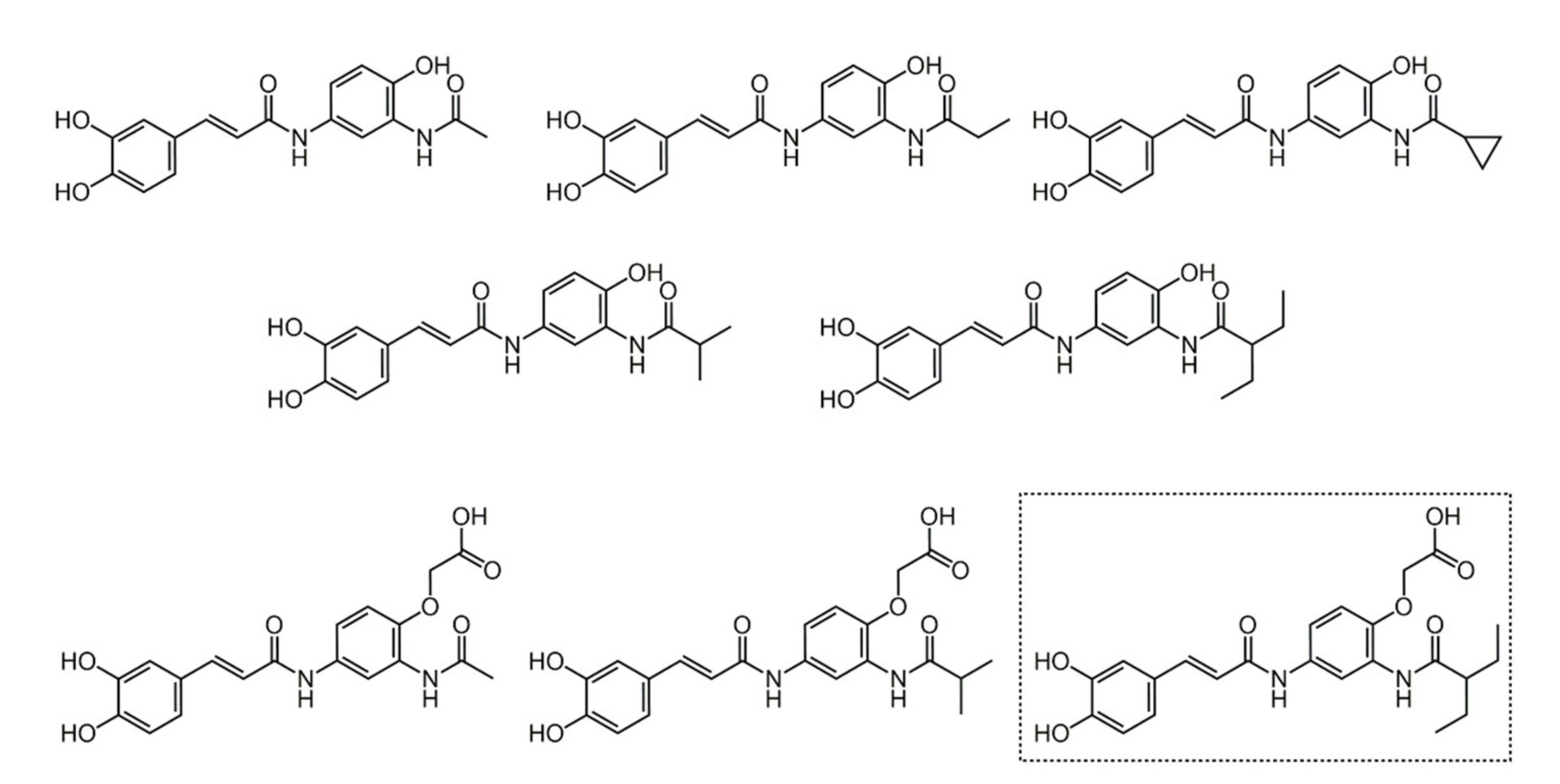





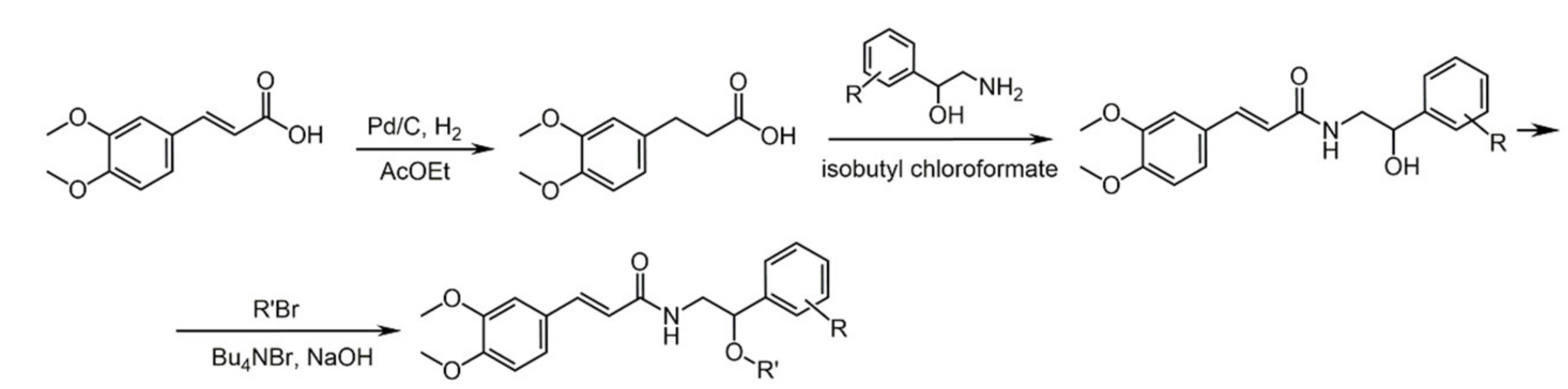
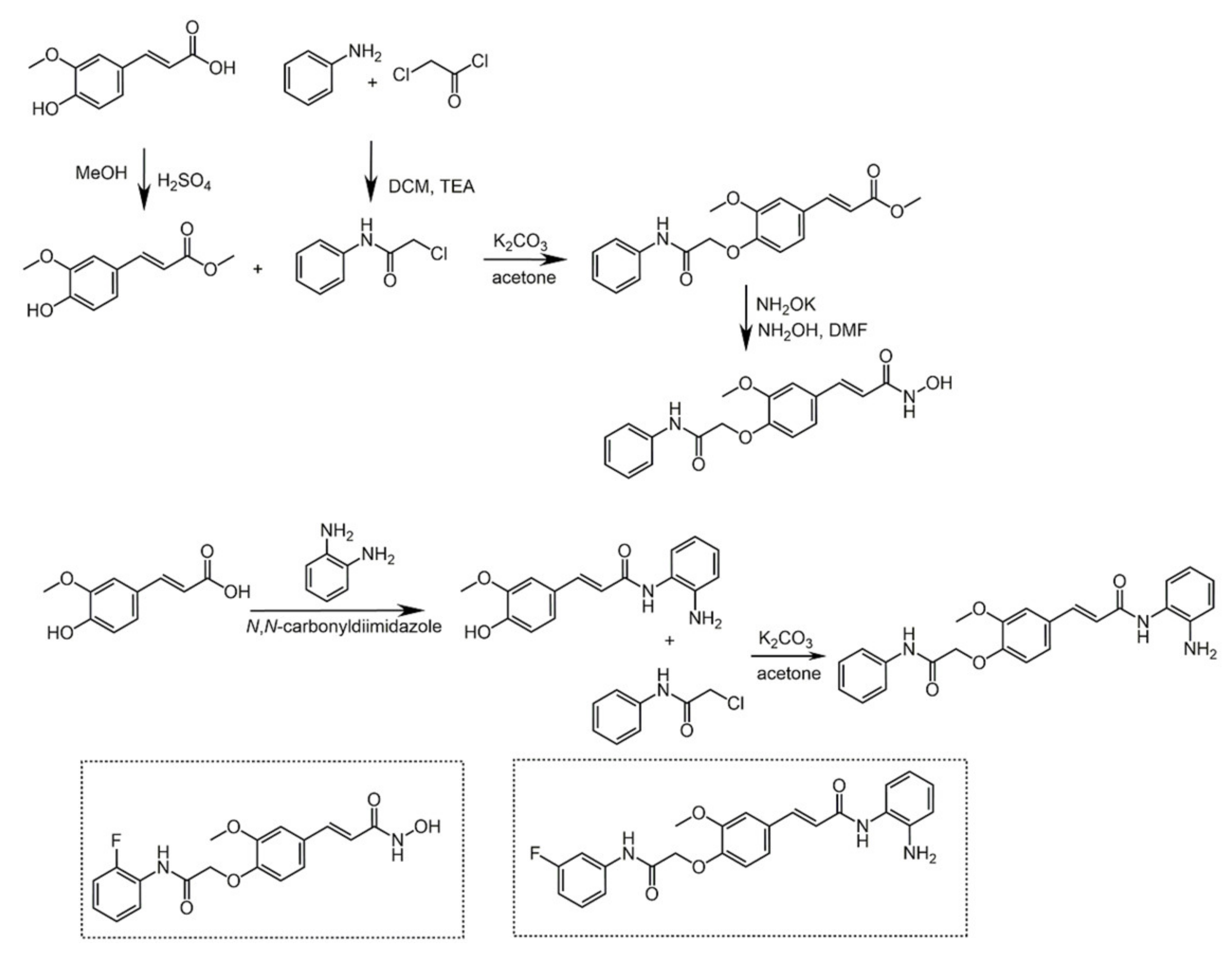
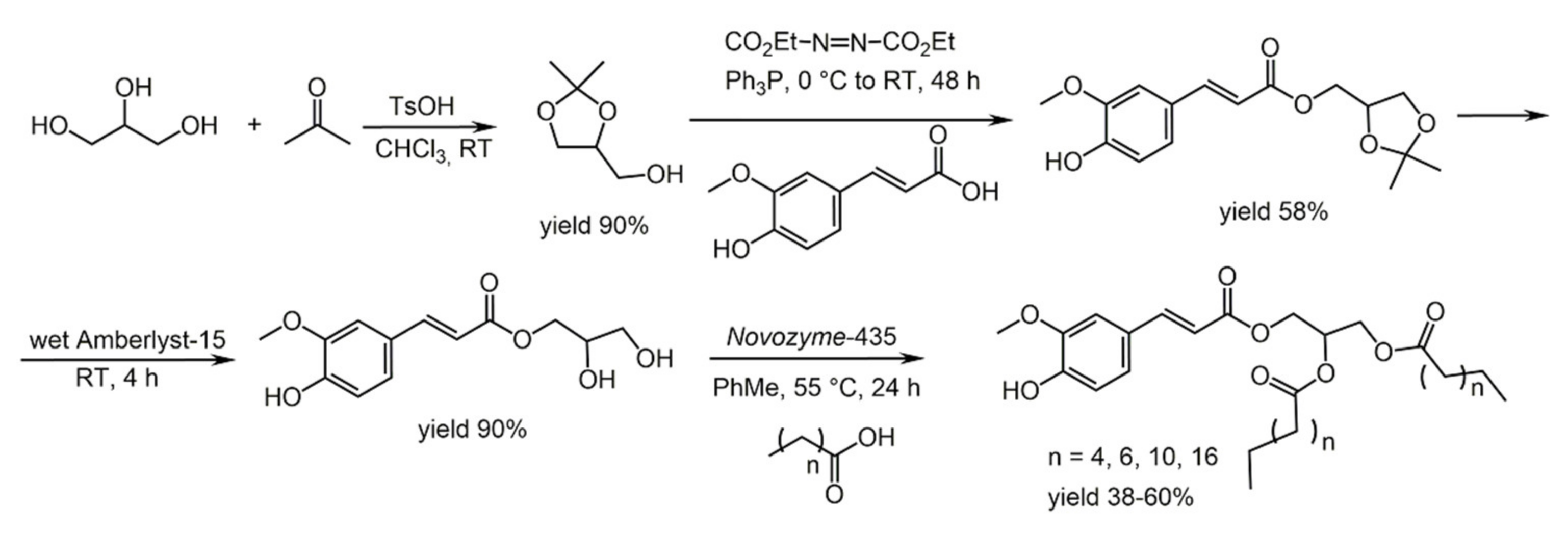
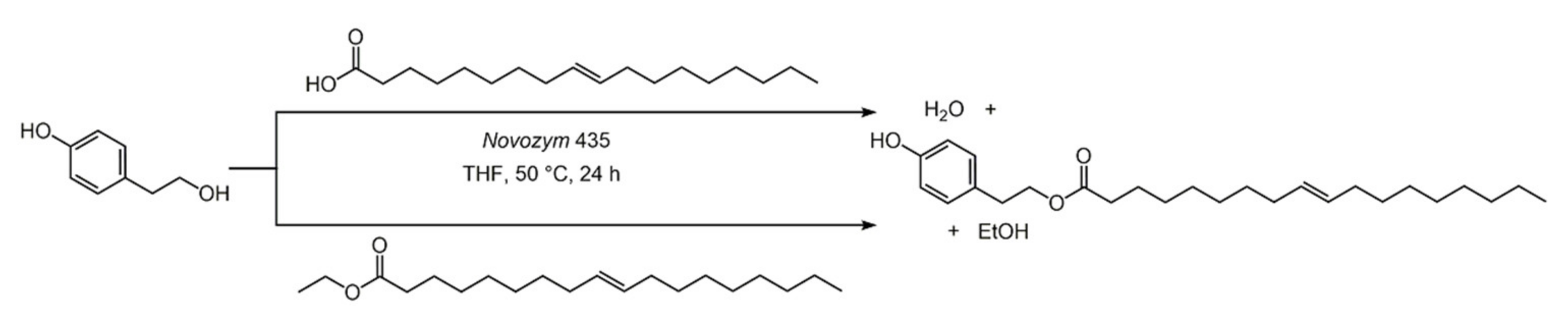
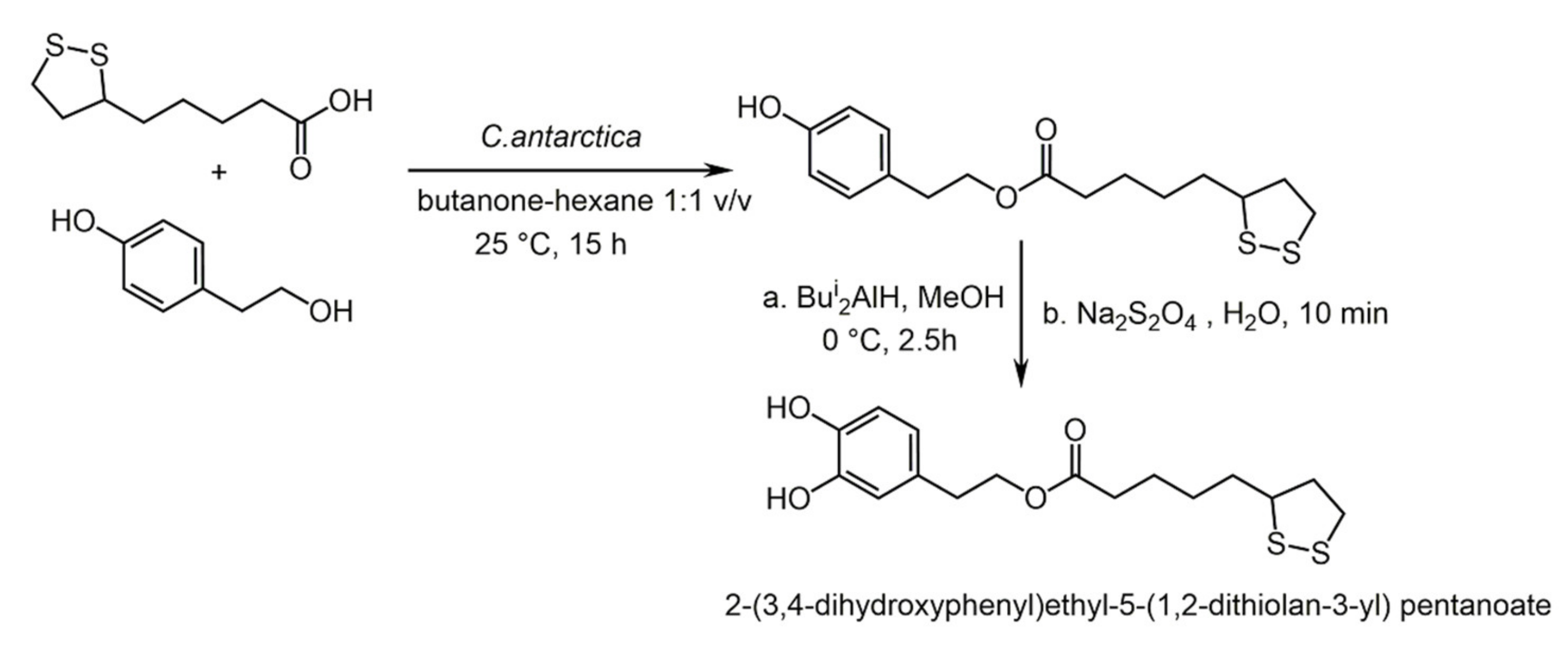
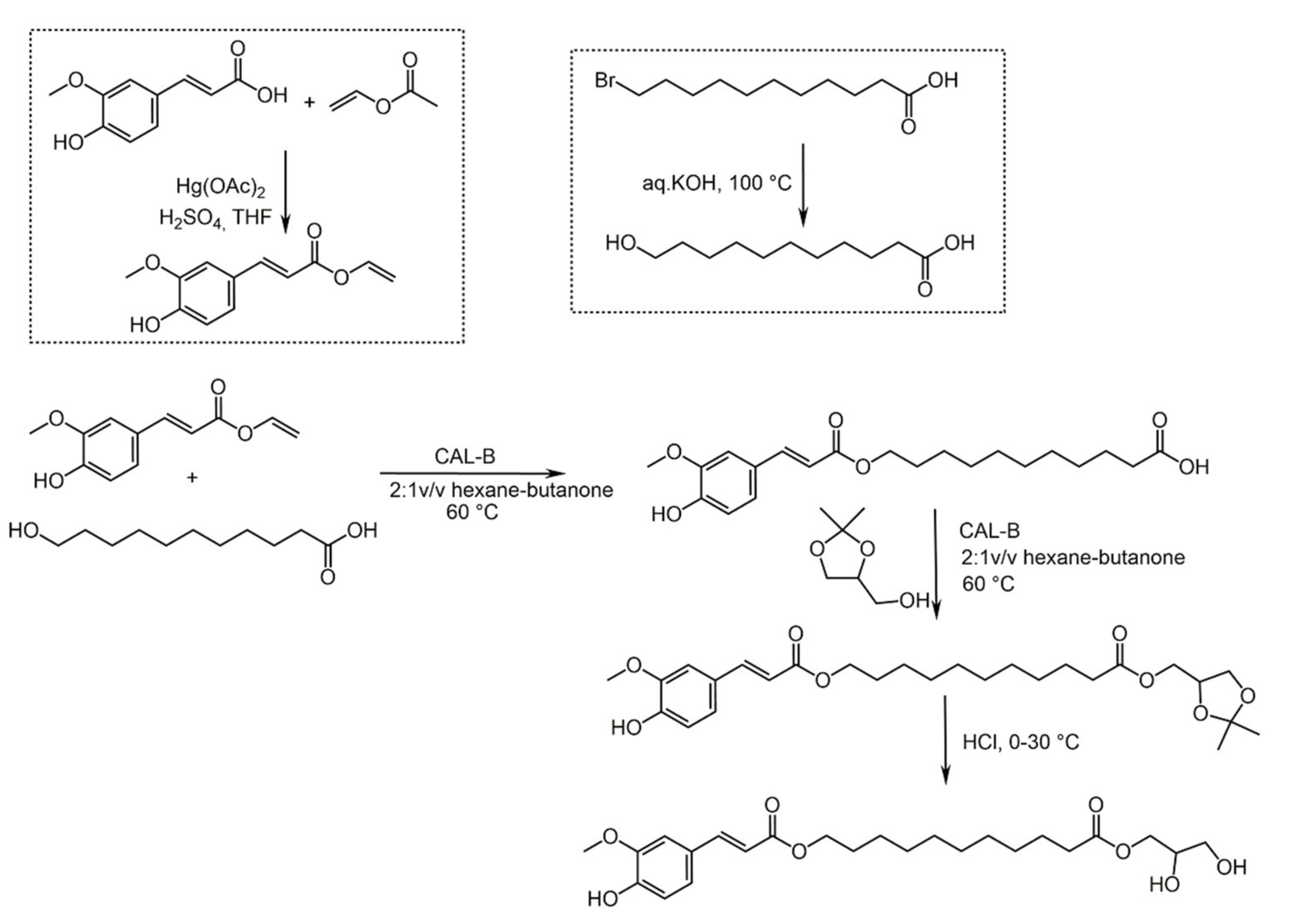


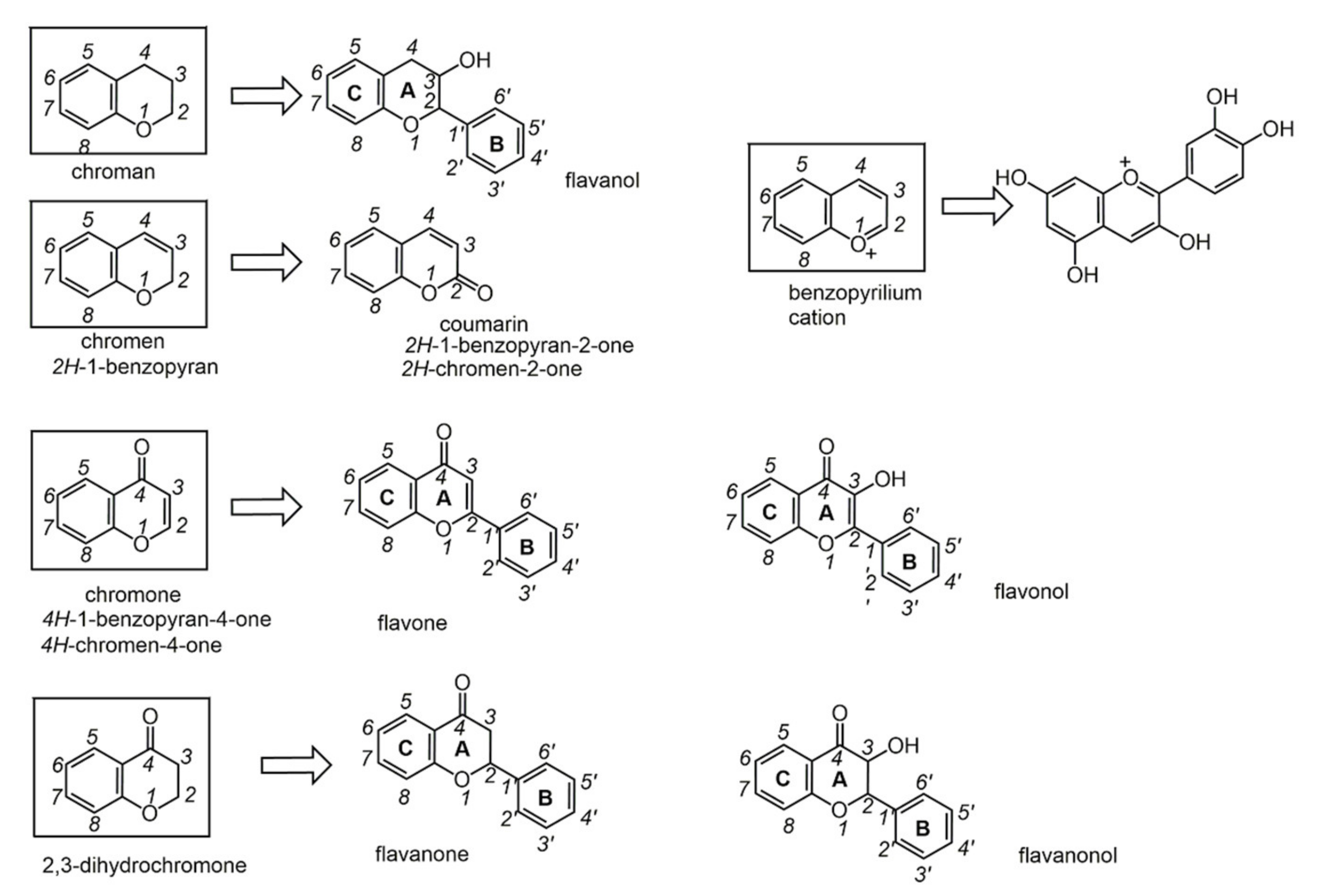
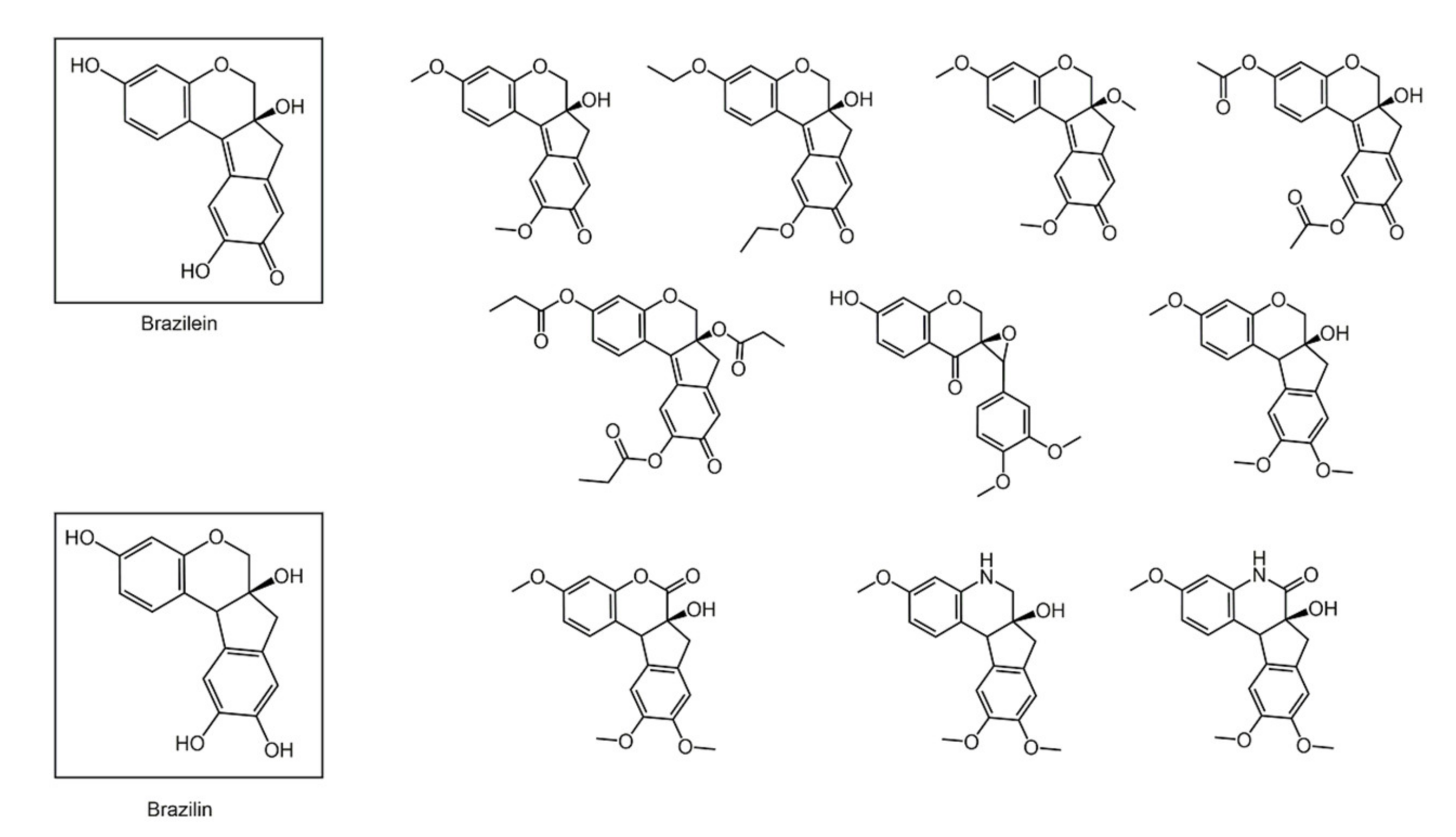
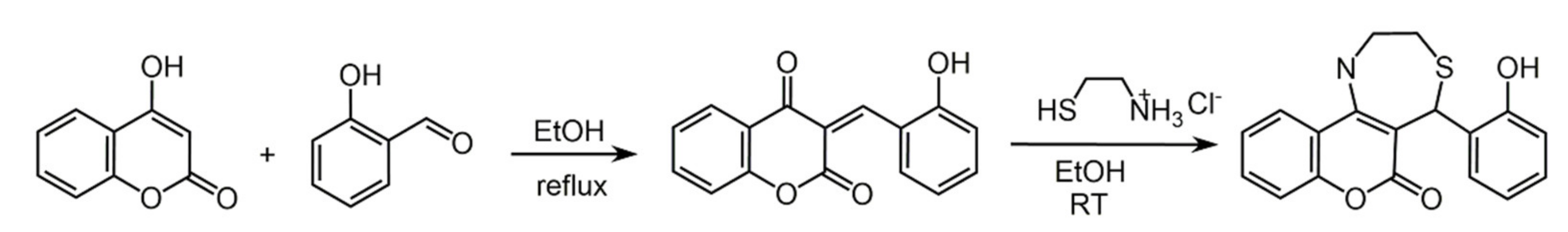


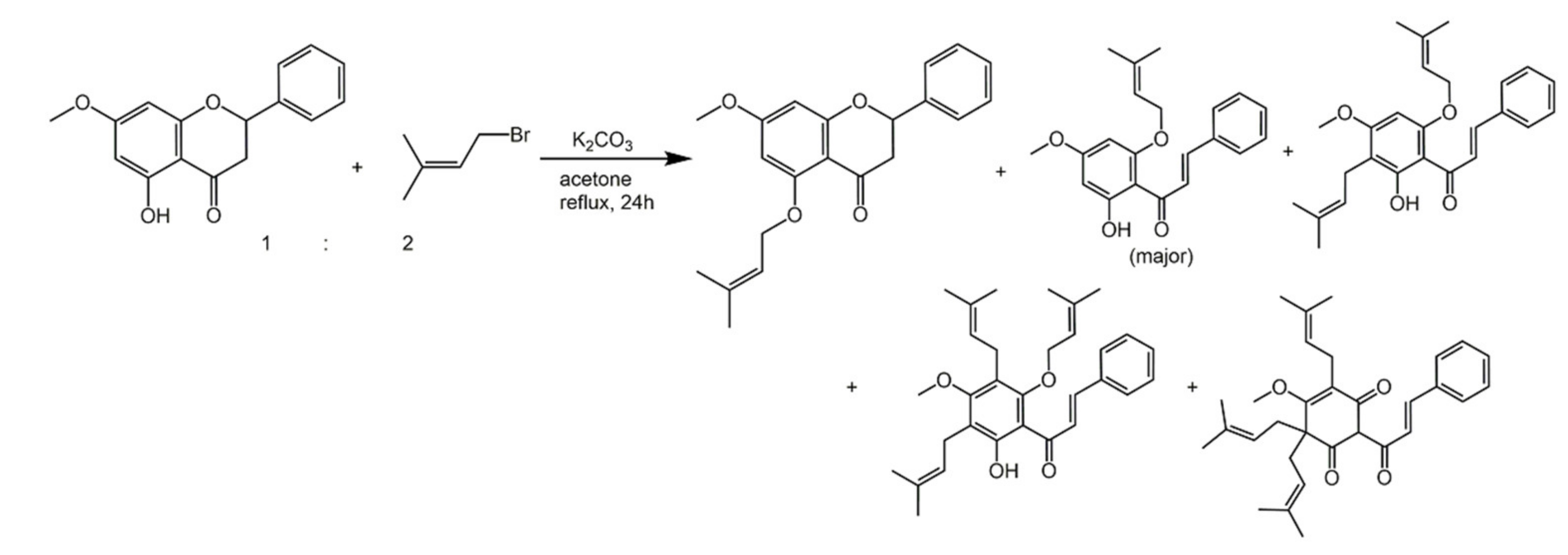
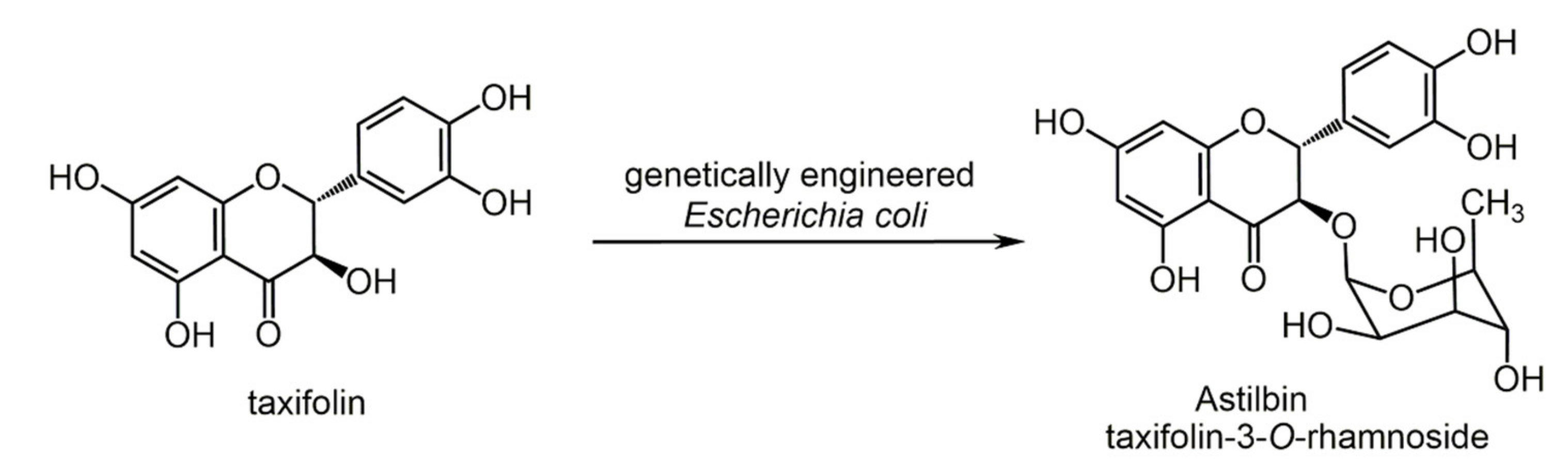
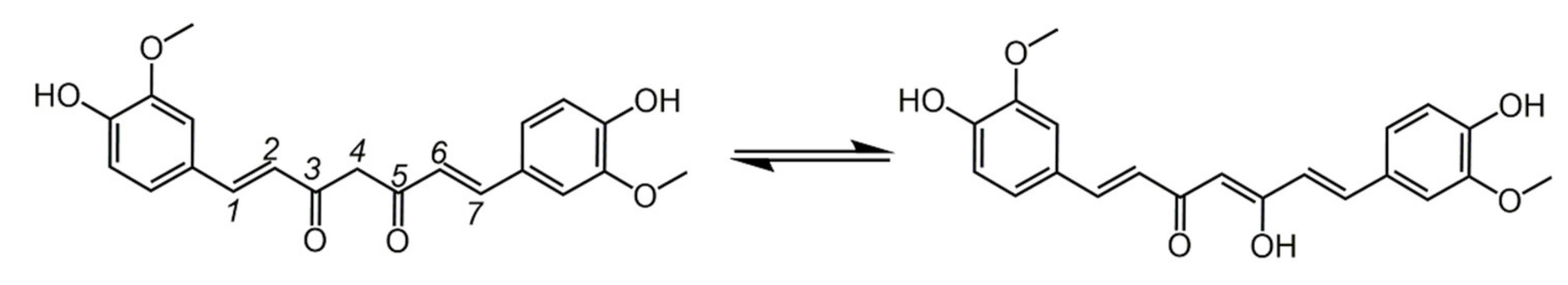
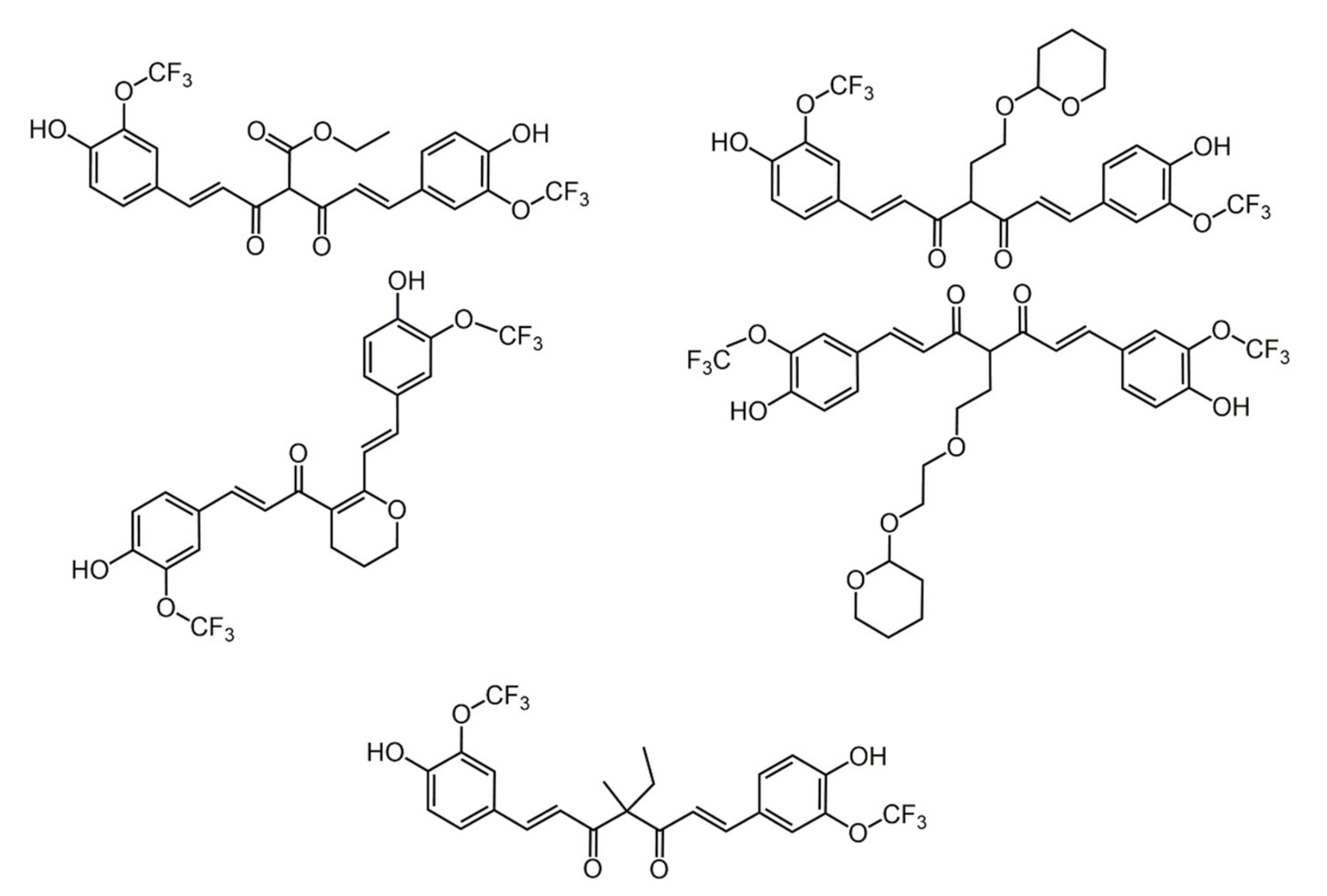
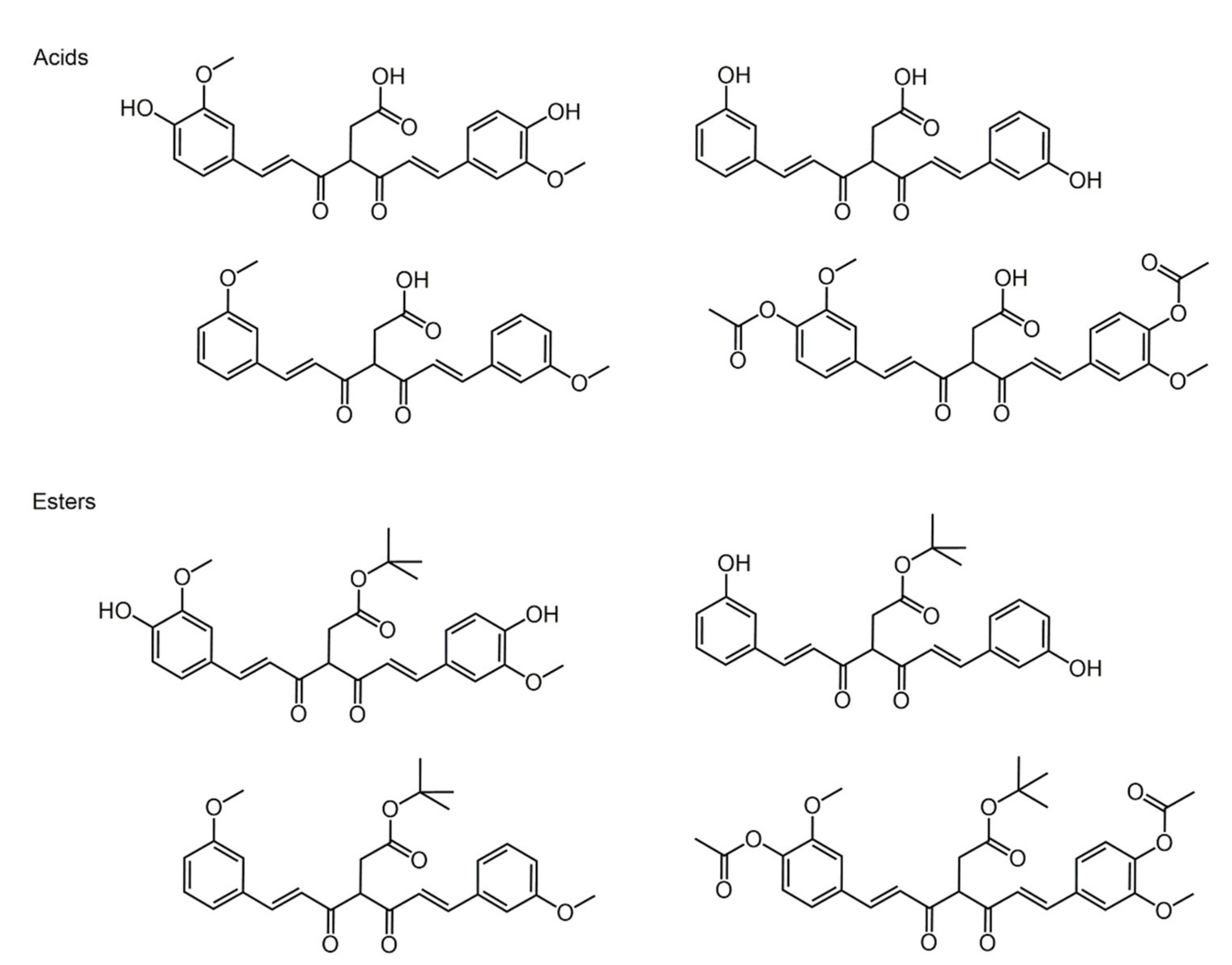
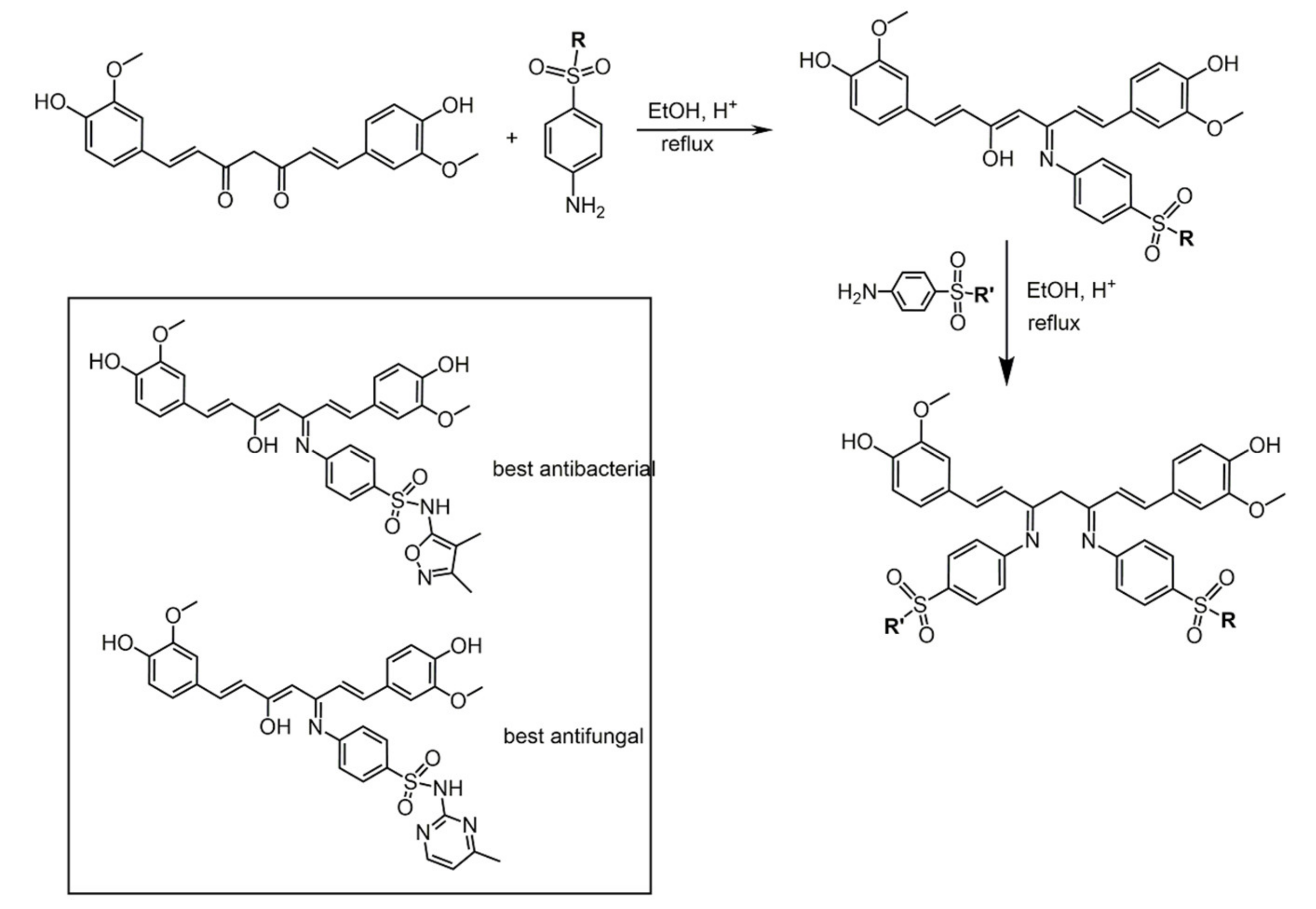
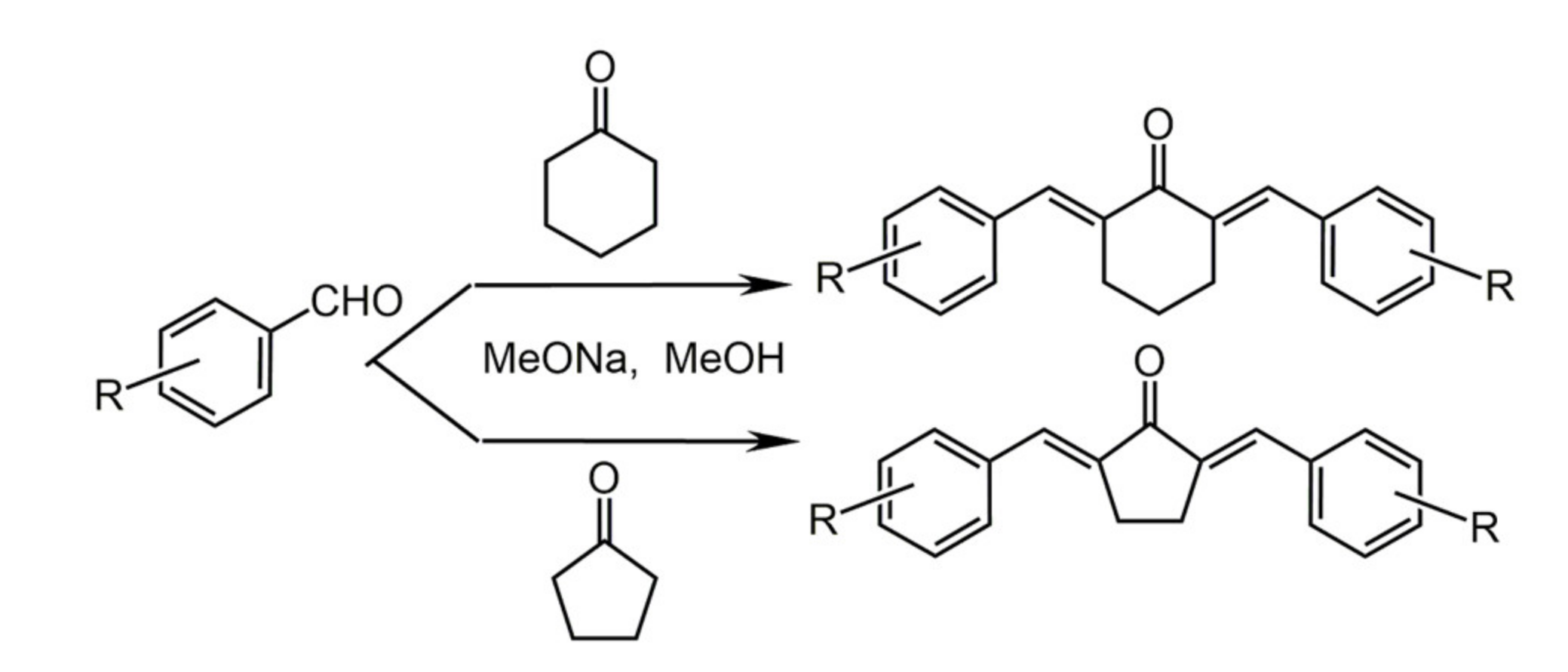


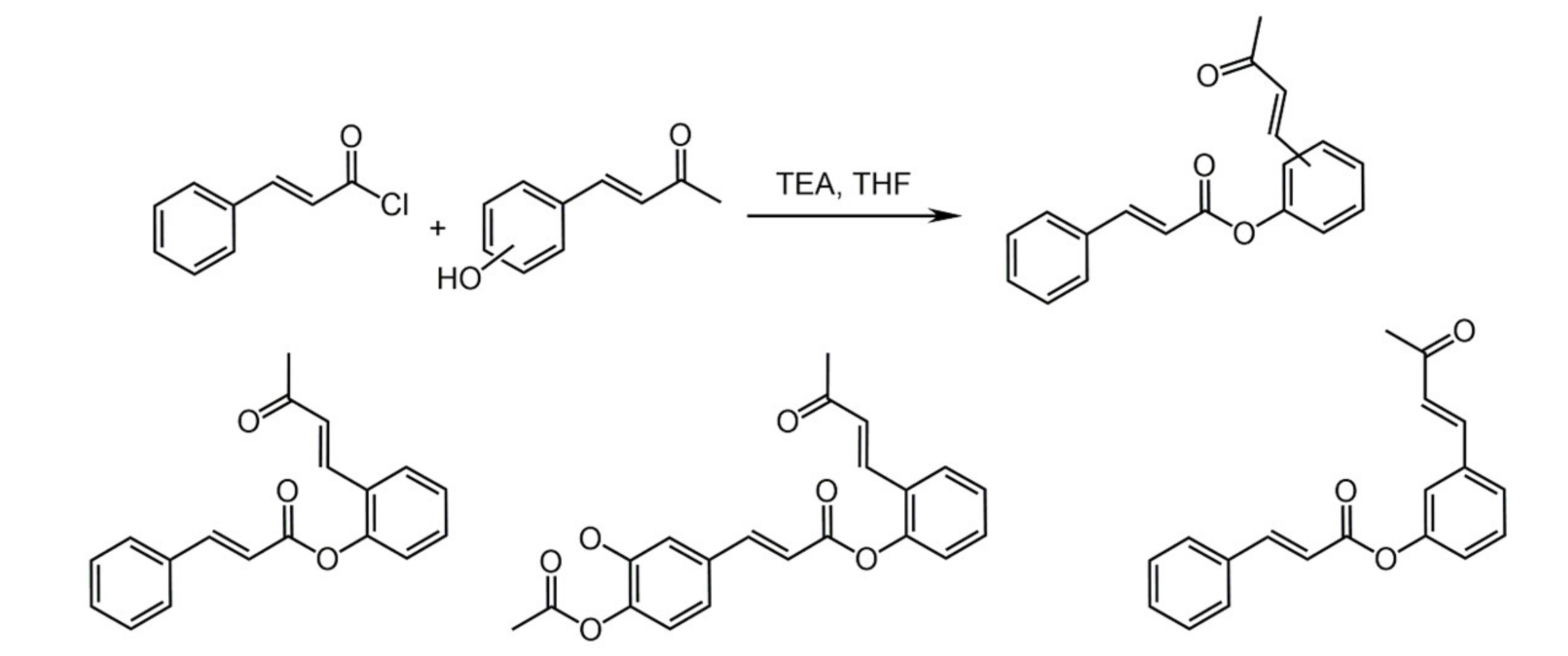
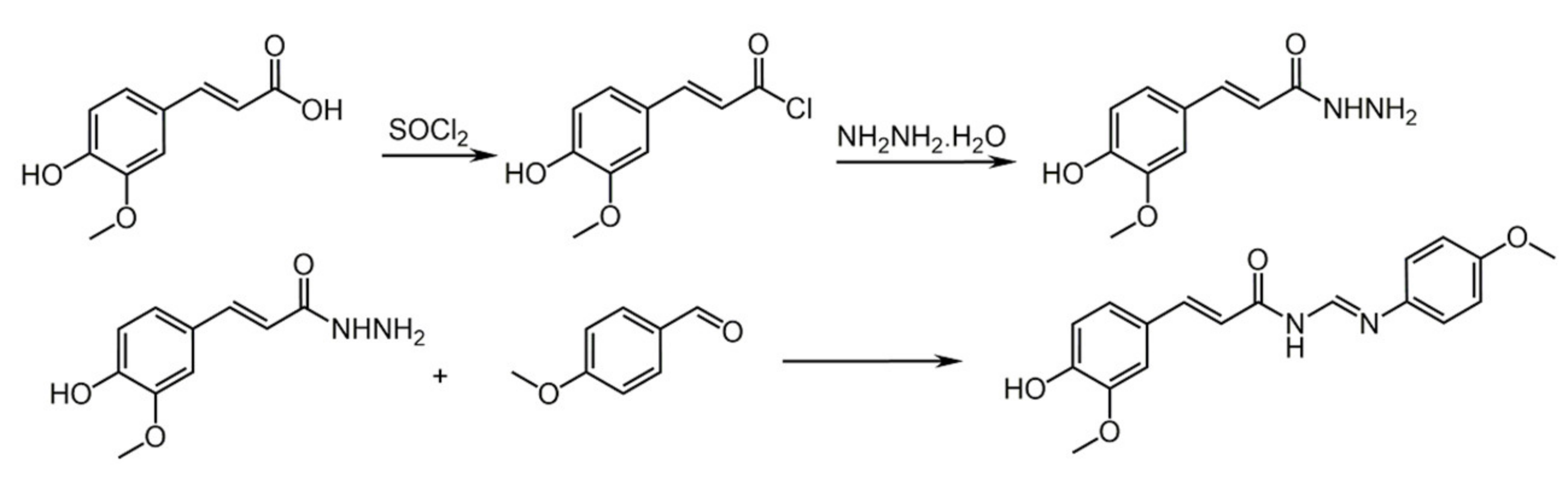

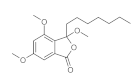 | 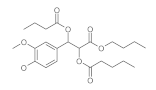 |
| chemotherapy adjuvant [353] | moderate antioxidant activity [354] |
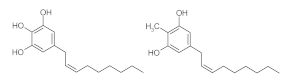 | |
| defense chemical [355] | |
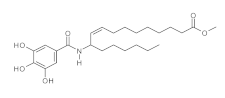 |  |
| antioxidant and anticancer activity [356] | anticoagulant and vasodilatation effects [357] |
 | |
| inhibit cell proliferation, induce cell death in human breast cancer cell lines [320,358] | |
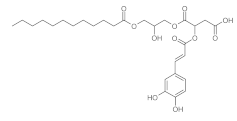 | |
| non-toxic antioxidant [359] | |
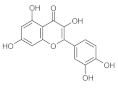 Quercetin antiviral activity against DENV-2 used in treating a dengue virus infection | |
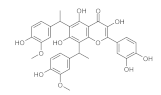 | 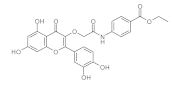 |
| anticancer/antiproliferative activity (possible treatment of oral cancer) | anticancer/antiproliferative activity (activity against human esophageal squamous cell EC109) |
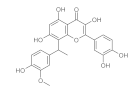 | 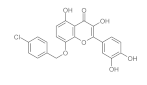 |
| anticancer/antiproliferative activity (quasi-drugs to reduce the risk of developing cancer) | antiviral activity (prophylaxis or treatment of hepatitis C virus) |
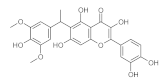 | 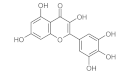 |
| anti-inflammatory activity (inhibiting the production of the inflammatory mediator TNF-α) | cardioprotective activity (suppressing the activity of the human chymase) |
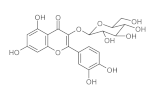 | 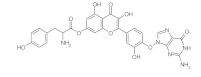 |
| cardioprotective activity (regulating the cholesterol synthesis) | skin protective activity |
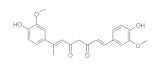 | 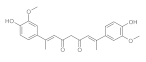 |
| 2-methylcurcumin | 2,7-dimethylcurcumin |
| enhanced antiangiogenesis activity, increased anti-inflammatory activity and stability against enzymatic reduction [410,411,412] | |
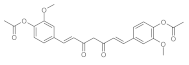 | 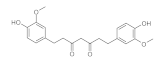 |
| diacetylcurcumin antiarthritic activity in mice [413,414] | tetrahydrocurcumin increased antioxidant activity [415] |
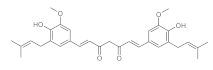 | |
| diprenylcurcumin antioxidant properties [416] | |
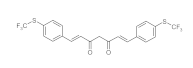 | 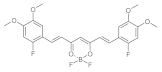 |
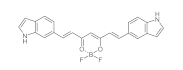 | 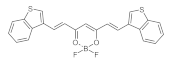 |
| anti-proliferative in vitro bioassay studies [417] | |
 |  |
| inhibition of TXNIP, protein associated with multiple diseases [418] | 4-benzylidenecurcumins [419,420] |
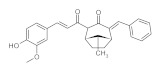 | 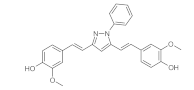 |
| versatile synthesis of 18 dicarbonyl curcumins containing tropane [421] | neuroprotective activity in cell culture; enhances memory; anti-neuroinflammatory effects [422,423,424] |
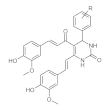 | 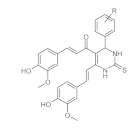 |
| antibacterial and antioxidant activity [425] | |
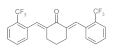 | |
| (2E,6E)-2,6-bis(2(trifluoromethyl)benzylidene)cyclohexanone promising for healing diabetic wound in mice [426] | |
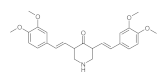 | 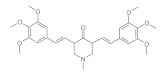 |
| better anticancer activity than curcumin against melanoma cells [427,428] | |
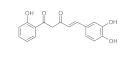 |  |
| 5-(3,4-dihydroxyphenyl)-3-hydroxy-1-(2-hydroxyphenyl)penta-2,4-dien-1-one anti-edematogenic/anti-granuloma [429] | effect on hepatic steatosis in mice with induced obesity [430] |
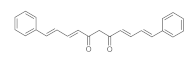 | 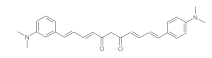 |
| efficient for photodynamic therapy [431] | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Floris, B.; Galloni, P.; Conte, V.; Sabuzi, F. Tailored Functionalization of Natural Phenols to Improve Biological Activity. Biomolecules 2021, 11, 1325. https://doi.org/10.3390/biom11091325
Floris B, Galloni P, Conte V, Sabuzi F. Tailored Functionalization of Natural Phenols to Improve Biological Activity. Biomolecules. 2021; 11(9):1325. https://doi.org/10.3390/biom11091325
Chicago/Turabian StyleFloris, Barbara, Pierluca Galloni, Valeria Conte, and Federica Sabuzi. 2021. "Tailored Functionalization of Natural Phenols to Improve Biological Activity" Biomolecules 11, no. 9: 1325. https://doi.org/10.3390/biom11091325
APA StyleFloris, B., Galloni, P., Conte, V., & Sabuzi, F. (2021). Tailored Functionalization of Natural Phenols to Improve Biological Activity. Biomolecules, 11(9), 1325. https://doi.org/10.3390/biom11091325