Attenuation of PITPNM1 Signaling Cascade Can Inhibit Breast Cancer Progression
Abstract
:1. Introduction
2. Materials and Methods
2.1. Cell Culture
2.2. SiRNA Transfection
2.3. CCK8 Cell Counting Assay
2.4. Colony Formation Assay
2.5. RNA Isolation and Real-Time Quantitative PCR
2.6. Data Extraction and Bioinformatics Analysis
2.7. Statistical Analysis
3. Results
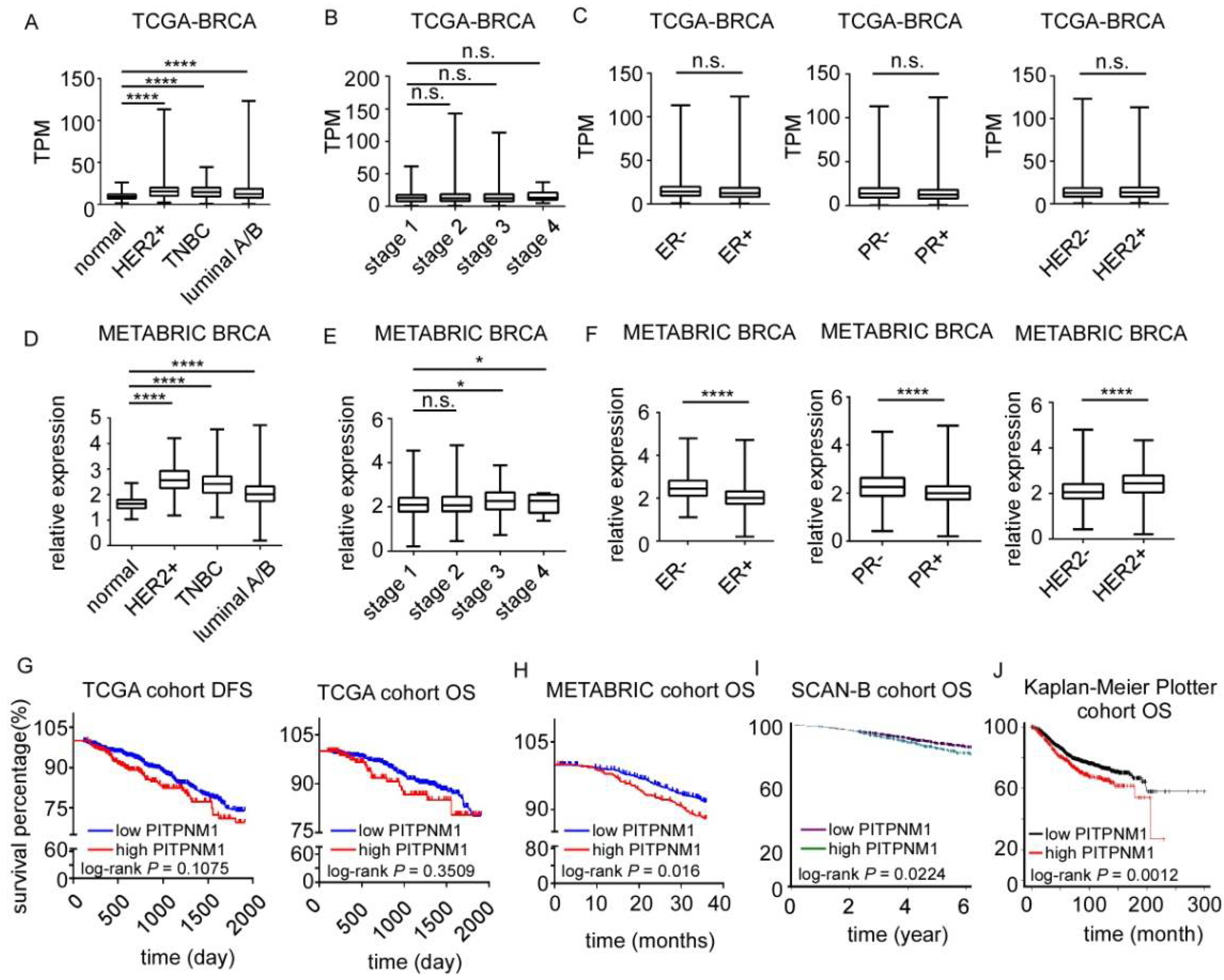
3.1. PITPNM1 Is Significantly Higher in Breast Cancer and Correlates with Poor Prognosis in Patients with Breast Cancer
3.2. PITPNM1 Over-Expression Is Associated with Carcinogenesis Gene Ontology and Pathway
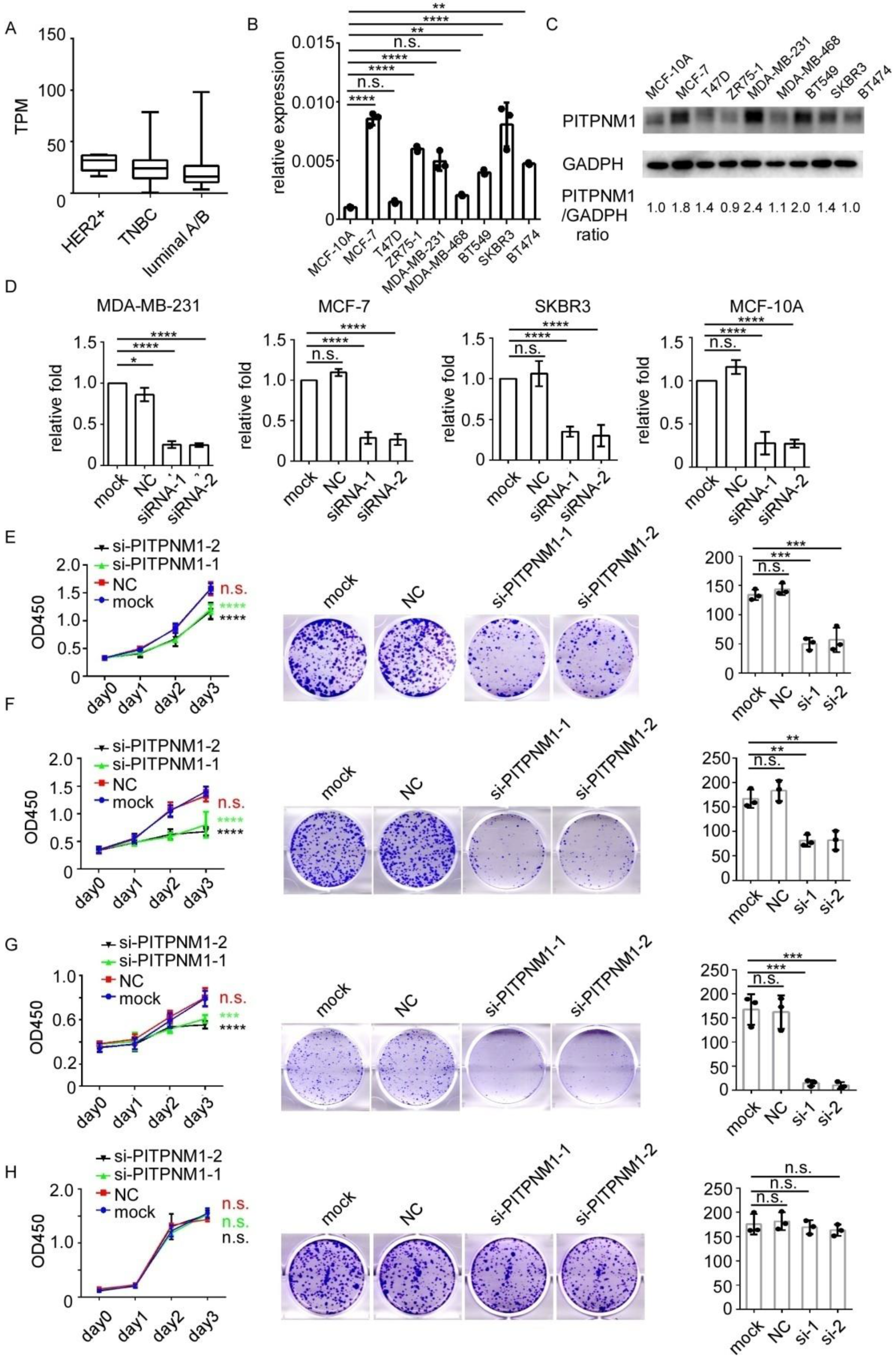
3.3. Silencing of PITPNM1 Inhibits Proliferation of Breast Cancer Cells
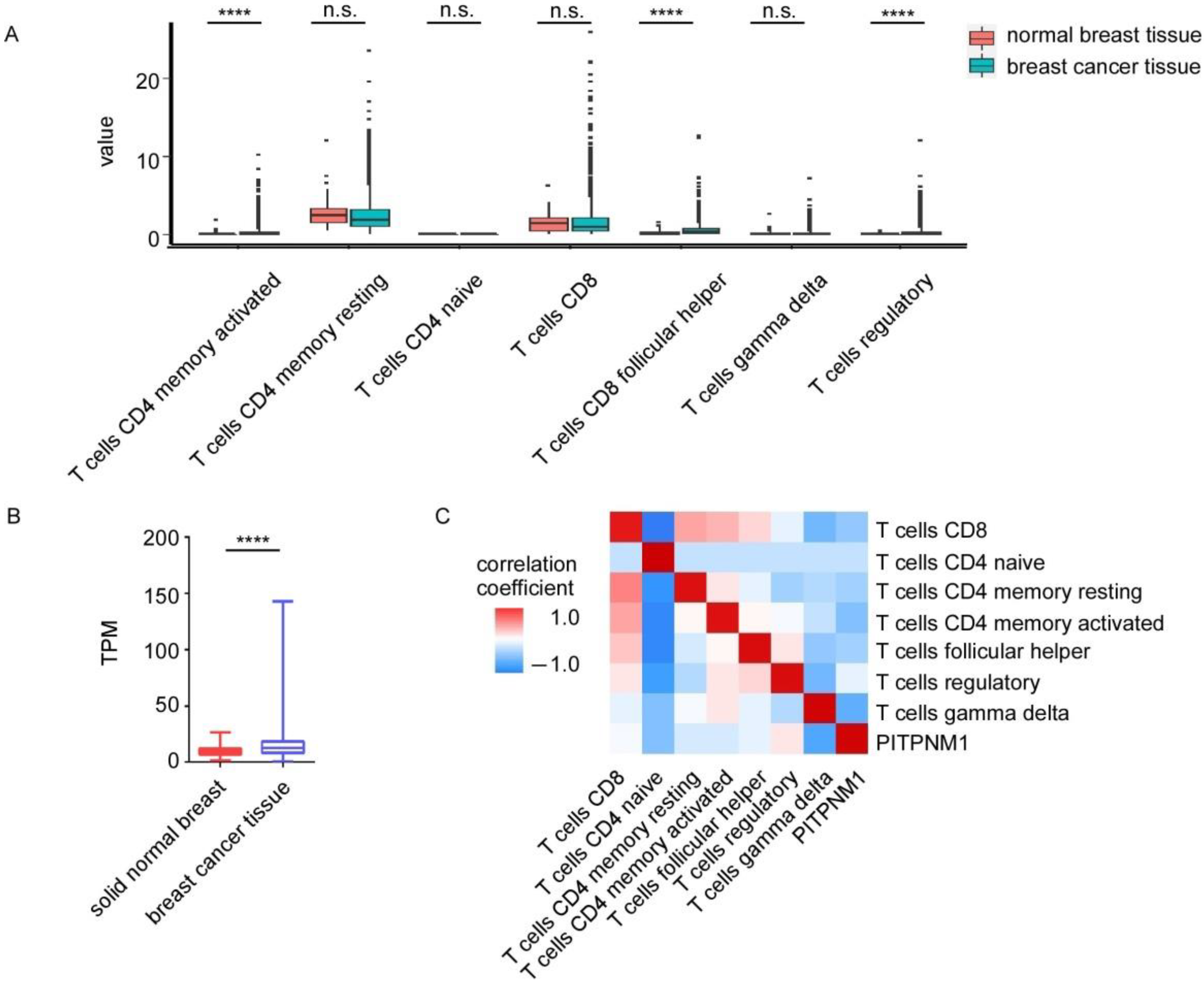
3.4. The Relationship between PITPNM1 Expression and T Cell Populations
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global cancer statistics 2020: Globocan estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA A Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer statistics, 2021. CA A Cancer J. Clin. 2021, 71, 7–33. [Google Scholar] [CrossRef] [PubMed]
- Trivedi, D.; Padinjat, R. Rdgb proteins: Functions in lipid homeostasis and signal transduction. Biochim. Biophys. Acta (BBA)-Mol. Cell Biol. Lipids 2007, 1771, 692–699. [Google Scholar] [CrossRef] [PubMed]
- Woodard, C.; Alcorta, E.; Carlson, J. The rdgb gene of drosophila: A link between vision and olfaction. J. Neurogenet. 2007, 21, 291–305. [Google Scholar] [CrossRef] [PubMed]
- Aikawa, Y.; Kuraoka, A.; Kondo, H.; Kawabuchi, M.; Watanabe, T. Involvement of pitpnm, a mammalian homologue of drosophila rdgb, in phosphoinositide synthesis on golgi membranes. J. Biol. Chem. 1999, 274, 20569–20577. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ocaka, L.; Spalluto, C.; Wilson, D.; Hunt, D.; Halford, S. Chromosomal localization, genomic organization and evolution of the genes encoding human phosphatidylinositol transfer protein membrane-associated (pitpnm) 1, 2 and 3. Cytogenet. Genome Res. 2005, 108, 293–302. [Google Scholar] [CrossRef]
- Lev, S.; Hernandez, J.; Martinez, R.; Chen, A.; Plowman, G.; Schlessinger, J. Identification of a novel family of targets of pyk2 related to drosophila retinal degeneration b (rdgb) protein. Mol. Cell. Biol. 1999, 19, 2278–2288. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reinis, A.; Golovleva, I.; Köhn, L.; Sandgren, O. Ocular phenotype of cord5, an autosomal dominant retinal dystrophy associated with pitpnm3 p. Q626h mutation. Acta Ophthalmol. 2013, 91, 259–266. [Google Scholar] [CrossRef]
- Carlisle, F.; Pearson, S.; Steel, K.; Lewis, M. Pitpnm1 is expressed in hair cells during development but is not required for hearing. Neuroscience 2013, 248, 620–625. [Google Scholar] [CrossRef] [Green Version]
- Li, H.; Zhao, X.; Wen, X.; Zeng, A.; Mao, G.; Lin, R.; Hu, S.; Liao, W.; Zhang, Z. Inhibition of mir-490-5p promotes human adipose-derived stem cells chondrogenesis and protects chondrocytes via the pitpnm1/pi3k/akt axis. Front. Cell Dev. Biol. 2020, 8. [Google Scholar] [CrossRef]
- Lewis, M.A.; Quint, E.; Glazier, A.M.; Fuchs, H.; de Angelis, M.H.; Langford, C.; van Dongen, S.; Abreu-Goodger, C.; Piipari, M.; Redshaw, N. An enu-induced mutation of mir-96 associated with progressive hearing loss in mice. Nat. Genet. 2009, 41, 614–618. [Google Scholar] [CrossRef] [Green Version]
- Keinan, O.; Kedan, A.; Gavert, N.; Selitrennik, M.; Kim, S.; Karn, T.; Becker, S.; Lev, S. The lipid-transfer protein nir2 enhances epithelial-mesenchymal transition and facilitates breast cancer metastasis. J. Cell Sci. 2014, 127, 4740–4749. [Google Scholar] [CrossRef] [Green Version]
- Hanahan, D.; Weinberg, R.A. Hallmarks of cancer: The next generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef] [Green Version]
- Subramanian, A.; Tamayo, P.; Mootha, V.K.; Mukherjee, S.; Ebert, B.L.; Gillette, M.A.; Paulovich, A.; Pomeroy, S.L.; Golub, T.R.; Lander, E.S. Gene set enrichment analysis: A knowledge-based approach for interpreting genome-wide expression profiles. Proc. Natl. Acad. Sci. USA 2005, 102, 15545–15550. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dennis, G.; Sherman, B.T.; Hosack, D.A.; Yang, J.; Gao, W.; Lane, H.C.; Lempicki, R.A. David: Database for annotation, visualization, and integrated discovery. GenomeBiol. 2003, 4, 1–11. [Google Scholar]
- Newman, A.M.; Steen, C.B.; Liu, C.L.; Gentles, A.J.; Chaudhuri, A.A.; Scherer, F.; Khodadoust, M.S.; Esfahani, M.S.; Luca, B.A.; Steiner, D. Determining cell type abundance and expression from bulk tissues with digital cytometry. Nat. Biotechnol. 2019, 37, 773–782. [Google Scholar] [CrossRef]
- Omori, Y.; Katoh, K.; Sato, S.; Muranishi, Y.; Chaya, T.; Onishi, A.; Minami, T.; Fujikado, T.; Furukawa, T. Analysis of transcriptional regulatory pathways of photoreceptor genes by expression profiling of the otx2-deficient retina. PLoS ONE 2011, 6, e19685. [Google Scholar] [CrossRef]
- Kim, Y.J.; Guzman-Hernandez, M.-L.; Wisniewski, E.; Balla, T. Phosphatidylinositol-phosphatidic acid exchange by nir2 at er-pm contact sites maintains phosphoinositide signaling competence. Dev. Cell 2015, 33, 549–561. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lev, S. The role of the nir/rdgb protein family in membrane trafficking and cytoskeleton remodeling. Exp. Cell Res. 2004, 297, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.-L.; Liou, J. Phosphatidylinositol 4, 5-bisphosphate homeostasis regulated by nir2 and nir3 proteins at endoplasmic reticulum-plasma membrane junctions. J. Biol. Chem. 2015, 290, 14289–14301. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Togashi, Y.; Shitara, K.; Nishikawa, H. Regulatory t cells in cancer immunosuppression—Implications for anticancer therapy. Nat. Rev. Clin. Oncol. 2019, 16, 356–371. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.-N.; Zhang, H.; Zhang, L.; Cai, T.-T.; Huang, D.-J.; He, J.; Ni, H.-H.; Zhou, F.-J.; Zhang, X.-S.; Li, J. Sphingosine 1 phosphate receptor-1 (s1p1) promotes tumor-associated regulatory t cell expansion: Leading to poor survival in bladder cancer. Cell Death Dis. 2019, 10, 1–11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, R.; Wu, M.; Liu, S.; Shang, W.; Li, R.; Xu, J.; Huang, L.; Wang, F. Glucose metabolism characteristics and tlr8-mediated metabolic control of cd4+ treg cells in ovarian cancer cells microenvironment. Cell Death Dis. 2021, 12, 1–11. [Google Scholar]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Z.; Shi, Y.; Lin, Q.; Yang, W.; Luo, Q.; Cen, Y.; Li, J.; Fang, X.; Jiang, W.G.; Gong, C. Attenuation of PITPNM1 Signaling Cascade Can Inhibit Breast Cancer Progression. Biomolecules 2021, 11, 1265. https://doi.org/10.3390/biom11091265
Liu Z, Shi Y, Lin Q, Yang W, Luo Q, Cen Y, Li J, Fang X, Jiang WG, Gong C. Attenuation of PITPNM1 Signaling Cascade Can Inhibit Breast Cancer Progression. Biomolecules. 2021; 11(9):1265. https://doi.org/10.3390/biom11091265
Chicago/Turabian StyleLiu, Zihao, Yu Shi, Qun Lin, Wenqian Yang, Qing Luo, Yinghuan Cen, Juanmei Li, Xiaolin Fang, Wen G. Jiang, and Chang Gong. 2021. "Attenuation of PITPNM1 Signaling Cascade Can Inhibit Breast Cancer Progression" Biomolecules 11, no. 9: 1265. https://doi.org/10.3390/biom11091265
APA StyleLiu, Z., Shi, Y., Lin, Q., Yang, W., Luo, Q., Cen, Y., Li, J., Fang, X., Jiang, W. G., & Gong, C. (2021). Attenuation of PITPNM1 Signaling Cascade Can Inhibit Breast Cancer Progression. Biomolecules, 11(9), 1265. https://doi.org/10.3390/biom11091265






