Metformin and Risk of Malignant Brain Tumors in Patients with Type 2 Diabetes Mellitus
Abstract
:1. Introduction
2. Materials and Methods
2.1. National Health Insurance
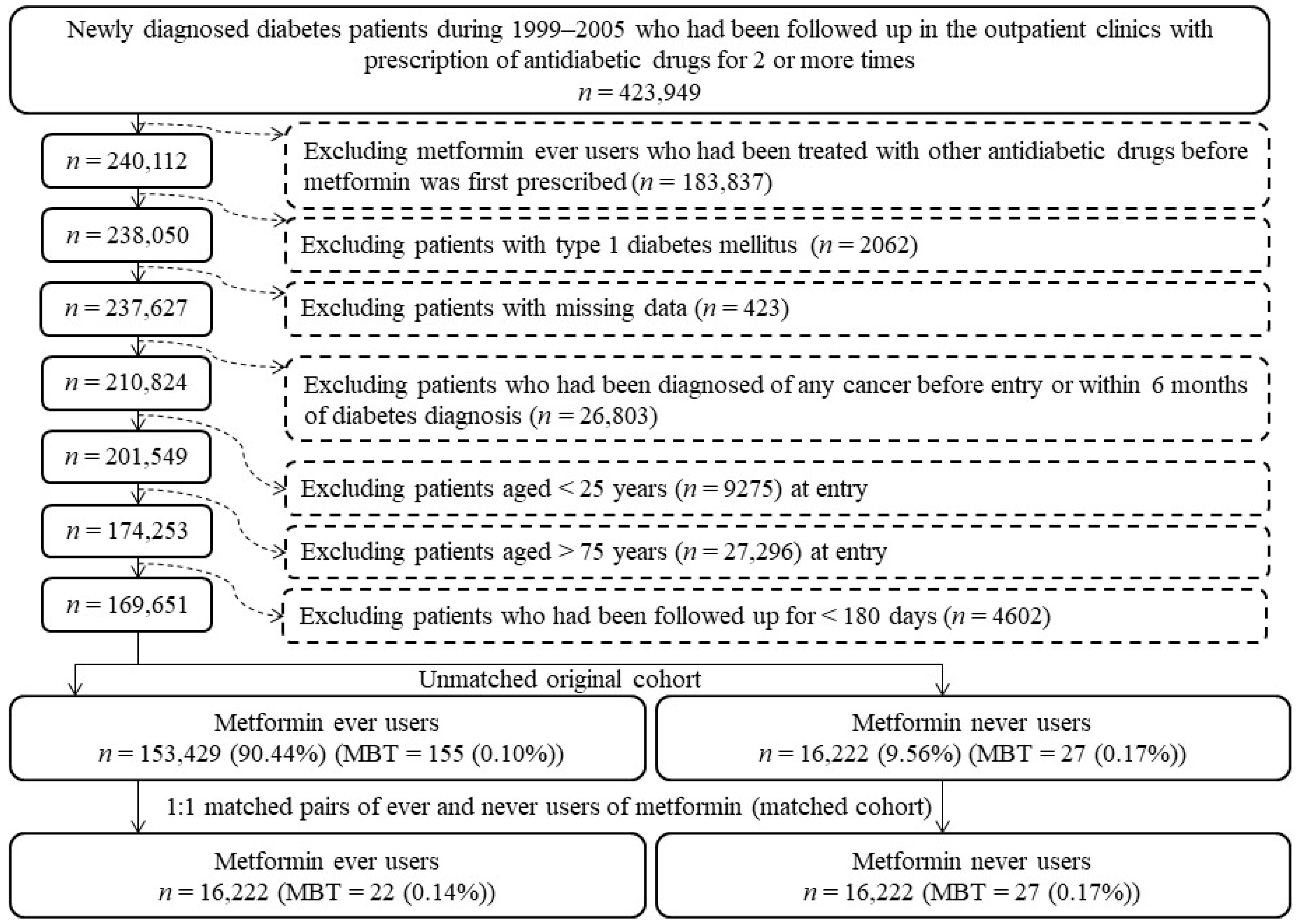
2.2. Study Population
2.3. Potential Confounders
2.4. Statistical Analyses
3. Results
4. Discussion
4.1. Main Findings
4.2. Limitations of an Early Study
4.3. Potential Mechanisms
4.4. Implications
4.5. Strengths
4.6. Limitations
5. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Nelson, J.S.; Burchfiel, C.M.; Fekedulegn, D.; Andrew, M.E. Potential risk factors for incident glioblastoma multiforme: The Honolulu Heart Program and Honolulu-Asia Aging Study. J. Neurooncol. 2012, 109, 315–321. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tamimi, A.F.; Juweid, M. Epidemiology and Outcome of Glioblastoma; De Vleeschouwer, S., Ed.; Exon Publications: Brisbane, Australia, 2017; Chapter 8. Available online: https://www.ncbi.nlm.nih.gov/books/NBK470003/ (accessed on 14 August 2021).
- Ostrom, Q.T.; Cioffi, G.; Gittleman, H.; Patil, N.; Waite, K.; Kruchko, C.; Barnholtz-Sloan, J.S. CBTRUS Statistical Report: Primary brain and other central nervous system tumors diagnosed in the United States in 2012-2016. Neuro. Oncol. 2019, 21, v1–v100. [Google Scholar] [CrossRef] [PubMed]
- Ostrom, Q.T.; Gittleman, H.; Farah, P.; Ondracek, A.; Chen, Y.; Wolinsky, Y.; Stroup, N.E.; Kruchko, C.; Barnholtz-Sloan, J.S. CBTRUS statistical report: Primary brain and central nervous system tumors diagnosed in the United States in 2006-2010. Neuro. Oncol. 2013, 15, ii1–ii56. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chiang, C.J.; Chen, Y.C.; Chen, C.J.; You, S.L.; Lai, M.S.; Taiwan Cancer Registry Task Force. Cancer trends in Taiwan. Jpn J. Clin. Oncol. 2010, 40, 897–904. [Google Scholar] [CrossRef] [PubMed]
- Louis, D.N.; Perry, A.; Reifenberger, G.; von Deimling, A.; Figarella-Branger, D.; Cavenee, W.K.; Ohgaki, H.; Wiestler, O.D.; Kleihues, P.; Ellison, D.W. The 2016 World Health Organization classification of tumors of the central nervous system: A summary. Acta Neuropathol. 2016, 131, 803–820. [Google Scholar] [CrossRef] [Green Version]
- Komori, T. The 2016 WHO classification of tumours of the central nervous system: The major points of revision. Neurol. Med.-Chir. 2017, 57, 301–311. [Google Scholar] [CrossRef] [Green Version]
- Gomes, J.; Al Zayadi, A.; Guzman, A. Occupational and environmental risk factors of adult primary brain cancers: A systematic review. Int. J. Occup. Environ. Med. 2011, 2, 82–111. [Google Scholar] [PubMed]
- Ostrom, Q.T.; Gittleman, H.; Stetson, L.; Virk, S.M.; Barnholtz-Sloan, J.S. Epidemiology of gliomas. Cancer Treat. Res. 2015, 163, 1–14. [Google Scholar]
- Johnson, K.J.; Hainfellner, J.A.; Lau, C.C.; Scheurer, M.E.; Woehrer, A.; Wiemels, J. Immune factors and viral interactions in brain cancer etiology and outcomes, the 2016 Brain Tumor Epidemiology Consortium Meeting report. Clin. Neuropathol. 2016, 35, 280–286. [Google Scholar] [CrossRef] [Green Version]
- Andersen, Z.J.; Pedersen, M.; Weinmayr, G.; Stafoggia, M.; Galassi, C.; Jørgensen, J.T.; Sommar, J.N.; Forsberg, B.; Olsson, D.; Oftedal, B.; et al. Long-term exposure to ambient air pollution and incidence of brain tumor: The European Study of Cohorts for Air Pollution Effects (ESCAPE). Neuro. Oncol. 2018, 20, 420–432. [Google Scholar] [CrossRef] [Green Version]
- Maniar, K.; Moideen, A.; Mittal, A.; Patil, A.; Chakrabarti, A.; Banerjee, D. A story of metformin-butyrate synergism to control various pathological conditions as a consequence of gut microbiome modification: Genesis of a wonder drug? Pharmacol. Res. 2017, 117, 103–128. [Google Scholar] [CrossRef] [PubMed]
- Rena, G.; Hardie, D.G.; Pearson, E.R. The mechanisms of action of metformin. Diabetologia 2017, 60, 1577–1585. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mbara, K.C.; Mofo Mato, P.E.; Driver, C.; Nzuza, S.; Mkhombo, N.T.; Gcwensa, S.K.; Mcobothi, E.N.; Owira, P.M. Metformin turns 62 in pharmacotherapy: Emergence of non-glycaemic effects and potential novel therapeutic applications. Eur. J. Pharmacol. 2021, 898. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Mao, W.; Zhai, Y.; Tong, C.; Liu, M.; Ma, L.; Yu, X.; Li, S. Anti-tumor activity of metformin: From metabolic and epigenetic perspectives. Oncotarget 2017, 8, 5619–5628. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Łabuzek, K.; Suchy, D.; Gabryel, B.; Bielecka, A.; Liber, S.; Okopień, B. Quantification of metformin by the HPLC method in brain regions, cerebrospinal fluid and plasma of rats treated with lipopolysaccharide. Pharmacol. Rep. 2010, 62, 956–965. [Google Scholar] [CrossRef]
- El-Arabey, A.A.; Abdalla, M.; Ali Eltayb, W. Metformin: Ongoing journey with superdrug revolution. Adv. Pharm. Bull. 2019, 9, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Takhwifa, F.; Aninditha, T.; Setiawan, H.; Sauriasari, R. The potential of metformin as an antineoplastic in brain tumors: A systematic review. Heliyon 2021, 7. [Google Scholar] [CrossRef]
- Seliger, C.; Meyer, A.L.; Renner, K.; Leidgens, V.; Moeckel, S.; Jachnik, B.; Dettmer, K.; Tischler, U.; Gerthofer, V.; Rauer, L.; et al. Metformin inhibits proliferation and migration of glioblastoma cells independently of TGF-β2. Cell Cycle 2016, 15, 1755–1766. [Google Scholar] [CrossRef] [Green Version]
- Kolesnik, D.L.; Pyaskovskaya, O.N.; Yurchenko, O.V.; Solyanik, G.I. Metformin enhances antitumor action of sodium dichloroacetate against glioma C6. Exp. Oncol. 2019, 41, 123–129. [Google Scholar] [CrossRef]
- Kinfe, T.M.; Stadlbauer, A.; Bozhkov, Y.; Kremenevski, N.; Brandner, S.; Buchfelder, M.; Chaudhry, S.R. The diagnostic and therapeutic role of leptin and its receptor ObR in glioblastoma multiforme. Cancers 2020, 12, 3691. [Google Scholar] [CrossRef] [PubMed]
- Porper, K.; Shpatz, Y.; Plotkin, L.; Pechthold, R.G.; Talianski, A.; Champ, C.E.; Furman, O.; Shimoni-Sebag, A.; Symon, Z.; Amit, U.; et al. A phase I clinical trial of dose-escalated metabolic therapy combined with concomitant radiation therapy in high-grade glioma. J. Neurooncol. 2021, 153, 487–496. [Google Scholar] [CrossRef] [PubMed]
- Seliger, C.; Ricci, C.; Meier, C.R.; Bodmer, M.; Jick, S.S.; Bogdahn, U.; Hau, P.; Leitzmann, M.F. Diabetes, use of antidiabetic drugs, and the risk of glioma. Neuro. Oncol. 2016, 18, 340–349. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tseng, C.H. Metformin and lung cancer risk in patients with type 2 diabetes mellitus. Oncotarget 2017, 8, 41132–41142. [Google Scholar] [CrossRef]
- Tseng, C.H. Metformin is associated with a lower risk of colorectal cancer in Taiwanese patients with type 2 diabetes: A retrospective cohort analysis. Diabetes Metab. 2017, 43, 438–445. [Google Scholar] [CrossRef]
- Parsons, L.S. Performing a 1:N Case-Control Match on Propensity Score. Available online: http://www.google.com.tw/url?sa=t&rct=j&q=&esrc=s&source=web&cd=1&ved=0CBsQFjAAahUKEwibi7HllcnIAhUDoJQKHVeZA9A&url=http%3A%2F%2Fwww2.sas.com%2Fproceedings%2Fsugi29%2F165-29.pdf&usg=AFQjCNFOHGWYu8E8Bn4-Bo1TUiJKtT987Q (accessed on 2 July 2021).
- Tseng, C.H. Diabetes, metformin use, and colon cancer: A population-based cohort study in Taiwan. Eur. J. Endocrinol. 2012, 167, 409–416. [Google Scholar] [CrossRef] [Green Version]
- Austin, P.C.; Stuart, E.A. Moving towards best practice when using inverse probability of treatment weighting (IPTW) using the propensity score to estimate causal treatment effects in observational studies. Stat. Med. 2015, 34, 3661–3679. [Google Scholar] [CrossRef]
- Austin, P.C. The performance of different propensity score methods for estimating marginal hazard ratios. Stat. Med. 2013, 32, 2837–2849. [Google Scholar] [CrossRef] [Green Version]
- Strickland, M.; Stoll, E.A. Metabolic reprogramming in glioma. Front. Cell Dev. Biol. 2017, 5. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sesen, J.; Dahan, P.; Scotland, S.J.; Saland, E.; Dang, V.T.; Lemarié, A.; Tyler, B.M.; Brem, H.; Toulas, C.; Cohen-Jonathan Moyal, E.; et al. Metformin inhibits growth of human glioblastoma cells and enhances therapeutic response. PLoS ONE 2015, 10. [Google Scholar] [CrossRef] [Green Version]
- Najbauer, J.; Kraljik, N.; Németh, P. Glioma stem cells: Markers, hallmarks and therapeutic targeting by metformin. Pathol. Oncol. Res. 2014, 20, 789–797. [Google Scholar] [CrossRef] [PubMed]
- Jaganjac, M.; Cindrić, M.; Jakovčević, A.; Žarković, K.; Žarković, N. Lipid peroxidation in brain tumors. Neurochem. Int. 2021, 149. [Google Scholar] [CrossRef]
- Ostrom, Q.T.; Adel Fahmideh, M.; Cote, D.J.; Muskens, I.S.; Schraw, J.M.; Scheurer, M.E.; Bondy, M.L. Risk factors for childhood and adult primary brain tumors. Neuro. Oncol. 2019, 21, 1357–1375. [Google Scholar] [CrossRef] [PubMed]
- Azmoonfar, R.; Amini, P.; Saffar, H.; Rezapoor, S.; Motevaseli, E.; Cheki, M.; Yahyapour, R.; Farhood, B.; Nouruzi, F.; Khodamoradi, E.; et al. Metformin protects against radiation-induced pneumonitis and fibrosis and attenuates upregulation of dual oxidase genes expression. Adv. Pharm. Bull. 2018, 8, 697–704. [Google Scholar] [CrossRef]
- Xiao, T.; Chen, Y.; Song, C.; Xu, S.; Lin, S.; Li, M.; Chen, X.; Gu, H. Possible treatment for UVB-induced skin injury: Anti-inflammatory and cytoprotective role of metformin in UVB-irradiated keratinocytes. J. Dermatol. Sci. 2021, 102, 25–35. [Google Scholar] [CrossRef] [PubMed]
- Tseng, C.H. Metformin may reduce breast cancer risk in Taiwanese women with type 2 diabetes. Breast Cancer Res. Treat. 2014, 145, 785–790. [Google Scholar] [CrossRef]
- Tseng, C.H. Metformin reduces thyroid cancer risk in Taiwanese patients with type 2 diabetes. PLoS ONE 2014, 9. [Google Scholar] [CrossRef]
- Tseng, C.H. Metformin may reduce bladder cancer risk in Taiwanese patients with type 2 diabetes. Acta Diabetol. 2014, 51, 295–303. [Google Scholar] [CrossRef] [PubMed]
- Tseng, C.H. Metformin significantly reduces incident prostate cancer risk in Taiwanese men with type 2 diabetes mellitus. Eur. J. Cancer 2014, 50, 2831–2837. [Google Scholar] [CrossRef] [PubMed]
- Tseng, C.H. Metformin and endometrial cancer risk in Chinese women with type 2 diabetes mellitus in Taiwan. Gynecol. Oncol. 2015, 138, 147–153. [Google Scholar] [CrossRef] [PubMed]
- Tseng, C.H. Metformin reduces ovarian cancer risk in Taiwanese women with type 2 diabetes mellitus. Diabetes Metab. Res. Rev. 2015, 31, 619–626. [Google Scholar] [CrossRef]
- Tseng, C.H. Metformin use and cervical cancer risk in female patients with type 2 diabetes. Oncotarget 2016, 7, 59548–59555. [Google Scholar] [CrossRef] [Green Version]
- Tseng, C.H. Use of metformin and risk of kidney cancer in patients with type 2 diabetes. Eur. J. Cancer 2016, 52, 19–25. [Google Scholar] [CrossRef]
- Tseng, C.H. Metformin may reduce oral cancer risk in patients with type 2 diabetes. Oncotarget 2016, 7, 2000–2008. [Google Scholar] [CrossRef] [Green Version]
- Tseng, C.H. Metformin reduces gastric cancer risk in patients with type 2 diabetes mellitus. Aging (Albany NY). 2016, 8, 1636–1649. [Google Scholar] [CrossRef] [Green Version]
- Tseng, C.H. Metformin and esophageal cancer risk in Taiwanese patients with type 2 diabetes mellitus. Oncotarget 2017, 8, 18802–18810. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tseng, C.H. Metformin and risk of developing nasopharyngeal cancer in patients with type 2 diabetes mellitus. Metabolism 2018, 85, 223–226. [Google Scholar] [CrossRef] [PubMed]
- Tseng, C.H. Metformin is associated with decreased skin cancer risk in Taiwanese patients with type 2 diabetes. J. Am. Acad. Dermatol. 2018, 78, 694–700. [Google Scholar] [CrossRef]
- Tseng, C.H. Metformin and pancreatic cancer risk in patients with type 2 diabetes. Pancreas 2018, 47, e57–e59. [Google Scholar] [CrossRef]
- Tseng, C.H. Metformin and risk of hepatocellular carcinoma in patients with type 2 diabetes. Liver Int. 2018, 38, 2018–2027. [Google Scholar] [CrossRef]
- Tseng, C.H. Metformin and biliary tract cancer in patients with type 2 diabetes. Front. Oncol. 2020, 10. [Google Scholar] [CrossRef] [PubMed]
- Tseng, C.H. Metformin is associated with a lower risk of non-Hodgkin lymphoma in patients with type 2 diabetes. Diabetes Metab. 2019, 45, 458–464. [Google Scholar] [CrossRef]
- Tseng, C.H. Metformin and primary bone cancer risk in Taiwanese patients with type 2 diabetes mellitus. Bone 2021, 151. [Google Scholar] [CrossRef]
- Hanahan, D.; Weinberg, R.A. Hallmarks of cancer: The next generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef] [Green Version]
- Baker, C.; Retzik-Stahr, C.; Singh, V.; Plomondon, R.; Anderson, V.; Rasouli, N. Should metformin remain the first-line therapy for treatment of type 2 diabetes? Ther. Adv. Endocrinol. Metab. 2021, 12. [Google Scholar] [CrossRef]
- Nathan, D.M.; Buse, J.B.; Davidson, M.B.; Ferrannini, E.; Holman, R.R.; Sherwin, R.; Zinman, B. Medical management of hyperglycemia in type 2 diabetes: A consensus algorithm for the initiation and adjustment of therapy. Diabetes Care. 2008, 31, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Tseng, C.H. Metformin and risk of hypertension in Taiwanese patients with type 2 diabetes mellitus. J. Am. Heart Assoc. 2018, 6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tseng, C.H. Metformin use is associated with a lower risk of hospitalization for heart failure in patients with type 2 diabetes mellitus: A retrospective cohort analysis. J. Am. Heart Assoc. 2019, 8. [Google Scholar] [CrossRef] [PubMed]
- Tseng, C.H. Metformin use is associated with a lower incidence of hospitalization for atrial fibrillation in patients with type 2 diabetes mellitus. Front. Med. 2021, 7. [Google Scholar] [CrossRef]
- Tseng, C.H. Metformin and risk of chronic obstructive pulmonary disease in diabetes patients. Diabetes Metab. 2019, 45, 184–190. [Google Scholar] [CrossRef]
- Tseng, C.H. Metformin decreases risk of tuberculosis infection in type 2 diabetes patients. J. Clin. Med. 2018, 7, 264. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tseng, C.H. Metformin and Helicobacter pylori infection in patients with type 2 diabetes. Diabetes Care. 2018, 41, e42–e43. [Google Scholar] [CrossRef] [Green Version]
- Tseng, C.H. Metformin reduces risk of varicose veins in patients with type 2 diabetes. Diabetes Metab. Res. Rev. 2020, 36. [Google Scholar] [CrossRef] [PubMed]
- Tseng, C.H. Metformin use is associated with a reduced risk of acute appendicitis in Taiwanese patients with type 2 diabetes mellitus. Sci. Rep. 2021, 11. [Google Scholar] [CrossRef] [PubMed]
- Tseng, C.H. Chronic metformin therapy is associated with a lower risk of hemorrhoid in patients with type 2 diabetes mellitus. Front. Pharmacol. 2021, 11. [Google Scholar] [CrossRef]
- Tseng, C.H. Metformin and the risk of dementia in type 2 diabetes patients. Aging Dis. 2019, 10, 37–48. [Google Scholar]
- Tseng, C.H. Dementia risk in type 2 diabetes patients: Acarbose use and its joint effects with metformin and pioglitazone. Aging Dis. 2020, 11, 658–667. [Google Scholar] [CrossRef]
- Tseng, C.H. Metformin reduces risk of benign nodular goiter in patients with type 2 diabetes mellitus. Eur. J. Endocrinol. 2019, 180, 367–374. [Google Scholar] [CrossRef]
- Tseng, C.H. Metformin use is associated with a lower risk of uterine leiomyoma in female type 2 diabetes patients. Ther Adv. Endocrinol. Metab. 2019, 10. [Google Scholar] [CrossRef]
- Tseng, C.H. Metformin use is associated with a lower risk of osteoporosis/vertebral fracture in Taiwanese patients with type 2 diabetes mellitus. Eur. J. Endocrinol. 2021, 184, 299–310. [Google Scholar] [CrossRef]
- Tseng, C.H. Metformin use is associated with a lower risk of inflammatory bowel disease in patients with type 2 diabetes mellitus. J. Crohns. Colitis. 2021, 15, 64–73. [Google Scholar] [CrossRef] [PubMed]
- Duraj, T.; García-Romero, N.; Carrión-Navarro, J.; Madurga, R.; Mendivil, A.O.; Prat-Acin, R.; Garcia-Cañamaque, L.; Ayuso-Sacido, A. Beyond the Warburg effect: Oxidative and glycolytic phenotypes coexist within the metabolic heterogeneity of glioblastoma. Cells 2021, 10, 202. [Google Scholar] [CrossRef]
- Louis, D.N.; Perry, A.; Wesseling, P.; Brat, D.J.; Cree, I.A.; Figarella-Branger, D.; Hawkins, C.; Ng, H.K.; Pfister, S.M.; Reifenberger, G.; et al. The 2021 WHO Classification of Tumors of the Central Nervous System: A summary. Neuro. Oncol. 2021, 23, 1231–1251. [Google Scholar] [CrossRef]
- Fangusaro, J.; Bandopadhayay, P. Advances in the classification and treatment of pediatric brain tumors. Curr. Opin. Pediatr. 2021, 33, 26–32. [Google Scholar] [CrossRef]
- Merchant, T.E.; Pollack, I.F.; Loeffler, J.S. Brain tumors across the age spectrum: Biology, therapy, and late effects. Semin. Radiat. Oncol. 2010, 20, 58–66. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Majd, N.; Penas-Prado, M. Updates on management of adult medulloblastoma. Curr. Treat. Options Oncol. 2019, 20. [Google Scholar] [CrossRef]
- Fetahu, I.S.; Taschner-Mandl, S. Neuroblastoma and the epigenome. Cancer Metastasis Rev. 2021, 40, 173–189. [Google Scholar] [CrossRef]
- Health Promotion Administration, Ministry of Health and Welfare, Taiwan. Cancer Registry Annual Report 2018. Taiwan. Available online: https://www.hpa.gov.tw/Pages/Detail.aspx?nodeid=269&pid=13498 (accessed on 4 August 2021).
- Alghamri, M.S.; McClellan, B.L.; Hartlage, M.S.; Haase, S.; Faisal, S.M.; Thalla, R.; Dabaja, A.; Banerjee, K.; Carney, S.V.; Mujeeb, A.A.; et al. Targeting neuroinflammation in brain cancer: Uncovering mechanisms, pharmacological targets, and neuropharmaceutical developments. Front. Pharmacol. 2021, 12. [Google Scholar] [CrossRef]
- Ohgaki, H.; Kleihues, P. The definition of primary and secondary glioblastoma. Clin. Cancer Res. 2013, 19, 764–772. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ward, P.S.; Thompson, C.B. Metabolic reprogramming: A cancer hallmark even Warburg did not anticipate. Cancer Cell. 2012, 21, 297–308. [Google Scholar] [CrossRef] [Green Version]
- Cantor, J.R.; Sabatini, D.M. Cancer cell metabolism: One hallmark, many faces. Cancer Discov. 2012, 2, 881–898. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van Stralen, K.J.; Dekker, F.W.; Zoccali, C.; Jager, K.J. Confounding. Nephron Clin. Pract. 2010, 116. [Google Scholar] [CrossRef] [PubMed]
- Kirbas Cilingir, E.; Seven, E.S.; Zhou, Y.; Walters, B.M.; Mintz, K.J.; Pandey, R.R.; Wikramanayake, A.H.; Chusuei, C.C.; Vanni, S.; Graham, R.M.; et al. Metformin derived carbon dots: Highly biocompatible fluorescent nanomaterials as mitochondrial targeting and blood-brain barrier penetrating biomarkers. J. Colloid Interface Sci. 2021, 592, 485–497. [Google Scholar] [CrossRef] [PubMed]
| Variables | Unmatched Cohort | Matched Cohort * | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Never Users | Ever Users | Standardized Difference | Never Users | Ever Users | Standardized Difference | |||||
| (n = 16,222) | (n = 15,3429) | (n = 16,222) | (n = 16,222) | |||||||
| n | % | n | % | n | % | n | % | |||
| Demographic and Basic Data | ||||||||||
| Age ** (years) | 63.62 ± 10.43 | 61.84 ± 10.03 | −17.75 | 63.62 ± 10.43 | 63.83 ± 9.79 | 2.72 | ||||
| Sex (men) | 9298 | 57.32 | 82,575 | 53.82 | −7.72 | 9298 | 57.32 | 9238 | 56.95 | −0.98 |
| Occupation | ||||||||||
| I | 6336 | 39.06 | 59,853 | 39.01 | 6336 | 39.06 | 6359 | 39.20 | ||
| II | 3229 | 19.91 | 35,286 | 23.00 | 8.08 | 3229 | 19.91 | 3177 | 19.58 | −0.81 |
| III | 3410 | 21.02 | 32,175 | 20.97 | 0.07 | 3410 | 21.02 | 3423 | 21.10 | 0.36 |
| IV | 3247 | 20.02 | 26,115 | 17.02 | −8.61 | 3247 | 20.02 | 3263 | 20.11 | 0.14 |
| Living Region | ||||||||||
| Taipei | 5453 | 33.61 | 48,388 | 31.54 | 5453 | 33.61 | 5414 | 33.37 | ||
| Northern | 1658 | 10.22 | 17,386 | 11.33 | 3.74 | 1658 | 10.22 | 1686 | 10.39 | 0.59 |
| Central | 2841 | 17.51 | 28,069 | 18.29 | 2.13 | 2841 | 17.51 | 2866 | 17.67 | 0.37 |
| Southern | 2806 | 17.30 | 26,174 | 17.06 | −0.65 | 2806 | 17.30 | 2852 | 17.58 | 0.82 |
| Kao-Ping and Eastern | 3464 | 21.35 | 33,412 | 21.78 | 1.22 | 3464 | 21.35 | 3404 | 20.98 | −0.75 |
| Major Comorbidities | ||||||||||
| Hypertension (401–405) | 13,309 | 82.04 | 125,955 | 82.09 | 0.23 | 13,309 | 82.04 | 13,315 | 82.08 | 0.33 |
| Dyslipidemia (272.0–272.4) | 11,723 | 72.27 | 127,387 | 83.03 | 28.45 | 11,723 | 72.27 | 11,751 | 72.44 | 0.72 |
| Obesity (278) | 440 | 2.71 | 6957 | 4.53 | 10.00 | 440 | 2.71 | 411 | 2.53 | −1.13 |
| Diabetes-related Complications | ||||||||||
| Nephropathy (580–589) | 5666 | 34.93 | 42,457 | 27.67 | −17.80 | 5666 | 34.93 | 5557 | 34.26 | −1.80 |
| Eye diseases (250.5, 362.0, 369, 366.41, and 365.44) | 3011 | 18.56 | 49,861 | 32.50 | 32.53 | 3011 | 18.56 | 2854 | 17.59 | −3.07 |
| Stroke (430–438) | 5401 | 33.29 | 45,899 | 29.92 | −8.11 | 5401 | 33.29 | 5352 | 32.99 | −0.59 |
| Ischemic Heart Disease (410–414) | 7773 | 47.92 | 70,789 | 46.14 | −3.78 | 7773 | 47.92 | 7800 | 48.08 | 0.48 |
| Peripheral arterial disease (250.7, 785.4, 443.81 and 440–448) | 3777 | 23.28 | 39,982 | 26.06 | 6.61 | 3777 | 23.28 | 3688 | 22.73 | −1.42 |
| Antidiabetic drugs | ||||||||||
| Insulin | 1351 | 8.33 | 3571 | 2.33 | −30.61 | 1351 | 8.33 | 1137 | 7.01 | −6.63 |
| Sulfonylurea | 11,790 | 72.68 | 111,546 | 72.70 | 5.66 | 11,790 | 72.68 | 12,199 | 75.20 | 5.89 |
| Meglitinide | 1340 | 8.26 | 6032 | 3.93 | −19.34 | 1340 | 8.26 | 1317 | 8.12 | −0.59 |
| Acarbose | 1835 | 11.31 | 8397 | 5.47 | −20.71 | 1835 | 11.31 | 1841 | 11.35 | −1.13 |
| Rosiglitazone | 479 | 2.95 | 7599 | 4.95 | 10.83 | 479 | 2.95 | 509 | 3.14 | 0.53 |
| Pioglitazone | 401 | 2.47 | 4049 | 2.64 | −20.71 | 401 | 2.47 | 429 | 2.64 | −1.13 |
| Commonly encountered comorbidities | ||||||||||
| Chronic obstructive pulmonary disease (490–496) | 8087 | 49.85 | 74,987 | 48.87 | −2.40 | 8087 | 49.85 | 8246 | 50.83 | 2.11 |
| Tobacco abuse (305.1, 649.0 and 989.84) | 460 | 2.84 | 6145 | 4.01 | 6.67 | 460 | 2.84 | 458 | 2.82 | −0.05 |
| Alcohol-related diagnoses (291, 303, 535.3, 571.0–571.3 and 980.0) | 1285 | 7.92 | 10,973 | 7.15 | −4.23 | 1285 | 7.92 | 1191 | 7.34 | −2.39 |
| Ocular pterygium (372.40–372.44) | 897 | 5.53 | 8990 | 5.86 | 1.44 | 897 | 5.53 | 894 | 5.51 | 0.02 |
| Medications that are commonly used in diabetes patients | ||||||||||
| Angiotensin converting enzyme inhibitor/angiotensin receptor blocker | 11,298 | 69.65 | 112,720 | 73.47 | 8.85 | 11,298 | 69.65 | 11,280 | 69.54 | −0.15 |
| Calcium channel blocker | 10,215 | 62.97 | 92,518 | 60.30 | −5.65 | 10,215 | 62.97 | 10,265 | 63.28 | 0.79 |
| Statin | 8768 | 54.05 | 101,371 | 66.07 | 26.41 | 8768 | 54.05 | 8730 | 53.82 | −0.33 |
| Fibrate | 5549 | 34.21 | 66,521 | 43.36 | 20.08 | 5549 | 34.21 | 5474 | 33.74 | −0.81 |
| Aspirin | 9333 | 57.53 | 95,058 | 61.96 | 9.38 | 9333 | 57.53 | 9290 | 57.27 | −0.32 |
| Cohort/Metformin Use | Incident Cases of Malignant Brain Tumors | Cases Followed | Person-Years | Incidence Rate (Per 100,000 Person-Years) | Hazard Ratio | 95% Confidence Interval | p Value |
|---|---|---|---|---|---|---|---|
| Unmatched Cohort | |||||||
| Never Users | 27 | 16,222 | 72,755.38 | 37.11 | 1.000 | ||
| Ever Users | 155 | 153,429 | 724,547.50 | 21.39 | 0.574 | (0.381–0.863) | 0.0077 |
| Tertiles of cumulative duration of metformin therapy (months) | |||||||
| Never Users | 27 | 16,222 | 72,755.38 | 37.11 | 1.000 | ||
| <27.13 | 59 | 50,605 | 178,095.31 | 33.13 | 0.897 | (0.567–1.421) | 0.6440 |
| 27.13–58.33 | 58 | 50,628 | 248,115.71 | 23.38 | 0.623 | (0.395–0.984) | 0.0426 |
| >58.33 | 38 | 52,196 | 298,336.48 | 12.74 | 0.316 | (0.192–0.518) | <0.0001 |
| Matched Cohort | |||||||
| Never Users | 27 | 16,222 | 72,755.38 | 37.11 | 1.000 | ||
| Ever Users | 9 | 16,222 | 76,004.89 | 11.84 | 0.317 | (0.149–0.673) | 0.0028 |
| Tertiles of cumulative duration of metformin therapy (months) | |||||||
| Never Users | 27 | 16,222 | 72,755.38 | 37.11 | 1.000 | ||
| <27.00 | 3 | 5343 | 18,587.13 | 16.14 | 0.427 | (0.129–1.412) | 0.1632 |
| 27.00–58.40 | 5 | 5361 | 26,025.97 | 19.21 | 0.509 | (0.196–1.322) | 0.1657 |
| >58.40 | 1 | 5518 | 31,391.79 | 3.19 | 0.087 | (0.012–0.639) | 0.0164 |
| Model/Metformin Use | Incident Cases of Malignant Brain Tumors | Cases Followed | Hazard Ratio | 95% Confidence Interval | p Value |
|---|---|---|---|---|---|
| I. After excluding patients who had received any two consecutive metformin prescriptions spanning a period of four or more months | |||||
| Never Users | 27 | 16,222 | 1.000 | ||
| Ever Users | 48 | 51,616 | 0.568 | (0.355–0.911) | 0.0188 |
| Tertiles of cumulative duration of metformin therapy (months) | |||||
| Never Users | 27 | 16,222 | 1.000 | ||
| <27.13 | 17 | 16,879 | 0.979 | (0.527–1.818) | 0.9463 |
| 27.13–58.33 | 16 | 14,014 | 0.675 | (0.363–1.254) | 0.2134 |
| >58.33 | 15 | 20,723 | 0.324 | (0.172–0.611) | 0.0005 |
| II. After excluding patients who happened to be treated with incretins during follow-up | |||||
| Never Users | 27 | 15,237 | 1.000 | ||
| Ever Users | 151 | 117,171 | 0.703 | (0.467–1.059) | 0.0916 |
| Tertiles of cumulative duration of metformin therapy (months) | |||||
| Never Users | 27 | 15,237 | 1.000 | ||
| <27.13 | 60 | 42,600 | 1.002 | (0.634–1.582) | 0.9940 |
| 27.13–58.33 | 53 | 38,328 | 0.710 | (0.446–1.128) | 0.1471 |
| >58.33 | 38 | 36,243 | 0.428 | (0.261–0.703) | 0.0008 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tseng, C.-H. Metformin and Risk of Malignant Brain Tumors in Patients with Type 2 Diabetes Mellitus. Biomolecules 2021, 11, 1226. https://doi.org/10.3390/biom11081226
Tseng C-H. Metformin and Risk of Malignant Brain Tumors in Patients with Type 2 Diabetes Mellitus. Biomolecules. 2021; 11(8):1226. https://doi.org/10.3390/biom11081226
Chicago/Turabian StyleTseng, Chin-Hsiao. 2021. "Metformin and Risk of Malignant Brain Tumors in Patients with Type 2 Diabetes Mellitus" Biomolecules 11, no. 8: 1226. https://doi.org/10.3390/biom11081226
APA StyleTseng, C.-H. (2021). Metformin and Risk of Malignant Brain Tumors in Patients with Type 2 Diabetes Mellitus. Biomolecules, 11(8), 1226. https://doi.org/10.3390/biom11081226






