Sex-Biased Expression of Pharmacogenes across Human Tissues
Abstract
:1. Introduction
2. Materials and Methods
2.1. Data Sources
2.2. Statistical Methods
2.3. Criteria for Pharmacogene Inclusion
3. Results
3.1. Sex Effects on Drug Response (SBDR) Genes
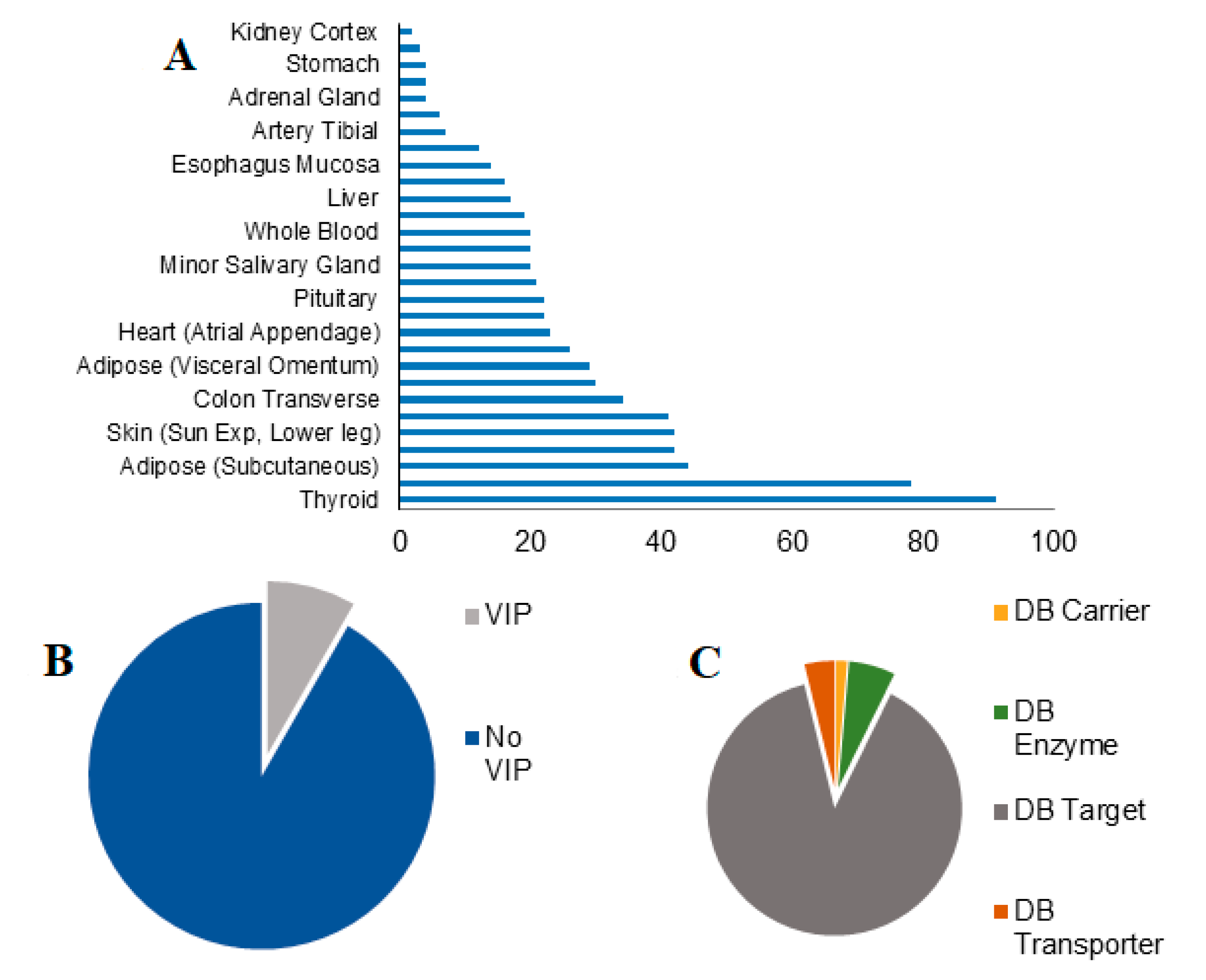
3.2. Effects of SBDR in 6 Tissues Most Relevant for Drug Pharmacokinetics
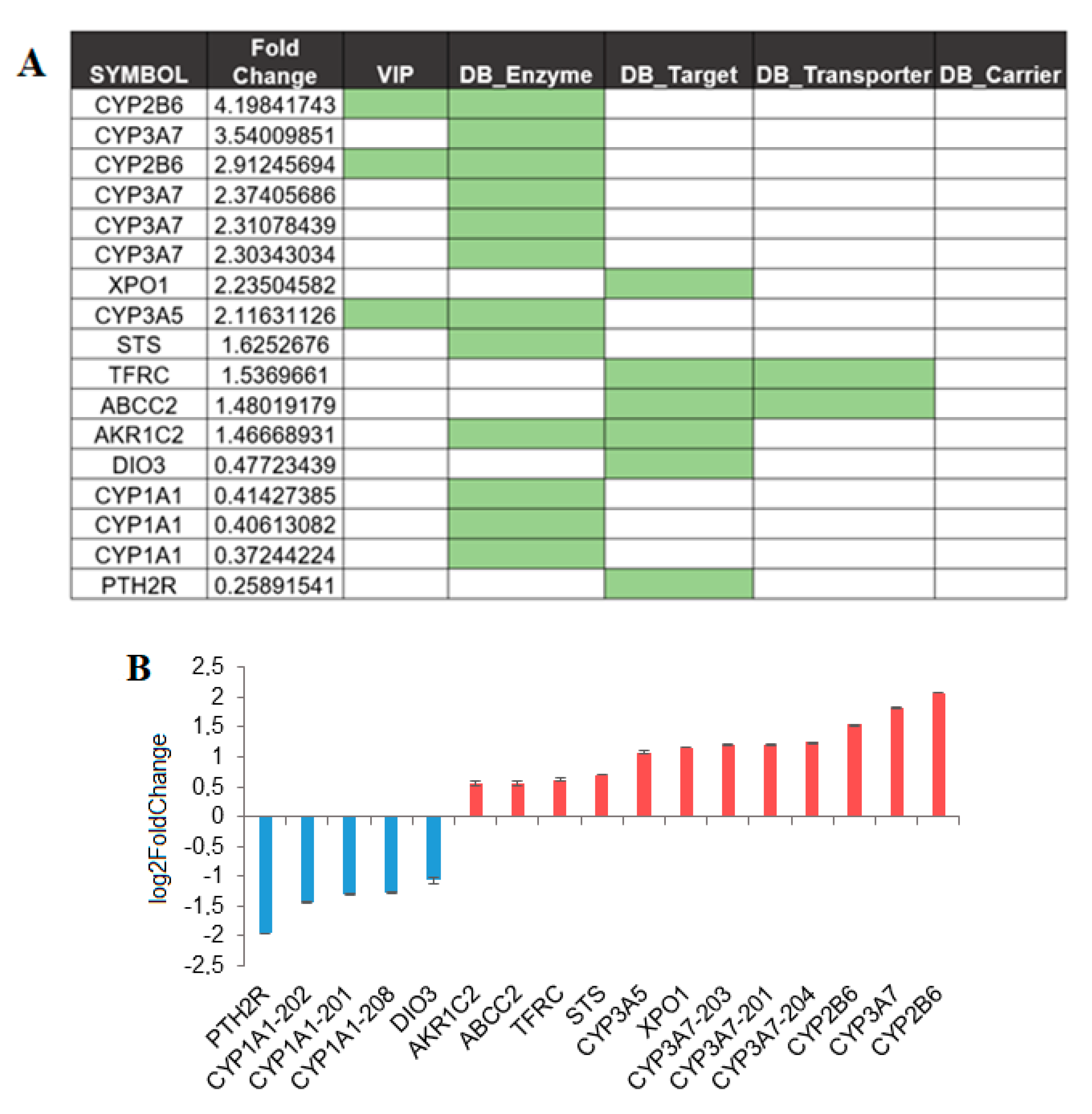
3.3. SBDR Genes in Liver
3.4. SBDR Genes in Other Key Organs Implicated in Drug Metabolism
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
- https://gtexportal.org/home/datasets (accessed on 13 August 2021)
- https://go.drugbank.com/releases/latest#protein-identifiers (accessed on 13 August 2021)
- https://www.pharmgkb.org/downloads (accessed on 13 August 2021).
Acknowledgments
Conflicts of Interest
References
- Akhondzadeh, S. Personalized Medicine: A Tailor Made Medicine. Avicenna J. Med. Biotechnol. 2014, 6, 191. [Google Scholar] [PubMed]
- Sim, S.C.; Kacevska, M.; Ingelmansundberg, M. Pharmacogenomics of drug-metabolizing enzymes: A recent update on clinical implications and endogenous effects. Pharmacogenom. J. 2013, 13, 1–11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- EUGenMed Cardiovascular Clinical Study Group; Regitz-Zagrosek, V.; Oertelt-Prigione, S.; Prescott, E.; Franconi, F.; Gerdts, E.; Foryst-Ludwig, A.; Maas, A.H.; Kautzky-Willer, A.; Knappe-Wegner, D.; et al. Gender in Cardiovascular Diseases: Impact on Clinical Manifestations, Management, and Outcomes. Eur. Heart J. 2016, 37, 24–34. [Google Scholar] [PubMed] [Green Version]
- Franconi, F.; Campesi, I. Sex Impact on Biomarkers, Pharmacokinetics and Pharmacodynamicsv. Curr. Med. Chem. 2017, 24, 2561–2575. [Google Scholar] [CrossRef]
- Yang, L.; Li, Y.; Hong, H.; Chang, C.W.; Guo, L.W.; Lyn-Cook, B.; Shi, L.; Ning, B. Sex Differences in the Expression of Drug-Metabolizing and Transporter Genes in Human Liver. J. Drug Metab. Toxicol. 2012, 3, 1000119. [Google Scholar] [CrossRef]
- Campesi, I.; Franconi, F.; Seghieri, G.; Meloni, M. Sex-gender-related therapeutic approaches for cardiovascular complications associated with diabetes. Pharmacol. Res. 2017, 119, 195–207. [Google Scholar] [CrossRef]
- Zanger, U.M.; Schwab, M. Cytochrome P450 Enzymes in Drug Metabolism: Regulation of Gene Expression, Enzyme Activities, and Impact of Genetic Variation. Pharmacol. Ther. 2013, 138, 103–141. [Google Scholar] [CrossRef]
- Fernández-Liz, E.; Modamio, P.; Catalán, A.; Lastra, C.F.; Rodríguez, T.; Mariño, E.L. Identifying how age and gender influence prescription drug use in a primary health care environment in Catalonia, Spain. Br. J. Clin. Pharmacol. 2008, 65, 407–417. [Google Scholar] [CrossRef] [Green Version]
- Orlando, V.; Mucherino, S.; Guarino, I.; Guerriero, F.; Trama, U.; Menditto, E. Gender Differences in Medication Use: A Drug Utilization Study Based on Real World Data. Int. J. Environ. Res. Public Health 2020, 17, 3926. [Google Scholar] [CrossRef] [PubMed]
- Greenblatt, D.J.; Harmatz, J.S.; Von Moltke, L.L.; Wright, C.E.; Durol, A.L.; Harrel-Joseph, L.M.; Shader, R.I. Comparative kinetics and response to the benzodiazepine agonists triazolam and zolpidem: Evaluation of sex-dependent differences. J. Pharmacol. Exp. Ther. 2000, 293, 435–443. [Google Scholar]
- Olubodun, J.O.; Ochs, H.R.; Von Moltke, L.L.; Roubenoff, R.; Hesse, L.M.; Harmatz, J.S.; Shader, R.I.; Greenblatt, D.J. Pharmacokinetic properties of zolpidem in elderly and young adults: Possible modulation by testosterone in men. Br. J. Clin. Pharmacol. 2003, 56, 297–304. [Google Scholar] [CrossRef] [Green Version]
- Greenblatt, D.J.; Harmatz, J.S.; Singh, N.N.; Steinberg, F.; Roth, T.; Moline, M.L.; Harris, S.C.; Kapil, R.P. Gender differences in pharmacokinetics and pharmacodynamics of zolpidem following sublingual administration. J. Clin. Pharmacol. 2014, 54, 282–290. [Google Scholar] [CrossRef] [PubMed]
- Madla, C.M.; Gavins, F.K.H.; Merchant, H.A.; Orlu, M.; Murdan, S.; Basit, A.W. Let’s talk about sex: Differences in drug therapy in males and females. Adv. Drug Deliv. Rev. 2021, 175, 113804. [Google Scholar] [CrossRef] [PubMed]
- Mauvais-Jarvis, F.; Berthold, H.K.; Campesi, I.; Carrero, J.J.; Dakal, S.; Franconi, F.; Gouni-Berthold, I.; Heiman, M.L.; Kautzky-Willer, A.; Klein, S.L.; et al. Sex- and Gender-Based Pharmacological Response to Drugs. Pharmacol. Rev. 2021, 73, 730–762. [Google Scholar] [CrossRef]
- McGready, R.; Stepniewska, K.; Seaton, E.; Cho, T.; Cho, D.; Ginsberg, A.; Edstein, M.D.; Ashley, E.; Looareesuwan, S.; White, N.J.; et al. Pregnancy and use of oral contraceptives reduces the biotransformation of proguanil to cycloguanil. Eur. J. Clin. Pharmacol. 2003, 59, 553–557. [Google Scholar] [CrossRef] [Green Version]
- Puangpetch, A.; Vanwong, N.; Nuntamool, N.; Hongkaew, Y.; Chamnanphon, M.; Sukasem, C. CYP2D6 polymorphisms and their influence on risperidone treatment. Pharmgenomics Pers. Med. 2016, 9, 131–147. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abduljalil, K.; Badhan, R.K.S. Drug dosing during pregnancy—opportunities for physiologically based pharmacokinetic models. J. Pharmacokinet. Pharmacodyn. 2020, 47, 319–340. [Google Scholar] [CrossRef]
- Hägg, S.; Spigset, O.; Dahlqvist, R. Influence of gender and oral contraceptives on CYP2D6 and CYP2C19 activity in healthy volunteers. Br. J. Clin. Pharmacol. 2001, 51, 169–177. [Google Scholar] [CrossRef] [PubMed]
- Seeman, M.V. The Pharmacodynamics of Antipsychotic Drugs in Women and Men. Front. Psychiatry 2021, 12, 650904. [Google Scholar] [CrossRef]
- Campesi, I.; Seghieri, G.; Franconi, F. Type 2 diabetic women are not small type 2 diabetic men: Sex-and-gender differences in antidiabetic drugs. Curr. Opin. Pharmacol. 2021, 60, 40–45. [Google Scholar] [CrossRef]
- Campesi, I.; Racagni, G.; Franconi, F. Just a Reflection: Does Drug Repurposing Perpetuate Sex-Gender Bias in the Safety Profile? Pharmaceuticals 2021, 14, 730. [Google Scholar] [CrossRef]
- The GTEx Consortium. The GTEx Consortium Atlas of Genetic Regulatory Effects across Human Tissues. Science 2020, 369, 1318–1330. [Google Scholar] [CrossRef] [PubMed]
- Love, M.I.; Huber, W.; Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014, 15, 550. [Google Scholar] [CrossRef] [Green Version]
- Leek, J.T.; Johnson, W.E.; Parker, H.S.; Jaffe, A.E.; Storey, J.D. The sva package for removing batch effects and other unwanted variation in high-throughput experiments. Bioinformatics 2012, 28, 882–883. [Google Scholar] [CrossRef]
- Whirl-Carrillo, M.; McDonagh, E.M.; Hebert, J.M.; Gong, L.; Sangkuhl, K.; Thorn, C.F.; Altman, R.B.; Klein, T.E. Pharmacogenomics Knowledge for Personalized Medicine. Clin. Pharmacol. Ther. 2012, 92, 414–417. [Google Scholar] [CrossRef] [PubMed]
- Wishart, D.S.; Knox, C.; Guo, A.C.; Shrivastava, S.; Hassanali, M.; Stothard, P.; Chang, Z.; Woolsey, J. Drugbank: A comprehensive resource for in silico drug discovery and exploration. Nucleic Acids Res. 2006, 34, D668–D672. [Google Scholar] [CrossRef]
- Oliva, M.; Muñoz-Aguirre, M.; Kim-Hellmuth, S.; Wucher, V.; Gewirtz, A.D.H.; Cotter, D.J.; Parsana, P.; Kasela, S.; Balliu, B.; Viñuela, A.; et al. The Impact of Sex on Gene Expression across Human Tissues. Science 2020, 369, eaba3066. [Google Scholar] [CrossRef]
- Kwon, Y.-J.; Shin, S.; Chun, Y.-J. Biological roles of cytochrome P450 1A1, 1A2, and 1B1 enzymes. Arch. Pharmacal Res. 2021, 44, 63–83. [Google Scholar] [CrossRef]
- Kim, J.; McMillan, E.; Kim, H.S.; Venkateswaran, N.; Makkar, G.; Rodriguez-Canales, J.; Villalobos, P.; Neggers, J.E.; Mendiratta, S.; Wei, S.; et al. XPO1-Dependent Nuclear Export Is a Druggable Vulnerability in KRAS-Mutant Lung Cancer. Nature 2016, 538, 114–117. [Google Scholar] [CrossRef] [Green Version]
- Reddy, A.; Zhang, J.; Davis, N.S.; Moffitt, A.B.; Love, C.L.; Waldrop, A.; Leppa, S.; Pasanen, A.; Meriranta, L.; Karjalainen-Lindsberg, M.L.; et al. Genetic and Functional Drivers of Diffuse Large B Cell Lymphoma. Cell 2017, 171, 481–494.e15. [Google Scholar] [CrossRef] [Green Version]
- Suzuki, N.; Ishizaki, J.; Yokota, Y.; Higashino, K.; Ono, T.; Ikeda, M.; Fujii, N.; Kawamoto, K.; Hanasaki, K. Structures, Enzymatic Properties, and Expression of Novel Human and Mouse Secretory Phospholipase A2s. J. Biol. Chem. 2000, 275, 5785–5793. [Google Scholar] [CrossRef] [Green Version]
- Kramer, R.M.; Hession, C.; Johansen, B.; Hayes, G.; McGray, P.; Chow, E.P.; Tizard, R.; Pepinsky, R.B. Structure and Properties of a Human Non-Pancreatic Phospholipase A2. J. Biol. Chem. 1989, 264, 5768–5775. [Google Scholar] [CrossRef]
- Barski, O.A.; Tipparaju, S.M.; Bhatnagar, A. The Aldo-Keto Reductase Superfamily and its Role in Drug Metabolism and Detoxification. Drug Metab. Rev. 2008, 40, 553–624. [Google Scholar] [CrossRef] [Green Version]
- Bartz, D.; Chitnis, T.; Kaiser, U.B.; Rich-Edwards, J.W.; Rexrode, K.M.; Pennell, P.B.; Goldstein, J.M.; O’Neal, M.A.; LeBoff, M.; Behn, M.; et al. Clinical Advances in Sex- and Gender-Informed Medicine to Improve the Health of All: A Review. JAMA Intern. Med. 2020, 180, 574–583. [Google Scholar] [CrossRef]
- Guo, S.; Zhou, Y.; Zeng, P.; Xu, G.; Wang, G.; Cui, Q. Identification and analysis of the human sex-biased genes. Brief. Bioinform. 2018, 19, 188–198. [Google Scholar] [CrossRef] [PubMed]
- Available online: https://doi.org/10.5281/zenodo.3939042 (accessed on 13 August 2021).
- Paulussen, A.; Lavrijsen, K.; Bohets, H.; Hendrickx, J.; Verhasselt, P.; Luyten, W.; Konings, F.; Armstrong, M. Two Linked Mutations in Transcriptional Regulatory Elements of the CYP3A5 Gene Constitute the Major Genetic Determinant of Polymorphic Activity in Humans. Pharmacogenetics 2000, 10, 415–424. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Tompkins, L.M. CYP2B6: New Insights into a Historically Overlooked Cytochrome P450 Isozyme. Curr. Drug Metab. 2008, 9, 598–610. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, X.; Zhang, B.; Molony, C.; Chudin, E.; Hao, K.; Zhu, J.; Gaedigk, A.; Suver, C.; Zhong, H.; Leeder, J.S.; et al. Systematic genetic and genomic analysis of cytochrome P450 enzyme activities in human liver. Genome Res. 2010, 20, 1020–1036. [Google Scholar] [CrossRef] [Green Version]
- Cotreau, M.M.; Von Moltke, L.L.; Greenblatt, D.J. The Influence of Age and Sex on the Clearance of Cytochrome P450 3A Substrates. Clin. Pharmacokinet. 2005, 44, 33–60. [Google Scholar] [CrossRef] [PubMed]
- Lamba, V.; Lamba, J.; Yasuda, K.; Strom, S.; Davila, J.; Hancock, M.L.; Fackenthal, J.D.; Rogan, P.K.; Ring, B.; Wrighton, S.A.; et al. Hepatic CYP2B6 Expression: Gender and Ethnic Differences and Relationship to CYP2B6 Genotype and CAR (Constitutive Androstane Receptor) Expression. J. Pharmacol. Exp. Ther. 2003, 307, 906–922. [Google Scholar] [CrossRef]
- Waxman, D.J.; Holloway, M.G. Sex Differences in the Expression of Hepatic Drug Metabolizing Enzymes. Mol. Pharmacol. 2009, 76, 215–228. [Google Scholar] [CrossRef] [Green Version]
- Li, X.M.; Alperin, E.S.; Salido, E.; Gong, Y.; Yen, P.; Shapiro, L.J. Characterization of the promoter region of human steroid sulfatase: A gene which escapes X inactivation. Somat. Cell Mol. Genet 1996, 22, 105–117. [Google Scholar] [CrossRef] [PubMed]
- Steckelbroeck, S.; Nassen, A.; Ugele, B.; Ludwig, M.; Watzka, M.; Reissinger, A.; Clusmann, H.; Lütjohann, D.; Siekmann, L.; Klingmüller, D.; et al. Steroid Sulfatase (STS) Expression in the Human Temporal Lobe: Enzyme Activity, MRNA Expression and Immunohistochemistry Study. J. Neurochem. 2004, 89, 403–417. [Google Scholar] [CrossRef] [PubMed]
- Bi, Y.; Jiang, M.; Guo, W.; Guan, X.; Xu, M.; Ren, S.; Yang, D.; Gaikwad, N.W.; Selcer, K.W.; Xie, W. Sex-Dimorphic and Sex Hormone–Dependent Role of Steroid Sulfatase in Adipose Inflammation and Energy Homeostasis. Endocrinology 2018, 159, 3365–3377. [Google Scholar] [CrossRef]
- Davies, W. Sulfation Pathways: The Steroid Sulfate Axis and Its Relationship to Maternal Behaviour and Mental Health. J. Mol. Endocrinol. 2018, 61, T199–T210. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Conklin, D.J.; Bhatnagar, A. 6.26—aldehydes and cardiovascular disease. In Comprehensive Toxicology, 2nd ed.; McQueen, C.A., Ed.; Elsevier: Oxford, UK, 2010; pp. 489–512. [Google Scholar]
- Sousa, A.; Ferreira, M.; Oliveira, C.; Ferreira, P.G. Gender Differential Transcriptome in Gastric and Thyroid Cancers. Front. Genet. 2020, 11, 808. [Google Scholar] [CrossRef]
- Rademaker, M. Do Women Have More Adverse Drug Reactions? Am. J. Clin. Dermatol. 2001, 2, 349–351. [Google Scholar] [CrossRef]
- Available online: https://academic.oup.com/nargab/article/2/1/lqz010/5609475#209718189 (accessed on 13 August 2021).
- Campesi, I.; Occhioni, S.; Capobianco, G.; Fois, M.; Montella, A.; Dessole, S.; Franconi, F. Sex-Specific Pharmacological Modulation of Autophagic Process in Human Umbilical Artery Smooth Muscle. Cells Pharmacol. Res. 2016, 113, 166–174. [Google Scholar] [CrossRef]
- Campesi, I.; Montella, A.; Sotgiu, G.; Dore, S.; Carru, C.; Zinellu, A.; Palermo, M.; Franconi, F. Combined Oral Contraceptives Modify the Effect of Smoking on Inflammatory Cellular Indexes and Endothelial Function in Healthy Subjects. Eur. J. Pharmacol. 2021, 891, 173762. [Google Scholar] [CrossRef]
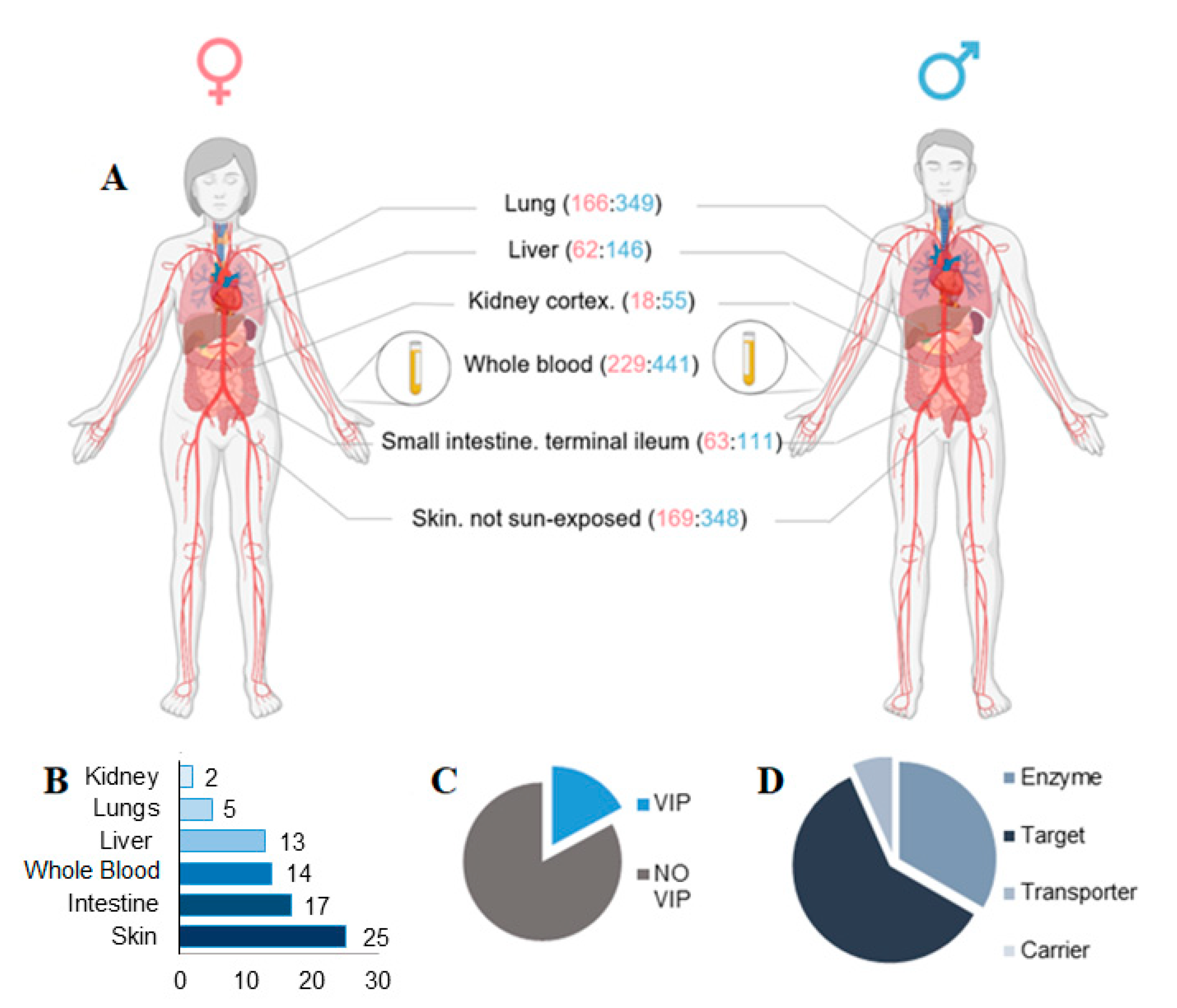

| Tissue | # Transcripts | # PKG-T | # of (%) Male | # of (%) Female | Mean Age |
|---|---|---|---|---|---|
| Liver | 208 | 24 | 146 (70.20%) | 62 (29.80%) | 54.25 |
| Lung | 515 | 27 | 349 (67.76%) | 166 (32.24%) | 53.31 |
| Kidney Cortex | 73 | 4 | 55 (75.34%) | 18 (24.66%) | 56.28 |
| Small Intestine | 174 | 37 | 111 (63.80%) | 63 (36.20%) | 48.12 |
| Skin | 517 | 397 | 348 (67.32%) | 169 (32.68%) | 52.7 |
| Whole Blood | 670 | 54 | 441 (65.82%) | 229 (34.18%) | 51.82 |
| Category | Source | Description |
|---|---|---|
| VIP | PharmGKB | Genes involved in metabolism and response to drugs. Often, VIP either play a role in the metabolism of many drugs or contain genetic variants which may contribute to severe drug responses. |
| Targets | DrugBank | Protein targets of drug action. |
| Enzymes | DrugBank | Proteins that are inhibited/induced or involved in drug metabolism. |
| Carriers | DrugBank | Endogenous proteins which bind to drugs and modify their pharmacokinetics and may facilitate transport in the bloodstream or across cell membranes (an example is albumin). |
| Transporters | DrugBank | Endogenous, membrane-bound, protein-based structure that physically moves drugs across cell membranes between the two sides of the cell membrane. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Idda, M.L.; Campesi, I.; Fiorito, G.; Vecchietti, A.; Urru, S.A.M.; Solinas, M.G.; Franconi, F.; Floris, M. Sex-Biased Expression of Pharmacogenes across Human Tissues. Biomolecules 2021, 11, 1206. https://doi.org/10.3390/biom11081206
Idda ML, Campesi I, Fiorito G, Vecchietti A, Urru SAM, Solinas MG, Franconi F, Floris M. Sex-Biased Expression of Pharmacogenes across Human Tissues. Biomolecules. 2021; 11(8):1206. https://doi.org/10.3390/biom11081206
Chicago/Turabian StyleIdda, Maria Laura, Ilaria Campesi, Giovanni Fiorito, Andrea Vecchietti, Silvana Anna Maria Urru, Maria Giuliana Solinas, Flavia Franconi, and Matteo Floris. 2021. "Sex-Biased Expression of Pharmacogenes across Human Tissues" Biomolecules 11, no. 8: 1206. https://doi.org/10.3390/biom11081206
APA StyleIdda, M. L., Campesi, I., Fiorito, G., Vecchietti, A., Urru, S. A. M., Solinas, M. G., Franconi, F., & Floris, M. (2021). Sex-Biased Expression of Pharmacogenes across Human Tissues. Biomolecules, 11(8), 1206. https://doi.org/10.3390/biom11081206











