Design and Evaluation of Synthetic RNA-Based Incoherent Feed-Forward Loop Circuits
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plasmid Construction and E. coli Strains Used
2.2. Cell Culture and Microplate Reader Analysis
2.3. Mathematical Modeling
3. Results
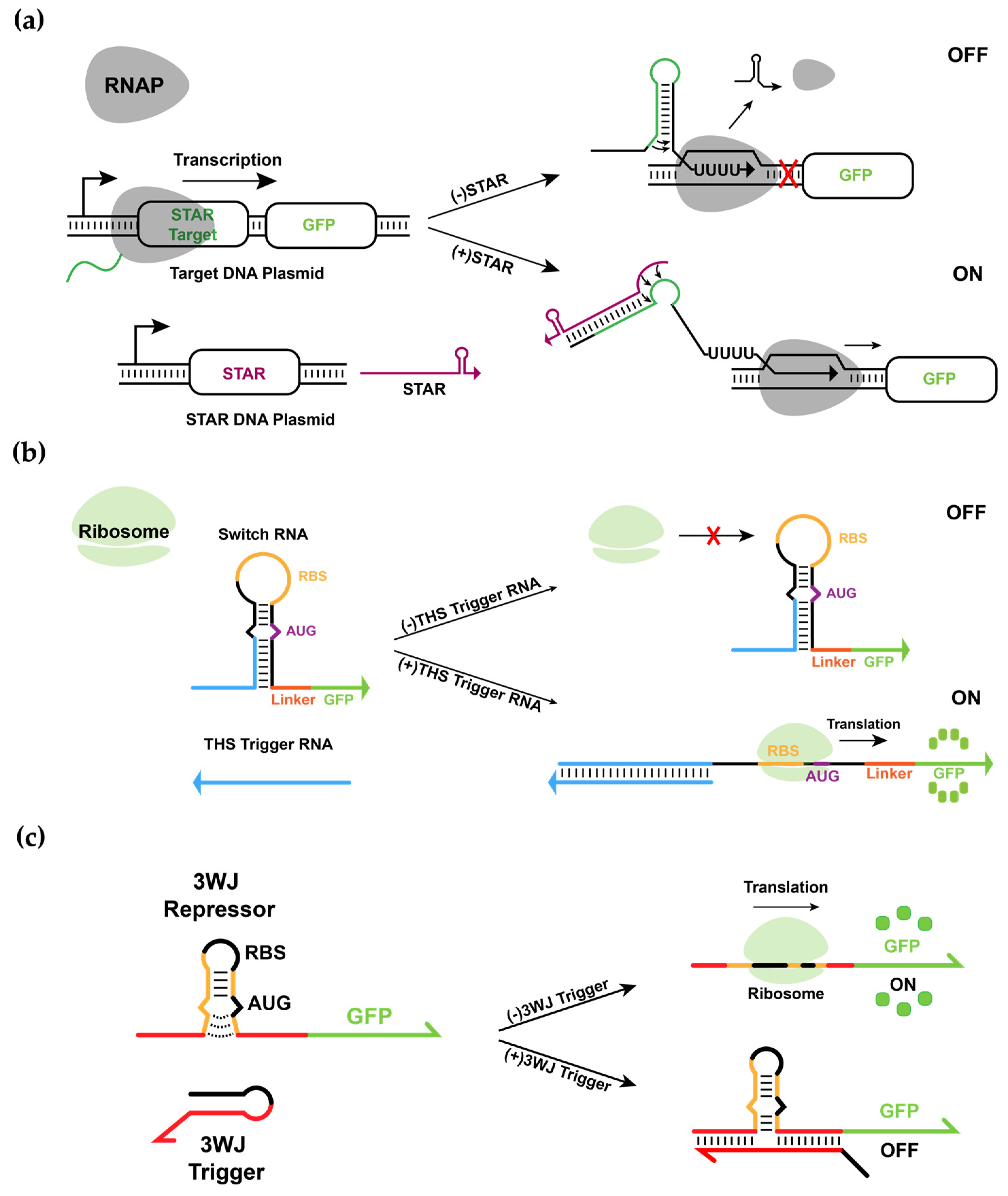
3.1. Design and Experimental Characterization of Synthetic RNA-Based IFFL Circuit
3.2. RNA-Only IFFL Modeling
3.3. RNA–Protein Hybrid IFFL Modeling
3.4. Experimental Realization and Validation of the RNA–Protein Hybrid IFFL Design
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cameron, D.E.; Bashor, C.J.; Collins, J.J. A brief history of synthetic biology. Nat. Rev. Microbiol. 2014, 12, 381–390. [Google Scholar] [CrossRef]
- Cheng, A.A.; Lu, T.K. Synthetic Biology: An Emerging Engineering Discipline. Annu. Rev. Biomed. Eng. 2012, 14, 155–178. [Google Scholar] [CrossRef] [Green Version]
- Elowitz, M.B.; Leibler, S. A synthetic oscillatory network of transcriptional regulators. Nature 2000, 403, 335–338. [Google Scholar] [CrossRef] [PubMed]
- Danino, T.; Mondragón-Palomino, O.; Tsimring, L.; Hasty, J. A synchronized quorum of genetic clocks. Nature 2010, 463, 326–330. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, J.; White, K.S.; Winfree, E. Construction of an in vitro bistable circuit from synthetic transcriptional switches. Mol. Syst. Biol. 2006, 2, 68. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gardner, T.S.; Cantor, C.R.; Collins, J.J. Construction of a genetic toggle switch in Escherichia coli. Nature 2000, 403, 339–342. [Google Scholar] [CrossRef]
- Xu, S.; Li, H.; Miao, Y.; Liu, Y.; Wang, E. Implementation of half adder and half subtractor with a simple and universal DNA-based platform. NPG Asia Mater. 2013, 5, e76. [Google Scholar] [CrossRef]
- Weinberg, B.H.; Pham, N.T.H.; Caraballo, L.D.; Lozanoski, T.; Engel, A.; Bhatia, S.; Wong, W.W. Large-scale design of robust genetic circuits with multiple inputs and outputs for mammalian cells. Nat. Biotechnol. 2017, 35, 453–462. [Google Scholar] [CrossRef]
- Jagadevan, S.; Banerjee, A.; Banerjee, C.; Guria, C.; Tiwari, R.; Baweja, M.; Shukla, P. Recent developments in synthetic biology and metabolic engineering in microalgae towards biofuel production. Biotechnol. Biofuels 2018, 11, 185. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nissim, L.; Wu, M.R.; Pery, E.; Binder-Nissim, A.; Suzuki, H.I.; Stupp, D.; Wehrspaun, C.; Tabach, Y.; Sharp, P.A.; Lu, T.K. Synthetic RNA-Based Immunomodulatory Gene Circuits for Cancer Immunotherapy. Cell 2017, 171, 1138–1150. [Google Scholar] [CrossRef] [Green Version]
- Bereza-Malcolm, L.T.; Mann, G.; Franks, A.E. Environmental Sensing of Heavy Metals through Whole Cell Microbial Biosensors: A Synthetic Biology Approach. ACS Synth. Biol. 2015, 4, 535–546. [Google Scholar] [CrossRef]
- Dimitrov, R.A.; Zuker, M. Prediction of hybridization and melting for double-stranded nucleic acids. Biophys. J. 2004, 87, 215–226. [Google Scholar] [CrossRef] [Green Version]
- Laalami, S.; Zig, L.; Putzer, H. Initiation of mRNA decay in bacteria. Cell. Mol. Life Sci. 2014, 71, 1799–1828. [Google Scholar] [CrossRef] [Green Version]
- Rodrigo, G.; Landrain, T.E.; Jaramillo, A. De novo automated design of small RNA circuits for engineering synthetic riboregulation in living cells. Proc. Natl. Acad. Sci. USA 2012, 109, 15271–15276. [Google Scholar] [CrossRef] [Green Version]
- Chappell, J.; Watters, K.E.; Takahashi, M.K.; Lucks, J.B. A renaissance in RNA synthetic biology: New mechanisms, applications and tools for the future. Curr. Opin. Chem. Biol. 2015, 28, 47–56. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chappell, J.; Takahashi, M.K.; Lucks, J.B. Creating small transcription activating RNAs. Nat. Chem. Biol. 2015, 11, 214–220. [Google Scholar] [CrossRef] [PubMed]
- Green, A.A.; Silver, P.A.; Collins, J.J.; Yin, P. Toehold switches: De-novo-designed regulators of gene expression. Cell 2014, 159, 925–939. [Google Scholar] [CrossRef] [Green Version]
- Green, A.A.; Kim, J.; Ma, D.; Silver, P.A.; Collins, J.J.; Yin, P. Complex cellular logic computation using ribocomputing devices. Nature 2017, 548, 117–121. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, J.; Zhou, Y.; Carlson, P.D.; Teichmann, M.; Chaudhary, S.; Simmel, F.C.; Silver, P.A.; Collins, J.J.; Lucks, J.B.; Yin, P.; et al. De novo-designed translation-repressing riboregulators for multi-input cellular logic. Nat. Chem. Biol. 2019, 15, 1173–1182. [Google Scholar] [CrossRef] [PubMed]
- Adler, M.; Alon, U. Fold-change detection in biological systems. Curr. Opin. Syst. Biol. 2018, 8, 81–89. [Google Scholar] [CrossRef]
- Kim, J.; Khetarpal, I.; Sen, S.; Murray, R.M. Synthetic circuit for exact adaptation and fold-change detection. Nucleic Acids Res. 2014, 42, 6078–6089. [Google Scholar] [CrossRef] [Green Version]
- Segall-Shapiro, T.H.; Sontag, E.D.; Voigt, C.A. Engineered promoters enable constant gene expression at any copy number in bacteria. Nat. Biotechnol. 2018, 36, 352–358. [Google Scholar] [CrossRef] [PubMed]
- Hart, Y.; Alon, U. The utility of paradoxical components in biological circuits. Mol. Cell 2013, 49, 213–221. [Google Scholar] [CrossRef] [Green Version]
- Levine, J.H.; Lin, Y.; Elowitz, M.B. Functional roles of pulsing in genetic circuits. Science 2013, 342, 1193–1200. [Google Scholar] [CrossRef] [Green Version]
- Westbrook, A.; Tang, X.; Marshall, R.; Maxwell, C.S.; Chappell, J.; Agrawal, D.K.; Dunlop, M.J.; Noireaux, V.; Beisel, C.L.; Lucks, J. Distinct timescales of RNA regulators enable the construction of a genetic pulse generator. Biotechnol. Bioeng. 2019, 116, 1139–1151. [Google Scholar] [CrossRef]
- Santos-Moreno, J.; Tasiudi, E.; Stelling, J.; Schaerli, Y. Multistable and dynamic CRISPRi-based synthetic circuits. Nat. Commun. 2020, 11, 2746. [Google Scholar] [CrossRef] [PubMed]
- Frei, T.; Cella, F.; Tedeschi, F.; Gutiérrez, J.; Stan, G.-B.; Khammash, M.; Siciliano, V. Characterization and mitigation of gene expression burden in mammalian cells. Nat. Commun. 2020, 11, 4641. [Google Scholar] [CrossRef] [PubMed]
- Kamionka, A.; Bogdanska-Urbaniak, J.; Scholz, O.; Hillen, W. Two mutations in the tetracycline repressor change the inducer anhydrotetracycline to a corepressor. Nucleic Acids Res. 2004, 32, 842–847. [Google Scholar] [CrossRef] [Green Version]
- Nielsen, A.A.K.; Der, B.S.; Shin, J.; Vaidyanathan, P.; Paralanov, V.; Strychalski, E.A.; Ross, D.; Densmore, D.; Voigt, C.A. Genetic circuit design automation. Science 2016, 352, aac7341. [Google Scholar] [CrossRef] [Green Version]
- Chen, Y.; Zhang, S.; Young, E.M.; Jones, T.S.; Densmore, D.; Voigt, C.A. Genetic circuit design automation for yeast. Nat. Microbiol. 2020, 5, 1349–1360. [Google Scholar] [CrossRef]
- Zong, D.M.; Cinar, S.; Shis, D.L.; Josić, K.; Ott, W.; Bennett, M.R. Predicting Transcriptional Output of Synthetic Multi-input Promoters. ACS Synth. Biol. 2018, 7, 1834–1843. [Google Scholar] [CrossRef]
- Hu, C.Y.; Takahashi, M.K.; Zhang, Y.; Lucks, J.B. Engineering a Functional Small RNA Negative Autoregulation Network with Model-Guided Design. ACS Synth. Biol. 2018, 7, 1507–1518. [Google Scholar] [CrossRef] [PubMed]
- Muldoon, J.J.; Kandula, V.; Hong, M.; Donahue, P.S.; Boucher, J.D.; Bagheri, N.; Leonard, J.N. Model-guided design of mammalian genetic programs. Sci. Adv. 2021, 7, eabe9375. [Google Scholar] [CrossRef]
- Barone, F.; Dorr, F.; Marasco, L.E.; Mildiner, S.; Patop, I.L.; Sosa, S.; Vattino, L.G.; Vignale, F.A.; Altszyler, E.; Basanta, B.; et al. Design and evaluation of an incoherent feed-forward loop for an arsenic biosensor based on standard iGEM parts. Synth. Biol. 2017, 2. [Google Scholar] [CrossRef] [Green Version]
- Reeves, G.T. The engineering principles of combining a transcriptional incoherent feedforward loop with negative feedback. J. Biol. Eng. 2019, 13, 62. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Belta, C.; Isaacson, S.A. How Retroactivity Affects the Behavior of Incoherent Feedforward Loops. iScience 2020, 23, 101779. [Google Scholar] [CrossRef]
- Chappell, J.; Westbrook, A.; Verosloff, M.; Lucks, J.B. Computational design of small transcription activating RNAs for versatile and dynamic gene regulation. Nat. Commun. 2017, 8, 1051. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lou, C.; Stanton, B.; Chen, Y.J.; Munsky, B.; Voigt, C.A. Ribozyme-based insulator parts buffer synthetic circuits from genetic context. Nat. Biotechnol. 2012, 30, 1137–1142. [Google Scholar] [CrossRef] [Green Version]
- Lagarias, J.C.; Reeds, J.A.; Wright, M.H.; Wright, P.E. Convergence Properties of the Nelder--Mead Simplex Method in Low Dimensions. SIAM J. Optim. 1998, 9, 112–147. [Google Scholar] [CrossRef] [Green Version]
- Andersen, J.B.; Sternberg, C.; Poulsen, L.K.; Bjørn, S.P.; Givskov, M.; Molin, S. New Unstable Variants of Green Fluorescent Protein for Studies of Transient Gene Expression in Bacteria. Appl. Environ. Microbiol. 1998, 64, 2240–2246. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cheng, Y.-Y.; Hirning, A.J.; Josić, K.; Bennett, M.R. The Timing of Transcriptional Regulation in Synthetic Gene Circuits. ACS Synth. Biol. 2017, 6, 1996–2002. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Entus, R.; Aufderheide, B.; Sauro, H.M. Design and implementation of three incoherent feed-forward motif based biological concentration sensors. Syst. Synth. Biol. 2007, 1, 119–128. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hong, S.; Kim, J.; Kim, J. Multilevel Gene Regulation Using Switchable Transcription Terminator and Toehold Switch in Escherichia coli. Appl. Sci. 2021, 11, 4532. [Google Scholar] [CrossRef]
- Lehr, F.-X.; Hanst, M.; Vogel, M.; Kremer, J.; Göringer, H.U.; Suess, B.; Koeppl, H. Cell-Free Prototyping of AND-Logic Gates Based on Heterogeneous RNA Activators. ACS Synth. Biol. 2019, 8, 2163–2173. [Google Scholar] [CrossRef] [PubMed]
- Stanton, B.C.; Nielsen, A.A.; Tamsir, A.; Clancy, K.; Peterson, T.; Voigt, C.A. Genomic mining of prokaryotic repressors for orthogonal logic gates. Nat. Chem. Biol. 2014, 10, 99–105. [Google Scholar] [CrossRef] [PubMed] [Green Version]






Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hong, S.; Jeong, D.; Ryan, J.; Foo, M.; Tang, X.; Kim, J. Design and Evaluation of Synthetic RNA-Based Incoherent Feed-Forward Loop Circuits. Biomolecules 2021, 11, 1182. https://doi.org/10.3390/biom11081182
Hong S, Jeong D, Ryan J, Foo M, Tang X, Kim J. Design and Evaluation of Synthetic RNA-Based Incoherent Feed-Forward Loop Circuits. Biomolecules. 2021; 11(8):1182. https://doi.org/10.3390/biom11081182
Chicago/Turabian StyleHong, Seongho, Dohyun Jeong, Jordan Ryan, Mathias Foo, Xun Tang, and Jongmin Kim. 2021. "Design and Evaluation of Synthetic RNA-Based Incoherent Feed-Forward Loop Circuits" Biomolecules 11, no. 8: 1182. https://doi.org/10.3390/biom11081182
APA StyleHong, S., Jeong, D., Ryan, J., Foo, M., Tang, X., & Kim, J. (2021). Design and Evaluation of Synthetic RNA-Based Incoherent Feed-Forward Loop Circuits. Biomolecules, 11(8), 1182. https://doi.org/10.3390/biom11081182






