Grape Pomace for Topical Application: Green NaDES Sustainable Extraction, Skin Permeation Studies, Antioxidant and Anti-Inflammatory Activities Characterization in 3D Human Keratinocytes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Red Pomace Materials and Extracts
2.3. HPLC-ESI-MS/MS Method
2.4. Ex Vivo Permeation Study by Franz Diffusion Cells
2.5. D Keratinocyte Cell Model
2.6. Quantification of Intracellular H2O2 in 3D HaCaT Cells
2.7. Antioxidant Activity of Red Pomace Extracts in HaCaT Spheroids
2.8. Cell Cytotoxicity: Lactate Dehydrogenase Release
2.9. Spectrophotometric Cell Viability Bioassay
2.10. Protein Release
2.11. Statistical Analysis
3. Results and Discussion
3.1. Extraction and Characterization of Anthocyanins in NaDES Extracts
3.2. Skin Permeation of Anthocyanins Derived from NaDES Extracts
3.3. Safety of NaDES Extracts In Vitro in Human 3D Keratinocytes
3.4. Evaluation of Intracellular H2O2 Production in Human 3D Keratinocytes
3.5. Antioxidant Activity of NaDES Extracts toward Human HaCaT Spheroids
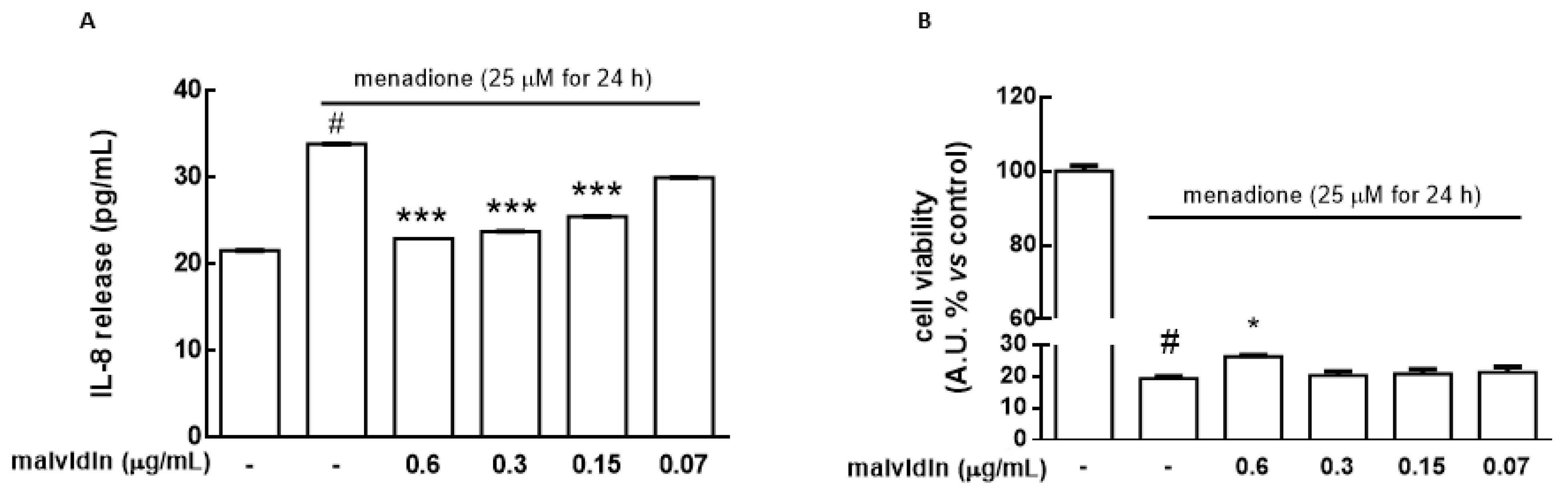
3.6. Protective Effects of NaDES Extracts on HaCat Spheroids in the Presence of the Cytotoxic Agent Menadione
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sustainable Development Goals United Nations Home Page. Available online: https://www.un.org/sustainabledevelopment/ (accessed on 25 May 2021).
- Pomarici, E.; Corsi, A.; Mazzarino, S.; Sardone, R. The Italian Wine Sector: Evolution, Structure, Competitiveness and Future Challenges of an Enduring Leader. Ital. Econ. J. 2021, 7, 259–295. [Google Scholar] [CrossRef]
- Schieber, A.; Stintzing, F.; Carle, R. By-products of plant food processing as a source of functional compounds—Recent developments. Trends Food Sci. Technol. 2001, 12, 401–413. [Google Scholar] [CrossRef]
- Samorì, C.; Mazzei, L.; Ciurli, S.; Cravotto, G.; Grillo, G.; Guidi, E.; Pasteris, A.; Tabasso, S.; Galletti, P. Urease Inhibitory Potential and Soil Ecotoxicity of Novel “Polyphenols–Deep Eutectic Solvents” Formulations. ACS Sustain. Chem. Eng. 2019, 7, 15558–15567. [Google Scholar] [CrossRef]
- Ruesgas-Ramón, M.; Figueroa-Espinoza, M.C.; Durand, E. Application of Deep Eutectic Solvents (DES) for Phenolic Compounds Extraction: Overview, Challenges, and Opportunities. J. Agric. Food Chem. 2017, 65, 3591–3601. [Google Scholar] [CrossRef] [PubMed]
- Faggian, M.; Sut, S.; Perissutti, B.; Baldan, V.; Grabnar, I.; Dall’Acqua, S. Natural Deep Eutectic Solvents (NADES) as a Tool for Bioavailability Improvement: Pharmacokinetics of Rutin Dissolved in Proline/Glycine after Oral Administration in Rats: Possible Application in Nutraceuticals. Molecules 2016, 21, 1531. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hänel, K.H.; Cornelissen, C.; Lüscher, B.; Baron, J.M. Cytokines and the Skin Barrier. Int. J. Mol. Sci. 2013, 14, 6720–6745. [Google Scholar] [CrossRef] [Green Version]
- Boukamp, P.; Petrussevska, R.T.; Breitkreutz, D.; Hornung, J.; Markham, A.; Fusenig, N.E. Normal keratinization in a spontaneously immortalized aneuploid human keratinocyte cell line. J. Cell Biol. 1988, 106, 761–771. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lehmann, B. HaCaT Cell Line as a Model System for Vitamin D3 Metabolism in Human Skin. J. Investig. Dermatol. 1997, 108, 78–82. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Klicks, J.; von Molitor, E.; Ertongur-Fauth, T.; Rudolf, R.; Hafner, M. In vitro skin three-dimensional models and their applications. J. Cell. Biotechnol. 2017, 3, 21–39. [Google Scholar] [CrossRef] [Green Version]
- Panić, M.; Stojković, M.R.; Kraljić, K.; Škevin, D.; Redovniković, I.R.; Srček, V.G.; Radošević, K. Ready-to-use green polyphenolic extracts from food by-products. Food Chem. 2019, 283, 628–636. [Google Scholar] [CrossRef] [PubMed]
- Klicks, J.; Maßlo, C.; Kluth, A.; Rudolf, R.; Hafner, M. A novel spheroid-based co-culture model mimics loss of keratinocyte differentiation, melanoma cell invasion, and drug-induced selection of ABCB5-expressing cells. BMC Cancer 2019, 19, 1–14. [Google Scholar] [CrossRef]
- Calabria, D.; Guardigli, M.; Mirasoli, M.; Punzo, A.; Porru, E.; Zangheri, M.; Simoni, P.; Pagnotta, E.; Ugolini, L.; Lazzeri, L.; et al. Selective chemiluminescent TURN-ON quantitative bioassay and imaging of intracellular hydrogen peroxide in human living cells. Anal. Biochem. 2020, 600, 113760. [Google Scholar] [CrossRef]
- Klotz, L.-O.; Hou, X.; Jacob, C. 1,4-Naphthoquinones: From Oxidative Damage to Cellular and Inter-Cellular Signaling. Mol. 2014, 19, 14902–14918. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Caliceti, C.; Capriotti, A.L.; Calabria, D.; Bonvicini, F.; Chiozzi, R.Z.; Montone, C.M.; Piovesana, S.; Zangheri, M.; Mirasoli, M.; Simoni, P.; et al. Peptides from Cauliflower By-Products, Obtained by an Efficient, Ecosustainable, and Semi-Industrial Method, Exert Protective Effects on Endothelial Function. Oxid. Med. Cell. Longev. 2019, 2019, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Antoniolli, A.; Fontana, A.R.; Piccoli, P.; Bottini, R. Characterization of polyphenols and evaluation of antioxidant capacity in grape pomace of the cv. Malbec. Food Chem. 2015, 178, 172–178. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Durst, R.W.; Wrolstad, R.E.; Eisele, T.; Giusti, M.M.; Hofsommer, H.; Koswig, S.; Krueger, D.A.; Kupina, S.; Martin, S.K.; et al. Determination of Total Monomeric Anthocyanin Pigment Content of Fruit Juices, Beverages, Natural Colorants, and Wines by the pH Differential Method: Collaborative Study. J. AOAC Int. 2005, 88, 1269–1278. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carlsen, C.; Stapelfeldt, H. Light sensitivity of elderberry extract. quantum yields for photodegradation in aqueous solution. Food Chem. 1997, 60, 383–387. [Google Scholar] [CrossRef]
- Ignat, I.; Volf, I.; Popa, V.I. A critical review of methods for characterisation of polyphenolic compounds in fruits and vegetables. Food Chem. 2011, 126, 1821–1835. [Google Scholar] [CrossRef] [PubMed]
- Qi, Q.M.; Duffy, M.; Curreri, A.M.; Balkaran, J.P.R.; Tanner, E.E.L.; Mitragotri, S. Comparison of Ionic Liquids and Chemical Permeation Enhancers for Transdermal Drug Delivery. Adv. Funct. Mater. 2020, 30, 2004257. [Google Scholar] [CrossRef]
- Benvenutti, L.; Sanchez-Camargo, A.D.P.; Zielinski, A.A.F.; Ferreira, S.R.S. NADES as potential solvents for anthocyanin and pectin extraction from Myrciaria cauliflora fruit by-product: In silico and experimental approaches for solvent selection. J. Mol. Liq. 2020, 315, 113761. [Google Scholar] [CrossRef]
- EUR-Lex, Access to European Union Law. Available online: http://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:32006D0257.pdf (accessed on 25 May 2021).
- Fruijtier-Pölloth, C. Safety assessment on polyethylene glycols (PEGs) and their derivatives as used in cosmetic products. Toxicology 2005, 214, 1–38. [Google Scholar] [CrossRef] [PubMed]
- Panuwet, P.; Hunter, R.E.; D’Souza, P.E.; Chen, X.; Radford, S.A.; Cohen, J.R.; Marder, M.E.; Kartavenka, K.; Ryan, P.B.; Barr, D.B. Biological Matrix Effects in Quantitative Tandem Mass Spectrometry-Based Analytical Methods: Advancing Biomonitoring. Crit. Rev. Anal. Chem. 2016, 46, 93–105. [Google Scholar] [CrossRef] [Green Version]
- Benson, H.A.; Watkinson, A.C. Topical and Transdermal Drug Delivery: Principles and Practice, 1st ed.; John Wiley & Sons: Hoboken, NJ, USA, 2012. [Google Scholar]
- Bharate, S.S.; Vishwakarma, R.A. Impact of preformulation on drug development. Expert Opin. Drug Deliv. 2013, 10, 1239–1257. [Google Scholar] [CrossRef] [PubMed]
- Moser, K. Passive skin penetration enhancement and its quantification in vitro. Eur. J. Pharm. Biopharm. 2001, 52, 103–112. [Google Scholar] [CrossRef]
- Nystedt, H.L.; Grønlien, K.G.; Tønnesen, H.H. Interactions of natural deep eutectic solvents (NADES) with artificial and natural membranes. J. Mol. Liq. 2021, 328, 115452. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, Y.; Tao, C.; Liu, M.; Pan, Y.; Lv, Z. Effect of temperature and pH on stability of anthocyanin obtained from blueberry. J. Food Meas. Charact. 2018, 12, 1744–1753. [Google Scholar] [CrossRef]
- Macário, I.; Oliveira, H.; Menezes, A.C.; Ventura, S.; Pereira, J.L.; Gonçalves, A.M.M.; Coutinho, J.; Gonçalves, F.J.M. Cytotoxicity profiling of deep eutectic solvents to human skin cells. Sci. Rep. 2019, 9, 3932. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ye, S.; Hananya, N.; Green, O.; Chen, H.; Zhao, A.Q.; Shen, J.; Shabat, D.; Yang, D. A Highly Selective and Sensitive Chemiluminescent Probe for Real-Time Monitoring of Hydrogen Peroxide in Cells and Animals. Angew. Chem. Int. Ed. 2020, 59, 14326–14330. [Google Scholar] [CrossRef]
- Kurutas, E.B. The importance of antioxidants which play the role in cellular response against oxidative/nitrosative stress: Current state. Nutr. J. 2015, 15, 1–22. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mohamadzadeh, M.; Müller, M.; Hultsch, T.; Enk, A.; Saloga, J.; Knop, J.; Miiller, M. Enhanced expression of IL-8 in normal human keratinocytes and human keratinocyte cell line HaCaT in vitro after stimulation with contact sensitizers., tolerogens and irritants. Exp. Dermatol. 1994, 3, 298–303. [Google Scholar] [CrossRef]
- Park, K.; Lee, J.-H.; Cho, H.-C.; Cho, S.-Y.; Cho, J.-W. Down-regulation of IL-6, IL-8, TNF-α and IL-1β by glucosamine in HaCaT cells, but not in the presence of TNF-α. Oncol. Lett. 2010, 1, 289–292. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hubner, A.; Sobreira, F.; Neto, A.V.; Pinto, C.A.S.D.O.; Dario, M.; Díaz, I.; Lourenço, F.R.; Rosado, C.; Baby, A.R.; Bacchi, E.M. The Synergistic Behavior of Antioxidant Phenolic Compounds Obtained from Winemaking Waste’s Valorization, Increased the Efficacy of a Sunscreen System. Antioxidants 2019, 8, 530. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hübner, A.A.; Sarruf, F.D.; Oliveira, C.A.; Neto, A.V.; Fischer, D.C.H.; Kato, E.T.M.; Lourenço, F.R.; Baby, A.R.; Bacchi, E.M. Safety and Photoprotective Efficacy of a Sunscreen System Based on Grape Pomace (Vitis vinifera L.) Phenolics from Winemaking. Pharmaceutics 2020, 12, 1148. [Google Scholar] [CrossRef]
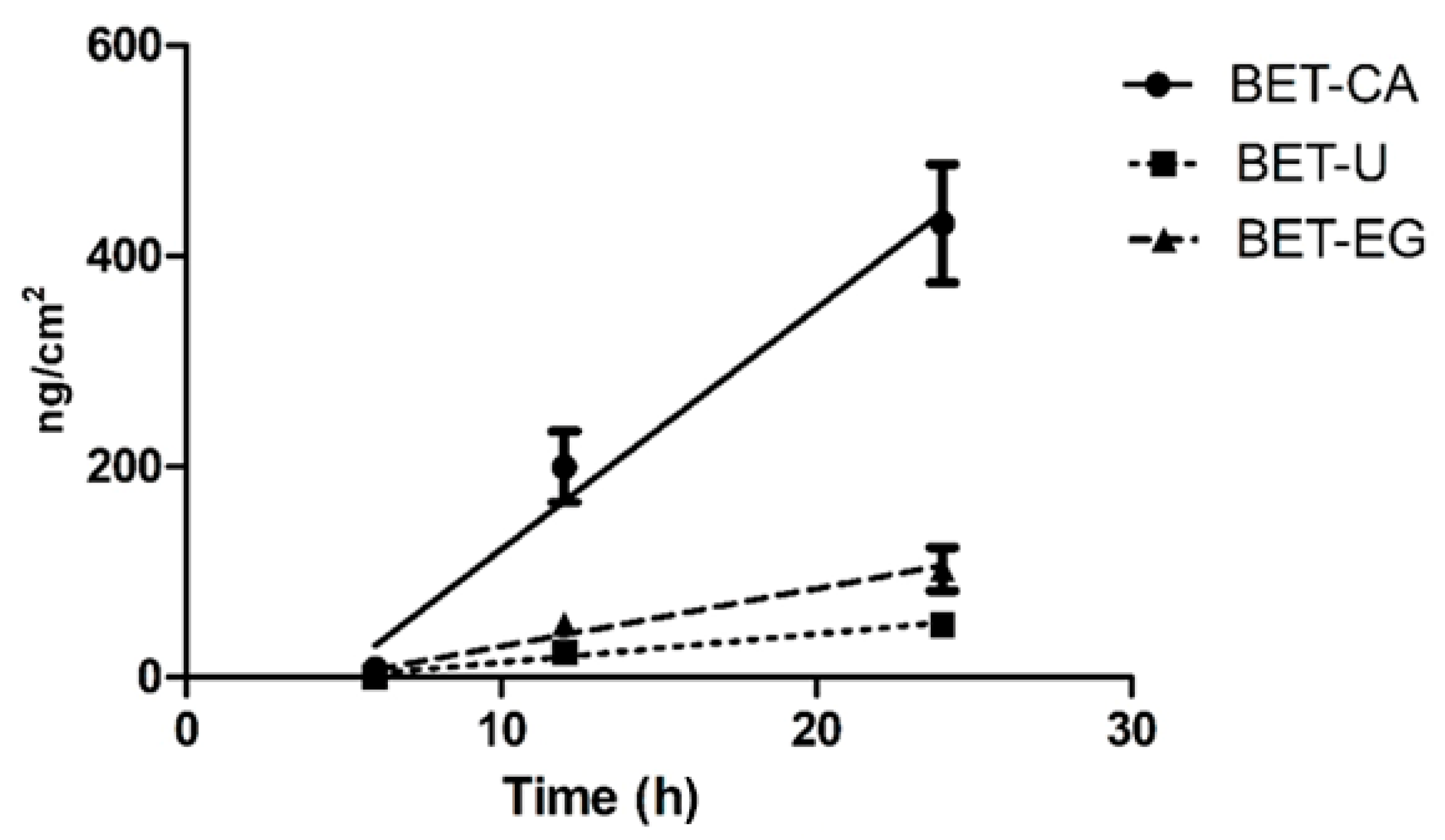


| Time (Hours) | BET-CA (ng cm−2) ± SD | BET-U (ng cm−2) ± SD | BET-EG (ng cm−2) ± SD |
|---|---|---|---|
| 6 | 8.35 ± 4.71 | 0.00 | 0.00 |
| 12 | 213 ± 43 | 28.1 ± 2.5 | 49.7 ± 10.2 |
| 24 | 431 ± 7 | 50.4 ± 14.9 | 103 ± 5 |
| Flow J (ng cm−2 h−1) | 23.4 ± 3.1 | 2.80 ± 0.48 | 5.71 ± 1.14 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Punzo, A.; Porru, E.; Silla, A.; Simoni, P.; Galletti, P.; Roda, A.; Tagliavini, E.; Samorì, C.; Caliceti, C. Grape Pomace for Topical Application: Green NaDES Sustainable Extraction, Skin Permeation Studies, Antioxidant and Anti-Inflammatory Activities Characterization in 3D Human Keratinocytes. Biomolecules 2021, 11, 1181. https://doi.org/10.3390/biom11081181
Punzo A, Porru E, Silla A, Simoni P, Galletti P, Roda A, Tagliavini E, Samorì C, Caliceti C. Grape Pomace for Topical Application: Green NaDES Sustainable Extraction, Skin Permeation Studies, Antioxidant and Anti-Inflammatory Activities Characterization in 3D Human Keratinocytes. Biomolecules. 2021; 11(8):1181. https://doi.org/10.3390/biom11081181
Chicago/Turabian StylePunzo, Angela, Emanuele Porru, Alessia Silla, Patrizia Simoni, Paola Galletti, Aldo Roda, Emilio Tagliavini, Chiara Samorì, and Cristiana Caliceti. 2021. "Grape Pomace for Topical Application: Green NaDES Sustainable Extraction, Skin Permeation Studies, Antioxidant and Anti-Inflammatory Activities Characterization in 3D Human Keratinocytes" Biomolecules 11, no. 8: 1181. https://doi.org/10.3390/biom11081181
APA StylePunzo, A., Porru, E., Silla, A., Simoni, P., Galletti, P., Roda, A., Tagliavini, E., Samorì, C., & Caliceti, C. (2021). Grape Pomace for Topical Application: Green NaDES Sustainable Extraction, Skin Permeation Studies, Antioxidant and Anti-Inflammatory Activities Characterization in 3D Human Keratinocytes. Biomolecules, 11(8), 1181. https://doi.org/10.3390/biom11081181







