A Novel Splice Variant of the Inhibitor of Growth 3 Lacks the Plant Homeodomain and Regulates Epithelial–Mesenchymal Transition in Prostate Cancer Cells
Abstract
:1. Introduction
2. Results
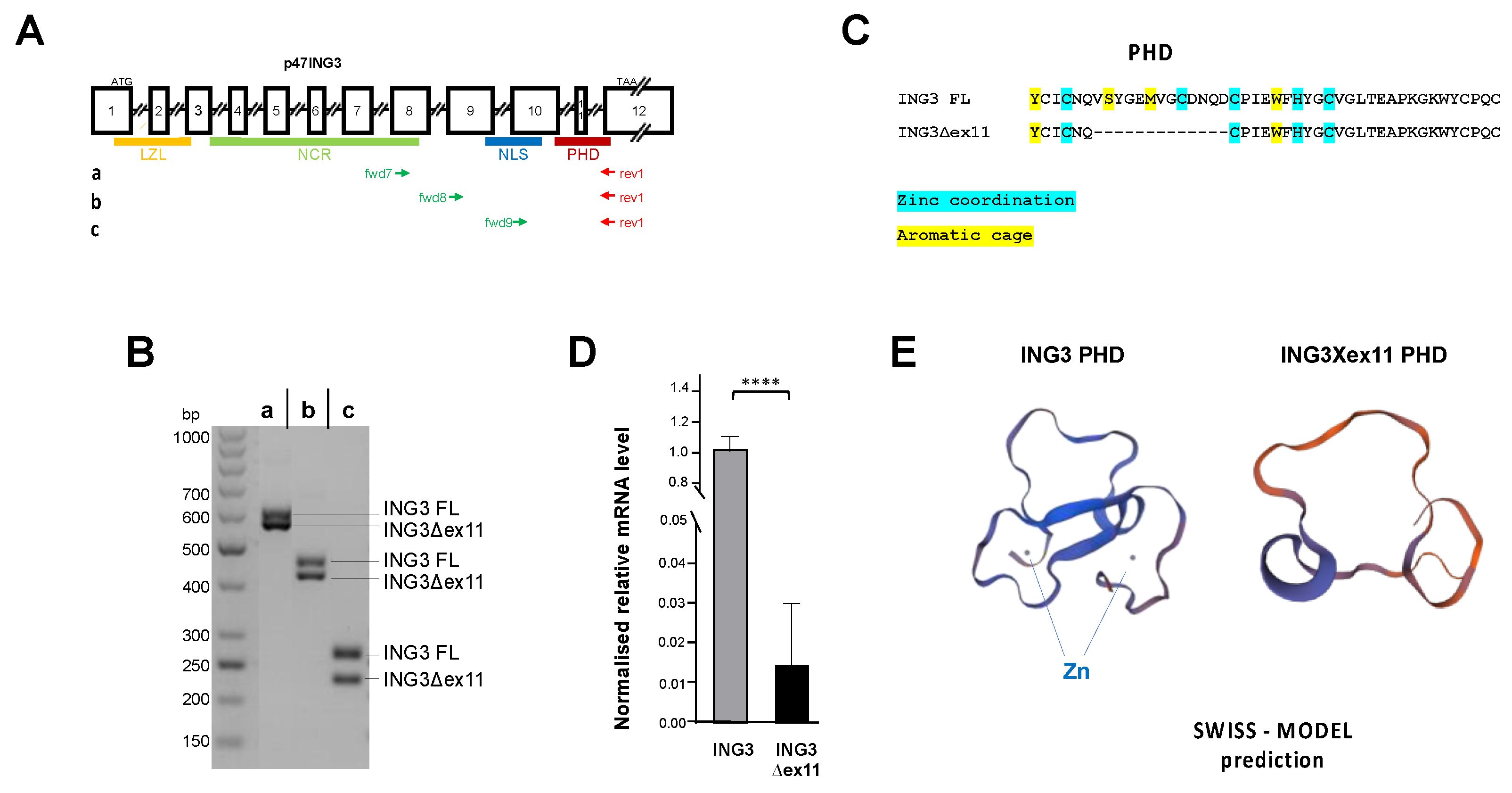
2.1. Identification and Functional Analysis of a Novel PHD-Lacking ING3 Splice Variant
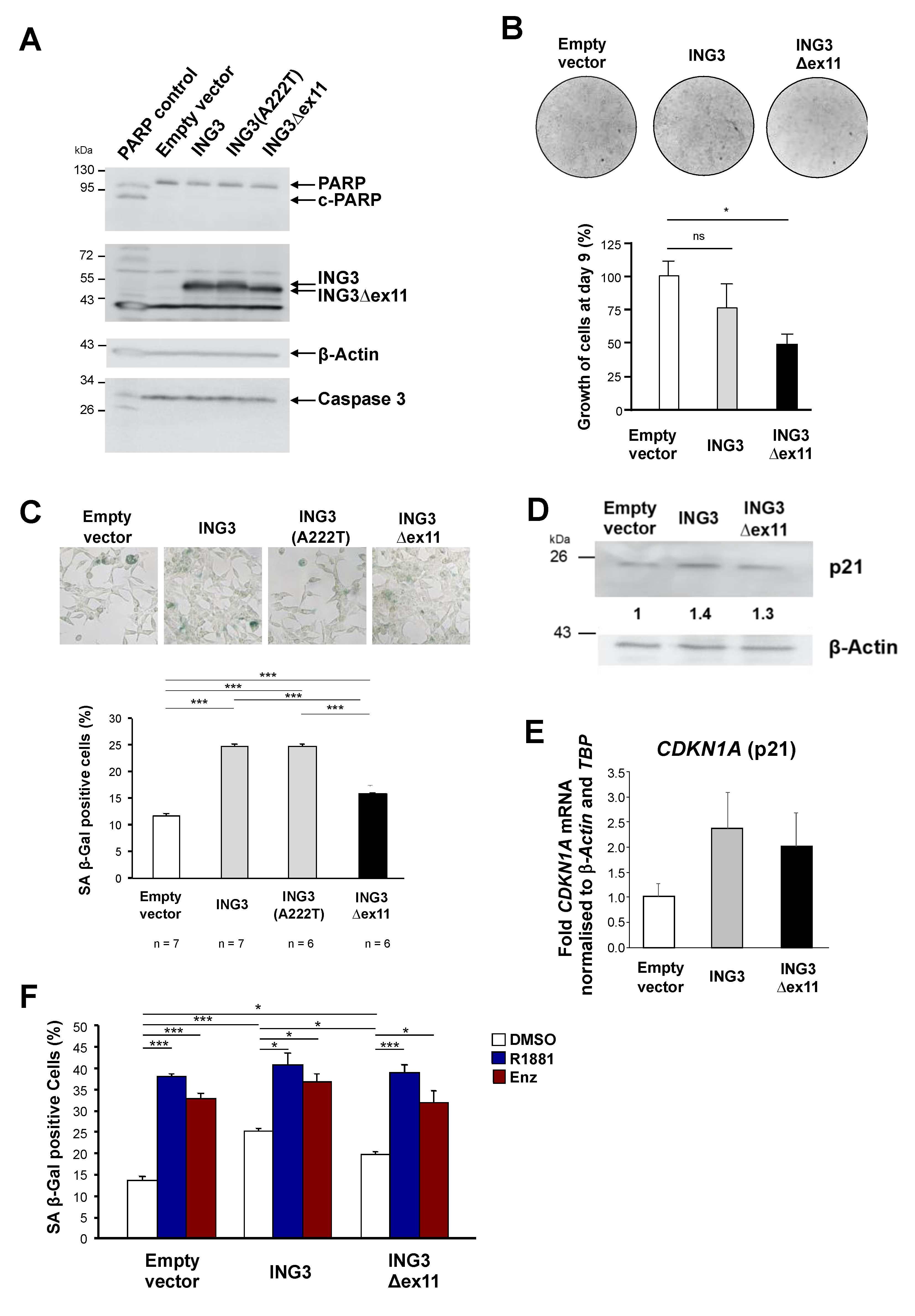
2.2. ING3∆ex11 Induces EMT
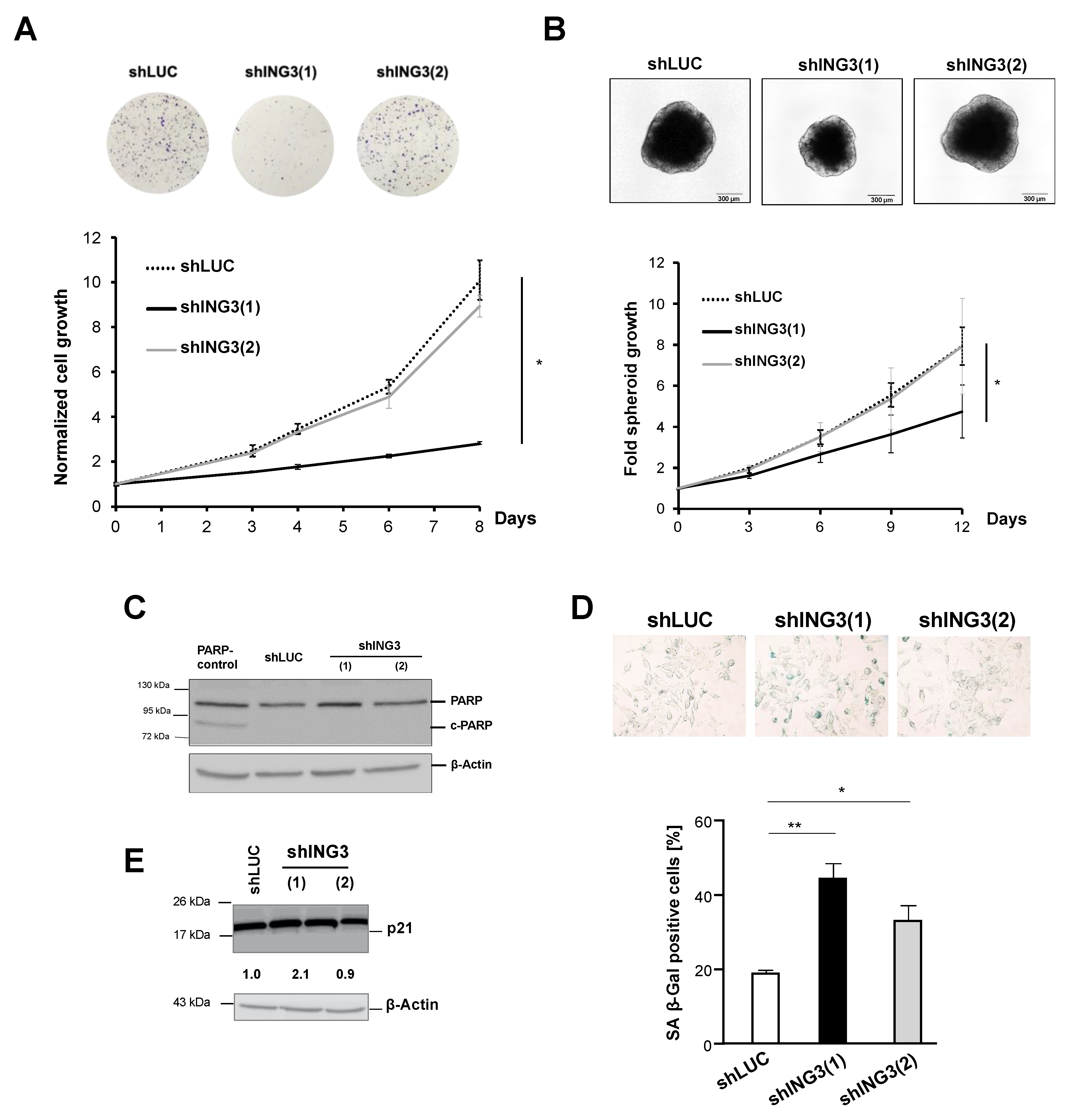
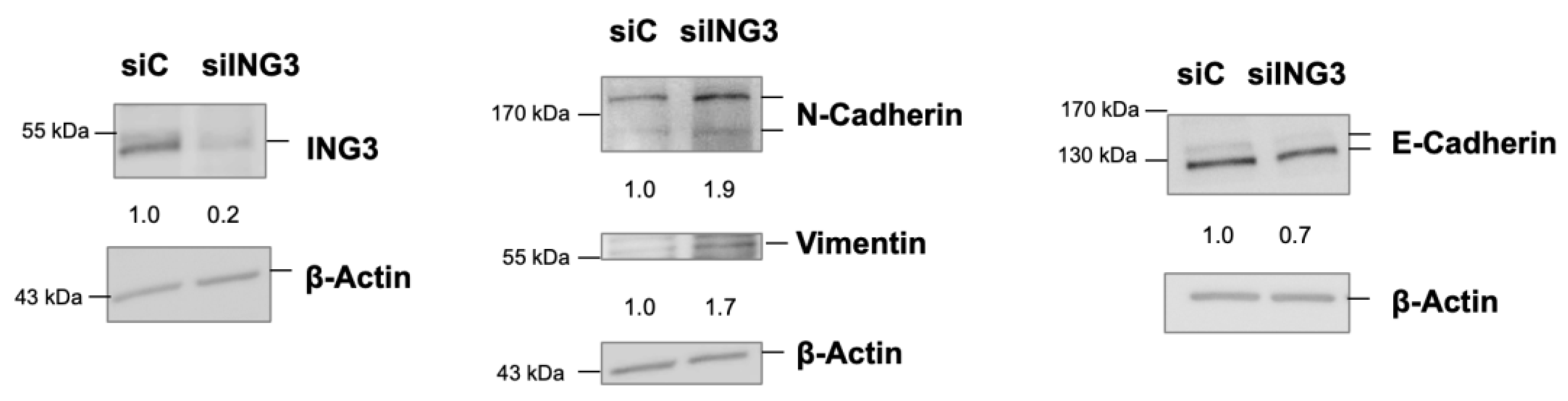
2.3. The Knockdown of ING3 Induces EMT
3. Discussion
4. Materials and Methods
4.1. Cell Culture, Retroviral Transduction, and Transient Knockdown with siRNA
4.2. Generation and Analyses of 3D Spheroids
4.3. Senescence Associated Beta-Galactosidase (SA β-Gal) Assays
4.4. Reverse Transcription Quantitative Real-Time PCR (qRT-PCR)
4.5. Antibodies and Western Blot Analyses
4.6. Ex Vivo Treatment of PCa Samples from Patients
4.7. ING3 Structure Predictions
4.8. The Genome Cancer Atlas (TCGA) Database Analysis
4.9. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Garkavtsev, I.I. Suppression of the novel growth inhibitor p33ING1 promotes neoplastic transformation. Nat. Genet. 1999, 23, 373. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Esmaeili, M.; Pungsrinont, T.; Schaefer, A.; Baniahmad, A. A novel crosstalk between the tumor suppressors ING1 and ING2 regulates androgen receptor signaling. J. Mol. Med. 2016, 94, 1167–1179. [Google Scholar] [CrossRef] [PubMed]
- Pungsrinont, T.; Baniahmad, A. Cellular Senescence by the Epigenetic Regulators Inhibitor of Growth. J. Aging Sci. 2016, 4. [Google Scholar] [CrossRef] [Green Version]
- Coles, A.H.; Jones, S.N. The ING gene family in the regulation of cell growth and tumorigenesis. J. Cell Physiol. 2009, 218, 45–57. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Y.; Li, G. ING3 promotes UV-induced apoptosis via Fas/caspase-8 pathway in melanoma cells. J. Biol. Chem. 2006, 281, 11887–11893. [Google Scholar] [CrossRef] [Green Version]
- Ludwig, S.; Klitzsch, A.; Baniahmad, A. The ING tumor suppressors in cellular senescence and chromatin. Cell Biosci. 2011, 1, 25. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Y.; Wang, J.; Li, G. Leucine zipper-like domain is required for tumor suppressor ING2-mediated nucleotide excision repair and apoptosis. FEBS Lett. 2006, 580, 3787–3793. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, H.K.; Pan, K.; Wang, H.; Weng, D.S.; Song, H.F.; Zhou, J.; Huang, W.; Li, J.J.; Chen, M.S.; Xia, J.C. Decreased expression of ING2 gene and its clinicopathological significance in hepatocellular carcinoma. Cancer Lett. 2008, 261, 183–192. [Google Scholar] [CrossRef]
- Cengiz, B.; Gunduz, E.; Gunduz, M.; Beder, L.B.; Tamamura, R.; Bagci, C.; Yamanaka, N.; Shimizu, K.; Nagatsuka, H. Tumor-specific mutation and downregulation of ING5 detected in oral squamous cell carcinoma. Int. J. Cancer 2010, 127, 2088–2094. [Google Scholar] [CrossRef]
- Gunduz, M.; Nagatsuka, H.; Demircan, K.; Gunduz, E.; Cengiz, B.; Ouchida, M.; Tsujigiwa, H.; Yamachika, E.; Fukushima, K.; Beder, L.; et al. Frequent deletion and down-regulation of ING4, a candidate tumor suppressor gene at 12p13, in head and neck squamous cell carcinomas. Gene 2005, 356, 109–117. [Google Scholar] [CrossRef]
- Gou, W.F.; Sun, H.Z.; Zhao, S.; Niu, Z.F.; Mao, X.Y.; Takano, Y.; Zheng, H.C. Downregulated inhibitor of growth 3 (ING3) expression during colorectal carcinogenesis. Indian J. Med. Res. 2014, 139, 561–567. [Google Scholar] [PubMed]
- Gunduz, M.; Ouchida, M.; Fukushima, K.; Ito, S.; Jitsumori, Y.; Nakashima, T.; Nagai, N.; Nishizaki, K.; Shimizu, K. Allelic loss and reduced expression of the ING3, a candidate tumor suppressor gene at 7q31, in human head and neck cancers. Oncogene 2002, 21, 4462–4470. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shah, S.; Smith, H.; Feng, X.; Rancourt, D.E.; Riabowol, K. ING function in apoptosis in diverse model systems. Biochem. Cell Biol. 2009, 87, 117–125. [Google Scholar] [CrossRef]
- Xu, M.; Xie, Y.; Sheng, W.; Miao, J.; Yang, J. Adenovirus-mediated ING4 Gene Transfer in Osteosarcoma Suppresses Tumor Growth via Induction of Apoptosis and Inhibition of Tumor Angiogenesis. Technol. Cancer Res. Treat. 2015, 14, 369–378. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mouche, A.; Archambeau, J.; Ricordel, C.; Chaillot, L.; Bigot, N.; Guillaudeux, T.; Grenon, M.; Pedeux, R. ING3 is required for ATM signaling and DNA repair in response to DNA double strand breaks. Cell Death Differ. 2019, 26, 2344–2357. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Natesan, S.; Cornilescu, G.; Carlson, S.; Tonelli, M.; McClurg, U.L.; Binda, O.; Robson, C.N.; Markley, J.L.; Balaz, S.; et al. Mechanism of Histone H3K4me3 Recognition by the Plant Homeodomain of Inhibitor of Growth 3. J. Biol. Chem. 2016, 291, 18326–18341. [Google Scholar] [CrossRef] [Green Version]
- Doyon, Y.; Cayrou, C.; Ullah, M.; Landry, A.J.; Cote, V.; Selleck, W.; Lane, W.S.; Tan, S.; Yang, X.J.; Cote, J. ING tumor suppressor proteins are critical regulators of chromatin acetylation required for genome expression and perpetuation. Mol. Cell 2006, 21, 51–64. [Google Scholar] [CrossRef]
- Almami, A.; Hegazy, S.A.; Nabbi, A.; Alshalalfa, M.; Salman, A.; Abou-Ouf, H.; Riabowol, K.; Bismar, T.A. ING3 is associated with increased cell invasion and lethal outcome in ERG-negative prostate cancer patients. Tumour Biol. 2016, 37, 9731–9738. [Google Scholar] [CrossRef]
- McClurg, U.L.; Nabbi, A.; Ricordel, C.; Korolchuk, S.; McCracken, S.; Heer, R.; Wilson, L.; Butler, L.M.; Irving-Hooper, B.K.; Pedeux, R.; et al. Human ex vivo prostate tissue model system identifies ING3 as an oncoprotein. Br. J. Cancer 2018, 118, 713–726. [Google Scholar] [CrossRef] [Green Version]
- Nabbi, A.; McClurg, U.L.; Thalappilly, S.; Almami, A.; Mobahat, M.; Bismar, T.A.; Binda, O.; Riabowol, K.T. ING3 promotes prostate cancer growth by activating the androgen receptor. BMC Med. 2017, 15, 103. [Google Scholar] [CrossRef] [Green Version]
- Li, X.; Zhang, Q.; Zhang, M.; Luo, Y.; Fu, Y. Downregulation of nuclear ING3 expression and translocalization to cytoplasm promotes tumorigenesis and progression in head and neck squamous cell carcinoma (HNSCC). Histol. Histopathol. 2020, 35, 681–690. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Dai, D.L.; Martinka, M.; Li, G. Prognostic significance of nuclear ING3 expression in human cutaneous melanoma. Clin. Cancer Res. 2007, 13, 4111–4116. [Google Scholar] [CrossRef] [Green Version]
- Yang, H.Y.; Liu, H.L.; Tian, L.T.; Song, R.P.; Song, X.; Yin, D.L.; Liang, Y.J.; Qu, L.D.; Jiang, H.C.; Liu, J.R.; et al. Expression and prognostic value of ING3 in human primary hepatocellular carcinoma. Exp. Biol. Med. 2012, 237, 352–361. [Google Scholar] [CrossRef] [PubMed]
- Fink, D.; Yau, T.; Nabbi, A.; Wagner, B.; Wagner, C.; Hu, S.M.; Lang, V.; Handschuh, S.; Riabowol, K.; Rulicke, T. Loss of Ing3 Expression Results in Growth Retardation and Embryonic Death. Cancers 2019, 12, 80. [Google Scholar] [CrossRef] [Green Version]
- Nagashima, M.; Shiseki, M.; Pedeux, R.M.; Okamura, S.; Kitahama-Shiseki, M.; Miura, K.; Yokota, J.; Harris, C.C. A novel PHD-finger motif protein, p47ING3, modulates p53-mediated transcription, cell cycle control, and apoptosis. Oncogene 2003, 22, 343–350. [Google Scholar] [CrossRef] [Green Version]
- Zhao, S.; Wang, L.; Zhang, C.; Deng, Y.; Zhao, B.; Ren, Y.; Fu, Y.; Meng, X. Inhibitor of growth 3 induces cell death by regulating cell proliferation, apoptosis and cell cycle arrest by blocking the PI3K/AKT pathway. Cancer Gene Ther. 2018, 25, 240–247. [Google Scholar] [CrossRef] [PubMed]
- Roediger, J.; Hessenkemper, W.; Bartsch, S.; Manvelyan, M.; Huettner, S.S.; Liehr, T.; Esmaeili, M.; Foller, S.; Petersen, I.; Grimm, M.O.; et al. Supraphysiological androgen levels induce cellular senescence in human prostate cancer cells through the Src-Akt pathway. Mol. Cancer 2014, 13, 214. [Google Scholar] [CrossRef] [Green Version]
- Pungsrinont, T.; Sutter, M.F.; Ertingshausen, M.; Lakshmana, G.; Kokal, M.; Khan, A.S.; Baniahmad, A. Senolytic compounds control a distinct fate of androgen receptor agonist- and antagonist-induced cellular senescent LNCaP prostate cancer cells. Cell Biosci. 2020, 10, 59. [Google Scholar] [CrossRef] [Green Version]
- Mosaad, E.O.; Chambers, K.F.; Futrega, K.; Clements, J.A.; Doran, M.R. The Microwell-mesh: A high-throughput 3D prostate cancer spheroid and drug-testing platform. Sci. Rep. 2018, 8, 253. [Google Scholar] [CrossRef]
- Zhou, Y. The Application of Ultrasound in 3D Bio-Printing. Molecules 2016, 21, 590. [Google Scholar] [CrossRef]
- Saxena, K.; Jolly, M.K.; Balamurugan, K. Hypoxia, partial EMT and collective migration: Emerging culprits in metastasis. Transl. Oncol. 2020, 13, 100845. [Google Scholar] [CrossRef] [PubMed]
- Rusthoven, C.G.; Carlson, J.A.; Waxweiler, T.V.; Yeh, N.; Raben, D.; Flaig, T.W.; Kavanagh, B.D. The prognostic significance of Gleason scores in metastatic prostate cancer. Urol. Oncol. 2014, 32, 707–713. [Google Scholar] [CrossRef] [PubMed]
- Dantas, A.; Al Shueili, B.; Yang, Y.; Nabbi, A.; Fink, D.; Riabowol, K. Biological Functions of the ING Proteins. Cancers 2019, 11, 1817. [Google Scholar] [CrossRef] [Green Version]
- Ma, Y.; Yan, R.; Wan, Q.; Lv, B.; Yang, Y.; Lv, T.; Xin, W. Inhibitor of growth 2 regulates the high glucose-induced cell cycle arrest and epithelial-to-mesenchymal transition in renal proximal tubular cells. J. Physiol. Biochem. 2020, 76, 373–382. [Google Scholar] [CrossRef]
- Wang, C.J.; Yang, D.; Luo, Y.W. Recombinant ING4 suppresses the migration of SW579 thyroid cancer cells via epithelial to mesenchymal transition. Exp. Ther. Med. 2015, 10, 603–607. [Google Scholar] [CrossRef] [Green Version]
- Qu, H.; Yin, H.; Yan, S.; Tao, M.; Xie, Y.; Chen, W. Inhibitor of growth 4 suppresses colorectal cancer growth and invasion by inducing G1 arrest, inhibiting tumor angiogenesis and reversing epithelial-mesenchymal transition. Oncol. Rep. 2016, 35, 2927–2935. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, Q.Y.; Ju, F.; Wang, Z.H.; Ma, X.Z.; Zhao, H. ING5 inhibits epithelial-mesenchymal transition in breast cancer by suppressing PI3K/Akt pathway. Int. J. Clin. Exp. Med. 2015, 8, 15498–15505. [Google Scholar]
- Gao, W.; Han, J. Overexpression of ING5 inhibits HGF-induced proliferation, invasion and EMT in thyroid cancer cells via regulation of the c-Met/PI3K/Akt signaling pathway. Biomed. Pharmacother. 2018, 98, 265–270. [Google Scholar] [CrossRef]



| Gene (Protein) | Primer sequence 5′-3′ |
|---|---|
| CDKN1A (p21) | fw: TCGACTTTGTCACCGAGACACCAC rev: CAGGTCCACATGGTCTTCCTCTG |
| CDKN2A (p16) | fw: CTTGCCTGGAAAGATACCG rev: CCCTCCTCTTTCTTCCTCC |
| GAPDH 5′ | fw: GTGAACCATGAGAAGTATGACAAC rev: GAGTCCTTCCACGATACC |
| ING3 (p47) | fw: CAGATGAAGGAGGGACGAAG rev: GCCGAAGATGATGAATAGCC |
| ING3Δex11 | fw: GCATTTGTAATCAGTGCCCTAT rev: TCTGCTGCCTCTTCTCTTCATTGC |
| SNAI1 (Snail) | fw: CAGTGCCTCGACCACTATGC rev: TGCTGGAAGGTAAACTCTGGAT |
| SNAI2 (Slug) | fw: TTCGGACCCACACATTACCT rev: CTTCTCCCCCGTGTGAGTTCT |
| TBP | fw: GGCGTGTGAAGATAACCCAAGG rev: CGCTGGAACTCGTCTCACT |
| a-Tubulin | fw: TGGAACCCACAGTCATTGATGA rev: TGATCTCCTTGCCAATGGTGTA |
| TWIST1 (Twist) | fw: CGGAGACCTAGATGTCATTGTTTC rev: CCCACGCCCTGTTTCTTTGAA |
| ZEB1 (Zeb1) | fw: ACTCAACTACGGTCAGCCCT rev: ATCTTGTCTTTCATCCTGATTTCCA |
| β-Actin | fw: CACCACACCTTCTACAATGAGC rev: CACAGCCTGGATAGCAACG |
| Primer | Localisation in Exon-Nr. | Sequence (5′-3′) | Localisation (bp) |
|---|---|---|---|
| fwd7 | 8 | CCACAGCCTCTTCTAACAATG | 709–729 |
| fwd8 | 9 | GGGACGAAGAACATCAAGT | 857–875 |
| fwd9 | 10 | CTTCAAGCCAGCAGTCATC | 1057–1075 |
| Primer | Localisation in Exon-Nr. | Sequence (5′-3′) | Localisation (bp) |
|---|---|---|---|
| rev1 | 12 | CTGTCAATCCAACGCAGC | 1317–1300 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Melekhova, A.; Leeder, M.; Pungsrinont, T.; Schmäche, T.; Kallenbach, J.; Ehsani, M.; Mirzakhani, K.; Rasa, S.M.M.; Neri, F.; Baniahmad, A. A Novel Splice Variant of the Inhibitor of Growth 3 Lacks the Plant Homeodomain and Regulates Epithelial–Mesenchymal Transition in Prostate Cancer Cells. Biomolecules 2021, 11, 1152. https://doi.org/10.3390/biom11081152
Melekhova A, Leeder M, Pungsrinont T, Schmäche T, Kallenbach J, Ehsani M, Mirzakhani K, Rasa SMM, Neri F, Baniahmad A. A Novel Splice Variant of the Inhibitor of Growth 3 Lacks the Plant Homeodomain and Regulates Epithelial–Mesenchymal Transition in Prostate Cancer Cells. Biomolecules. 2021; 11(8):1152. https://doi.org/10.3390/biom11081152
Chicago/Turabian StyleMelekhova, Anna, Mirjam Leeder, Thanakorn Pungsrinont, Tim Schmäche, Julia Kallenbach, Marzieh Ehsani, Kimia Mirzakhani, Seyed Mohammad Mahdi Rasa, Francesco Neri, and Aria Baniahmad. 2021. "A Novel Splice Variant of the Inhibitor of Growth 3 Lacks the Plant Homeodomain and Regulates Epithelial–Mesenchymal Transition in Prostate Cancer Cells" Biomolecules 11, no. 8: 1152. https://doi.org/10.3390/biom11081152
APA StyleMelekhova, A., Leeder, M., Pungsrinont, T., Schmäche, T., Kallenbach, J., Ehsani, M., Mirzakhani, K., Rasa, S. M. M., Neri, F., & Baniahmad, A. (2021). A Novel Splice Variant of the Inhibitor of Growth 3 Lacks the Plant Homeodomain and Regulates Epithelial–Mesenchymal Transition in Prostate Cancer Cells. Biomolecules, 11(8), 1152. https://doi.org/10.3390/biom11081152







