High Mobility Group Box 1 in Pig Amniotic Membrane Experimentally Infected with E. coli O55
Abstract
1. Introduction
2. Materials and Methods
2.1. Bacterial Inoculum
2.2. Animals and Intra-Amniotic Infection
2.3. Microbiological Examination of Pre-Infected Amniotic Fluids
2.4. Post-Infection Sampling
2.5. Total RNA Purification and Reverse Transcription
2.6. Real-Time PCR
2.7. Immunofluorescent Detection of HMGB1 in Amniotic Membrane
2.8. Immunochemical Detection of RAGE and TLR4 in the Amniotic Membrane
2.9. ELISA
2.10. Statistical Analysis
3. Results
3.1. Survival of Infected Pig Fetuses and Translocation of E. coli O55
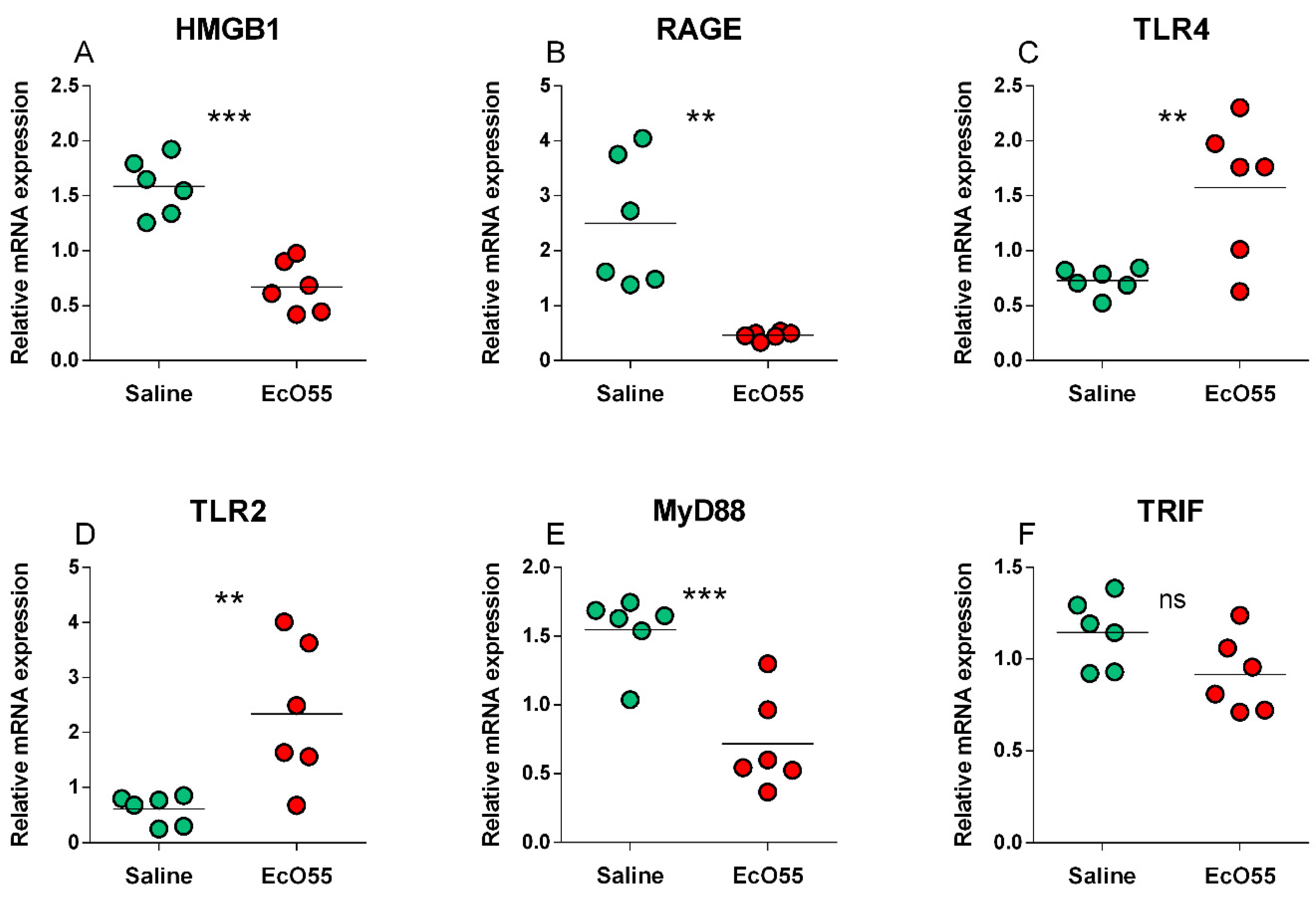
3.2. Expression of HMGB1, RAGE, TLR2, TLR4, MyD88, and TRIF mRNA in the Amniotic Membrane
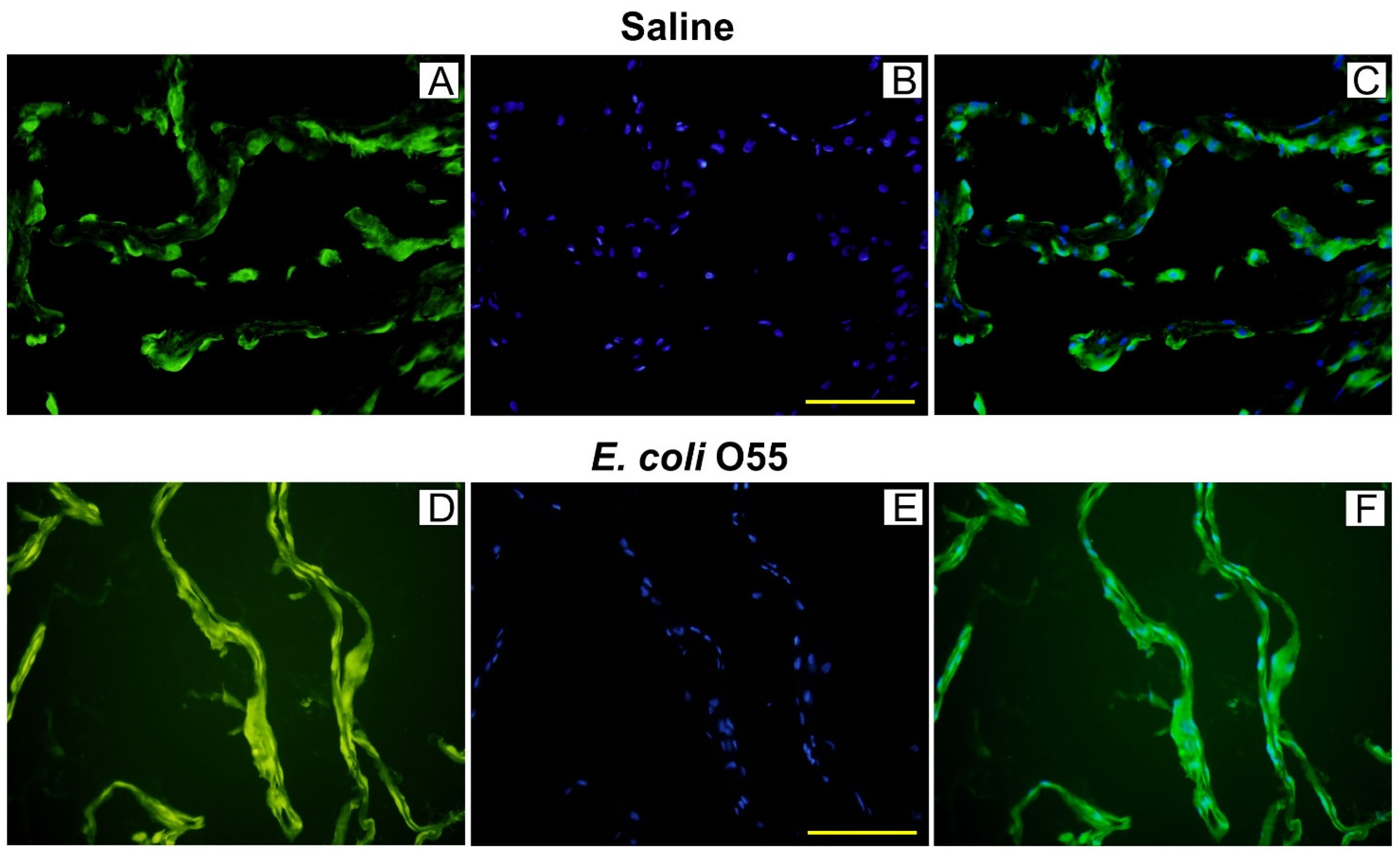
3.3. Colocalization of HMGB1 and Cell Nuclei in Amniotic Membrane
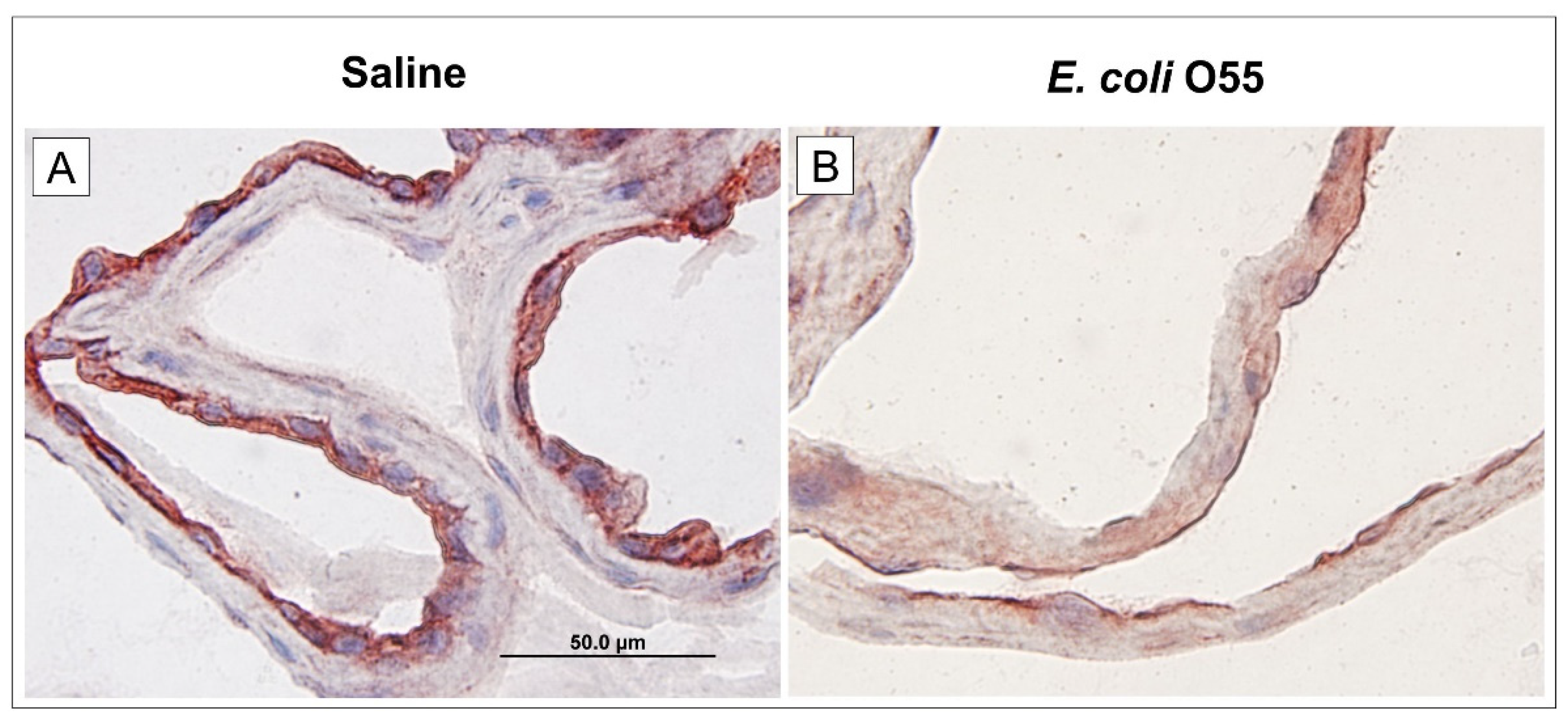
3.4. Expression of RAGE in Amniotic Membrane
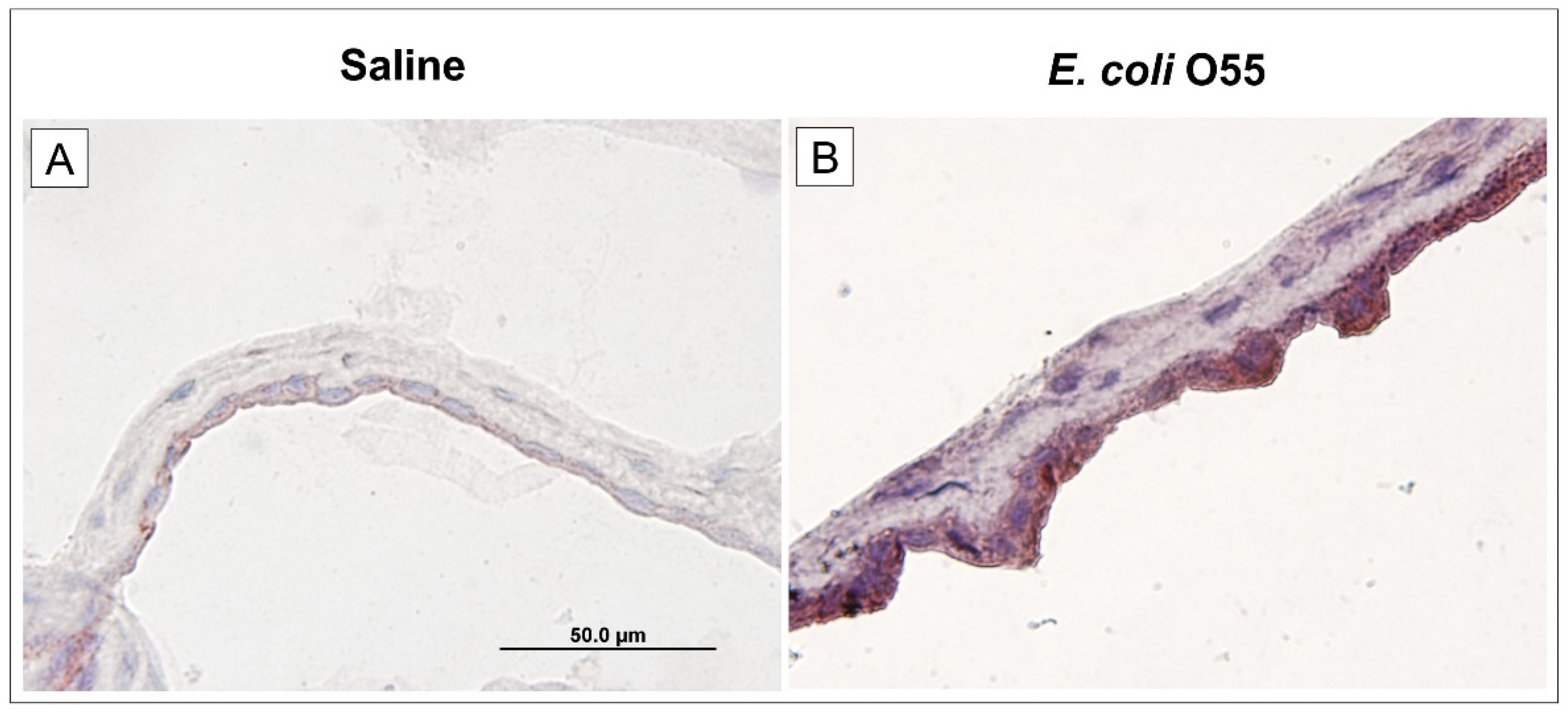
3.5. Expression of TLR4 in Amniotic Membrane
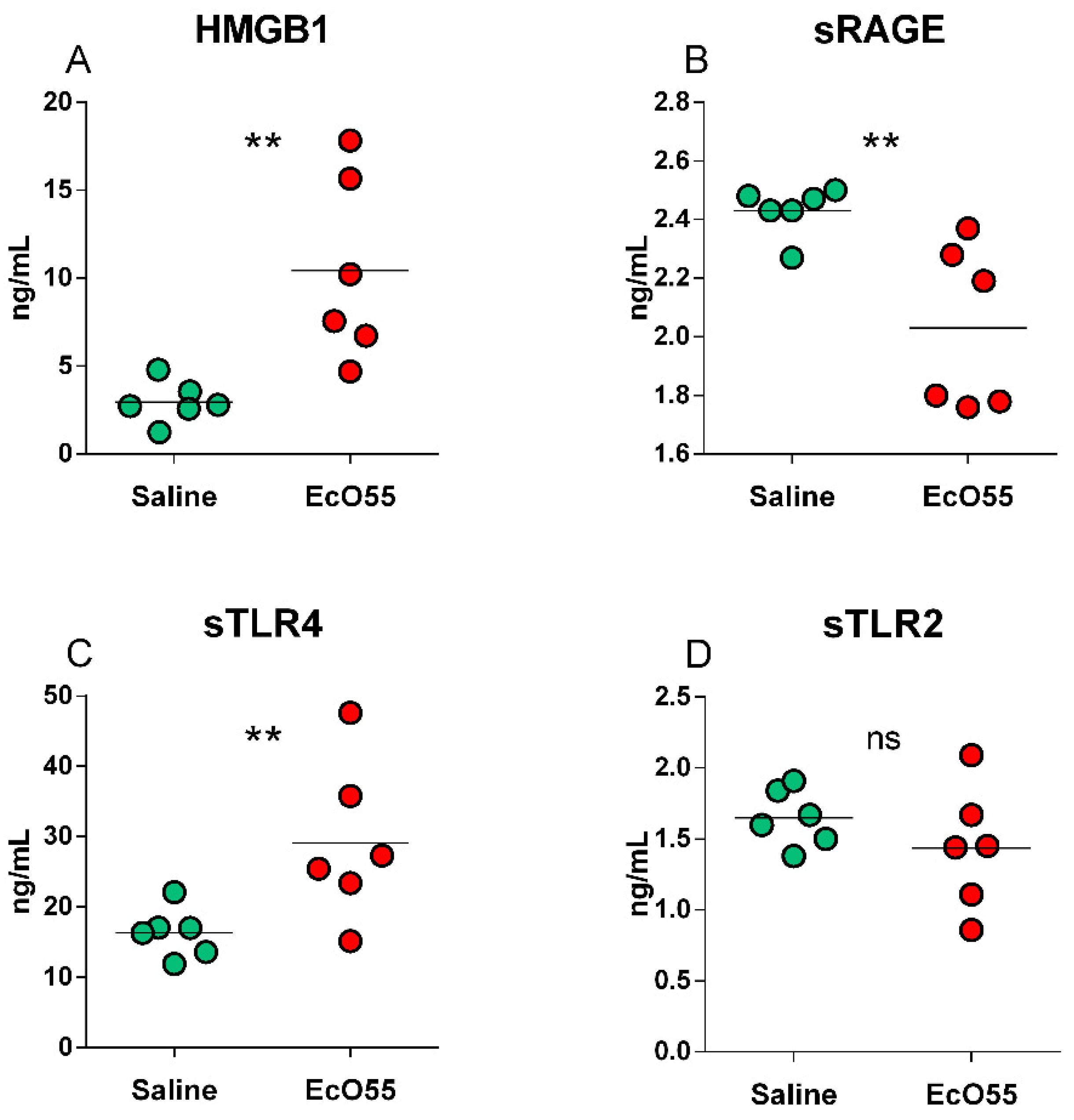
3.6. Levels of HMGB1, sRAGE, sTLR2, and sTLR4 in the Amniotic Fluid
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Goldenberg, R.L.; Culhane, J.F.; Iams, J.D.; Romero, R. Epidemiology and causes of preterm birth. Lancet 2008, 371, 75–84. [Google Scholar] [CrossRef]
- Romero, R.; Dey, S.K.; Fisher, S.J. Preterm labor: One syndrome, many causes. Science 2014, 345, 760–765. [Google Scholar] [CrossRef]
- Gravett, M.G. Successful treatment of intraamniotic infection/inflammation: A paradigm shift. Am. J. Obstet. Gynecol. 2019, 221, 83–85. [Google Scholar] [CrossRef]
- Romero, R.; Grivel, J.C.; Tarca, A.L.; Chaemsaithong, P.; Xu, Z.; Fitzgerald, W.; Hassan, S.S.; Chaiworapongsa, T.; Margolis, L. Evidence of perturbations of the cytokine network in preterm labor. Am. J. Obstet. Gynecol. 2015, 213, 836.e1–836.e18. [Google Scholar] [CrossRef]
- Elovitz, M.A.; Mrinalini, C. Animal models of preterm birth. Trends Endocrinol. Metab. 2004, 15, 479–487. [Google Scholar] [CrossRef] [PubMed]
- Theis, K.R.; Romero, R.; Winters, A.D.; Jobe, A.H.; Gomez-Lopez, N. Lack of Evidence for Microbiota in the Placental and Fetal Tissues of Rhesus Macaques. mSphere 2020, 5, e00210-20. [Google Scholar] [CrossRef] [PubMed]
- Splichalova, A.; Splichal, I.; Trebichavsky, I.; Hojna, H. Expression of inflammatory markers in pig amnion after intraamniotic infection with nonpathogenic or enteropathogenic Escherichia coli. Folia Microbiol. 2004, 49, 751–756. [Google Scholar] [CrossRef]
- Roberts, R.M.; Green, J.A.; Schulz, L.C. The evolution of the placenta. Reproduction 2016, 152, R179–R189. [Google Scholar] [CrossRef]
- Mitchell, B.F.; Taggart, M.J. Are animal models relevant to key aspects of human parturition? Am. J. Physiol. Regul. Integr. Comp. Physiol. 2009, 297, R525–R545. [Google Scholar] [CrossRef]
- Simister, N.E. Placental transport of immunoglobulin G. Vaccine 2003, 21, 3365–3369. [Google Scholar] [CrossRef]
- Salmon, H.; Berri, M.; Gerdts, V.; Meurens, F. Humoral and cellular factors of maternal immunity in swine. Dev. Comp. Immunol. 2009, 33, 384–393. [Google Scholar] [CrossRef] [PubMed]
- Peter, A.T. Bovine placenta: A review on morphology, components, and defects from terminology and clinical perspectives. Theriogenology 2013, 80, 693–705. [Google Scholar] [CrossRef] [PubMed]
- Collins, J.J.; Kallapur, S.G.; Knox, C.L.; Nitsos, I.; Polglase, G.R.; Pillow, J.J.; Kuypers, E.; Newnham, J.P.; Jobe, A.H.; Kramer, B.W. Inflammation in fetal sheep from intra-amniotic injection of Ureaplasma parvum. Am. J. Physiol. Lung. Cell. Mol. Physiol. 2010, 299, L852–L860. [Google Scholar] [CrossRef] [PubMed]
- Regan, J.K.; Kannan, P.S.; Kemp, M.W.; Kramer, B.W.; Newnham, J.P.; Jobe, A.H.; Kallapur, S.G. Damage-Associated Molecular Pattern and Fetal Membrane Vascular Injury and Collagen Disorganization in Lipopolysaccharide-Induced Intra-amniotic Inflammation in Fetal Sheep. Reprod. Sci. 2016, 23, 69–80. [Google Scholar] [CrossRef]
- Janeway, C.A., Jr. Approaching the asymptote? Evolution and revolution in immunology. Cold Spring Harb. Symp. Quant. Biol. 1989, 54, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Gong, T.; Liu, L.; Jiang, W.; Zhou, R. DAMP-sensing receptors in sterile inflammation and inflammatory diseases. Nat. Rev. Immunol. 2020, 20, 95–112. [Google Scholar] [CrossRef]
- Kawai, T.; Akira, S. Toll-like receptors and their crosstalk with other innate receptors in infection and immunity. Immunity 2011, 34, 637–650. [Google Scholar] [CrossRef]
- Chen, G.Y.; Nunez, G. Sterile inflammation: Sensing and reacting to damage. Nat. Rev. Immunol. 2010, 10, 826–837. [Google Scholar] [CrossRef]
- Andersson, U.; Yang, H.; Harris, H. High-mobility group box 1 protein (HMGB1) operates as an alarmin outside as well as inside cells. Semin. Immunol. 2018, 38, 40–48. [Google Scholar] [CrossRef]
- Bianchi, M.E.; Crippa, M.P.; Manfredi, A.A.; Mezzapelle, R.; Rovere, Q.P.; Venereau, E. High-mobility group box 1 protein orchestrates responses to tissue damage via inflammation, innate and adaptive immunity, and tissue repair. Immunol. Rev. 2017, 280, 74–82. [Google Scholar] [CrossRef]
- Nadeau-Vallee, M.; Obari, D.; Palacios, J.; Brien, M.E.; Duval, C.; Chemtob, S.; Girard, S. Sterile inflammation and pregnancy complications: A review. Reproduction 2016, 152, R277–R292. [Google Scholar] [CrossRef]
- Dumitriu, I.E.; Baruah, P.; Manfredi, A.A.; Bianchi, M.E.; Rovere-Querini, P. HMGB1: Guiding immunity from within. Trends Immunol. 2005, 26, 381–387. [Google Scholar] [CrossRef] [PubMed]
- Deng, M.; Scott, M.J.; Fan, J.; Billiar, T.R. Location is the key to function: HMGB1 in sepsis and trauma-induced inflammation. J. Leukoc. Biol. 2019, 106, 161–169. [Google Scholar] [CrossRef] [PubMed]
- Bertheloot, D.; Latz, E. HMGB1, IL-1alpha, IL-33 and S100 proteins: Dual-function alarmins. Cell. Mol. Immunol. 2017, 14, 43–64. [Google Scholar] [CrossRef]
- Paudel, Y.N.; Angelopoulou, E.; Piperi, C.; Balasubramaniam, V.R.; Othman, I.; Shaikh, M.F. Enlightening the role of high mobility group box 1 (HMGB1) in inflammation: Updates on receptor signalling. Eur. J. Pharmacol. 2019, 858, 172487. [Google Scholar] [CrossRef]
- Yang, H.; Wang, H.; Andersson, U. Targeting Inflammation Driven by HMGB1. Front. Immunol. 2020, 11, 484. [Google Scholar] [CrossRef]
- Gibbs, R.S.; Duff, P. Progress in pathogenesis and management of clinical intraamniotic infection. Am. J. Obstet. Gynecol. 1991, 164, 1317–1326. [Google Scholar] [CrossRef]
- Splichalova, A.; Splichal, I.; Chmelarova, P.; Trebichavsky, I. Alarmin HMGB1 is released in the small intestine of gnotobiotic piglets infected with enteric pathogens and its level in plasma reflects severity of sepsis. J. Clin. Immunol. 2011, 31, 488–497. [Google Scholar] [CrossRef]
- Splichalova, A.; Splichal, I. Local and systemic occurrences of HMGB1 in gnotobiotic piglets infected with E. coli O55 are related to bacterial translocation and inflammatory cytokines. Cytokine 2012, 60, 597–600. [Google Scholar] [CrossRef]
- Splichalova, A.; Trebichavsky, I.; Muneta, Y.; Mori, Y.; Splichal, I. Effect of bacterial virulence on IL-18 expression in the amnion infected with Escherichia coli. Am. J. Reprod. Immunol. 2005, 53, 255–260. [Google Scholar] [CrossRef]
- Splichal, I.; Trebichavsky, I.; Splichalova, A.; Ditetova, L.; Zahradnickova, M. Escherichia coli administered into pig amniotic cavity appear in fetal airways and attract macrophages into fetal lungs. Physiol. Res. 2002, 51, 523–528. [Google Scholar] [PubMed]
- Splichalova, A.; Slavikova, V.; Splichalova, Z.; Splichal, I. Preterm Life in Sterile Conditions: A Study on Preterm, Germ-Free Piglets. Front. Immunol. 2018, 9, 220. [Google Scholar] [CrossRef]
- Splichal, I.; Donovan, S.M.; Jenistova, V.; Splichalova, I.; Salmonova, H.; Vlkova, E.; Neuzil, B.V.; Sinkora, M.; Killer, J.; Skrivanova, E.; et al. High Mobility Group Box 1 and TLR4 Signaling Pathway in Gnotobiotic Piglets Colonized/Infected with L. amylovorus, L. mucosae, E. coli Nissle 1917 and S. Typhimurium. Int. J. Mol. Sci. 2019, 20, 6294. [Google Scholar] [CrossRef] [PubMed]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef]
- Splichal, I.; Rychlik, I.; Splichalova, I.; Karasova, D.; Splichalova, A. Toll-Like Receptor 4 Signaling in the Ileum and Colon of Gnotobiotic Piglets Infected with Salmonella Typhimurium or Its Isogenic rfa Mutants. Toxins 2020, 12, 545. [Google Scholar] [CrossRef]
- Looi, K.; Evans, D.J.; Garratt, L.W.; Ang, S.; Hillas, J.K.; Kicic, A.; Simpson, S.J. Preterm birth: Born too soon for the developing airway epithelium? Paediatr. Respir. Rev. 2019, 31, 82–88. [Google Scholar] [CrossRef]
- Coffey, L.L.; Keesler, R.I.; Pesavento, P.A.; Woolard, K.; Singapuri, A.; Watanabe, J.; Cruzen, C.; Christe, K.L.; Usachenko, J.; Yee, J.; et al. Intraamniotic Zika virus inoculation of pregnant rhesus macaques produces fetal neurologic disease. Nat. Commun. 2018, 9, 2414. [Google Scholar] [CrossRef]
- Grigsby, P.L.; Novy, M.J.; Sadowsky, D.W.; Morgan, T.K.; Long, M.; Acosta, E.; Duffy, L.B.; Waites, K.B. Maternal azithromycin therapy for Ureaplasma intraamniotic infection delays preterm delivery and reduces fetal lung injury in a primate model. Am. J. Obstet. Gynecol. 2012, 207, 475.e1–475.e14. [Google Scholar] [CrossRef]
- Gravett, M.G.; Adams, K.M.; Sadowsky, D.W.; Grosvenor, A.R.; Witkin, S.S.; Axthelm, M.K.; Novy, M.J. Immunomodulators plus antibiotics delay preterm delivery after experimental intraamniotic infection in a nonhuman primate model. Am. J. Obstet. Gynecol. 2007, 197, 518.e1–518.e8. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, D.N.; Thymann, T.; Goericke-Pesch, S.K.; Ren, S.; Wei, W.; Skovgaard, K.; Damborg, P.; Brunse, A.; van Gorp, C.; Kramer, B.W.; et al. Prenatal Intra-Amniotic Endotoxin Induces Fetal Gut and Lung Immune Responses and Postnatal Systemic Inflammation in Preterm Pigs. Am. J. Pathol. 2018, 188, 2629–2643. [Google Scholar] [CrossRef]
- Trebichavsky, I.; Splichal, I.; Zahradnickova, M.; Splichalova, A.; Mori, Y. Lipopolysaccharide induces inflammatory cytokines in the pig amnion. Vet. Immunol. Immunopathol. 2002, 87, 11–18. [Google Scholar] [CrossRef]
- Stinson, L.F.; Payne, M.S. Infection-mediated preterm birth: Bacterial origins and avenues for intervention. Aust. N. Z. J. Obstet. Gynaecol. 2019, 59, 781–790. [Google Scholar] [CrossRef]
- Matzinger, P. Tolerance, danger, and the extended family. Annu. Rev. Immunol. 1994, 12, 991–1045. [Google Scholar] [CrossRef]
- Kim, C.J.; Romero, R.; Chaemsaithong, P.; Chaiyasit, N.; Yoon, B.H.; Kim, Y.M. Acute chorioamnionitis and funisitis: Definition, pathologic features, and clinical significance. Am. J. Obstet. Gynecol. 2015, 213, S29–S52. [Google Scholar] [CrossRef] [PubMed]
- Gomez-Lopez, N.; Romero, R.; Plazyo, O.; Panaitescu, B.; Furcron, A.E.; Miller, D.; Roumayah, T.; Flom, E.; Hassan, S.S. Intra-Amniotic Administration of HMGB1 Induces Spontaneous Preterm Labor and Birth. Am. J. Reprod. Immunol. 2016, 75, 3–7. [Google Scholar] [CrossRef]
- Wang, H.; Bloom, O.; Zhang, M.; Vishnubhakat, J.M.; Ombrellino, M.; Che, J.; Frazier, A.; Yang, H.; Ivanova, S.; Borovikova, L.; et al. HMG-1 as a late mediator of endotoxin lethality in mice. Science 1999, 285, 248–251. [Google Scholar] [CrossRef]
- Cheng, Z.; Abrams, S.T.; Toh, J.; Wang, S.S.; Wang, Z.; Yu, Q.; Yu, W.; Toh, C.H.; Wang, G. The Critical Roles and Mechanisms of Immune Cell Death in Sepsis. Front. Immunol. 2020, 11, 1918. [Google Scholar] [CrossRef]
- Wang, H.; Yang, H.; Czura, C.J.; Sama, A.E.; Tracey, K.J. HMGB1 as a late mediator of lethal systemic inflammation. Am. J. Respir. Crit. Care Med. 2001, 164, 1768–1773. [Google Scholar] [CrossRef]
- Karlsson, S.; Pettila, V.; Tenhunen, J.; Laru-Sompa, R.; Hynninen, M.; Ruokonen, E. HMGB1 as a predictor of organ dysfunction and outcome in patients with severe sepsis. Intensive Care Med. 2008, 34, 1046–1053. [Google Scholar] [CrossRef] [PubMed]
- Akira, S.; Uematsu, S.; Takeuchi, O. Pathogen recognition and innate immunity. Cell 2006, 124, 783–801. [Google Scholar] [CrossRef]
- Hudson, B.I.; Lippman, M.E. Targeting RAGE Signaling in Inflammatory Disease. Annu. Rev. Med. 2018, 69, 349–364. [Google Scholar] [CrossRef]
- Bonaldi, T.; Talamo, F.; Scaffidi, P.; Ferrera, D.; Porto, A.; Bachi, A.; Rubartelli, A.; Agresti, A.; Bianchi, M.E. Monocytic cells hyperacetylate chromatin protein HMGB1 to redirect it towards secretion. EMBO J. 2003, 22, 5551–5560. [Google Scholar] [CrossRef]
- Youn, J.H.; Shin, J.S. Nucleocytoplasmic shuttling of HMGB1 is regulated by phosphorylation that redirects it toward secretion. J. Immunol. 2006, 177, 7889–7897. [Google Scholar] [CrossRef]
- Ito, I.; Fukazawa, J.; Yoshida, M. Post-translational methylation of high mobility group box 1 (HMGB1) causes its cytoplasmic localization in neutrophils. J. Biol. Chem. 2007, 282, 16336–16344. [Google Scholar] [CrossRef]
- Gardella, S.; Andrei, C.; Ferrera, D.; Lotti, L.V.; Torrisi, M.R.; Bianchi, M.E.; Rubartelli, A. The nuclear protein HMGB1 is secreted by monocytes via a non-classical, vesicle-mediated secretory pathway. EMBO Rep. 2002, 3, 995–1001. [Google Scholar] [CrossRef] [PubMed]
- Romero, R.; Chaiworapongsa, T.; Alpay, S.Z.; Xu, Y.; Hussein, Y.; Dong, Z.; Kusanovic, J.P.; Kim, C.J.; Hassan, S.S. Damage-associated molecular patterns (DAMPs) in preterm labor with intact membranes and preterm PROM: A study of the alarmin HMGB1. J. Matern. Fetal Neonatal Med. 2011, 24, 1444–1455. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, K.; Miwa, H.; Yasui, M. Inflammatory mediators weaken the amniotic membrane barrier through disruption of tight junctions. J. Physiol. 2010, 588, 4859–4869. [Google Scholar] [CrossRef]
- Romero, R.; Chaiworapongsa, T.; Savasan, Z.A.; Hussein, Y.; Dong, Z.; Kusanovic, J.P.; Kim, C.J.; Hassan, S.S. Clinical chorioamnionitis is characterized by changes in the expression of the alarmin HMGB1 and one of its receptors, sRAGE. J. Matern. Fetal Neonatal Med. 2012, 25, 558–567. [Google Scholar] [CrossRef] [PubMed]
- Baumbusch, M.A.; Buhimschi, C.S.; Oliver, E.A.; Zhao, G.; Thung, S.; Rood, K.; Buhimschi, I.A. High Mobility Group-Box 1 (HMGB1) levels are increased in amniotic fluid of women with intra-amniotic inflammation-determined preterm birth, and the source may be the damaged fetal membranes. Cytokine 2016, 81, 82–87. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Choltus, H.; Lavergne, M.; Belville, C.; Gallot, D.; Minet-Quinard, R.; Durif, J.; Blanchon, L.; Sapin, V. Occurrence of a RAGE-Mediated Inflammatory Response in Human Fetal Membranes. Front. Physiol. 2020, 11, 581. [Google Scholar] [CrossRef] [PubMed]
- Romero, R.; Miranda, J.; Chaiworapongsa, T.; Korzeniewski, S.J.; Chaemsaithong, P.; Gotsch, F.; Dong, Z.; Ahmed, A.I.; Yoon, B.H.; Hassan, S.S.; et al. Prevalence and clinical significance of sterile intra-amniotic inflammation in patients with preterm labor and intact membranes. Am. J. Reprod. Immunol. 2014, 72, 458–474. [Google Scholar] [CrossRef] [PubMed]
- Kang, R.; Chen, R.; Zhang, Q.; Hou, W.; Wu, S.; Cao, L.; Huang, J.; Yu, Y.; Fan, X.G.; Yan, Z.; et al. HMGB1 in health and disease. Mol. Asp. Med. 2014, 40, 1–116. [Google Scholar] [CrossRef]
- Vijay, K. Toll-like receptors in immunity and inflammatory diseases: Past, present, and future. Int. Immunopharmacol. 2018, 59, 391–412. [Google Scholar] [CrossRef] [PubMed]
- Caroff, M.; Karibian, D. Structure of bacterial lipopolysaccharides. Carbohydr. Res. 2003, 338, 2431–2447. [Google Scholar] [CrossRef]
- Yamamoto, Y.; Harashima, A.; Saito, H.; Tsuneyama, K.; Munesue, S.; Motoyoshi, S.; Han, D.; Watanabe, T.; Asano, M.; Takasawa, S.; et al. Septic shock is associated with receptor for advanced glycation end products ligation of LPS. J. Immunol. 2011, 186, 3248–3257. [Google Scholar] [CrossRef]
- Palanissami, G.; Paul, S.F.D. RAGE and Its Ligands: Molecular Interplay Between Glycation, Inflammation, and Hallmarks of Cancer—A Review. Horm. Cancer 2018, 9, 295–325. [Google Scholar] [CrossRef] [PubMed]
- Romero, R.; Espinoza, J.; Hassan, S.; Gotsch, F.; Kusanovic, J.P.; Avila, C.; Erez, O.; Edwin, S.; Schmidt, A.M. Soluble receptor for advanced glycation end products (sRAGE) and endogenous secretory RAGE (esRAGE) in amniotic fluid: Modulation by infection and inflammation. J. Perinat. Med. 2008, 36, 388–398. [Google Scholar] [CrossRef]
- Buhimschi, I.A.; Zhao, G.; Pettker, C.M.; Bahtiyar, M.O.; Magloire, L.K.; Thung, S.; Fairchild, T.; Buhimschi, C.S. The receptor for advanced glycation end products (RAGE) system in women with intraamniotic infection and inflammation. Am. J. Obstet. Gynecol. 2007, 196, 181.e1–181.e13. [Google Scholar] [CrossRef]
- Cao, X. Self-regulation and cross-regulation of pattern-recognition receptor signalling in health and disease. Nat. Rev. Immunol. 2016, 16, 35–50. [Google Scholar] [CrossRef] [PubMed]
- Takeuchi, O.; Akira, S. Pattern recognition receptors and inflammation. Cell 2010, 140, 805–820. [Google Scholar] [CrossRef]
- Wu, Z.; Zhang, Z.; Lei, Z.; Lei, P. CD14: Biology and role in the pathogenesis of disease. Cytokine Growth Factor Rev. 2019, 48, 24–31. [Google Scholar] [CrossRef]
- Dulay, A.T.; Buhimschi, C.S.; Zhao, G.; Oliver, E.A.; Abdel-Razeq, S.S.; Shook, L.L.; Bahtiyar, M.O.; Buhimschi, I.A. Amniotic Fluid Soluble Myeloid Differentiation-2 (sMD-2) as Regulator of Intra-amniotic Inflammation in Infection-induced Preterm Birth. Am. J. Reprod. Immunol. 2015, 73, 507–521. [Google Scholar] [CrossRef]
- Dziarski, R.; Viriyakosol, S.; Kirkland, T.N.; Gupta, D. Soluble CD14 enhances membrane CD14-mediated responses to peptidoglycan: Structural requirements differ from those for responses to lipopolysaccharide. Infect. Immun. 2000, 68, 5254–5260. [Google Scholar] [CrossRef][Green Version]
- Adams, K.M.; Lucas, J.; Kapur, R.P.; Stevens, A.M. LPS induces translocation of TLR4 in amniotic epithelium. Placenta 2007, 28, 477–481. [Google Scholar] [CrossRef]
- Kacerovsky, M.; Andrys, C.; Hornychova, H.; Pliskova, L.; Lancz, K.; Musilova, I.; Drahosova, M.; Bolehovska, R.; Tambor, V.; Jacobsson, B. Amniotic fluid soluble Toll-like receptor 4 in pregnancies complicated by preterm prelabor rupture of the membranes. J. Matern. Fetal Neonatal Med. 2012, 25, 1148–1155. [Google Scholar] [CrossRef]
- Robertson, S.A.; Wahid, H.H.; Chin, P.Y.; Hutchinson, M.R.; Moldenhauer, L.M.; Keelan, J.A. Toll-like Receptor-4: A New Target for Preterm Labour Pharmacotherapies? Curr. Pharm. Des. 2018, 24, 960–973. [Google Scholar] [CrossRef] [PubMed]
- Robertson, S.A.; Hutchinson, M.R.; Rice, K.C.; Chin, P.Y.; Moldenhauer, L.M.; Stark, M.J.; Olson, D.M.; Keelan, J.A. Targeting Toll-like receptor-4 to tackle preterm birth and fetal inflammatory injury. Clin. Transl. Immunol. 2020, 9, e1121. [Google Scholar] [CrossRef]
- Wahid, H.H.; Dorian, C.L.; Chin, P.Y.; Hutchinson, M.R.; Rice, K.C.; Olson, D.M.; Moldenhauer, L.M.; Robertson, S.A. Toll-Like Receptor 4 Is an Essential Upstream Regulator of On-Time Parturition and Perinatal Viability in Mice. Endocrinology 2015, 156, 3828–3841. [Google Scholar] [CrossRef]
- Raby, A.C.; Holst, B.; Le, B.E.; Diaz, C.; Ferran, E.; Conraux, L.; Guillemot, J.C.; Coles, B.; Kift-Morgan, A.; Colmont, C.S.; et al. Targeting the TLR co-receptor CD14 with TLR2-derived peptides modulates immune responses to pathogens. Sci. Transl. Med. 2013, 5, 185ra64. [Google Scholar] [CrossRef] [PubMed]
- Dziarski, R.; Wang, Q.; Miyake, K.; Kirschning, C.J.; Gupta, D. MD-2 enables Toll-like receptor 2 (TLR2)-mediated responses to lipopolysaccharide and enhances TLR2-mediated responses to Gram-positive and Gram-negative bacteria and their cell wall components. J. Immunol. 2001, 166, 1938–1944. [Google Scholar] [CrossRef] [PubMed]
- Lembo, A.; Kalis, C.; Kirschning, C.J.; Mitolo, V.; Jirillo, E.; Wagner, H.; Galanos, C.; Freudenberg, M.A. Differential contribution of Toll-like receptors 4 and 2 to the cytokine response to Salmonella enterica serovar Typhimurium and Staphylococcus aureus in mice. Infect. Immun. 2003, 71, 6058–6062. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, Z.A.; Armour, C.L.; Phipps, S.; Sukkar, M.B. RAGE and TLRs: Relatives, friends or neighbours? Mol. Immunol. 2013, 56, 739–744. [Google Scholar] [CrossRef] [PubMed]
- Dulay, A.T.; Buhimschi, C.S.; Zhao, G.; Oliver, E.A.; Mbele, A.; Jing, S.; Buhimschi, I.A. Soluble TLR2 is present in human amniotic fluid and modulates the intraamniotic inflammatory response to infection. J. Immunol. 2009, 182, 7244–7253. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Splichal, I.; Splichalova, A. High Mobility Group Box 1 in Pig Amniotic Membrane Experimentally Infected with E. coli O55. Biomolecules 2021, 11, 1146. https://doi.org/10.3390/biom11081146
Splichal I, Splichalova A. High Mobility Group Box 1 in Pig Amniotic Membrane Experimentally Infected with E. coli O55. Biomolecules. 2021; 11(8):1146. https://doi.org/10.3390/biom11081146
Chicago/Turabian StyleSplichal, Igor, and Alla Splichalova. 2021. "High Mobility Group Box 1 in Pig Amniotic Membrane Experimentally Infected with E. coli O55" Biomolecules 11, no. 8: 1146. https://doi.org/10.3390/biom11081146
APA StyleSplichal, I., & Splichalova, A. (2021). High Mobility Group Box 1 in Pig Amniotic Membrane Experimentally Infected with E. coli O55. Biomolecules, 11(8), 1146. https://doi.org/10.3390/biom11081146






