Pathological Roles and Clinical Usefulness of Periostin in Type 2 Inflammation and Pulmonary Fibrosis
Abstract
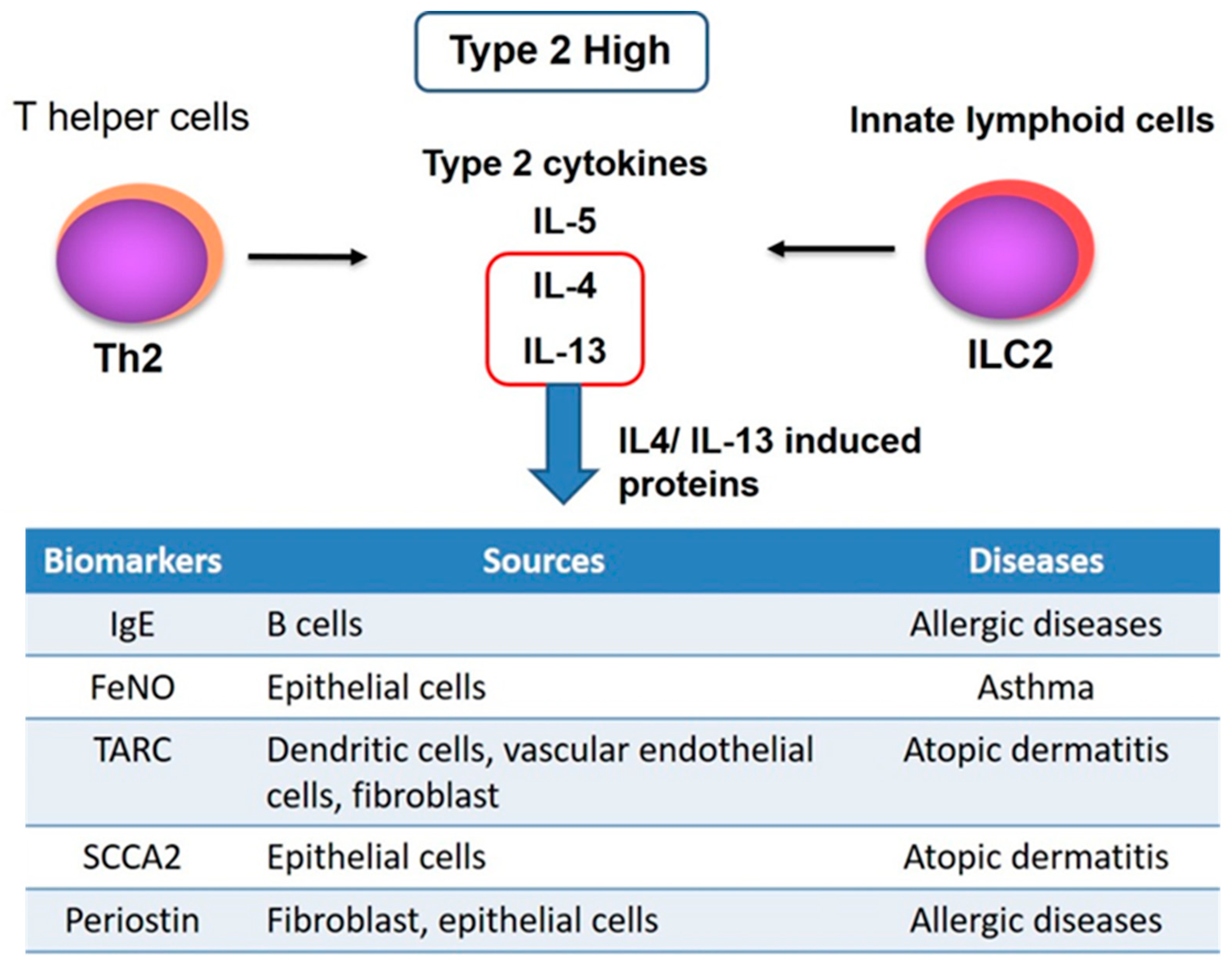
1. Introduction
2. Importance of Development of a Biomarker for Type2 Inflammation
2.1. IgE
2.2. Eosinophil
2.3. Fractional Exhaled Nitric Oxide (FeNO)
2.4. Thymus and Activation-Regulated Chemokine (TARC)
2.5. Squamous Cell Carcinoma Antigens (SCCA2)
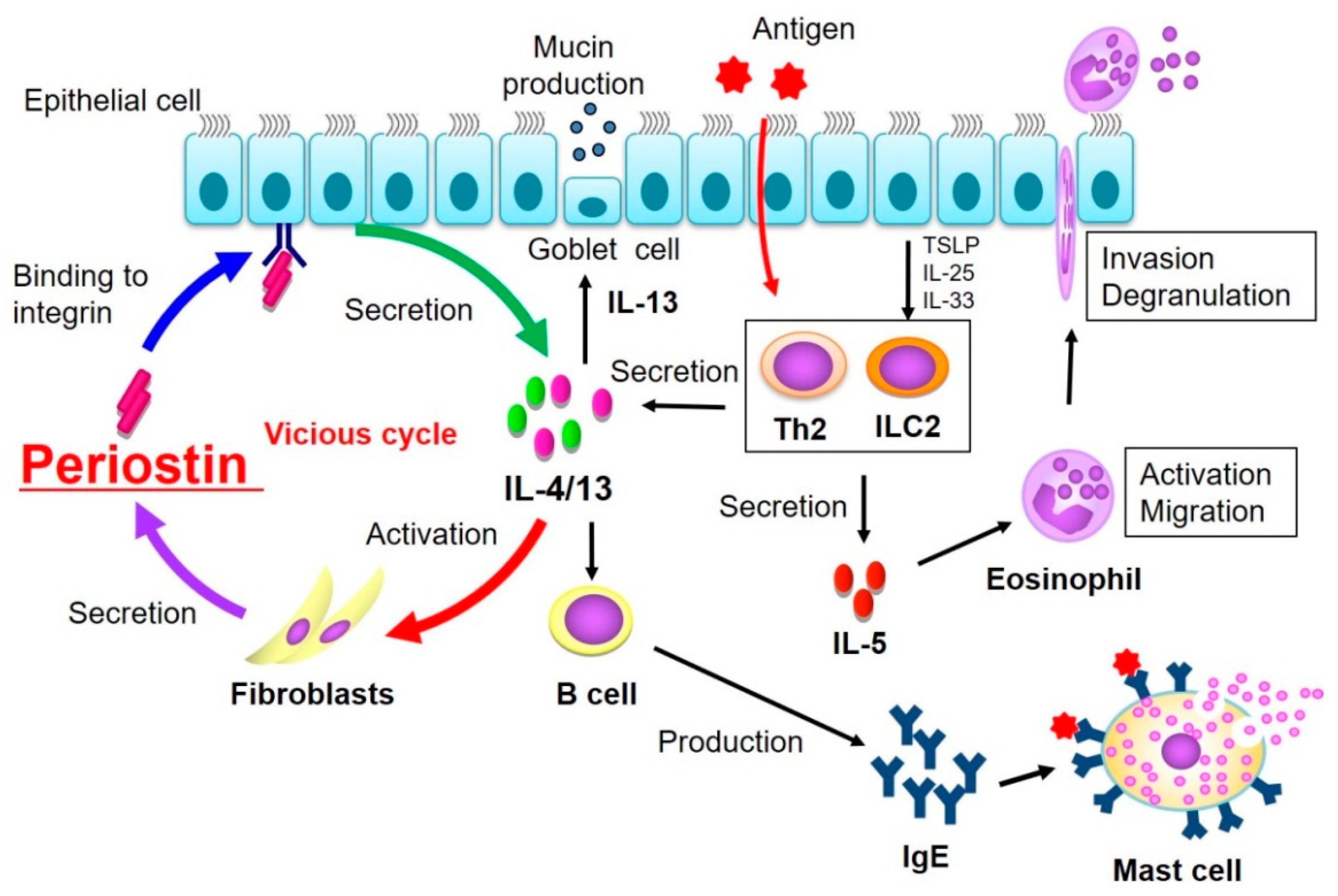
3. Pathogenic Role of Periostin
3.1. Discovery of Periostin
3.2. Pathological Role of Periostin in Allergic Inflammation and Interstitial Lung Disease
4. Periostin as a Biomarker
4.1. Periostin Assays
4.2. Clinical Significance of Serum Periostin
4.2.1. Utility as a Biomarker for Eosinophilic Chronic Rhinosinusitis (ECRS)
4.2.2. Utility as a Biomarker for Idiopathic Pulmonary Fibrosis
4.3. Utility of Periostin in Local Biological Samples
Clinical Usefulness of Tear Periostin
4.4. Periostin as a Biomarker in Therapy
4.5. Variation of Serum Periostin in Healthy Subjects
5. Future Development as Periostin Biomarker
6. Conclusions and Perspectives
Supplementary Materials
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Takeshita, S.; Kikuno, R.; Tezuka, K.-I.; Amann, E. Osteoblast-specific factor 2: Cloning of a putative bone adhesion protein with homology with the insect protein fasciclin I. Biochem. J. 1993, 294, 271–278. [Google Scholar] [CrossRef]
- Horiuchi, K.; Amizuka, N.; Takeshita, S.; Takamatsu, H.; Katsuura, M.; Ozawa, H.; Toyama, Y.; Bonewald, L.F.; Kudo, A. Identification and characterization of a novel protein, periostin, with restricted expression to periosteum and periodontal ligament and increased expression by transforming growth factor beta. J. Bone Miner. Res. 1999, 14, 1239–1249. [Google Scholar] [CrossRef]
- Conway, S.J.; Izuhara, K.; Kudo, Y.; Litvin, J.; Markwald, R.; Ouyang, G.; Arron, J.R.; Holweg, C.T.; Kudo, A. The role of periostin in tissue remodeling across health and disease. Cell. Mol. Life Sci. 2014, 71, 1279–1288. [Google Scholar] [CrossRef]
- Izuhara, K.; Arima, K.; Ohta, S.; Suzuki, S.; Inamitsu, M.; Yamamoto, K. Periostin in allergic inflammation. Allergol. Int. 2014, 63, 143–151. [Google Scholar] [CrossRef]
- Takayama, G.; Arima, K.; Kanaji, T.; Toda, S.; Tanaka, H.; Shoji, S.; McKenzie, A.N.; Nagai, H.; Izuhara, T.H.K. Periostin: A novel component of subepithelial fibrosis of bronchial asthma downstream of IL-4 and IL-13 signals. J. Allergy Clin. Immunol. 2006, 118, 98–104. [Google Scholar] [CrossRef] [PubMed]
- Kii, I.; Nishiyama, T.; Li, M.; Matsumoto, K.; Saito, M.; Amizuka, N.; Kudo, A. Incorporation of tenascin-C into the extracellular matrix by periostin underlies an extracellular meshwork architecture. J. Biol. Chem. 2010, 285, 2028–2039. [Google Scholar] [CrossRef]
- Norris, R.A.; Damon, B.; Mironov, V.; Kasyanov, V.; Ramamurthi, A.; Moreno-Rodriguez, R.; Trusk, T.; Potts, J.D.; Goodwin, R.L.; Davis, J.; et al. Periostin regulates collagen fibrillogenesis and the biomechanical properties of connective tissues. J. Cell. Biochem. 2007, 101, 695–711. [Google Scholar] [CrossRef] [PubMed]
- Butcher, J.T.; Norris, R.A.; Hoffman, S.; Mjaatvedt, C.H.; Markwald, R.R. Periostin promotes atrioventricular mesenchyme matrix invasion and remodeling mediated by integrin signaling through Rho/PI 3-kinase. Dev. Biol. 2007, 302, 256–266. [Google Scholar] [CrossRef] [PubMed]
- Kühn, B.; del Monte, F.; Hajjar, R.J.; Chang, Y.S.; Lebeche, D.; Arab, S.; Keating, M.T. Periostin induces proliferation of differentiated cardiomyocytes and promotes cardiac repair. Nat. Med. 2007, 13, 962–969. [Google Scholar] [CrossRef]
- Gillan, L.; Matei, D.; Fishman, D.A.; Gerbin, C.S.; Karlan, B.Y.; Chang, D.D. Periostin secreted by epithelial ovarian carcinoma is a ligand for αVβ3 and αVβ5 integrins and promotes cell motility. Cancer Res. 2002, 62, 5358–5364. [Google Scholar]
- Baril, P.; Gangeswaran, R.; Mahon, P.C.; Caulee, K.; Kocher, H.M.; Harada, T.; Zhu, M.; Kalthoff, H.; Crnogorac-Jurcevic, T.; Lemoine, N.R. Periostin promotes invasiveness and resistance of pancreatic cancer cells to hypoxia-induced cell death: Role of the beta4 integrin and the PI3k pathway. Oncogene 2007, 26, 2082–2094. [Google Scholar] [CrossRef] [PubMed]
- Johansson, M.W.; Annis, D.S.; Mosher, D.F. αMβ2 integrin-mediated adhesion and motility of IL-5-stimulated eosinophils on periostin. Am. J. Respir. Cell Mol. Biol. 2013, 48, 503–510. [Google Scholar] [CrossRef]
- Shimazaki, M.; Nakamura, K.; Kii, I.; Kashima, T.; Amizuka, N.; Li, M.; Saito, M.; Fukuda, K.; Nishiyama, T.; Kitajima, S.; et al. Periostin is essential for cardiac healing after acute myocardial infarction. J. Exp. Med. 2008, 205, 295–303. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Serada, S.; Fujimoto, M.; Terao, M.; Kotobuki, Y.; Kitaba, S.; Matsui, S.; Kudo, A.; Naka, T.; Murota, H.; et al. Periostin facilitates skin sclerosis via PI3K/Akt dependent mechanism in a mouse model of scleroderma. PLoS ONE 2012, 7, e41994. [Google Scholar] [CrossRef]
- Bao, S.; Ouyang, G.; Bai, X.; Huang, Z.; Ma, C.; Liu, M.; Shao, R.; Anderson, R.M.; Rich, J.N.; Wang, X.F. Periostin potently promotes metastatic growth of colon cancer by augmenting cell survival via the Akt/PKB pathway. Cancer Cell. 2004, 5, 329–339. [Google Scholar] [CrossRef]
- Ontsuka, K.; Kotobuki, Y.; Shiraishi, H.; Serada, S.; Ohta, S.; Tanemura, A.; Yang, L.; Fujimoto, M.; Arima, K.; Suzuki, S.; et al. Periostin, a matricellular protein, accelerates cutaneous wound repair by activating dermal fibroblasts. Exp. Dermatol. 2012, 21, 331–336. [Google Scholar] [CrossRef] [PubMed]
- Masuoka, M.; Shiraishi, H.; Ohta, S.; Suzuki, S.; Arima, K.; Aoki, S.; Toda, S.; Inagaki, N.; Kurihara, Y.; Hayashida, S.; et al. Periostin promotes chronic allergic inflammation in response to Th2 cytokines. J. Clin. Investig. 2012, 122, 2590–2600. [Google Scholar] [CrossRef]
- Nishiyama, T.; Kii, I.; Kashima, T.G.; Kikuchi, Y.; Ohazama, A.; Shimazaki, M.; Fukayama, M.; Kudo, A. Delayed re-epithelialization in periostin-deficient mice during cutaneous wound healing. PLoS ONE 2011, 6, e18410. [Google Scholar] [CrossRef]
- Woodruff, P.G.; Modrek, B.; Choy, D.F.; Jia, G.; Abbas, A.R.; Ellwanger, A.; Koth, L.L.; Arron, J.R.; Fahy, J.V. T-helper type 2-driven inflammation defines major subphenotypes of asthma. Am. J. Respir. Crit. Care Med. 2009, 180, 388–395. [Google Scholar] [CrossRef]
- Corren, J.; Lemanske, R.F.; Hanania, N.A.; Korenblat, P.E.; Parsey, M.V.; Arron, J.R.; Harris, J.M.; Scheerens, H.; Wu, L.C.; Su, Z.; et al. Lebrikizumab treatment in adults with asthma. N. Engl. J. Med. 2011, 365, 1088–1098. [Google Scholar] [CrossRef] [PubMed]
- Kou, K.; Okawa, T.; Yamaguchi, Y.; Ono, J.; Inoue, Y.; Kohno, M.; Matsukura, S.; Kambara, T.; Ohta, S.; Izuhara, K.; et al. Periostin levels correlate with disease severity and chronicity in patients with atopic dermatitis. Br. J. Dermatol. 2014, 171, 283–291. [Google Scholar] [CrossRef]
- Ozceker, D.; Yucel, E.; Sipahi, S.; Dilek, F.; Ozkaya, E.; Guler, E.M.; Kocyigit, A.; Guler, N.; Tamay, Z. Evaluation of periostin level for predicting severity and chronicity of childhood atopic dermatitis. Postepy Dermatol. Alergol. 2019, 36, 616–619. [Google Scholar] [CrossRef]
- Kanemitsu, Y.; Matsumoto, H.; Izuhara, K.; Tohda, Y.; Kita, H.; Horiguchi, T.; Kuwabara, K.; Tomii, K.; Otsuka, K.; Fujimura, M.; et al. Increased periostin associates with greater airflow limitation in patients receiving inhaled corticosteroids. J. Allergy Clin. Immunol. 2013, 132, 305–312. [Google Scholar] [CrossRef] [PubMed]
- Matsusaka, M.; Kabata, H.; Fukunaga, K.; Suzuki, Y.; Masaki, K.; Mochimaru, T.; Sakamak78i, F.; Oyamada, Y.; Inoue, T.; Oguma, T.; et al. Phenotype of asthma related with high serum periostin levels. Allergol. Int. 2015, 64, 175–180. [Google Scholar] [CrossRef] [PubMed]
- Izuhara, K.; Nunomura, S.; Nanri, Y.; Ono, J.; Takai, M.; Kawaguchi, A. Periostin: An emerging biomarker for allergic diseases. Allergy 2019, 74, 2116–2128. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, H. Serum periostin: A novel biomarker for asthma management. Allergol. Int. 2014, 63, 153–160. [Google Scholar] [CrossRef]
- Okamoto, M.; Hoshino, T.; Kitasato, Y.; Sakazaki, Y.; Kawayama, T.; Fujimoto, K.; Ohshima, K.; Shiraishi, H.; Uchida, M.; Ono, J.; et al. Periostin, a matrix protein, is a novel biomarker for idiopathic interstitial pneumonias. Eur. Respir. J. 2011, 37, 1119–1127. [Google Scholar] [CrossRef]
- Tajiri, M.; Okamoto, M.; Fujimoto, K.; Johkoh, T.; Ono, J.; Tominaga, M.; Azuma, K.; Kawayama, T.; Ohta, S.; Izuhara, K.; et al. Serum level of periostin can predict long-term outcome of idiopathic pulmonary fibrosis. Respir. Investig. 2015, 53, 73–81. [Google Scholar] [CrossRef]
- Ohta, S.; Okamoto, M.; Fujimoto, K.; Sakamoto, N.; Takahashi, K.; Yamamoto, H.; Kushima, H.; Ishii, H.; Akasaka, K.; Ono, J.; et al. The usefulness of monomeric periostin as a biomarker for idiopathic pulmonary fibrosis. PLoS ONE 2017, 12, e0174547. [Google Scholar] [CrossRef]
- Okamoto, M.; Izuhara, K.; Ohta, S.; Ono, J.; Hoshino, T. Ability of Periostin as a New Biomarker of Idiopathic Pulmonary Fibrosis. Adv. Exp. Med. Biol. 2019, 1132, 79–87. [Google Scholar]
- Yuyama, N.; Davies, D.E.; Akaiwa, M.; Matsui, K.; Hamasaki, Y.; Suminami, Y.; Yoshida, N.L.; Maeda, M.; Pandit, A.; Lordan, J.L.; et al. Analysis of novel disease-related genes in bronchial asthma. Cytokine 2002, 19, 287–296. [Google Scholar] [CrossRef]
- Arima, K.; Sato, K.; Tanaka, G.; Kanaji, S.; Terada, T.; Honjo, E.; Kuroki, R.; Matsuo, Y.; Izuhara, K. Characterization of the interaction between interleukin-13 and interleukin-13 receptors. J. Biol. Chem. 2005, 280, 24915–24922. [Google Scholar] [CrossRef] [PubMed]
- Gour, N.; Wills-Karp, M. IL-4 and IL-13 signaling in allergic airway disease. Cytokine 2015, 75, 68–78. [Google Scholar] [CrossRef]
- Izuhara, K.; Conway, S.J.; Moore, B.B.; Matsumoto, H.; Holweg, C.T.; Matthews, J.G.; Arron, J.R. Roles of Periostin in Respiratory Disorders. Am. J. Respir. Crit. Care Med. 2016, 193, 949–956. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Z.; Homer, R.J.; Wang, Z.; Chen, Q.; Geba, G.P.; Wang, J.; Zhang, Y.; Elias, J.A. Pulmonary expression of interleukin-13 causes inflammation, mucus hypersecretion, subepithelial fibrosis, physiologic abnormalities, and eotaxin production. J. Clin Investig. 1999, 103, 779–788. [Google Scholar] [CrossRef]
- Rael, E.L.; Lockey, R.F. Interleukin-13 signaling and its role in asthma. World Allergy Organ. J. 2011, 4, 54–64. [Google Scholar] [CrossRef]
- Grünig, G.; Warnock, M.; Wakil, A.E.; Venkayya, R.; Brombacher, F.; Rennick, D.M.; Sheppard, D.; Mohrs, M.; Donaldson, D.D.; Locksley, R.M.; et al. Requirement for IL-13 independently of IL-4 in experimental asthma. Science 1998, 282, 5397. [Google Scholar] [CrossRef]
- Wills-Karp, M.; Luyimbazi, J.; Xu, X.; Schofield, B.; Neben, T.Y.; Karp, C.L.; Donaldson, D.D. Interleukin-13: Central mediator of allergic asthma. Science 1998, 282, 2258–2261. [Google Scholar] [CrossRef] [PubMed]
- Tomlinson, K.L.; Davies, G.C.; Sutton, D.J.; Palframan, R.T. Neutralisation of interleukin-13 in mice prevents airway pathology caused by chronic exposure to house dust mite. PLoS ONE 2010, 5, e13136. [Google Scholar] [CrossRef] [PubMed]
- Berry, M.A.; Parker, D.; Neale, N.; Woodman, L.; Morgan, A.; Monk, P.; Bradding, P.; Wardlaw, A.J.; Pavord, I.D.; Brightling, C.E. Sputum and bronchial submucosal IL-13 expression in asthma and eosinophilic bronchitis. J. Allergy Clin. Immunol. 2004, 114, 1106–1109. [Google Scholar] [CrossRef]
- Truyen, E.; Coteur, L.; Dilissen, E.; Overbergh, L.; Dupont, L.J.; Ceuppens, J.L.; Bullens, D.M. Evaluation of airway inflammation by quantitative Th1/Th2 cytokine mRNA measurement in sputum of asthma patients. Thorax 2006, 61, 202–208. [Google Scholar] [CrossRef]
- Karaman, O.; Arli, O.; Uzuner, N.; Islekel, H.; Babayigit, A.; Olmez, D.; Kose, S.; Tezcan, D. The effectiveness of asthma therapy alternatives and evaluating the effectivity of asthma therapy by interleukin-13 and interferon gamma levels in children. Allergy Asthma. Proc. 2007, 28, 204–209. [Google Scholar] [CrossRef] [PubMed]
- Oettgen, H.C.; Geha, R.S. IgE in asthma and atopy: Cellular and molecular connections. J. Clin. Investig. 1999, 104, 829–835. [Google Scholar] [CrossRef] [PubMed]
- Peters, M.C.; Kerr, S.; Dunican, E.M.; Woodruff, P.G.; Fajt, M.L.; Levy, B.D.; Israel, E.; Phillips, B.R.; Mauger, D.T.; Comhair, S.A.; et al. Refractory airway type 2 inflammation in a large subgroup of asthmatic patients treated with inhaled corticosteroids. J. Allergy Clin. Immunol. 2019, 143, 104–113. [Google Scholar] [CrossRef]
- Hamilton, R.G.; Marcotte, G.V.; Saini, S.S. Immunological methods for quantifying free and total serum IgE levels in allergy patients receiving omalizumab (Xolair) therapy. J. Immunol. Methods 2005, 303, 81–91. [Google Scholar] [CrossRef]
- Takatsu, K. Interleukin-5 and IL-5 receptor in health and diseases. Proc. Jpn. Acad. Ser. B Phys. Biol. Sci. 2011, 87, 463–485. [Google Scholar] [CrossRef] [PubMed]
- Green, R.H.; Brightling, C.E.; McKenna, S.; Hargadon, B.; Parker, D.; Bradding, P.; Wardlaw, A.J.; Pavord, I.D. Asthma exacerbations and sputum eosinophil counts: A randomised controlled trial. Lancet 2002, 360, 1715–1721. [Google Scholar] [CrossRef]
- Jayaram, L.; Pizzichini, M.M.; Cook, R.J.; Boulet, L.P.; Lemière, C.; Pizzichini, E.; Cartier, A.; Hussack, P.; Goldsmith, C.H.; Laviolette, M.; et al. Determining asthma treatment by monitoring sputum cell counts: Effect on exacerbations. Eur. Respir. J. 2006, 27, 483–494. [Google Scholar] [CrossRef]
- Buhl, R.; Humbert, M.; Bjermer, L.; Chanez, P.; Heaney, L.G.; Pavord, I.; Quirce, S.; Virchow, J.C.; Holgate, S. Severe eosinophilic asthma: A roadmap to consensus. Eur. Respir. J. 2017, 49, 1700634. [Google Scholar] [CrossRef] [PubMed]
- Katz, L.E.; Gleich, G.J.; Hartley, B.F.; Yancey, S.W.; Ortega, H.G. Blood eosinophil count is a useful biomarker to identify patients with severe eosinophilic asthma. Ann. Am. Thorac. Soc. 2014, 11, 531–536. [Google Scholar] [CrossRef]
- FitzGerald, J.M.; Bleecker, E.R.; Menzies-Gow, A.; Zangrilli, J.G.; Hirsch, I.; Metcalfe, P.; Newbold, P.; Goldman, M. Predictors of enhanced response with benralizumab for patients with severe asthma: Pooled analysis of the SIROCCO and CALIMA studies. Lancet Respir. Med. 2018, 6, 51–64. [Google Scholar] [CrossRef]
- Alderton, W.K.; Cooper, C.E.; Knowles, R.G. Nitric oxide synthases: Structure, function and inhibition. Biochem. J. 2001, 357 Pt 3, 593–615. [Google Scholar] [CrossRef]
- Kharitonov, S.A.; Yates, D.; Robbins, R.A.; Logan-Sinclair, R.; Shinebourne, E.A.; Barnes, P.J. Increased nitric oxide in exhaled air of asthmatic patients. Lancet 1994, 343, 133–135. [Google Scholar] [CrossRef]
- Chibana, K.; Trudeau, J.B.; Mustovich, A.T.; Hu, H.; Zhao, J.; Balzar, S.; Chu, H.W.; Wenzel, S.E. IL-13 induced increases in nitrite levels are primarily driven by increases in inducible nitric oxide synthase as compared with effects on arginases in human primary bronchial epithelial cells. Clin. Exp. Allergy 2008, 38, 936–946. [Google Scholar] [CrossRef]
- Modena, B.D.; Tedrow, J.R.; Milosevic, J.; Bleecker, E.R.; Meyers, D.A.; Wu, W.; Bar-Joseph, Z.; Erzurum, S.C.; Gaston, B.M.; Busse, W.W.; et al. Gene expression in relation to exhaled nitric oxide identifies novel asthma phenotypes with unique biomolecular pathways. Am. J. Respir. Crit. Care Med. 2014, 190, 1363–1372. [Google Scholar] [CrossRef]
- Saito, J.; Gibeon, D.; Macedo, P.; Menzies-Gow, A.; Bhavsar, P.K.; Chung, K.F. Domiciliary diurnal variation of exhaled nitric oxide fraction for asthma control. Eur. Respir. J. 2014, 43, 474–484. [Google Scholar] [CrossRef] [PubMed]
- Hirano, T.; Matsunaga, K.; Sugiura, H.; Minakata, Y.; Koarai, A.; Akamatsu, K.; Ichikawa, T.; Furukawa, K.; Ichinose, M. Persistent elevation of exhaled nitric oxide and modification of corticosteroid therapy in asthma. Respir. Investig. 2013, 51, 84–91. [Google Scholar] [CrossRef] [PubMed]
- Hanania, N.A.; Wenzel, S.; Rosén, K.; Hsieh, H.J.; Mosesova, S.; Choy, D.F.; Lal, P.; Arron, J.R.; Harris, J.M.; Busse, W. Exploring the effects of omalizumab in allergic asthma: An analysis of biomarkers in the EXTRA study. Am. J. Respir. Crit. Care Med. 2013, 187, 804–811. [Google Scholar] [CrossRef] [PubMed]
- Hanania, N.A.; Noonan, M.; Corren, J.; Korenblat, P.; Zheng, Y.; Fischer, S.K.; Cheu, M.; Putnam, W.S.; Murray, E.; Scheerens, H.; et al. Lebrikizumab in moderate-to-severe asthma: Pooled data from two randomised placebo-controlled studies. Thorax 2015, 70, 748–756. [Google Scholar] [CrossRef]
- Castro, M.; Corren, J.; Pavord, I.D.; Maspero, J.; Wenzel, S.; Rabe, K.F.; Busse, W.W.; Ford, L.; Sher, L.; FitzGerald, J.M.; et al. Dupilumab Efficacy and Safety in Moderate-to-Severe Uncontrolled Asthma. N. Engl. J. Med. 2018, 378, 2486–2496. [Google Scholar] [CrossRef]
- Imai, T.; Yoshida, T.; Baba, M.; Nishimura, M.; Kakizaki, M.; Yoshie, O. Molecular cloning of a novel T cell-directed CC chemokine expressed in thymus by signal sequence trap using Epstein-Barr virus vector. J. Biol. Chem. 1996, 271, 21514–21521. [Google Scholar] [CrossRef] [PubMed]
- Kataoka, Y. Thymus and activation-regulated chemokine as a clinical biomarker in atopic dermatitis. J. Dermatol. 2014, 41, 221–229. [Google Scholar] [CrossRef] [PubMed]
- Soumelis, V.; Reche, P.A.; Kanzler, H.; Yuan, W.; Edward, G.; Homey, B.; Gilliet, M.; Ho, S.; Antonenko, S.; Lauerma, A.; et al. Human epithelial cells trigger dendritic cell mediated allergic inflammation by producing TSLP. Nat. Immunol. 2002, 3, 673–680. [Google Scholar] [CrossRef] [PubMed]
- Horikawa, T.; Nakayama, T.; Hikita, I.; Yamada, H.; Fujisawa, R.; Bito, T.; Harada, S.; Fukunaga, A.; Chantry, D.; Gray, P.W.; et al. IFN-gamma-inducible expression of thymus and activation-regulated chemokine/CCL17 and macrophage-derived chemokine/CCL22 in epidermal keratinocytes and their roles in atopic dermatitis. Int. Immunol. 2002, 14, 767–773. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Shoda, T.; Futamura, K.; Kobayashi, F.; Saito, H.; Matsumoto, K.; Matsuda, A. Expression of thymus and activation-regulated chemokine (TARC) by human dermal cells, but not epidermal keratinocytes. J. Dermatol. Sci. 2014, 76, 90–95. [Google Scholar] [CrossRef] [PubMed]
- Sekiya, T.; Miyamasu, M.; Imanishi, M.; Yamada, H.; Nakajima, T.; Yamaguchi, M.; Fujisawa, T.; Pawankar, R.; Sano, Y.; Ohta, K.; et al. Inducible expression of a Th2-type CC chemokine thymus- and activation-regulated chemokine by human bronchial epithelial cells. J. Immunol. 2000, 165, 2205–2213. [Google Scholar] [CrossRef]
- Fujisawa, T.; Fujisawa, R.; Kato, Y.; Nakayama, T.; Morita, A.; Katsumata, H.; Nishimori, H.; Iguchi, K.; Kamiya, H.; Gray, P.W.; et al. Presence of high contents of thymus and activation-regulated chemokine in platelets and elevated plasma levels of thymus and activation-regulated chemokine and macrophage-derived chemokine in patients with atopic dermatitis. J. Allergy Clin. Immunol. 2002, 110, 139–146. [Google Scholar] [CrossRef]
- Fujisawa, T.; Nagao, M.; Hiraguchi, Y.; Katsumata, H.; Nishimori, H.; Iguchi, K.; Kato, Y.; Higashiura, M.; Ogawauchi, I.; Tamaki, K. Serum measurement of thymus and activation-regulated chemokine/CCL17 in children with atopic dermatitis: Elevated normal levels in infancy and age-specific analysis in atopic dermatitis. Pediatr. Allergy Immunol. 2009, 20, 633–641. [Google Scholar] [CrossRef]
- Adkins, B. Development of neonatal Th1/Th2 function. Int. Rev. Immunol. 2000, 19, 157–171. [Google Scholar] [CrossRef]
- Izuhara, K.; Yamaguchi, Y.; Ohta, S.; Nunomura, S.; Nanri, Y.; Azuma, Y.; Nomura, N.; Noguchi, Y.; Aihara, M. Squamous Cell Carcinoma Antigen 2 (SCCA2, SERPINB4): An Emerging Biomarker for Skin Inflammatory Diseases. Int. J. Mol. Sci. 2018, 19, 1102. [Google Scholar] [CrossRef]
- Ohta, S.; Shibata, R.; Nakao, Y.; Azuma, Y.; Taniguchi, K.; Arima, K.; Suzuki, S.; Shiraishi, H.; Iwasaka, T.; Izuhara, K. The usefulness of combined measurements of squamous cell carcinoma antigens 1 and 2 in diagnosing atopic dermatitis. Ann. Clin. Biochem. 2012, 49, 277–284. [Google Scholar] [CrossRef] [PubMed]
- Okawa, T.; Yamaguchi, Y.; Kou, K.; Ono, J.; Azuma, Y.; Komitsu, N.; Inoue, Y.; Kohno, M.; Matsukura, S.; Kambara, T.; et al. Serum levels of squamous cell carcinoma antigens 1 and 2 reflect disease severity and clinical type of atopic dermatitis in adult patients. Allergol. Int. 2018, 67, 124–130. [Google Scholar] [CrossRef] [PubMed]
- Nagao, M.; Inagaki, S.; Kawano, T.; Azuma, Y.; Nomura, N.; Noguchi, Y.; Ohta, S.; Kawaguchi, A.; Odajima, H.; Ohya, Y.; et al. SCCA2 is a reliable biomarker for evaluating pediatric atopic dermatitis. J. Allergy Clin. Immunol. 2018, 141, 1934–1936. [Google Scholar] [CrossRef] [PubMed]
- González-González, L.; Alonso, J. Periostin: A Matricellular Protein with Multiple Functions in Cancer Development and Progression. Front Oncol. 2018, 8, 225. [Google Scholar] [CrossRef] [PubMed]
- Ishida, A.; Ohta, N.; Suzuki, Y.; Kakehata, S.; Okubo, K.; Ikeda, H.; Shiraishi, H.; Izuhara, K. Expression of pendrin and periostin in allergic rhinitis and chronic rhinosinusitis. Allergol. Int. 2012, 61, 589–595. [Google Scholar] [CrossRef]
- Yamaguchi, Y.; Ono, J.; Masuoka, M.; Ohta, S.; Izuhara, K.; Ikezawa, Z.; Aihara, M.; Takahashi, K. Serum periostin levels are correlated with progressive skin sclerosis in patients with systemic sclerosis. Br. J. Dermatol. 2013, 168, 717–725. [Google Scholar] [CrossRef]
- Fujimoto, K.; Kawaguchi, T.; Nakashima, O.; Ono, J.; Ohta, S.; Kawaguchi, A.; Tonan, T.; Ohshima, K.; Yano, H.; Hayabuchi, N.; et al. Periostin, a matrix protein, has potential as a novel serodiagnostic marker for cholangiocarcinoma. Oncol. Rep. 2011, 25, 1211–1216. [Google Scholar] [CrossRef]
- Fujishima, H.; Okada, N.; Matsumoto, K.; Fukagawa, K.; Igarashi, A.; Matsuda, A.; Ono, J.; Ohta, S.; Mukai, H.; Yoshikawa, M.; et al. The usefulness of measuring tear periostin for the diagnosis and management of ocular allergic diseases. J. Allergy Clin. Immunol. 2016, 138, 459–467. [Google Scholar] [CrossRef][Green Version]
- Wallace, D.P. Periostin in the Kidney. Adv. Exp. Med. Biol. 2019, 1132, 99–112. [Google Scholar]
- Prakoura, N.; Chatziantoniou, C. Periostin in kidney diseases. Cell. Mol. Life Sci. 2017, 74, 4315–4320. [Google Scholar] [CrossRef]
- Khurshid, Z.; Mali, M.; Adanir, N.; Zafar, M.S.; Khan, R.S.; Latif, M. Periostin: Immunomodulatory Effects on Oral Diseases. Eur. J. Dent. 2020, 14, 462–466. [Google Scholar] [CrossRef]
- Moody, R.G.; Williamson, M.P. Structure and function of a bacterial Fasciclin I Domain Protein elucidates function of related cell adhesion proteins such as TGFBIp and periostin. FEBS Open Bio 2013, 3, 71–77. [Google Scholar] [CrossRef] [PubMed]
- Naik, P.K.; Bozyk, P.D.; Bentley, J.K.; Popova, A.P.; Birch, C.M.; Wilke, C.A.; Fry, C.D.; White, E.S.; Sisson, T.H.; Tayob, N.; et al. Periostin promotes fibrosis and predicts progression in patients with idiopathic pulmonary fibrosis. Am. J. Physiol. Lung Cell. Mol. Physiol. 2012, 303, L1046–L1056. [Google Scholar] [CrossRef]
- Nanri, Y.; Nunomura, S.; Terasaki, Y.; Yoshihara, T.; Hirano, Y.; Yokosaki, Y.; Yamaguchi, Y.; Feghali-Bostwick, C.; Ajito, K.; Murakami, S.; et al. Cross-Talk between Transforming Growth Factor-β and Periostin Can Be Targeted for Pulmonary Fibrosis. Am. J. Respir. Cell Mol. Biol. 2020, 62, 204–216. [Google Scholar] [CrossRef] [PubMed]
- Yoshihara, T.; Nanri, Y.; Nunomura, S.; Yamaguchi, Y.; Feghali-Bostwick, C.; Ajito, K.; Murakami, S.; Mawatari, M.; Izuhara, K. Periostin plays a critical role in the cell cycle in lung fibroblasts. Respir. Res. 2020, 21, 38. [Google Scholar] [CrossRef]
- Izuhara, K.; Matsumoto, H.; Ohta, S.; Ono, J.; Arima, K.; Ogawa, M. Recent developments regarding periostin in bronchial asthma. Allergol. Int. 2015, 64, S3–S10. [Google Scholar] [CrossRef]
- Muraro, A.; Lemanske, R.F., Jr.; Hellings, P.W.; Akdis, C.A.; Bieber, T.; Casale, T.B.; Jutel, M.; Ong, P.Y.; Poulsen, L.K.; Schmid-Grendelmeier, P.; et al. Precision medicine in patients with allergic diseases: Airway diseases and atopic dermatitis-PRACTALL document of the European Academy of Allergy and Clinical Immunology and the American Academy of Allergy, Asthma & Immunology. J. Allergy Clin. Immunol. 2016, 137, 1347–1358. [Google Scholar] [PubMed]
- Agache, I.; Akdis, C.A. Precision medicine and phenotypes, endotypes, genotypes, regiotypes, and theratypes of allergic diseases. J. Clin. Investig. 2019, 129, 1493–1503. [Google Scholar] [CrossRef]
- Izuhara, K.; Nunomura, S.; Nanri, Y.; Ogawa, M.; Ono, J.; Mitamura, Y.; Yoshihara, T. Periostin in inflammation and allergy. Cell. Mol. Life Sci. 2017, 74, 4293–4303. [Google Scholar] [CrossRef]
- Taniguchi, K.; Arima, K.; Masuoka, M.; Ohta, S.; Shiraishi, H.; Ontsuka, K.; Suzuki, S.; Inamitsu, M.; Yamamoto, K.I.; Simmons, O.; et al. Periostin controls keratinocyte proliferation and differentiation by interacting with the paracrine IL-1α/IL-6 loop. J. Investig. Dermatol. 2014, 134, 1295–1304. [Google Scholar] [CrossRef] [PubMed]
- Moore, M.W.; Herzog, E.L. Regulation and Relevance of Myofibroblast Responses in Idiopathic Pulmonary Fibrosis. Curr. Pathobiol. Rep. 2013, 1, 199–208. [Google Scholar] [CrossRef]
- Fernandez, I.E.; Eickelberg, O. The impact of TGF-β on lung fibrosis: From targeting to biomarkers. Proc. Am. Thorac. Soc. 2012, 9, 111–116. [Google Scholar] [CrossRef] [PubMed]
- Fingleton, J.; Braithwaite, I.; Travers, J.; Bowles, D.; Strik, R.; Siebers, R.; Holweg, C.; Matthews, J.; Weatherall, M.; Beasley, R. Serum periostin in obstructive airways disease. Eur. Respir. J. 2016, 47, 1383–1391. [Google Scholar] [CrossRef] [PubMed]
- Contié, S.; Voorzanger-Rousselot, N.; Litvin, J.; Clézardin, P.; Garnero, P. Increased expression and serum levels of the stromal cell-secreted protein periostin in breast cancer bone metastases. Int. J. Cancer 2011, 128, 352–360. [Google Scholar] [CrossRef]
- Hong, L.; Sun, H.; Lv, X.; Yang, D.; Zhang, J.; Shi, Y. Expression of periostin in the serum of NSCLC and its function on proliferation and migration of human lung adenocarcinoma cell line (A549) in vitro. Mol. Biol. Rep. 2010, 37, 2285–2293. [Google Scholar] [CrossRef]
- Sasaki, H.; Yu, C.Y.; Dai, M.; Tam, C.; Loda, M.; Auclair, D.; Chen, L.B.; Elias, A. Elevated serum periostin levels in patients with bone metastases from breast but not lung cancer. Breast Cancer Res. Treat. 2003, 77, 245–252. [Google Scholar] [CrossRef]
- Ono, J.; Takai, M.; Kamei, A.; Nunomura, S.; Nanri, Y.; Yoshihara, T.; Ohta, S.; Yasuda, K.; Conway, S.J.; Yokosaki, Y.; et al. Periostin forms a functional complex with IgA in human serum. Allergol. Int. 2020, 69, 111–120. [Google Scholar] [CrossRef] [PubMed]
- Palme, S.; Christenson, R.H.; Jortani, S.A.; Ostlund, R.E.; Kolm, R.; Kopal, G.; Laubender, R.P. Multicenter evaluation of analytical characteristics of the Elecsys® Periostin immunoassay. Clin. Biochem. 2017, 50, 139–144. [Google Scholar] [CrossRef] [PubMed]
- Jeanblanc, N.M.; Hemken, P.M.; Datwyler, M.J.; Brophy, S.E.; Manetz, T.S.; Lee, R.; Liang, M.; Chowdhury, P.S.; Varkey, R.; Grant, E.P.; et al. Development of a new ARCHITECT automated periostin immunoassay. Clin. Chim. Acta 2017, 464, 228–235. [Google Scholar] [CrossRef]
- Gadermaier, E.; Tesarz, M.; Suciu, A.A.; Wallwitz, J.; Berg, G.; Himmler, G. Characterization of a sandwich ELISA for the quantification of all human periostin isoforms. J. Clin. Lab. Anal. 2018, 32, e22252. [Google Scholar] [CrossRef] [PubMed]
- James, A.; Janson, C.; Malinovschi, A.; Holweg, C.; Alving, K.; Ono, J.; Ohta, S.; Ek, A.; Middelveld, R.; Dahlén, B.; et al. Serum periostin relates to type-2 inflammation and lung function in asthma: Data from the large population-based cohort Swedish GA(2)LEN. Allergy 2017, 72, 1753–1760. [Google Scholar] [CrossRef]
- Arron, J.R.; Izuhara, K. Asthma biomarkers: What constitutes a ‘gold standard’? Thorax 2015, 70, 105–107. [Google Scholar] [CrossRef]
- Izuhara, K.; Ohta, S.; Ono, J. Using Periostin as a Biomarker in the Treatment of Asthma. Allergy Asthma Immunol. Res. 2016, 8, 491–498. [Google Scholar] [CrossRef]
- Fujieda, S.; Imoto, Y.; Kato, Y.; Ninomiya, T.; Tokunaga, T.; Tsutsumiuchi, T.; Yoshida, K.; Kidoguchi, M.; Takabayashi, T. Eosinophilic chronic rhinosinusitis. Allergol. Int. 2019, 68, 403–412. [Google Scholar] [CrossRef] [PubMed]
- Esu, Y.; Masuda, M.; Yoshida, N. Periostin in middle ear mucosa according to eosinophilic otitis media severity: Middle ear pathology-based treatment. Auris Nasus Larynx 2020, 47, 527–535. [Google Scholar] [CrossRef]
- Inoue, T.; Akashi, K.; Watanabe, M.; Ikeda, Y.; Ashizuka, S.; Motoki, T.; Suzuki, R.; Sagara, N.; Yanagida, N.; Sato, S.; et al. Periostin as a biomarker for the diagnosis of pediatric asthma. Pediatr. Allergy Immunol. 2016, 27, 521–526. [Google Scholar] [CrossRef] [PubMed]
- Yavuz, S.T.; Bagci, S.; Bolat, A.; Akin, O.; Ganschow, R. Association of serum periostin levels with clinical features in children with asthma. Pediatr. Allergy Immunol. 2020. [Google Scholar] [CrossRef]
- Yildiz, H.; Alp, H.H.; Sünnetçioğlu, A.; Ekin, S.; Çilingir, B.M. Evaluation serum levels of YKL-40, Periostin, and some inflammatory cytokines together with IL-37, a new anti-inflammatory cytokine, in patients with stable and exacerbated asthma. Heart Lung 2021, 50, 177–183. [Google Scholar] [CrossRef] [PubMed]
- Hoshino, M.; Ohtawa, J.; Akitsu, K. Effect of treatment with inhaled corticosteroid on serum periostin levels in asthma. Respirology 2016, 21, 297–303. [Google Scholar] [CrossRef]
- Asano, T.; Ohbayashi, H.; Ariga, M.; Furuta, O.; Kudo, S.; Ono, J.; Izuhara, K. Serum periostin reflects dynamic hyperinflation in patients with asthma. ERJ Open Res. 2020, 6, 00347–02019. [Google Scholar] [CrossRef] [PubMed]
- Hur, G.Y.; Ye, Y.M.; Yang, E.; Park, H.S. Serum potential biomarkers according to sputum inflammatory cell profiles in adult asthmatics. Korean J. Intern. Med. 2020, 35, 988–997. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.A.; Izuhara, K.; Ohta, S.; Ono, J.; Yoon, M.K.; Ban, G.Y.; Yoo, H.S.; Shin, Y.S.; Ye, Y.M.; Nahm, D.H.; et al. Association of serum periostin with aspirin-exacerbated respiratory disease. Ann. Allergy Asthma Immunol. 2014, 113, 314–320. [Google Scholar] [CrossRef] [PubMed]
- Hoshino, M.; Ohtawa, J.; Akitsu, K. Association of airway wall thickness with serum periostin in steroid-naive asthma. Allergy Asthma Proc. 2016, 37, 225–230. [Google Scholar] [CrossRef]
- Maxfield, A.Z.; Landegger, L.D.; Brook, C.D.; Lehmann, A.E.; Campbell, A.P.; Bergmark, R.W.; Stankovic, K.M.; Metson, R. Periostin as a Biomarker for Nasal Polyps in Chronic Rhinosinusitis. Otolaryngol. Head Neck Surg. 2018, 158, 181–186. [Google Scholar] [CrossRef]
- Sobkowiak, P.; Narożna, B.; Wojsyk-Banaszak, I.; Bręborowicz, A.; Szczepankiewicz, A. Expression of proteins associated with airway fibrosis differs between children with allergic asthma and allergic rhinitis. Int. J. Immunopathol. Pharmacol. 2021, 35. [Google Scholar] [CrossRef]
- Hoshino, M.; Akitsu, K.; Kubota, K.; Ohtawa, J. Serum Periostin as a Biomarker for Predicting Clinical Response to House Dust Mite Sublingual Immunotherapy in Allergic Rhinitis. J. Allergy Clin. Immunol. Pract. 2021, 9, 1864–1870. [Google Scholar] [CrossRef]
- Kanemitsu, Y.; Kurokawa, R.; Ono, J.; Fukumitsu, K.; Takeda, N.; Fukuda, S.; Uemura, T.; Tajiri, T.; Ohkubo, H.; Maeno, K.; et al. Increased Serum Periostin Levels and Eosinophils in Nasal Polyps Are Associated with the Preventive Effect of Endoscopic Sinus Surgery for Asthma Exacerbations in Chronic Rhinosinusitis. Patients Int. Arch. Allergy Immunol. 2020, 181, 862–870. [Google Scholar] [CrossRef] [PubMed]
- Shirai, T.; Hirai, K.; Gon, Y.; Maruoka, S.; Mizumura, K.; Hikichi, M.; Holweg, C.; Itoh, K.; Inoue, H.; Hashimoto, S. Combined Assessment of Serum Periostin and YKL-40 May Identify Asthma-COPD Overlap. J. Allergy Clin. Immunol. Pract. 2019, 7, 134–145. [Google Scholar] [CrossRef] [PubMed]
- 119Ji, L.; Liu, Y.; Liu, P.; Ji, G.; He, J.; Gan, Y.; Zhu, S.; Chen, B.; Zhang, W. Serum periostin and TNF-α levels in patients with obstructive sleep apnea-hypopnea syndrome. Sleep Breath. 2021, 25, 331–337. [Google Scholar]
- Yamauchi, M.; Kinjo, T.; Parrott, G.; Miyagi, K.; Haranaga, S.; Nakayama, Y.; Chibana, K.; Fujita, K.; Nakamoto, A.; Higa, F.; et al. Diagnostic performance of serum interferon gamma, matrix metalloproteinases, and periostin measurements for pulmonary tuberculosis in Japanese patients with pneumonia. PLoS ONE 2020, 15, e0227636. [Google Scholar] [CrossRef] [PubMed]
- Wu, G.; Meng, X.; Zheng, P.; Zhang, X.D.; Li, L.; Hu, H.; Sun, B. Elevated serum levels of periostin in patients with allergic bronchopulmonary aspergillosis. Mycoses 2019, 62, 780–789. [Google Scholar] [CrossRef]
- Ariëns, L.F.M.; van der Schaft, J.; Bakker, D.S.; Balak, D.; Romeijn, M.L.E.; Kouwenhoven, T.; Kamsteeg, M.; Giovannone, B.; Drylewicz, J.; van Amerongen, C.C.A.; et al. Dupilumab is very effective in a large cohort of difficult-to-treat adult atopic dermatitis patients: First clinical and biomarker results from the BioDay registry. Allergy 2020, 75, 116–126. [Google Scholar] [CrossRef]
- Xu, C.H.; Wang, W.; Lin, Y.; Qian, L.H.; Zhang, X.W.; Wang, Q.B.; Yu, L.K. Diagnostic and prognostic value of serum periostin in patients with non-small cell lung cancer. Oncotarget 2017, 8, 18746–18753. [Google Scholar] [CrossRef]
- Zhu, J.Z.; Zhu, H.T.; Dai, Y.N.; Li, C.X.; Fang, Z.Y.; Zhao, D.J.; Wan, X.Y.; Wang, Y.M.; Wang, F.; Yu, C.H.; et al. Serum periostin is a potential biomarker for non-alcoholic fatty liver disease: A case-control study. Endocrine 2016, 51, 91–100. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Zhang, H.; Niu, Y.; Zhang, W.; Zhu, L.; Li, X.; Lu, S.; Fan, J.; Li, X.; Ning, G.; et al. Circulating periostin in relation to insulin resistance and nonalcoholic fatty liver disease among overweight and obese subjects. Sci. Rep. 2016, 6, 37886. [Google Scholar] [CrossRef] [PubMed]
- Lv, Y.; Wang, W.; Jia, W.D.; Sun, Q.K.; Huang, M.; Zhou, H.C.; Xia, H.H.; Liu, W.B.; Chen, H.; Sun, S.N.; et al. High preoparative levels of serum periostin are associated with poor prognosis in patients with hepatocellular carcinoma after hepatectomy. Eur. J. Surg. Oncol. 2013, 39, 1129–1135. [Google Scholar] [CrossRef]
- Thuwajit, C.; Thuwajit, P.; Jamjantra, P.; Pairojkul, C.; Wongkham, S.; Bhudhisawasdi, V.; Ono, J.; Ohta, S.; Fujimoto, K.; Izuhara, K. Clustering of patients with intrahepatic cholangiocarcinoma based on serum periostin may be predictive of prognosis. Oncol. Lett. 2017, 14, 623–634. [Google Scholar] [CrossRef]
- Ding, Y.; Ge, Q.; Qu, H.; Feng, Z.; Long, J.; Wei, Q.; Zhou, Q.; Wu, R.; Yao, L.; Deng, H. Increased serum periostin concentrations are associated with the presence of diabetic retinopathy in patients with type 2 diabetes mellitus. J. Endocrinol. Investig. 2018, 41, 937–945. [Google Scholar] [CrossRef]
- Cañas, J.A.; Tabares, A.; Barbero, C.; García-Sánchez, D.; Sastre, B.; Rodrigo-Muñoz, J.M.; Mahíllo-Fernández, I.; Rayo, A.; Borrell, B.; Cilleruelo, M.L.; et al. Proton-pump Inhibitor Response Prediction Using Esophageal microRNAs in Children With Eosinophilic Esophagitis. J. Pediatr. Gastroenterol. Nutr. 2020, 71, 755–763. [Google Scholar] [CrossRef]
- Conway, S.J.; Molkentin, J.D. Periostin as a heterofunctional regulator of cardiac development and disease. Curr. Genom. 2008, 9, 548–555. [Google Scholar] [CrossRef] [PubMed]
- Kudo, A. Periostin in fibrillogenesis for tissue regeneration: Periostin actions inside and outside the cell. Cell. Mol. Life Sci. 2011, 68, 3201–3207. [Google Scholar] [CrossRef]
- Ninomiya, T.; Noguchi, E.; Haruna, T.; Hasegawa, M.; Yoshida, T.; Yamashita, Y.; Okano, M.; Yoshida, N.; Haruna, S.; Sakuma, Y.; et al. Periostin as a novel biomarker for postoperative recurrence of chronic rhinosinitis with nasal polyps. Sci. Rep. 2018, 8, 11450. [Google Scholar] [CrossRef]
- Tokunaga, T.; Sakashita, M.; Haruna, T.; Asaka, D.; Takeno, S.; Ikeda, H.; Nakayama, T.; Seki, N.; Ito, S.; Murata, J.; et al. Novel scoring system and algorithm for classifying chronic rhinosinusitis: The JESREC Study. Allergy 2015, 70, 995–1003. [Google Scholar] [CrossRef] [PubMed]
- Graney, B.A.; Lee, J.S. Impact of novel antifibrotic therapy on patient outcomes in idiopathic pulmonary fibrosis: Patient selection and perspectives. Patient Relat. Outcome Meas. 2018, 9, 321–328. [Google Scholar] [CrossRef]
- Tzouvelekis, A.; Ntolios, P.; Karampitsakos, T.; Tzilas, V.; Anevlavis, S.; Bouros, E.; Steiropoulos, P.; Koulouris, N.; Stratakos, G.; Froudarakis, M.; et al. Safety and efficacy of pirfenidone in severe Idiopathic Pulmonary Fibrosis: A real-world observational study. Pulm. Pharmacol. Ther. 2017, 46, 48–53. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, M.; Okamoto, M.; Fujimoto, K.; Ebata, T.; Tominaga, M.; Nouno, T.; Zaizen, Y.; Kaieda, S.; Tsuda, T.; Kawayama, T.; et al. A retrospective study of the tolerability of nintedanib for severe idiopathic pulmonary fibrosis in the real world. Ann. Transl. Med. 2019, 7, 262. [Google Scholar] [CrossRef]
- Caminati, A.; Harari, S. IPF: New insight in diagnosis and prognosis. Respir. Med. 2010, 104, S2–S10. [Google Scholar] [CrossRef][Green Version]
- Flaherty, K.R.; Mumford, J.A.; Murray, S.; Kazerooni, E.A.; Gross, B.H.; Colby, T.V.; Travis, W.D.; Flint, A.; Toews, G.B.; Lynch, J.P., 3rd; et al. Prognostic implications of physiologic and radiographic changes in idiopathic interstitial pneumonia. Am. J. Respir. Crit. Care Med. 2003, 168, 543–548. [Google Scholar] [CrossRef]
- Shimizu, H.; Sakamoto, S.; Okamoto, M.; Isshiki, T.; Ono, J.; Shimizu, S.; Hoshino, T.; Izuhara, K.; Homma, S. Association of serum monomeric periostin level with outcomes of acute exacerbation of idiopathic pulmonary fibrosis and fibrosing nonspecific interstitial pneumoni. Ann. Transl. Med. 2021, 9, 1–11. [Google Scholar] [CrossRef] [PubMed]
- George, P.M.; Spagnolo, P.; Kreuter, M.; Altinisik, G.; Bonifazi, M.; Martinez, F.J.; Molyneaux, P.L.; Renzoni, E.A.; Richeldi, L.; Tomassetti, S.; et al. Progressive fibrosing interstitial lung disease: Clinical uncertainties, consensus recommendations, and research priorities. Lancet Respir. Med. 2020, 8, 925–934. [Google Scholar] [CrossRef]
- Kobayashi, Y.; Yoshida, S.; Nakama, T.; Zhou, Y.; Ishikawa, K.; Arita, R.; Nakao, S.; Miyazaki, M.; Sassa, Y.; Oshima, Y.; et al. Overexpression of CD163 in vitreous and fibrovascular membranes of patients with proliferative diabetic retinopathy: Possible involvement of periostin. Br. J. Ophthalmol. 2015, 99, 451–456. [Google Scholar] [CrossRef]
- Yoshida, S.; Ishikawa, K.; Asato, R.; Arima, M.; Sassa, Y.; Yoshida, A.; Yoshikawa, H.; Narukawa, K.; Obika, S.; Ono, J.; et al. Increased expression of periostin in vitreous and fibrovascular membranes obtained from patients with proliferative diabetic retinopathy. Investig. Ophthalmol. Vis. Sci. 2011, 52, 5670–5678. [Google Scholar] [CrossRef]
- Ishikawa, K.; Yoshida, S.; Nakao, S.; Nakama, T.; Kita, T.; Asato, R.; Sassa, Y.; Arita, R.; Miyazaki, M.; Enaida, H.; et al. Periostin promotes the generation of fibrous membranes in proliferative vitreoretinopathy. FASEB J. 2014, 28, 131–142. [Google Scholar] [CrossRef] [PubMed]
- Ohta, N.; Ishida, A.; Kurakami, K.; Suzuki, Y.; Kakehata, S.; Ono, J.; Ikeda, H.; Okubo, K.; Izuhara, K. Expressions and roles of periostin in otolaryngological diseases. Allergol. Int. 2014, 63, 171–180. [Google Scholar] [CrossRef]
- Jakiela, B.; Soja, J.; Sladek, K.; Przybyszowski, M.; Plutecka, H.; Gielicz, A.; Rebane, A.; Bochenek, G. Heterogeneity of lower airway inflammation in patients with NSAID-exacerbated respiratory disease. J. Allergy Clin. Immunol. 2021, 147, 1269–1280. [Google Scholar] [CrossRef] [PubMed]
- Nakagome, K.; Nakamura, Y.; Kobayashi, T.; Ohta, S.; Ono, J.; Kobayashi, K.; Ikebuchi, K.; Noguchi, T.; Soma, T.; Yamauchi, K.; et al. Elevated Periostin Concentrations in the Bronchoalveolar Lavage Fluid of Patients with Eosinophilic Pneumonia. Int. Arch. Allergy Immunol. 2019, 178, 264–271. [Google Scholar] [CrossRef]
- Katoh, S.; Matsumoto, N.; Tanaka, H.; Yasokawa, N.; Kittaka, M.; Kurose, K.; Abe, M.; Yoshioka, D.; Shirai, R.; Nakazato, M.; et al. Elevated levels of periostin and TGF-β1 in the bronchoalveolar lavage fluid of patients with idiopathic eosinophilic pneumonia. Asian Pac. J. Allergy Immunol. 2020, 38, 208–213. [Google Scholar]
- Yormaz, B.; Menevse, E.; Cetin, N.; Celik, Z.E.; Bakir, H.; Tulek, B.; Korez, M.K.; Suerdem, M. Diagnostic value of thymus and activation-regulated chemokine and of periostin in eosinophilic asthma: A prospective study. Allergy Asthma Proc. 2020, 42, 30–39. [Google Scholar] [CrossRef]
- Refaat, M.M.; el Sayed, E.; El-Fattah, W.A.; Elbanna, A.H.; Sayed, H.M.E. Relationship between sputum periostin level and inflammatory asthma phenotypes in Egyptian patients. J. Asthma 2020, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Cianchetti, S.; Cardini, C.; Puxeddu, I.; Latorre, M.; Bartoli, M.L.; Bradicich, M.; Dente, F.; Bacci, E.; Celi, A.; Paggiaro, P. Distinct profile of inflammatory and remodelling biomarkers in sputum of severe asthmatic patients with or without persistent airway obstruction. World Allergy Organ. J. 2019, 12, 100078. [Google Scholar] [CrossRef]
- Bobolea, I.; Barranco, P.; del Pozo, V.; Romero, D.; Sanz, V.; López-Carrasco, V.; Canabal, J.; Villasante, C.; Quirce, S. Sputum periostin in patients with different severe asthma phenotypes. Allergy 2015, 70, 540–546. [Google Scholar] [CrossRef] [PubMed]
- Kurokawa, R.; Kanemitsu, Y.; Fukumitsu, K.; Takeda, N.; Yap, J.M.; Ozawa, Y.; Masaki, A.; Ono, J.; Izuhara, K.; Nishiyama, H.; et al. Nasal polyp eosinophilia and FeNO may predict asthma symptoms development after endoscopic sinus surgery in CRS patients without asthma. J. Asthma 2021, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Kanemitsu, Y.; Suzuki, M.; Fukumitsu, K.; Asano, T.; Takeda, N.; Nakamura, Y.; Ozawa, Y.; Masaki, A.; Ono, J.; Kurokawa, R.; et al. A novel pathophysiologic link between upper and lower airways in patients with chronic rhinosinusitis: Association of sputum periostin levels with upper airway inflammation and olfactory function. World Allergy Organ. J. 2020, 13, 100094. [Google Scholar] [CrossRef]
- Carpagnano, G.E.; Scioscia, G.; Lacedonia, D.; Soccio, P.; Lepore, G.; Saetta, M.; Barbaro, M.P.F.; Barnes, P.J. Looking for Airways Periostin in Severe Asthma: Could It Be Useful for Clustering Type 2 Endotype? Chest 2018, 154, 1083–1090. [Google Scholar] [CrossRef]
- Górska, K.; Maskey-Warzęchowska, M.; Nejman-Gryz, P.; Korczyński, P.; Prochorec-Sobieszek, M.; Krenke, R. Comparative study of periostin expression in different respiratory samples in patients with asthma and chronic obstructive pulmonary disease. Pol. Arch. Med. Wewn. 2016, 126, 124–137. [Google Scholar] [CrossRef]
- Hachim, M.Y.; Elemam, N.M.; Ramakrishnan, R.K.; Hachim, I.Y.; Salameh, L.; Mahboub, B.; al Heialy, S.; Halwani, R.; Hamoudi, R.; Hamid, Q. Confounding Patient Factors Affecting the Proper Interpretation of the Periostin Level as a Biomarker in Asthma Development. J. Asthma Allergy 2020, 13, 23–37. [Google Scholar] [CrossRef] [PubMed]
- Esfahrood, Z.R.; Vardian, S.T.; Yadegari, Z.; Adhim, M.; Saravi, N.S.V. Periostin levels in saliva of patients with chronic periodontitis. J. Indian Soc. Periodontol. 2018, 22, 25–27. [Google Scholar] [PubMed]
- Aral, C.A.; Köseoğlu, S.; Sağlam, M.; Pekbağrıyanık, T.; Gingival, L.S. Crevicular Fluid and Salivary Periostin Levels in Non-Smoker Subjects With Chronic and Aggressive Periodontitis: Periostin Levels in Chronic and Aggressive Periodontitis. Inflammation 2016, 39, 986–993. [Google Scholar] [CrossRef]
- Sophia, K., 2nd; Suresh, S.; Sudhakar, U., Sr.; Cader, S.A., Jr.; Vardhini, V.M.; Arunachalam, L.T.; Jean, S.C. Comparative Evaluation of Serum and Gingival Crevicular Fluid Periostin Levels in Periodontal Health and Disease: A Biochemical Study. Cureus 2020, 12, e7218. [Google Scholar] [CrossRef]
- Ghafar, M.T.A.; Shalaby, K.H.; Okda, H.I.; el Gheit, R.E.A.; Soliman, N.A.; Keshk, W.A. Assessment of two novel renal tubular proteins in type 2 diabetic patients with nephropathy. J. Investig. Med. 2020, 68, 748–755. [Google Scholar] [CrossRef] [PubMed]
- Hwang, J.H.; Lee, J.P.; Kim, C.T.; Yang, S.H.; Kim, J.H.; An, J.N.; Moon, K.C.; Lee, H.; Oh, Y.K.; Joo, K.W.; et al. Urinary Periostin Excretion Predicts Renal Outcome in IgA Nephropathy. Am. J. Nephrol. 2016, 44, 481–492. [Google Scholar] [CrossRef]
- Satirapoj, B.; Tassanasorn, S.; Charoenpitakchai, M.; Supasyndh, O. Periostin as a tissue and urinary biomarker of renal injury in type 2 diabetes mellitus. PLoS ONE 2015, 10, e0124055. [Google Scholar] [CrossRef]
- Satirapoj, B.; Witoon, R.; Ruangkanchanasetr, P.; Wantanasiri, P.; Charoenpitakchai, M.; Choovichian, P. Urine periostin as a biomarker of renal injury in chronic allograft nephropathy. Transplant. Proc. 2014, 46, 135–140. [Google Scholar] [CrossRef]
- Takamura, E.; Uchio, E.; Ebihara, N.; Ohno, S.; Ohashi, Y.; Okamoto, S.; Kumagai, N.; Satake, Y.; Shoji, J.; Nakagawa, Y.; et al. Japanese guidelines for allergic conjunctival diseases 2017. Allergol. Int. 2017, 66, 220–229. [Google Scholar] [CrossRef]
- Hagan, S.; Martin, E.; Enríquez-de-Salamanca, A. Tear fluid biomarkers in ocular and systemic disease: Potential use for predictive, preventive and personalised medicine. EPMA J. 2016, 7, 15. [Google Scholar] [CrossRef] [PubMed]
- Shoji, J.; Kitazawa, M.; Inada, N.; Sawa, M.; Ono, T.; Kawamura, M.; Kato, H. Efficacy of tear eosinophil cationic protein level measurement using filter paper for diagnosing allergic conjunctival disorders. JPN J. Ophthalmol. 2003, 47, 64–68. [Google Scholar] [CrossRef]
- Gottlow, M.; Svensson, D.J.; Lipkovich, I.; Huhn, M.; Bowen, K.; Wessman, P.; Colice, G. Application of structured statistical analyses to identify a biomarker predictive of enhanced tralokinumab efficacy in phase III clinical trials for severe, uncontrolled asthma. BMC Pulm. Med. 2019, 19, 129. [Google Scholar] [CrossRef]
- Chung, K.F.; Wenzel, S.E.; Brozek, J.L.; Bush, A.; Castro, M.; Sterk, P.J.; Adcock, I.M.; Bateman, E.D.; Bel, E.H.; Bleecker, E.R.; et al. International ERS/ATS guidelines on definition, evaluation and treatment of severe asthma. Eur. Respir. J. 2014, 43, 343–373. [Google Scholar] [CrossRef] [PubMed]
- Shaw, D.E.; Sousa, A.R.; Fowler, S.J.; Fleming, L.J.; Roberts, G.; Corfield, J.; Pandis, I.; Bansal, A.T.; Bel, E.H.; Auffray, C.; et al. Clinical and inflammatory characteristics of the European U-BIOPRED adult severe asthma cohort. Eur. Respir. J. 2015, 46, 1308–1321. [Google Scholar] [CrossRef]
- Novosad, J.; Krčmová, I.; Bartoš, V.; Drahošová, M.; Vaník, P.; Růžičková-Kirchnerová, O.; Teřl, M.; Krejsek, J. Serum periostin levels in asthma patients in relation to omalizumab therapy and presence of chronic rhinosinusitis with nasal polyps. Postepy Dermatol. Alergol. 2020, 37, 240–249. [Google Scholar] [CrossRef] [PubMed]
- Scheerens, H.; Arron, J.R.; Zheng, Y.; Putnam, W.S.; Erickson, R.W.; Choy, D.F.; Harris, J.M.; Lee, J.; Jarjour, N.N.; Matthews, J.G. The effects of lebrikizumab in patients with mild asthma following whole lung allergen challenge. Clin. Exp. Allergy 2014, 44, 38–46. [Google Scholar] [CrossRef]
- de Schryver, E.; Derycke, L.; Calus, L.; Holtappels, G.; Hellings, P.W.; van Zele, T.; Bachert, C.; Gevaert, P. The effect of systemic treatments on periostin expression reflects their interference with the eosinophilic inflammation in chronic rhinosinusitis with nasal polyps. Rhinology 2017, 55, 152–160. [Google Scholar] [CrossRef] [PubMed]
- Tajiri, T.; Matsumoto, H.; Gon, Y.; Ito, R.; Hashimoto, S.; Izuhara, K.; Suzukawa, M.; Ohta, K.; Ono, J.; Ohta, S.; et al. Utility of serum periostin and free IgE levels in evaluating responsiveness to omalizumab in patients with severe asthma. Allergy 2016, 71, 1472–1479. [Google Scholar] [CrossRef]
- Parker, J.M.; Glaspole, I.N.; Lancaster, L.H.; Haddad, T.J.; She, D.; Roseti, S.L.; Fiening, J.P.; Grant, E.P.; Kell, C.M.; Flaherty, K.R. A Phase 2 Randomized Controlled Study of Tralokinumab in Subjects with Idiopathic Pulmonary Fibrosis. Am. J. Respir. Crit. Care Med. 2018, 197, 94–103. [Google Scholar] [CrossRef] [PubMed]
- Neighbors, M.; Cabanski, C.R.; Ramalingam, T.R.; Sheng, X.R.; Tew, G.W.; Gu, C.; Jia, G.; Peng, K.; Ray, J.M.; Ley, B.; et al. Prognostic and predictive biomarkers for patients with idiopathic pulmonary fibrosis treated with pirfenidone: Post-hoc assessment of the CAPACITY and ASCEND trials. Lancet Respir. Med. 2018, 6, 615–626. [Google Scholar] [CrossRef]
- Lehtonen, S.T.; Veijola, A.; Karvonen, H.; Lappi-Blanco, E.; Sormunen, R.; Korpela, S.; Zagai, U.; Sköld, M.C.; Kaarteenaho, R. Pirfenidone and nintedanib modulate properties of fibroblasts and myofibroblasts in idiopathic pulmonary fibrosis. Respir. Res. 2016, 17, 14. [Google Scholar] [CrossRef]
- Inoue, Y.; Izuhara, K.; Ohta, S.; Ono, J.; Shimojo, N. No increase in the serum periostin level is detected in elementary school-age children with allergic diseases. Allergol. Int. 2015, 64, 289–290. [Google Scholar] [CrossRef] [PubMed]
- Walsh, J.S.; Gossiel, F.; Scott, J.R.; Paggiosi, M.A.; Eastell, R. Effect of age and gender on serum periostin: Relationship to cortical measures, bone turnover and hormones. Bone 2017, 99, 8–13. [Google Scholar] [CrossRef] [PubMed]
- Kimura, H.; Konno, S.; Makita, H.; Taniguchi, N.; Kimura, H.; Goudarzi, H.; Shimizu, K.; Suzuki, M.; Shijubo, N.; Shigehara, K.; et al. Serum periostin is associated with body mass index and allergic rhinitis in healthy and asthmatic subjects. Allergol. Int. 2018, 67, 357–363. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.Y.; Kim, J.H.; Lee, K.H.; Hong, S.C.; Lee, H.S.; Kang, J.W. Serum periostin level is not associated with allergic rhinitis or allergic sensitization in Korean children. Int. J. Pediatr. Otorhinolaryngol. 2017, 93, 24–29. [Google Scholar] [CrossRef]
- Sung, M.; Baek, H.S.; Yon, D.K.; Lee, S.W.; Ha, E.K.; Lee, K.S.; Jee, H.M.; Sheen, Y.H.; Ono, J.; Izuhara, K.; et al. Serum Periostin Level Has Limited Usefulness as a Biomarker for Allergic Disease in 7-Year-Old Children. Int. Arch. Allergy Immunol. 2019, 180, 195–201. [Google Scholar]
- Caswell-Smith, R.; Cripps, T.; Charles, T.; Hosking, A.; Handigol, M.; Holweg, C.; Matthews, J.; Holliday, M.; Maillot, C.; Fingleton, J.; et al. Day-time variation of serum periostin in asthmatic adults treated with ICS/LABA and adults without asthma. Allergy Asthma Clin. Immunol. 2017, 13, 8. [Google Scholar] [CrossRef] [PubMed]
- Cho, J.H.; Kim, K.; Yoon, J.W.; Choi, S.H.; Sheen, Y.H.; Han, M.; Ono, J.; Izuhara, K.; Baek, H. Serum levels of periostin and exercise-induced bronchoconstriction in asthmatic children. World Allergy Organ. J. 2019, 12, 100004. [Google Scholar] [CrossRef]
- Song, J.S.; You, J.S.; Jeong, S.I.; Yang, S.; Hwang, I.T.; Im, Y.G.; Baek, H.S.; Kim, H.Y.; Suh, D.I.; Lee, H.B.; et al. Serum periostin levels correlate with airway hyper-responsiveness to methacholine and mannitol in children with asthma. Allergy 2015, 70, 674–681. [Google Scholar] [CrossRef] [PubMed]
- Nukui, Y.; Miyazaki, Y.; Masuo, M.; Okamoto, T.; Furusawa, H.; Tateishi, T.; Kishino, M.; Tateishi, U.; Ono, J.; Ohta, S.; et al. Periostin as a predictor of prognosis in chronic bird-related hypersensitivity pneumonitis. Allergol. Int. 2019, 68, 363–369. [Google Scholar] [CrossRef]
- Nakamura, Y.; Yokoyama, Y.; Nakajima, K.; Enomoto, T.; Fujiwara, K.; Takeuchi, H. Serum periostin levels in adolescents. Asian Pac. J. Allergy Immunol. 2020, 1–6. [Google Scholar] [CrossRef]
- Zhang, Z.R.; Chen, L.Y.; Qi, H.Y.; Sun, S.H. Expression and clinical significance of periostin in oral lichen planus. Exp. Ther. Med. 2018, 15, 5141–5147. [Google Scholar] [CrossRef] [PubMed]
- Wardzyńska, A.; Makowska, J.S.; Pawełczyk, M.; Piechota-Polańczyk, A.; Kurowski, M.; Kowalski, M.L. Periostin in Exhaled Breath Condensate and in Serum of Asthmatic Patients: Relationship to Upper and Lower Airway Disease. Allergy Asthma Immunol. Res. 2017, 9, 126–132. [Google Scholar] [CrossRef]
- Guañabens, N.; Filella, X.; Florez, H.; Ruiz-Gaspá, S.; Conesa, A.; Peris, P.; Monegal, A.; Torres, F. Tartrate-resistant acid phosphatase 5b, but not periostin, is useful for assessing Paget’s disease of bone. Bone 2019, 124, 132–136. [Google Scholar] [CrossRef]
- Lemaire, H.G.; Merle, B.; Borel, O.; Gensburger, D.; Chapurlat, R. Serum periostin levels and severity of fibrous dysplasia of bone. Bone 2019, 121, 68–71. [Google Scholar] [CrossRef]
- Pavlidis, S.; Takahashi, K.; Kwong, F.N.K.; Xie, J.; Hoda, U.; Sun, K.; Elyasigomari, V.; Agapow, P.; Loza, M.; Baribaud, F.; et al. T2-high in severe asthma related to blood eosinophil, exhaled nitric oxide and serum periostin. Eur. Respir. J. 2019, 53, 1800938. [Google Scholar] [CrossRef] [PubMed]
- Caswell-Smith, R.; Hosking, A.; Cripps, T.; Holweg, C.; Matthews, J.; Holliday, M.; Maillot, C.; Fingleton, J.; Weatherall, M.; Braithwaite, I.; et al. Reference ranges for serum periostin in a population without asthma or chronic obstructive pulmonary disease. Clin. Exp. Allergy 2016, 46, 1303–1314. [Google Scholar] [CrossRef] [PubMed]
- Chacón-Solano, E.; León, C.; Díaz, F.; García-García, F.; García, M.; Escámez, M.J.; Guerrero-Aspizua, S.; Conti, C.J.; Mencía, Á.; Martínez-Santamaría, L.; et al. Fibroblast activation and abnormal extracellular matrix remodelling as common hallmarks in three cancer-prone genodermatoses. Br. J. Dermatol. 2019, 181, 512–522. [Google Scholar] [CrossRef] [PubMed]
- Kalinauskaite-Zukauske, V.; Januskevicius, A.; Janulaityte, I.; Miliauskas, S.; Malakauskas, K. Expression of eosinophil β chain-signaling cytokines receptors, outer-membrane integrins, and type 2 inflammation biomarkers in severe non-allergic eosinophilic asthma. BMC Pulm. Med. 2019, 19, 158. [Google Scholar] [CrossRef] [PubMed]
- Kerschan-Schindl, K.; Ebenbichler, G.; Föeger-Samwald, U.; Leiss, H.; Gesslbauer, C.; Herceg, M.; Stummvoll, G.; Marculescu, R.; Crevenna, R.; Pietschmann, P. Rheumatoid arthritis in remission: Decreased myostatin and increased serum levels of periostin. Wien. Klin. Wochenschr. 2019, 131, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Yuan, D.; Yao, Y.; Sun, W.; Shi, Y.; Su, X. Predictive and prognostic value of serum periostin in advanced non-small cell lung cancer patients receiving chemotherapy. Tumour. Biol. 2017, 39. [Google Scholar] [CrossRef]
- Morra, L.; Rechsteiner, M.; Casagrande, S.; von Teichman, A.; Schraml, P.; Moch, H.; Soltermann, A. Characterization of periostin isoform pattern in non-small cell lung cancer. Lung Cancer 2012, 76, 183–190. [Google Scholar] [CrossRef]

| Study Groups | Age | Type of Biomarker (BM) | Potential Use As Biomarker of Serum Periostin Levels | |
|---|---|---|---|---|
| Ear | Eosinophilic otitis medeia | adult | diagnostic BM | Significantly higher in EOM patients than in COM patients [105]. |
| Under/under airway | Asthma | children | diagnostic BM | Significantly higher in patients with athma than healthy control [106]. |
| severity BM | Significantly higher in children with severe asthma than with mild to moderate asthma [107]. | |||
| adult | severity BM | Significantly higher in the exacerbated asthma group than stable asthma and healthy control groups [108]. Correlated with airway wall thickness and sputum eosinophilia, and inversely correlated with airflow limitation [109]. High periostin level associated with a decline in FEV1 of 30 mL or greater per year [23]. | ||
| severity BM, stratification BM | Correlated with a specific phenotype of eosinophilic asthma, late-onset and often complicated by obstructive pulmonary dysfunction and nasal disorders [24]. Significantly higher in patients with positive DH than patients with negative DH [110]. Discriminated the eosinophilic asthma group from patients having eosinophilic or mixed granulocytic asthma [111]. Predicted AERD phenotype together with severe asthma phenotype [112]. Correlated with blood eosinophil counts and sputum eosinophil counts [112]. Significantly higher in comorbid AERD patients with more severe chronic rhinosinusitis than with less severe [112]. | |||
| monitoring BM | Significantly elevated in patients with steroid-naive asthma compared with the controls and patients with asthma who were treated with steroids [113]. | |||
| predictive BM | Patients with high pretreatment levels had greater improvement in lung function with lebrikizumab [20]. | |||
| Chronic rhinosinusitis with nasal polyps | adult | diagnostic BM | Significantly elevated in patients with CRS than controls [114]. Significantly higher in CRS patients with nasal polyps than with those without polyps [114]. | |
| prognostic BM | Associated with the severity of CRSwNP, and postoperative recurrence of CRSwNP [107]. | |||
| Allergic rhinitis | children | differential diagnostic BM | Significantly higher in AR patients than AA patients [115]. | |
| adult | monitoring BM | Associated with an effective response to SLIT [116]. Correlated with improvement the magnitude of RQLQ [116]. | ||
| Asthma with CRS | adult | predictive BM | Associated with the preventive effect of ESS for asthma exacerbations in CRS patients comorbid with asthma [117]. | |
| Asthma-chronic obstructive pulmonary disease overlap | adult | differential diagnostic BM | Combined assessment of serum periostin and YKL-40 may identify ACO [118]. | |
| Obstructive sleep apnea-hypopnea syndrome | adult | severity BM | Significantly increased in patients with OSAHS and positively correlated with the apnea-hypopnea index (AHI) and negatively correlated with the lowest saturation oxygen (LSaO2) and mean saturation oxygen (MSaO2) [119]. | |
| Pulmonary tuberculosis | adult | differential diagnostic BM | Combination of MMP-1, MMP-9, and periostin data distinguished PTB from non-PTB patients [120]. | |
| Allergic bronchopulmonary aspergillosis | adult | differential diagnostic BM | Significantly higher in ABPA patients than in SAFS patients [121]. | |
| Skin | Atopic dermatitis | children adult | diagnostic BM | Significantly higher in AD patients than in healthy controls [17,22]. |
| adult | diagnostic BM, severity BM | Significantly higher in patients with AD than in patients with healthy controls [21]. Positively correlated with disease severity [21]. | ||
| monitoring BM | Significant decreased during dupilumab treatment [122]. | |||
| Systemic sclerosis | adult | severity BM | Significantly elevated with dSSc compared with lSSc and control subjects [76]. MRSS was positively correlated with periostin levels in patients with SSc [76]. | |
| Lung | Idiopathic interstitial pneumonias | adult | diagnostic BM, prognosis BM | Significantly higher in IPF than healthy subjects and COP [27]. Inversely correlated with pulmonary functions [27,29]. |
| prognostic BM | Significantly correlated with the increase of honeycombing score on HRCT during a 6-month period [28]. Higher serum periostin level was a predictor of a shortened OS and TTE [28]. | |||
| Non-small cell lung cancer | adult | diagnostic BM | Significantly elevated in NSCLC patients than in healthy controls and/or BLD patients [95,123]. | |
| Liver | Non-alcoholic fatty liver disease | adult | diagnostic BM | Significantly higher in NAFLD than in control [124,125]. Among overweight and obese subjects, it was significantly higher in NAFLD subjects than those without NAFLD [124]. Associated with a higher risk for NAFLD among overweight and obese subjects [125]. |
| Hepatocellular carcinoma | adult | diagnostic BM | Significantly increased in HCC patients than healthy controls, patients with hepatolithiasis, and patients with liver cirrhosis [126]. Significantly associated with Edmondson grade [126]. | |
| Cholangiocarcinoma | adult | differential diagnostic BM | significantly increased in patients with CCA than in healthy controls, patients with benign liver diseases and patients with breast cancer [127]. Significantly higher in patients with CCA compared to those patients with normal liver, liver cirrhosis, HCC and other malignancies [77]. | |
| Renal | Diabetic retinopathy | adult | diagnostic BM | Significantly associated with the presence of DR in patients with T2DM and is an independent risk factor of DR [128]. |
| Esophagus | Eosinophilic esophagitis | adult | monitoring BM | Significant decrease after Proton-pump inhibitors [129]. |
| Specimens | Study Groups | Significant Differences (High Group vs. Low Group) |
|---|---|---|
| Tear | Ocular allergic diseases | AKC, VKC vs. SAC, healthy control [78]. |
| Vitreous fluid | Diabetic retinopathy | PDR vs. non-diabetic ocular diseases [141,142]. |
| PDR, PVR vs. RRD, MH [143]. | ||
| PDR vs. MH, ERM [142]. | ||
| Nasal lavage fluid | Chronic rhinosinusitis | CRSwNP vs. AR [144]. |
| Bronchoalveolar Lavage Fluid | NSAIDs-tolerant asthma | N-ERD vs. NTA [145]. |
| Eosinophilic Pneumonia | AEP, CEP vs. healthy volunteers, sarcoidosis [146]. | |
| Idiopathic eosinophilic pneumonia | EP vs. IPF, sarcoidosis [147]. | |
| Sputum | Asthma | asthma vs. healthy control [148]. |
| severe asthma vs. moderate to mild asthma [149]. | ||
| patients with persistent obstruction vs. without persistent obstruction [150]. | ||
| eosinophilic type vs. mixed granulocytic phenotype [151]. | ||
| ESS in CRS | with asthma vs. without asthma [152]. | |
| Chronic rhinosinusitis comorbid asthma | CRS with asthma vs. CRS without asthma [153]. | |
| Exhaled breath condensate | Asthma | severe asthma vs. mild to moderate asthma [154]. |
| Asthma, COPD | asthma vs. COPD [155]. | |
| Saliva | Asthma | mild to moderate asthma vs. severe asthma [156]. |
| Chronic periodontitis | healthy control vs. chronic periodontitis [157]. | |
| Chronic and aggressive periodontitis | non-periodontitis vs. chronic periodontitis [158]. | |
| Gingival crevicular fluid | Chronic periodontitis, gingivitis | gingivitis, healthy vs. chronic periodontitis [159]. |
| Urine | Type 2 diabetic patients with nephropathy | DN vs. T2DM with normoalbuminuria [160]. |
| IgA nephropathy | IgA nephropathy vs. healthy control [161]. | |
| Type 2 diabetes mellitus | microalbuminuria, macroalbuminuria vs. normoalbuminuria [162]. | |
| Chronic allograft nephropathy | CAN vs. transplant and healthy controls [163]. |
| Drug | Manufacture | Target | Diseases | Specimens | Outcomes of Periostin As Predictive or Monitoring Biomarker |
|---|---|---|---|---|---|
| Lebrikizumab | Genentech | IL-13 | Asthma | Serum | Patients with high pretreatment levels of serum periostin had greater improvement in lung function with lebrikizumab than did patients with low periostin levels [20]. |
| Serum | Lebrikizumab-treated subjects with elevated baseline levels of peripheral blood eosinophils, serum IgE, or periostin exhibited a greater reduction in late asthmatic response [171]. | ||||
| Serum | Treatment with lebrikizumab reduced the rate of asthma exacerbations, which was more pronounced in the periostin-high patients (60% reduction) than in the periostin-low patients (5% reduction) [59]. | ||||
| Serum | Lung function also improved the following: lebrikizumab treatment, with greatest increase in FEV1 in periostin-high patients [58]. | ||||
| Omalizumab | Genentech | IgE | Asthma | Serum | There was a large difference in reduction in exacerbations after 48 weeks with omalizumab treatment between the high and low periostin groups (30% vs. 3%) [172]. |
| Serum | Omalizumab significantly reduced serum periostin levels at 4 and 8 weeks after the start of the treatment [170]. | ||||
| Serum | Serum periostin levels decreased in omalizumab-treated patients in comparison to conventionally treated patients. This effect was remarkably apparent only if CRSwNP was not present [173]. | ||||
| Mepolizumab | GlaxoSmithKline | IL-5 | Asthma | Nasal secretion | Nasal periostin levels decreased significantly after 8 weeks of treatment with mepolizumab [172]. |
| Dupilumab | Regeneron/Sanofi | IL-4 Rα | Asthma | Serum | Peripheral blood eosinophil and FeNO concentrations were associated with lower exacerbation frequency and improved respiratory function, whereas periostin concentrations were associated only with improved respiratory function [60]. |
| AD | Serum | During dupilumab treatment, disease severity-related serum periostin significantly decreased [122]. | |||
| Tralokinumab | AstraZeneca | IL-13 | Asthma | Serum | FeNO (>32.3 ppb) and periostin (>27.4 ng/mL) were identified as the only biomarkers potentially predictive of treatment effect [167]. |
| IPF | Serum | Tralokinumab did not demonstrate a significant change in percent predicted FVC from baseline to Week 52 for either the periostin-high or the periostin-low group [174]. | |||
| Pirfenidone | Genentech | TGF-β1, TNF-α | IPF | Serum | Several baseline biomarkers (CCL13, CCL18, CXCL13, CXCL14, periostin, and YKL40) were prognostic for progression outcomes with pirfenidon treatment [175]. |
| Kit Manufacture | Method | Age | Subject Number | Periostin (ng/mL) |
|---|---|---|---|---|
| Shino-Test Corp. | ELISA | Children | 30 | 74.0 a) [69.75–80.0] e) [183] |
| 23 | 71.0 a) [57.5–80.0] e) [184] | |||
| Adult | 113 | 89.7 b) ± 21.2 c) [185] | ||
| 230 | 66.1 a) (0.14) e) [179] | |||
| 11 | 57.0 a) [39.0–63.0] e) [24] | |||
| 25 | 56 a) [49–63] e) [21] | |||
| 66 | 39.1 b) ± 3.0 d) [27] | |||
| R&D Systems | ELISA | Children | 20 | 93.4 a)± 22.3 c) [115] |
| 60 | 32.7 b) ± 2.6 d) [186] | |||
| Adult | 110 | 37.4 a) [32.9–48.4] e) [187] | ||
| 17 | 24.6 b) ± 11.3 c) [188] | |||
| Biomedica | ELISA | Adult | 45 | 966.9 b) ± 195.4 c) pmol/L [189] |
| 128 | 958 pmol/L b) [190] | |||
| 24 | 864 a) ± 269 c) pmol/L [100] | |||
| Genentech, Inc., | ELISA | Adult | 44 | NS [191] |
| Roche Diagnostics | ECLIA | 480 | 51.2 a) (11.9) e) [192] | |
| Abbott Laboratories | CLIA | Adult | 757 | 16 b) (5–56) f) [99] |
| Thermo Fisher Scientific | ELISA | Adult | 10 | 3.72 b) ± 0.33 d) [193] |
| 10 | 40.0 b) [30.3–45.0] e) [194] | |||
| Cloud-Clone-Corp. | ELISA | Adult | 24 | 1021 a) [807–1941] e) [195] |
| Bioassay Technology | ELISA | Children | 31 | 23.6 b) ± 7.3 c) [22] |
| eBioscience | ELISA | Adult | 160 | 21.27 b) ± 3.42 c) [123] |
| USCN Life Science Inc. | ELISA | Adult | 40 | 299.29 b) ± 42.32 c) [196] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ono, J.; Takai, M.; Kamei, A.; Azuma, Y.; Izuhara, K. Pathological Roles and Clinical Usefulness of Periostin in Type 2 Inflammation and Pulmonary Fibrosis. Biomolecules 2021, 11, 1084. https://doi.org/10.3390/biom11081084
Ono J, Takai M, Kamei A, Azuma Y, Izuhara K. Pathological Roles and Clinical Usefulness of Periostin in Type 2 Inflammation and Pulmonary Fibrosis. Biomolecules. 2021; 11(8):1084. https://doi.org/10.3390/biom11081084
Chicago/Turabian StyleOno, Junya, Masayuki Takai, Ayami Kamei, Yoshinori Azuma, and Kenji Izuhara. 2021. "Pathological Roles and Clinical Usefulness of Periostin in Type 2 Inflammation and Pulmonary Fibrosis" Biomolecules 11, no. 8: 1084. https://doi.org/10.3390/biom11081084
APA StyleOno, J., Takai, M., Kamei, A., Azuma, Y., & Izuhara, K. (2021). Pathological Roles and Clinical Usefulness of Periostin in Type 2 Inflammation and Pulmonary Fibrosis. Biomolecules, 11(8), 1084. https://doi.org/10.3390/biom11081084







