The Aging Kidney—As Influenced by Heavy Metal Exposure and Selenium Supplementation
Abstract
:1. Introduction
2. Mercury, Cadmium and Lead—Nephrotoxic Environmental Pollutants
3. Functional Changes in Aging Kidneys and the Role of Environmental Pollutants
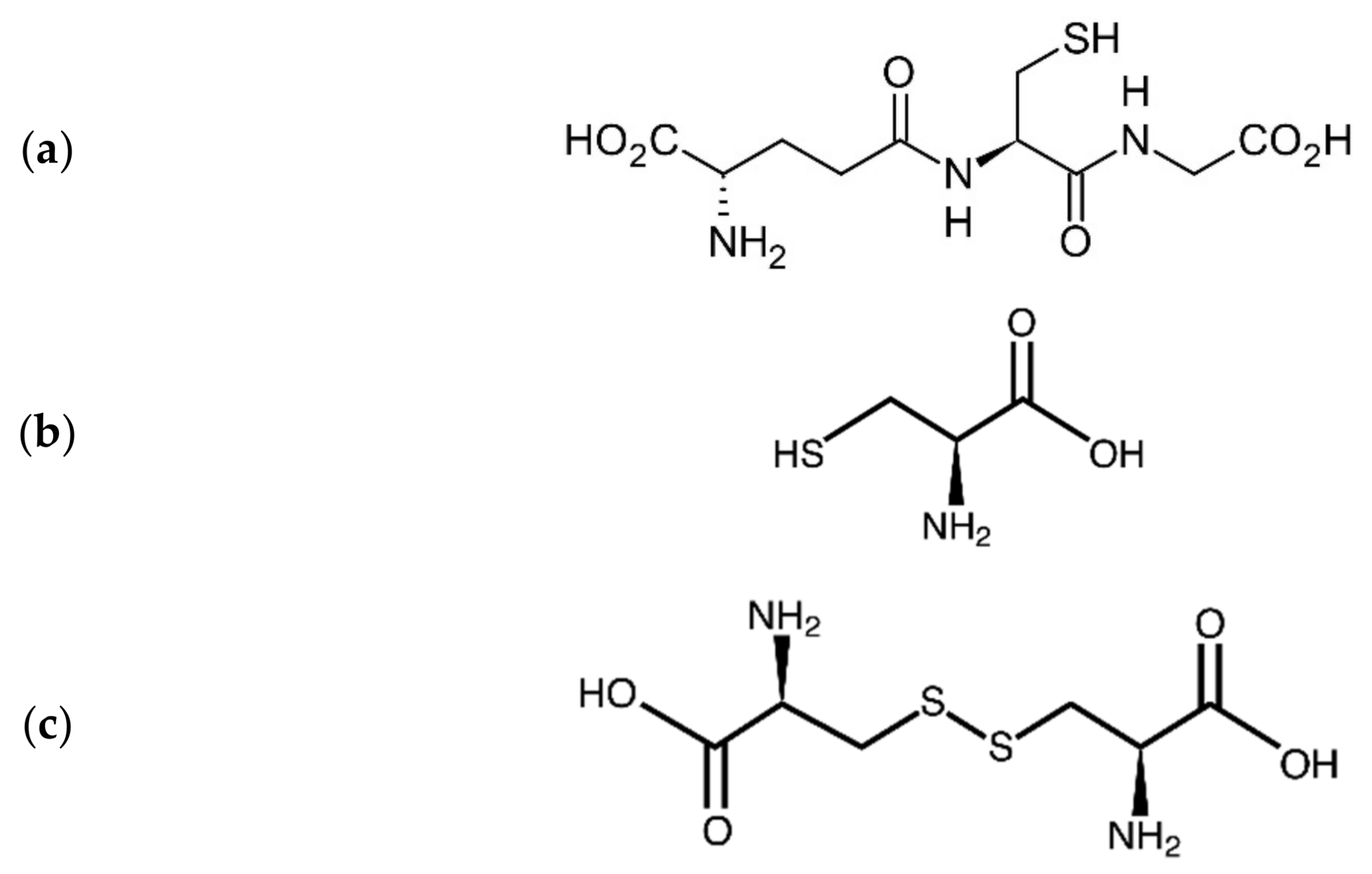
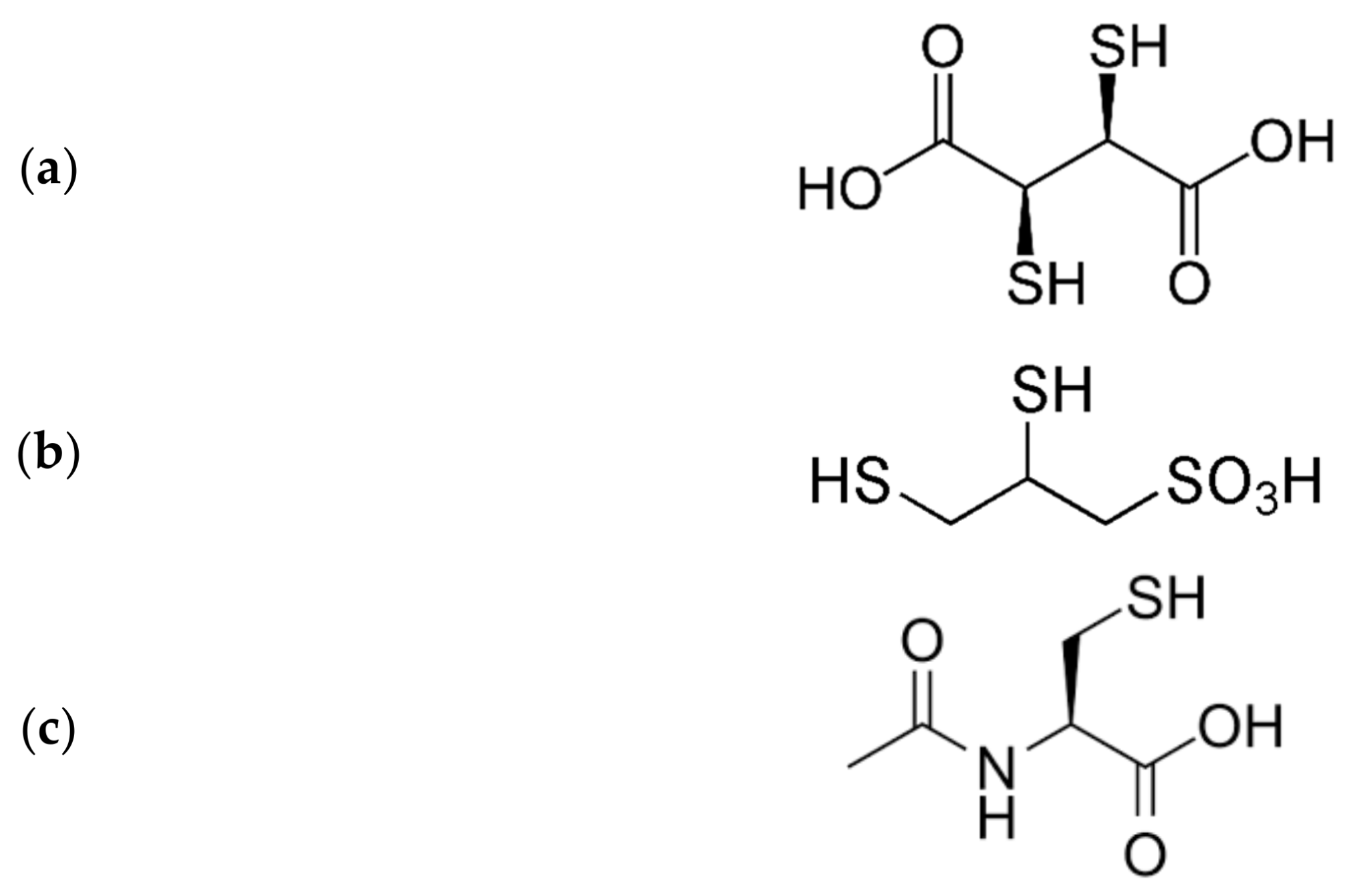
4. Interactions of Heavy Metals with Endogenous Thiols
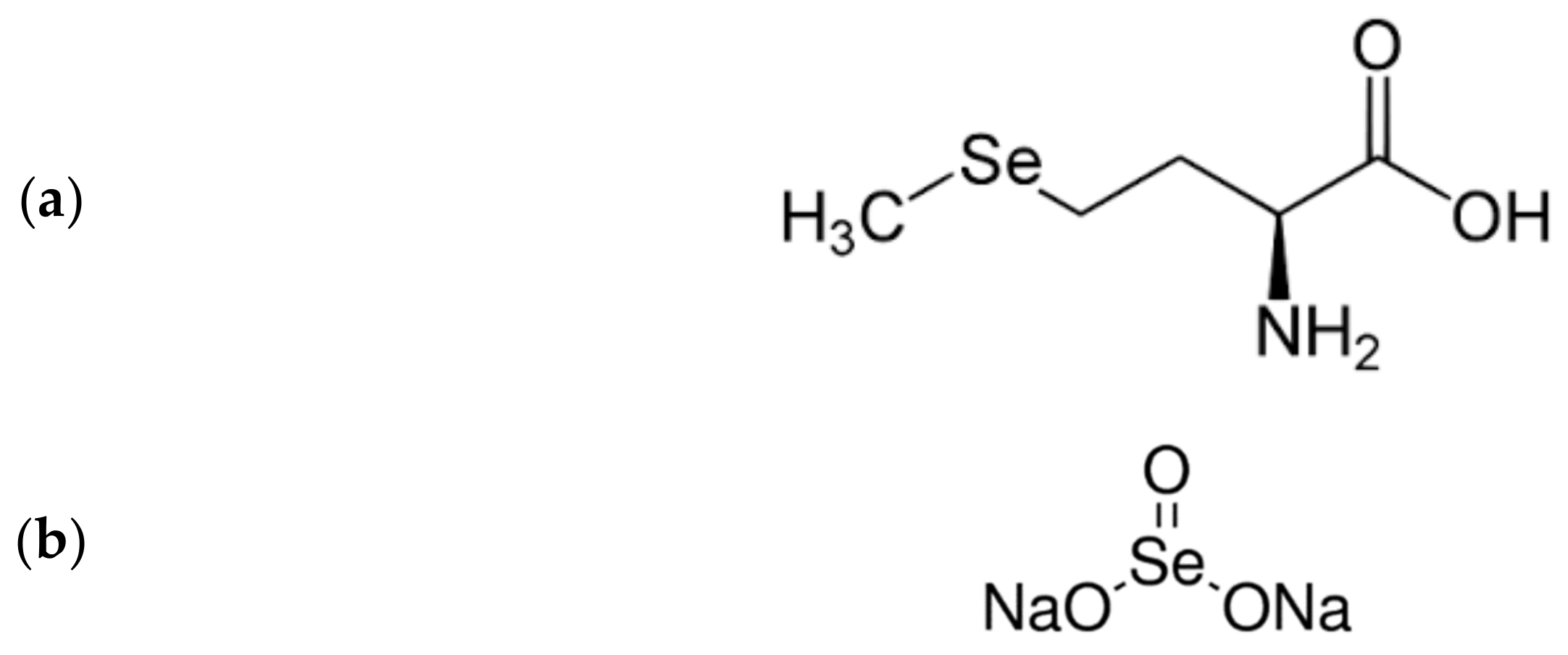
5. Selenium—A Renal Protector with Chelating Properties
6. Discussion and Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Xu, X.; Nie, S.; Ding, H.; Hou, F.F. Environmental pollution and kidney diseases. Nat. Rev. Nephrol. 2018, 14, 313. [Google Scholar] [CrossRef]
- Pigott, C.A. World Population Ageing, 1950–2050; No. 207; United Nations Publications: New York, NY, USA, 2002. [Google Scholar]
- Schmitt, R.; Cantley, L.G. The impact of aging on kidney repair. Am. J. Physiol. Renal. Physiol. 2008, 294, F1265–F1272. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- O’Brown, Z.K.; Van Nostrand, E.L.; Higgins, J.P.; Kim, S.K. The inflammatory transcription factors NFkappaB, STAT1 and STAT3 drive age-associated transcriptional changes in the human kidney. PLoS Genet. 2015, 11, e1005734. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liochev, S.I. Reactive oxygen species and the free radical theory of aging. Free Radic. Biol. Med. 2013, 60, 1–4. [Google Scholar] [CrossRef]
- Poulose, N.; Raju, R. Aging and injury: Alterations in cellular energetics and organ function. Aging Dis. 2014, 5, 101–108. [Google Scholar]
- Lim, J.H.; Kim, E.N.; Kim, M.Y.; Chung, S.; Shin, S.J.; Kim, H.W.; Yang, C.W.; Kim, Y.S.; Chang, Y.S.; Park, C.W.; et al. Age-associated molecular changes in the kidney in aged mice. Oxid. Med. Cell. Longev. 2012, 2012, 171383. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Teixeira, F.B.; de Oliveira, A.C.; Leão, L.K.; Fagundes, N.C.; Fernandes, R.M.; Fernandes, L.M.; Crespo-Lopez, M.E. Exposure to inorganic mercury causes oxidative stress, cell death, and functional deficits in the motor cortex. Front. Mol. Neurosci. 2018, 11, 125. [Google Scholar] [CrossRef] [Green Version]
- Bjørklund, G.; Aaseth, J.; Crisponi, G.; Rahman, M.M.; Chirumbolo, S. Insights on alpha lipoic and dihydrolipoic acids as promising scavengers of oxidative stress and possible chelators in mercury toxicology. J. Inorg. Biochem. 2019, 195, 111–119. [Google Scholar] [CrossRef]
- Bridges, C.C.; Zalups, R.K. The aging kidney and the nephrotoxic effects of mercury. J. Toxicol. Environ. Health 2017, 20, 55–80. [Google Scholar] [CrossRef]
- Moriguchi, J.; Ezaki, T.; Tsukahara, T.; Fukui, Y.; Ukai, H.; Okamoto, S.; Shimbo, S.; Sakurai, H.; Ikeda, M. Effects of aging on cadmium and tubular dysfunction markers in urine from adult women in non-polluted areas. Int. Arch. Occup. Environ. Health 2005, 78, 446–451. [Google Scholar] [CrossRef]
- Bjørklund, G.; Lindh, U.; Aaseth, J.; Mutter, J.; Chirumbolo, S. Mercury in dental amalgams: A great concern for clinical toxicology in developing countries. J. Trace Elem. Med. Biol. 2019, 51, 9–11. [Google Scholar] [CrossRef]
- Ye, B.J.; Kim, B.G.; Jeon, M.J.; Kim, S.Y.; Kim, H.C.; Jang, T.W.; Hong, Y.S. Evaluation of mercury exposure level, clinical diagnosis and treatment for mercury intoxication. Ann. Occup. Environ. Med. 2016, 28, 5. [Google Scholar] [CrossRef] [Green Version]
- Farina, M.; Avila, D.S.; Da Rocha, J.B.T.; Aschner, M. Metals, oxidative stress and neurodegeneration: A focus on iron, manganese and mercury. Neurochem. Int. 2013, 62, 575–594. [Google Scholar] [CrossRef] [Green Version]
- Syversen, T.; Kaur, P. The toxicology of mercury and its compounds. J. Trace Elem. Med. Biol. 2012, 26, 215–226. [Google Scholar] [CrossRef]
- Clarkson, T.W. The toxicology of mercury. Crit. Rev. Clin. Lab. Sci. 1997, 34, 369–403. [Google Scholar] [CrossRef]
- Bjørklund, G.; Crisponi, G.; Nurchi, V.M.; Cappai, R.; Djordjevic, A.B.; Aaseth, J. A review on coordination properties of thiol-containing chelating agents towards mercury, cadmium, and lead. Molecules 2019, 24, 3247. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eide, I.; Syversen, T.L. Relationship between catalase activity and uptake of elemental mercury by rat brain. Acta Pharmacol. Toxicol. 1983, 52, 217–223. [Google Scholar] [CrossRef] [PubMed]
- Ha, E.; Basu, N.; Bose-O’Reilly, S.; Dórea, J.G.; McSorley, E.; Sakamoto, M.; Chan, H.M. Current progress on understanding the impact of mercury on human health. Environ. Res. 2017, 152, 419–433. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pollack, A.Z.; Mumford, S.L.; Mendola, P.; Perkins, N.J.; Rotman, Y.; Wactawski-Wende, J.; Schisterman, E.F. Kidney biomarkers associated with blood lead, mercury, and cadmium in premenopausal women: A prospective cohort study. J. Toxicol. Environ. Health Sci. 2015, 78, 119–131. [Google Scholar] [CrossRef] [Green Version]
- Crisponi, G.; Nurchi, V.M. Metal Ion Toxicity. In Encyclopedia of Inorganic and Bioinorganic Chemistry; John Wiley & Sons Ltd.: Hoboken, NJ, USA, 2015. [Google Scholar]
- Joshi, D.; Kumar, M.D.; Kumar, S.A.; Sangeeta, S. Reversal of methylmercury-induced oxidative stress, lipid peroxidation, and DNA damage by the treatment of N-acetyl cysteine: A protective approach. J. Environ. Pathol. Toxicol. Oncol. 2014, 33, 167–182. [Google Scholar] [CrossRef]
- Al Bakheet, S.A.; Attafi, I.M.; Maayah, Z.H.; Abd-Allah, A.R.; Asiri, Y.A.; Korashy, H.M. Effect of long-term human exposure to environmental heavy metals on the expression of detoxification and DNA repair genes. Environ. Pollut. 2013, 181, 226–232. [Google Scholar] [CrossRef]
- Agrawal, S.; Flora, G.; Bhatnagar, P.; Flora, S. Comparative oxidative stress, metallothionein induction and organ toxicity following chronic exposure to arsenic, lead and mercury in rats. Cell. Mol. Biol. 2014, 60, 13–21. [Google Scholar] [PubMed]
- Nordberg, G.F. Historical perspectives on cadmium toxicology. Toxicol. Appl. Pharmacol. 2009, 238, 192–200. [Google Scholar] [CrossRef] [PubMed]
- Eybl, V.; Kotyzova, D.; Koutensky, J. Comparative study of natural antioxidants—curcumin, resveratrol and melatonin—in cadmium-induced oxidative damage in mice. Toxicology 2006, 225, 150–156. [Google Scholar] [CrossRef] [PubMed]
- Dua, T.K.; Dewanjee, S.; Khanra, R.; Bhattacharya, N.; Bhaskar, B.; Zia-Ul-Haq, M.; De Feo, V. The effects of two common edible herbs, Ipomoea aquatica and Enhydra fluctuans, on cadmium-induced pathophysiology: A focus on oxidative defence and anti-apoptotic mechanism. J. Transl. Med. 2015, 13, 245. [Google Scholar] [CrossRef] [Green Version]
- Liang, Y.; Lei, L.; Nilsson, J.; Li, H.; Nordberg, M.; Bernard, A.; Jin, T. Renal function after reduction in cadmium exposure: An 8-year follow-up of residents in cadmium-polluted areas. Environ. Health Perspect. 2012, 120, 223–228. [Google Scholar] [CrossRef] [Green Version]
- Åkesson, A.; Lundh, T.; Vahter, M.; Bjellerup, P.; Lidfeldt, J.; Nerbrand, C.; Samsioe, G.; Strömberg, U.; Skerfving, S. Tubular and glomerular kidney effects in Swedish women with low environmental cadmium exposure. Environ. Health Perspect. 2005, 113, 1627–1631. [Google Scholar] [CrossRef]
- Wallin, M.; Sallsten, G.; Lundh, T.; Barregard, L. Low-level cadmium exposure and effects on kidney function. Occup. Environ. Med. 2014, 71, 848–854. [Google Scholar] [CrossRef] [Green Version]
- Chen, L.; Lei, L.; Jin, T.; Nordberg, M.; Gunnar, F.; Nordberg, M.D. Plasma metallothionein antibody, urinary cadmium, and renal dysfunction in a Chinese type 2 diabetic population. Diabetes Care 2006, 29, 2682–2687. [Google Scholar] [CrossRef] [Green Version]
- An, H.C.; Sung, J.H.; Lee, J.; Sim, C.S.; Kim, S.H.; Kim, Y. The association between cadmium and lead exposure and blood pressure among workers of a smelting industry: A cross-sectional study. Ann. Occup. Environ. Med. 2017, 29, 47. [Google Scholar] [CrossRef]
- Reyes, J.L.; Molina-Jijón, E.; Rodríguez-Muñoz, R.; Bautista-García, P.; Debray-García, Y.; Namorado, M.D.C. Tight junction proteins and oxidative stress in heavy metals-induced nephrotoxicity. BioMed Res. Int. 2013, 2013, 730789. [Google Scholar] [CrossRef] [Green Version]
- Ponce-Canchihuamán, J.C.; Pérez-Méndez, O.; Hernández-Muñoz, R.; Torres-Durán, P.V.; Juárez-Oropeza, M.A. Protective effects of Spirulina maxima on hyperlipidemia and oxidative-stress induced by lead acetate in the liver and kidney. Lipids Health Dis. 2010, 9, 35. [Google Scholar] [CrossRef] [Green Version]
- Wang, H.; Wang, Z.-K.; Jiao, P.; Zhou, X.-P.; Yang, D.-B.; Wang, Z.-Y.; Wang, L. Redistribution of subcellular calcium and its effect on apoptosis in primary cultures of rat proximal tubular cells exposed to lead. Toxicology 2015, 333, 137–146. [Google Scholar] [CrossRef]
- Gidlow, D.A. Lead toxicity. Occup. Med. 2015, 65, 348–356. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tsaih, S.W.; Korrick, S.; Schwartz, J.; Amarasiriwardena, C.; Aro, A.; Sparrow, D.; Hu, H. Lead, diabetes, hypertension, and renal function: The normative aging study. Environ. Health Perspect. 2004, 112, 1178–1182. [Google Scholar] [CrossRef] [PubMed]
- Harari, F.; Sallsten, G.; Christensson, A.; Petkovic, M.; Hedblad, B.; Forsgard, N.; Melander, O.; Nilsson, P.M.; Borné, Y.; Engström, G.; et al. Blood Lead Levels and Decreased Kidney Function in a Population-Based Cohort. Am. J. Kidney Dis. 2018, 72, 381–389. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Denic, A.; Glassock, R.J.; Rule, A.D. Structural and functional changes within the aging kidney. Adv. Chronic Kidney Dis. 2016, 23, 19–28. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wiggins, J.E.; Patel, S.R.; Shedden, K.A.; Goyal, M.; Wharram, B.L.; Martini, S.; Kretzler, M.; Wiggins, R.C. NFkappaB promotes inflammation, coagulation, and fibrosis in the aging glomerulus. J. Am. Soc. Nephrol. 2010, 21, 587–597. [Google Scholar] [CrossRef]
- Houtkooper, R.H.; Pirinen, E.; Auwerx, J. Sirtuins as regulators of metabolism and healthspan. Nat. Rev. Mol. Cell Biol. 2012, 13, 225–238. [Google Scholar] [CrossRef] [Green Version]
- Tinkov, A.A.; Nguyen, T.T.; Santamaria, A.; Bowman, A.B.; Djordjevic, A.B.; Paoliello, M.M.B.; Skalny, A.V.; Aschner, M. Sirtuins as molecular targets, mediators, and protective agents in metal-induced toxicity. Arch. Toxicol. 2021, 95, 2263–2278. [Google Scholar]
- Weinstein, J.R.; Anderson, S. The aging kidney: Physiological changes. Adv. Chronic Kidney Dis. 2010, 17, 302–307. [Google Scholar] [CrossRef] [Green Version]
- Karam, Z.; Tuazon, J. Anatomic and physiologic changes of the aging kidney. Clin. Geriatr. Med. 2013, 29, 555–564. [Google Scholar] [CrossRef]
- Rule, A.D.; Cornell, L.D.; Poggio, E.D. Senile nephrosclerosis—Does it explain the decline in glomerular filtration rate with aging? Nephron Physiol. 2011, 119 (Suppl. 1), 6–11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lerma, E.V. Anatomic and physiologic changes of the aging kidney. Clin. Geriatr. Med. 2009, 25, 325–329. [Google Scholar] [CrossRef] [PubMed]
- Pecly, I.M.; Genelhu, V.; Francischetti, E.A. Renal functional reserve in obesity hypertension. Int. J. Clin. Pract. 2006, 60, 1198–1203. [Google Scholar] [CrossRef] [PubMed]
- Abdelhafiz, A.H.; Brown, S.H.; Bello, A.; El Nahas, M. Chronic kidney disease in older people: Physiology, pathology or both? Nephron Clin. Pract. 2010, 116, c19–c24. [Google Scholar] [CrossRef]
- Nwankwo, T.; Yoon, S.S.; Burt, V.; Gu, Q. Hypertension among adults in the United States: National Health and Nutrition Examination Survey, 2011–2012. NCHS Data Brief 2013, 133, 1–8. [Google Scholar]
- Ruge, T.; Carlsson, A.C.; Larsson, T.E.; Carrero, J.J.; Larsson, A.; Lind, L.; ÄrnlFv, J. Endostatin level is associated with kidney injury in the elderly: Findings from two community-based cohorts. Am. J. Nephrol. 2014, 40, 417–424. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, H.; Wallin, M.; Barregard, L.; Sallsten, G.; Lundh, T.; Ohlsson, C.; Andersson, E.M. Smoking-induced risk of osteoporosis is partly mediated by cadmium from tobacco smoke: The MrOS Sweden Study. J. Bone Miner. Res. 2020, 35, 1424–1429. [Google Scholar] [CrossRef] [Green Version]
- Clarkson, T.W.; Magos, L. The toxicology of mercury and its chemical compounds. Crit. Rev. Toxicol. 2006, 36, 609–662. [Google Scholar] [CrossRef]
- Aaseth, J.; Wallace, D.R.; Vejrup, K.; Alexander, J. Methylmercury and developmental neurotoxicity: A global concern. Curr. Opin. Toxicol. 2020, 19, 80–87. [Google Scholar] [CrossRef]
- Bjørklund, G.; Dadar, M.; Mutter, J.; Aaseth, J. The toxicology of mercury: Current research and emerging trends. Environ. Res. 2017, 159, 545–554. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Lee, C.K.; Kim, K.H.; Lee, J.T.; Suh, C.; Kim, S.Y.; Kim, J.H.; Son, B.C.; Kim, D.H.; Lee, S. Factors associated with total mercury concentrations in maternal blood, cord blood, and breast milk among pregnant women in Busan, Korea. Asia Pac. J. Clin. Nutr. 2016, 25, 340–349. [Google Scholar] [PubMed]
- Rajaee, M.; Sanchez, B.N.; Renne, E.P.; Basu, N. An investigation of organic and inorganic mercury exposure and blood pressure in a small-scale gold mining community in Ghana. Int. J. Environ. Res. Public Health 2015, 12, 10020–10038. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, Y.; Zhang, B.; Yang, L.; Li, H. Blood mercury concentration among residents of a historic mercury mine and possible effects on renal function: A cross-sectional study in southwestern China. Environ. Monit. Assess. 2013, 185, 3049–3055. [Google Scholar] [CrossRef]
- Sommar, J.N.; Svensson, M.K.; Bjor, B.M.; Elmstahl, S.I.; Hallmans, G.; Lundh, T.; Schon, S.M.; Skerfving, S.; Bergdahl, I.A. End-stage renal disease and low-level exposure to lead, cadmium and mercury: A population-based, prospective nested case-referent study in Sweden. Environ. Health 2013, 12, 9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, N.H.; Hyun, Y.Y.; Lee, K.B.; Chang, Y.; Ryu, S.; Oh, K.H.; Ahn, C. Environmental heavy metal exposure and chronic kidney disease in the general population. J. Korean Med. Sci. 2015, 30, 272–277. [Google Scholar] [CrossRef] [Green Version]
- Rooney, J.P. The role of thiols, dithiols, nutritional factors and interacting ligands in the toxicology of mercury. Toxicology 2007, 234, 145–156. [Google Scholar] [CrossRef]
- Cannon, V.T.; Zalups, R.K.; Barfuss, D.W. Amino acid transporters involved in luminal transport of mercuric conjugates of cysteine in rabbit proximal tubule. J. Pharmacol. Exp. Ther. 2001, 298, 780–789. [Google Scholar]
- Bridges, C.C.; Zalups, R.K. System b0,+ and the transport of thiol-s-conjugates of methylmercury. J. Pharmacol. Exp. Ther. 2006, 319, 948–956. [Google Scholar] [CrossRef]
- Sabolić, I.; Breljak, D.; Škarica, M.; Herak-Kramberger, C.M. Role of metallothionein in cadmium traffic and toxicity in kidneys and other mammalian organs. Biometals 2010, 23, 897–926. [Google Scholar] [CrossRef] [PubMed]
- Berlin, M.; Zalups, R.K.; Fowler, B.A. Mercury. In Handbook on the Toxicology of Metals, Specific Metals II; Nordber, G.F., Fowler, B.A., Nordberg, M., Eds.; Academic Press: Amsterdam, The Netherlands, 2015; pp. 1013–1075. [Google Scholar]
- Bridges, C.C.; Joshee, L.; van den Heuvel, J.J.; Russel, F.G.; Zalups, R.K. Glutathione status and the renal elimination of inorganic mercury in the Mrp2(−/−) mouse. PLoS ONE 2013, 8, e73559. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Šalamon, Š.; Kramar, B.; Marolt, T.P.; Poljšak, B.; Milisav, I. Medical and dietary uses of N-acetylcysteine. Antioxidants 2019, 8, 111. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ng, C.F.; Schafer, F.Q.; Buettner, G.R.; Rodgers, V.G.J. The rate of cellular hydrogen peroxide removal shows dependency on GSH: Mathematical insight into in vivo H2O2 and GPx concentrations. Free Radic. Res. 2007, 41, 1201–1211. [Google Scholar] [CrossRef] [Green Version]
- Cao, Y.; Skaug, M.A.; Andersen, O.; Aaseth, J. Chelation therapy in intoxications with mercury, lead and opper. J. Trace Elem. Med. Biol. 2015, 31, 188–192. [Google Scholar] [CrossRef] [PubMed]
- Kornhauser, C.; Garcia-Ramirez, J.R.; Wrobel, K.; Pérez-Luque, E.L.; Garay-Sevilla, M.E.; Wrobel, K. Serum selenium and glutathione peroxidase concentrations in type 2 diabetes mellitus patients. Prim. Care Diabetes 2008, 2, 81–85. [Google Scholar] [CrossRef]
- Olson, G.E.; Whitin, J.C.; Hill, K.E.; Winfrey, V.P.; Motley, A.K.; Austin, L.M.; Deal, J.; Cohen, H.J.; Burk, R.F. Extracellular glutathione peroxidase (Gpx3) binds specifically to basement membranes of mouse renal cortex tubule cells. Am. J. Physiol. Ren. Physiol. 2010, 298, F1244–F1253. [Google Scholar] [CrossRef] [Green Version]
- Alehagen, U.; Aaseth, J.; Alexander, J.; Brismar, K.; Larsson, A. Selenium and Coenzyme Q10 Supplementation Improves Renal Function in Elderly Deficient in Selenium: Observational Results and Results from a Subgroup Analysis of a Prospective Randomised Double-Blind Placebo-Controlled Trial. Nutrients 2020, 12, 3780. [Google Scholar] [CrossRef]
- Bjørklund, G.; Aaseth, J.; Ajsuvakova, O.P.; Nikonorov, A.A.; Skalny, A.V.; Skalnaya, M.G.; Tinkov, A.A. Molecular interaction between mercury and selenium in neurotoxicity. Coord. Chem. Rev. 2017, 332, 30–37. [Google Scholar] [CrossRef]
- Kieliszek, M.; Błażejak, S.; Bzducha-Wróbel, A.; Kot, A.M. Effect of selenium on growth and antioxidative system of yeast cells. Mol. Biol. Rep. 2019, 46, 1797–1808. [Google Scholar] [CrossRef] [Green Version]
- Rayman, M.P.; Stranges, S. Epidemiology of selenium and type 2 diabetes: Can we make sense of it? Free Radic. Biol. Med. 2013, 65, 1557–1564. [Google Scholar] [CrossRef] [PubMed]
- Iglesias, P.; Selgas, R.; Romero, S.; Díez, J.J. Selenium and kidney disease. J. Nephrol. 2013, 26, 266. [Google Scholar] [CrossRef] [PubMed]
- Pakfetrat, M.; Malekmakan, L.; Hasheminasab, M. Diminished selenium levels in hemodialysis and continuous ambulatory peritoneal dialysis patients. Biol. Trace Elem. Res. 2010, 137, 335–339. [Google Scholar] [CrossRef] [PubMed]
- Ruiz, A.A.; Jiménez, E.M.; Bermejo-Barrera, P.; Lozano, R.; Seijas, V.M.E. Selenium and All-cause Mortality in End-Stage Renal Disease. Retrospective Observational Cohort Study. J. Ren. Nutr. 2020, 30, 484–492. [Google Scholar] [CrossRef] [PubMed]
- Burk, R.F.; Hill, K.E. Regulation of Selenium Metabolism and Transport. Annu. Rev. Nutr. 2015, 35, 109–134. [Google Scholar] [CrossRef]
- Willnow, T.E.; Christ, A. Endocytic receptor LRP2/megalin-of holoprosencephaly and renal Fanconi syndrome. Pflug. Arch. 2017, 469, 907–916. [Google Scholar] [CrossRef] [PubMed]
- Negri, A.L. Proximal tubule endocytic apparatus as the specific renal uptake mechanism for vitamin D-binding protein/25-(OH)D3 complex. Nephrology 2006, 11, 510–515. [Google Scholar] [CrossRef]
- Bjørklund, G. Selenium as an antidote in the treatment of mercury intoxication. Biometals 2015, 28, 605–614. [Google Scholar] [CrossRef] [PubMed]
- Sugiura, Y.; Tamai, Y.; Tanaka, H. Selenium Protection against Mercury Toxicity; High Binding Affinity of Methylmercury by Selenium-containing Ligands in Comparison with Sulfur-containing Ligands. Bioinorg. Chem. 1978, 9, 167–180. [Google Scholar] [CrossRef]
- Kuria, A.; Fang, X.; Li, M.; Han, H.; He, J.; Aaseth, J.O.; Cao, Y. Does dietary intake of selenium protect against cancer? A systematic review and meta-analysis of population-based prospective studies. Crit. Rev. Food Sci. Nutr. 2020, 60, 684–694. [Google Scholar] [CrossRef]
- El-Khairy, L.; Ueland, P.M.; Refsum, H.; Graham, I.M.; Vollset, S.E. Plasma total cysteine as a risk factor for vascular disease: The European Concerted Action Project. Circulation 2001, 103, 2544–2549. [Google Scholar] [CrossRef] [Green Version]
- Spiller, H.A.; Hays, H.L.; Casavant, M.J. Rethinking treatment of mercury poisoning: The roles of selenium, acetylcysteine, and thiol chelators in the treatment of mercury poisoning: A narrative review. Toxicol. Commun. 2021, 5, 19–59. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aaseth, J.; Alexander, J.; Alehagen, U.; Tinkov, A.; Skalny, A.; Larsson, A.; Crisponi, G.; Nurchi, V.M. The Aging Kidney—As Influenced by Heavy Metal Exposure and Selenium Supplementation. Biomolecules 2021, 11, 1078. https://doi.org/10.3390/biom11081078
Aaseth J, Alexander J, Alehagen U, Tinkov A, Skalny A, Larsson A, Crisponi G, Nurchi VM. The Aging Kidney—As Influenced by Heavy Metal Exposure and Selenium Supplementation. Biomolecules. 2021; 11(8):1078. https://doi.org/10.3390/biom11081078
Chicago/Turabian StyleAaseth, Jan, Jan Alexander, Urban Alehagen, Alexey Tinkov, Anatoly Skalny, Anders Larsson, Guido Crisponi, and Valeria Marina Nurchi. 2021. "The Aging Kidney—As Influenced by Heavy Metal Exposure and Selenium Supplementation" Biomolecules 11, no. 8: 1078. https://doi.org/10.3390/biom11081078
APA StyleAaseth, J., Alexander, J., Alehagen, U., Tinkov, A., Skalny, A., Larsson, A., Crisponi, G., & Nurchi, V. M. (2021). The Aging Kidney—As Influenced by Heavy Metal Exposure and Selenium Supplementation. Biomolecules, 11(8), 1078. https://doi.org/10.3390/biom11081078










