Cannabinoquinones: Synthesis and Biological Profile
Abstract
:1. Introduction
2. Timeline of Studies
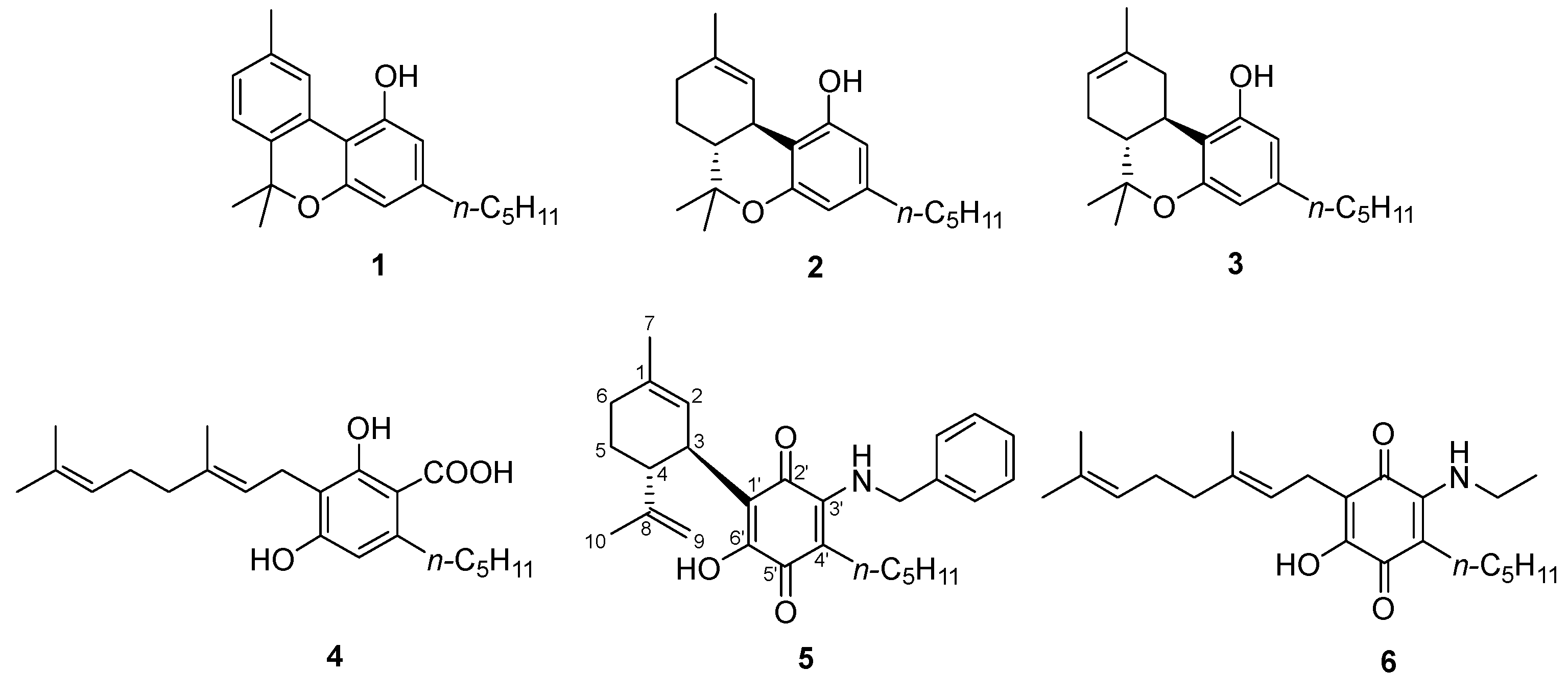
3. Synthesis and Chemistry of Cannabinoquinoids
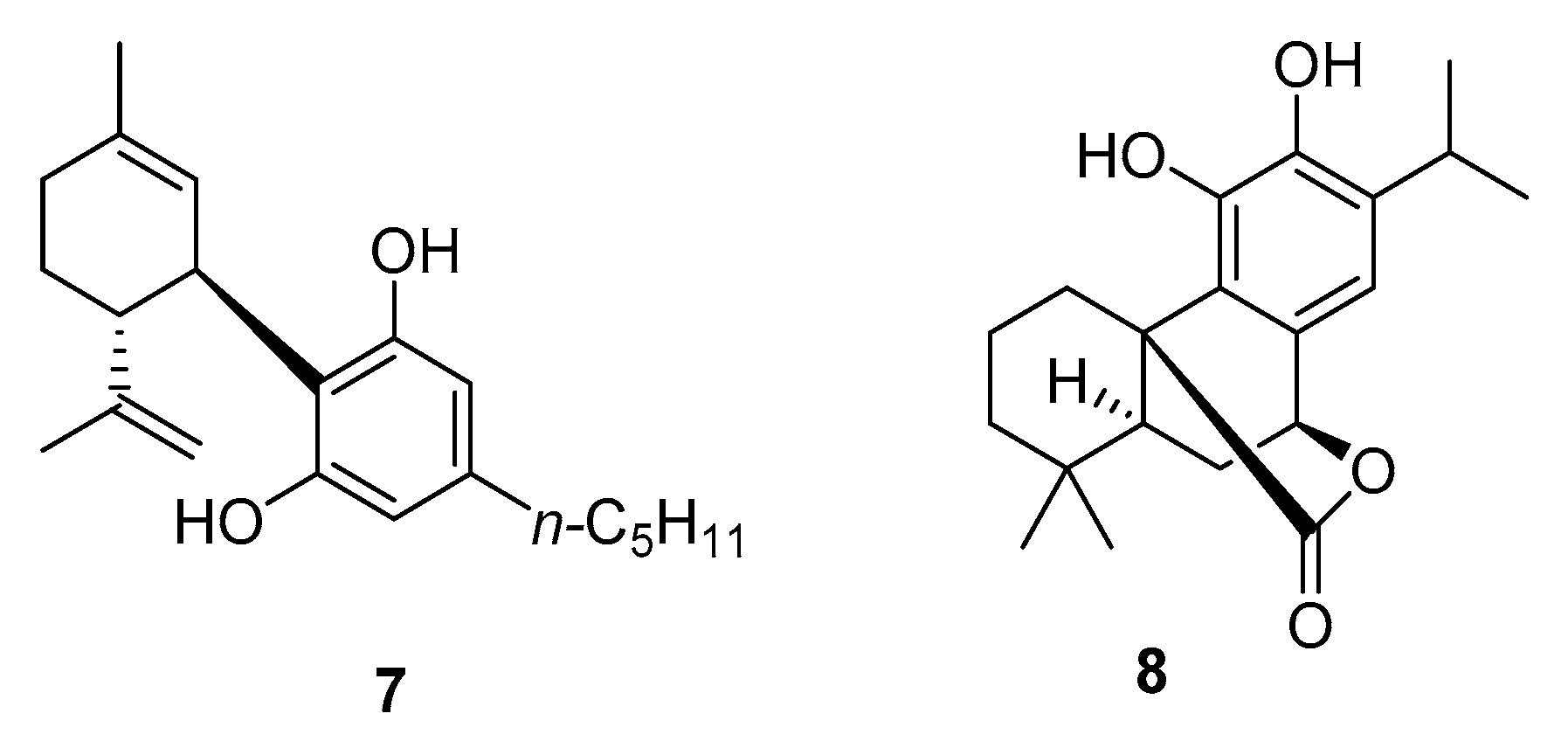
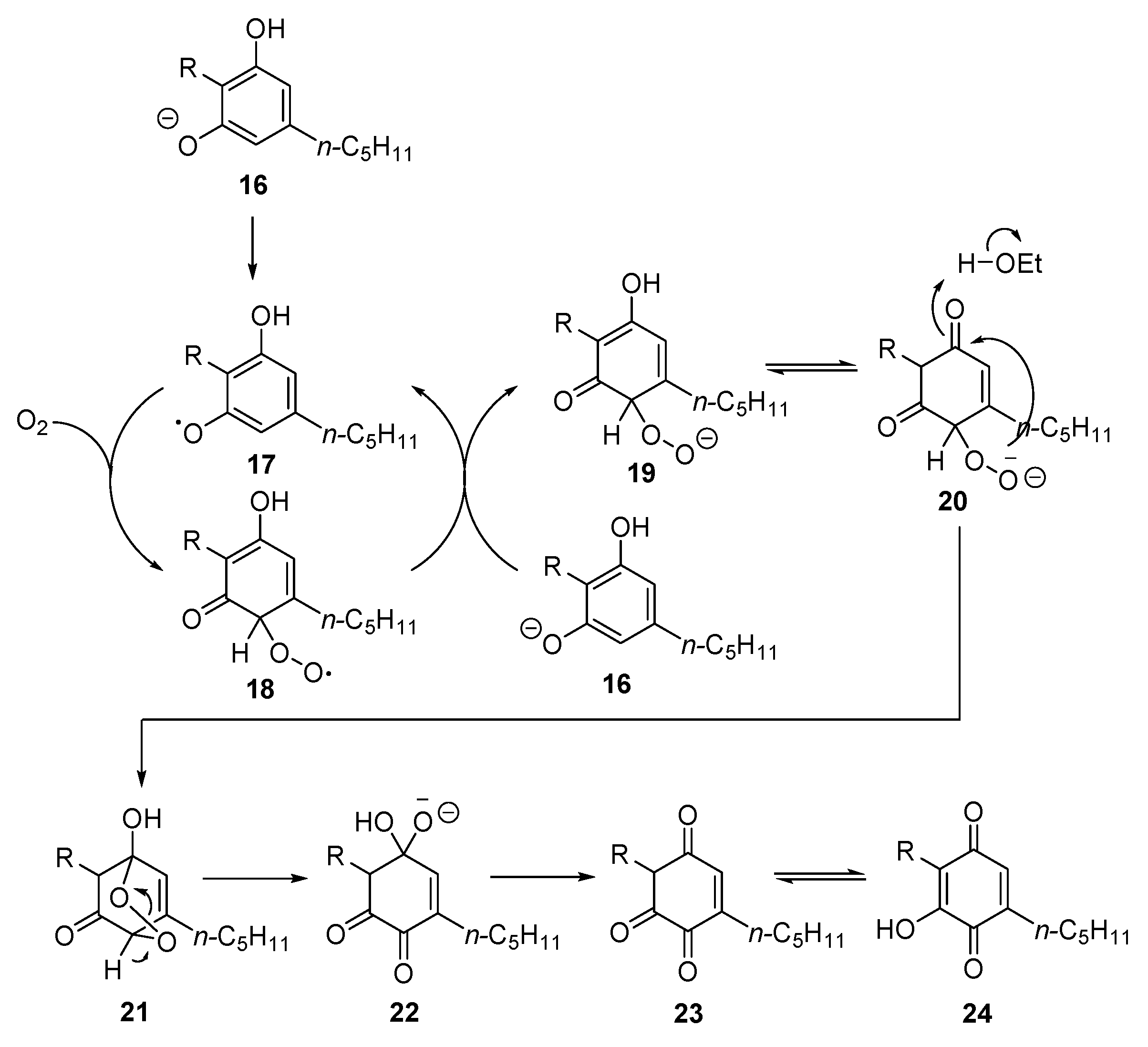
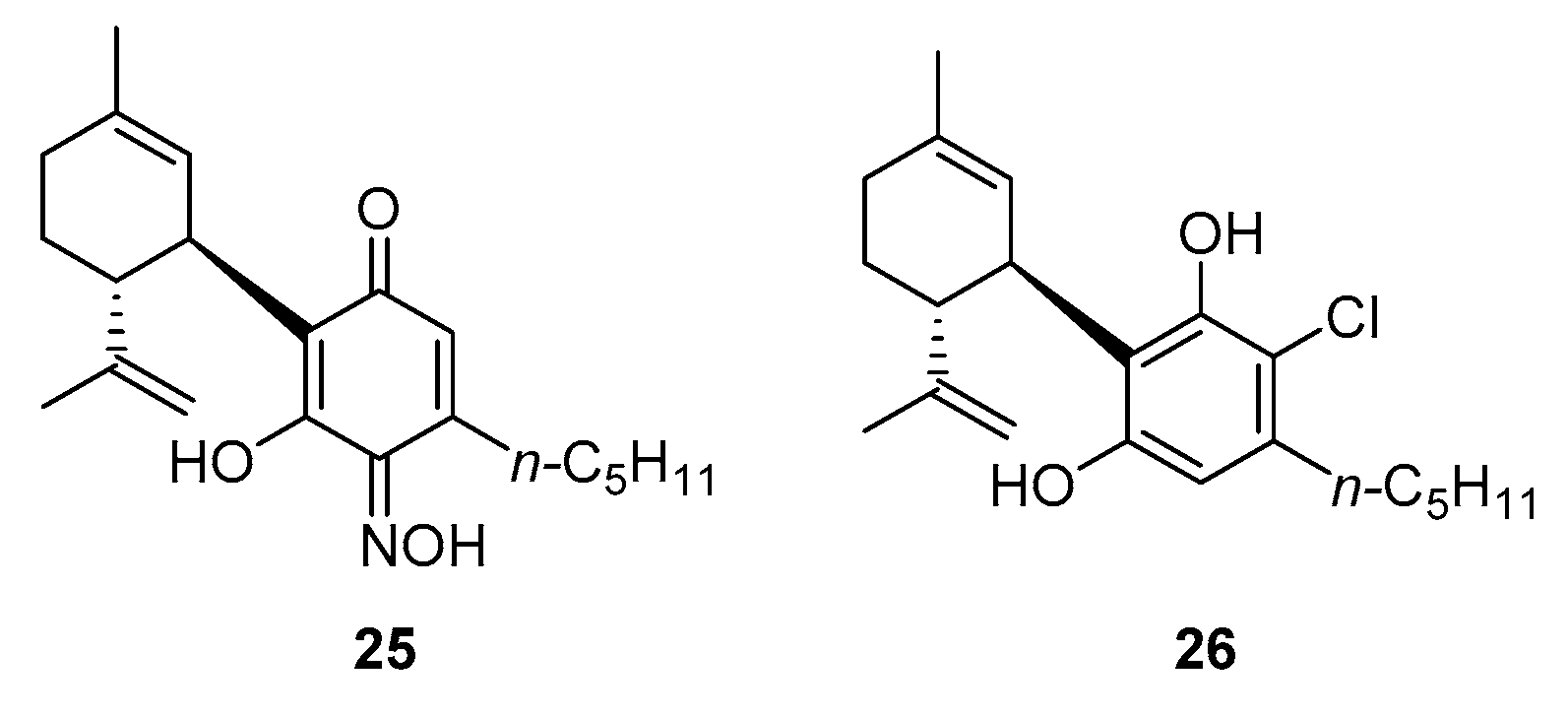
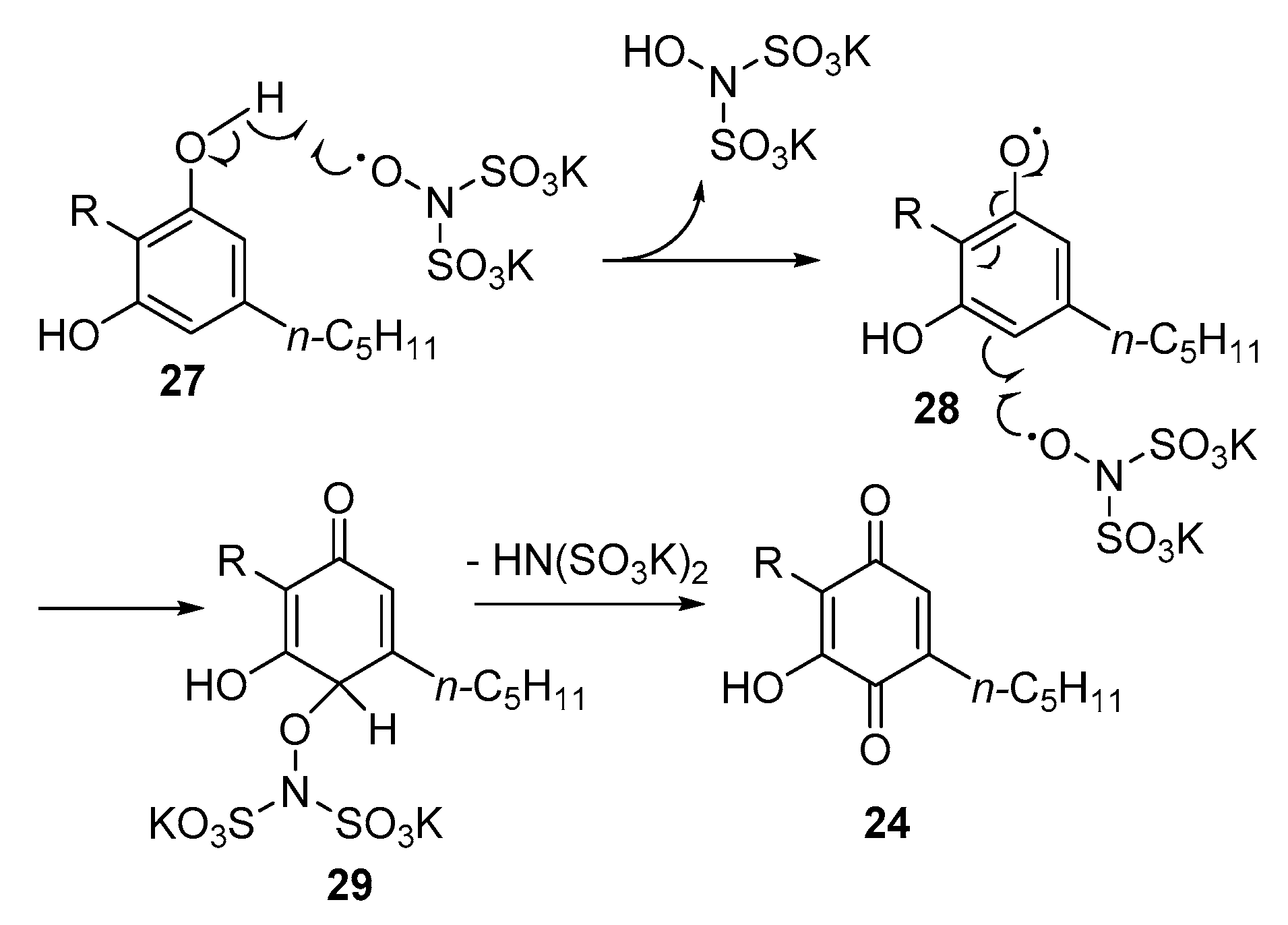
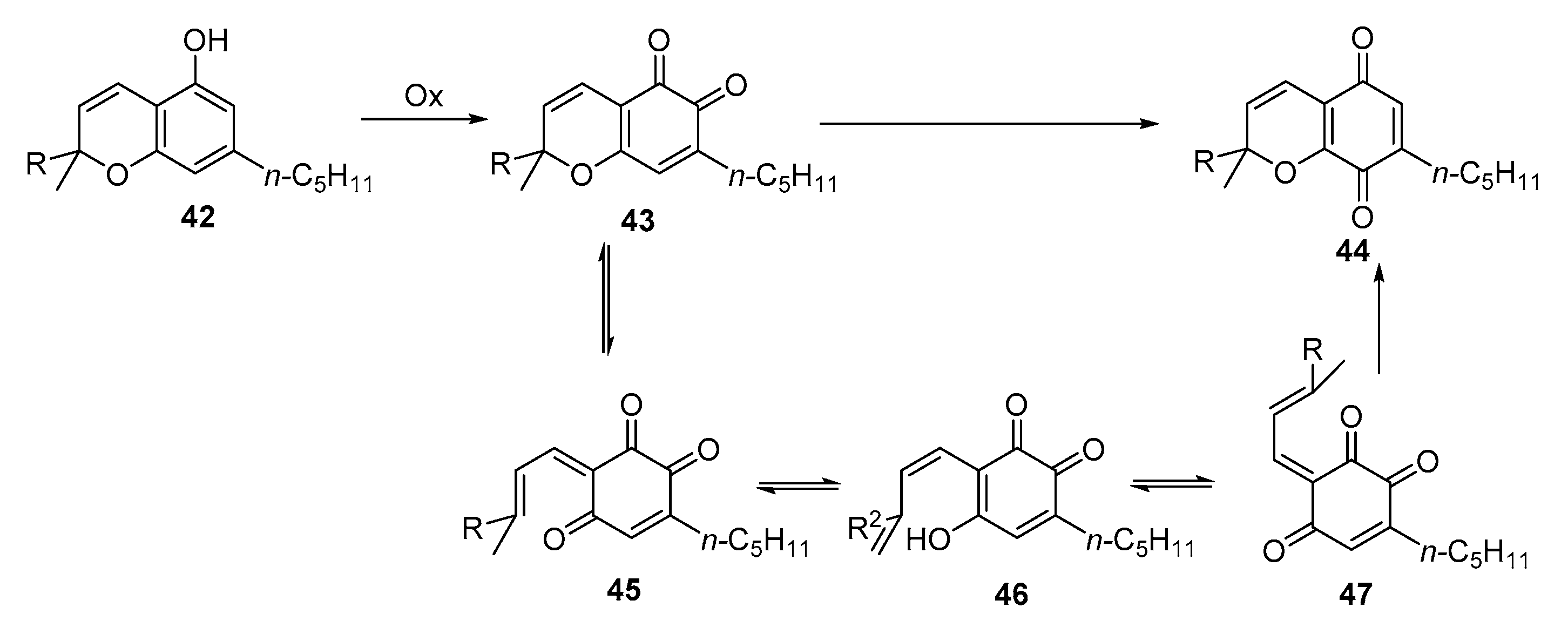
3.1. Synthesis
3.2. Reactivity
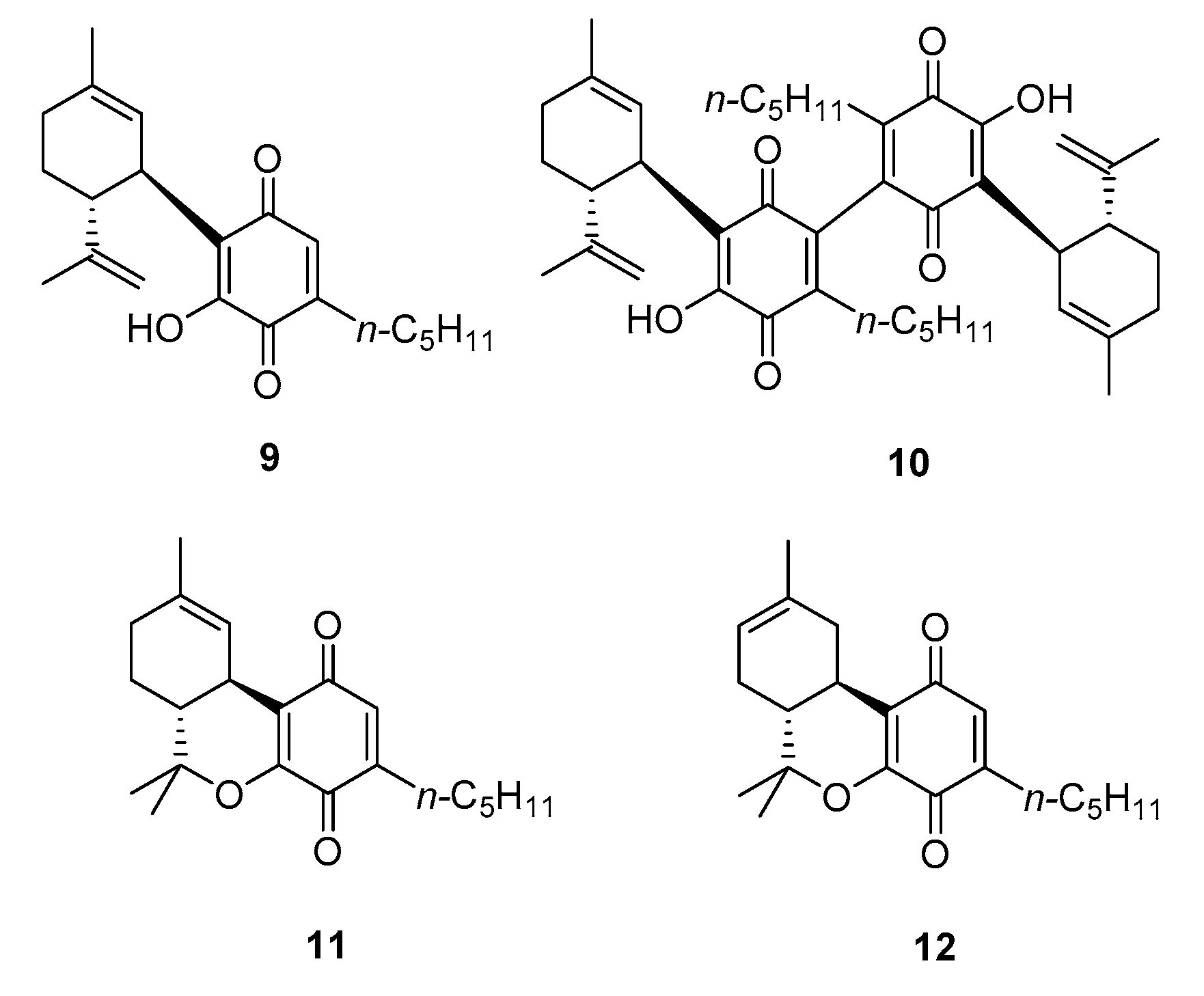
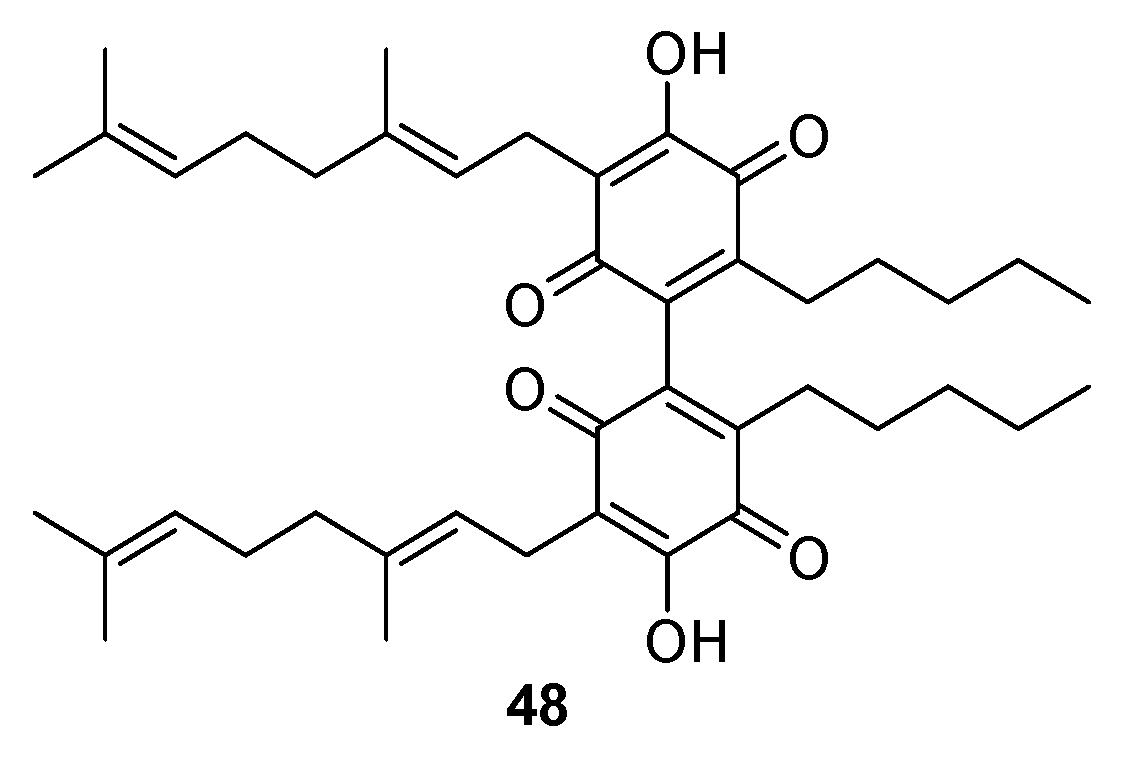
3.2.1. Dimerization
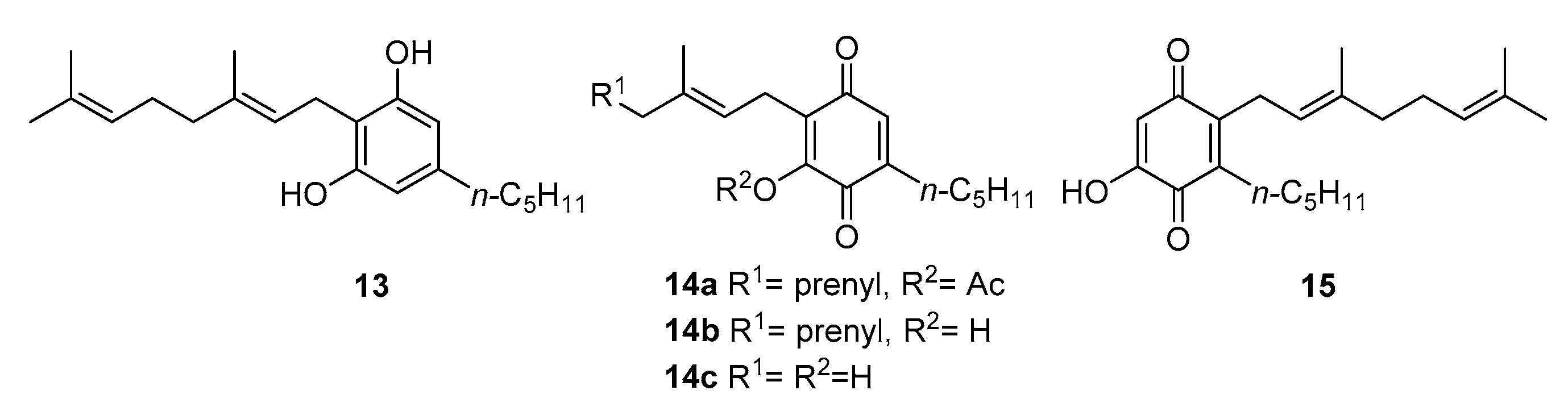
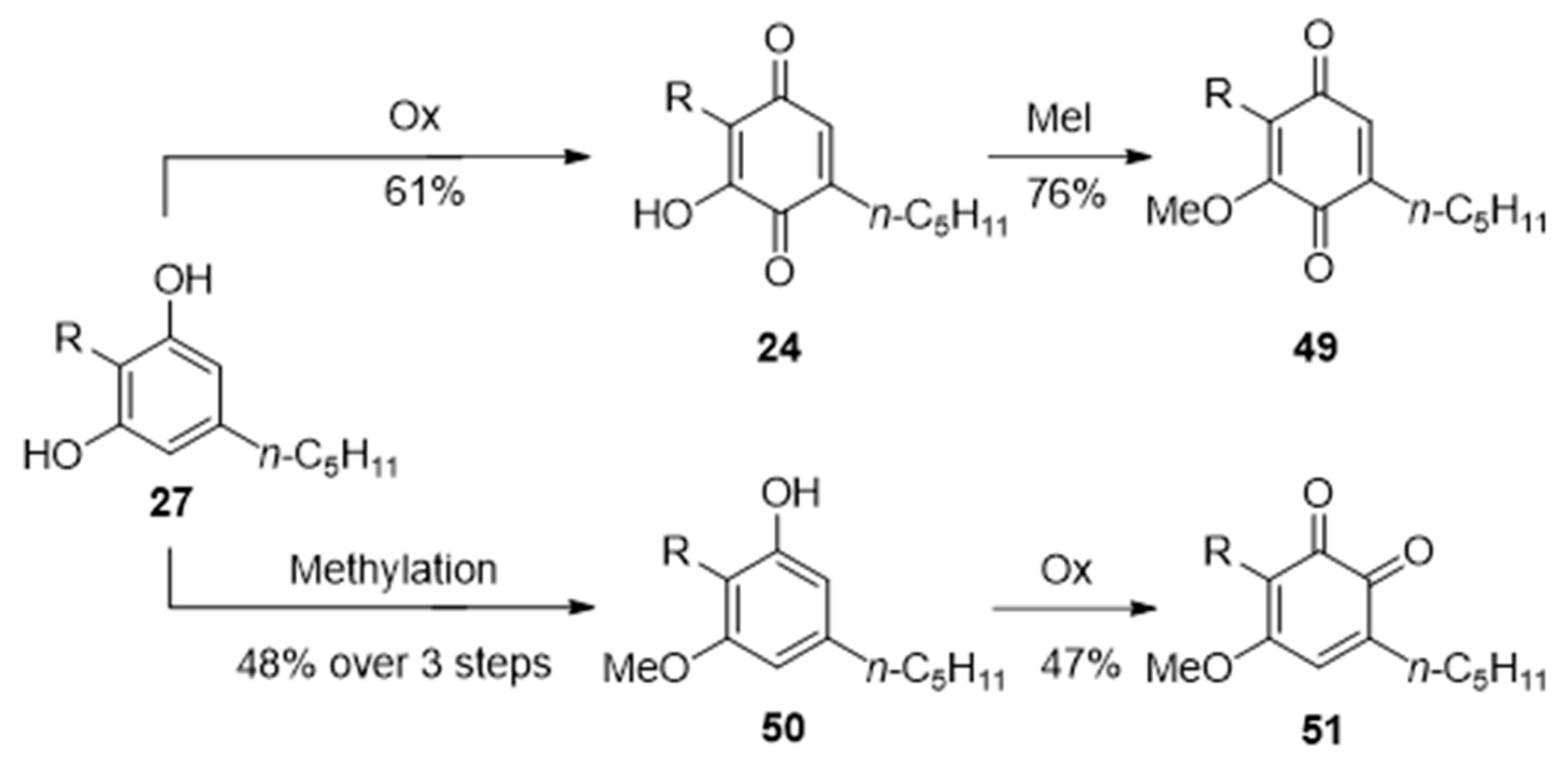
3.2.2. O-Methylation
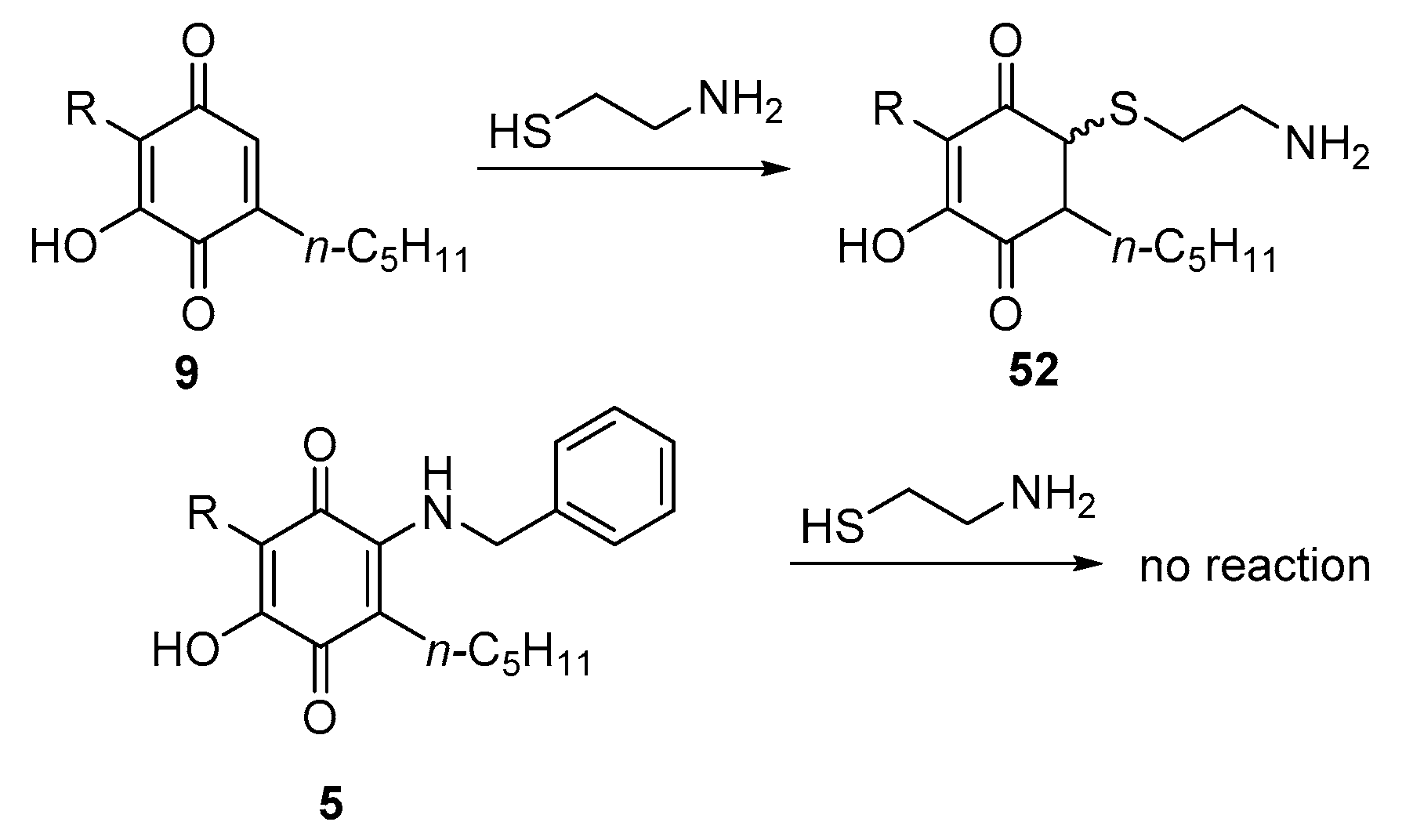
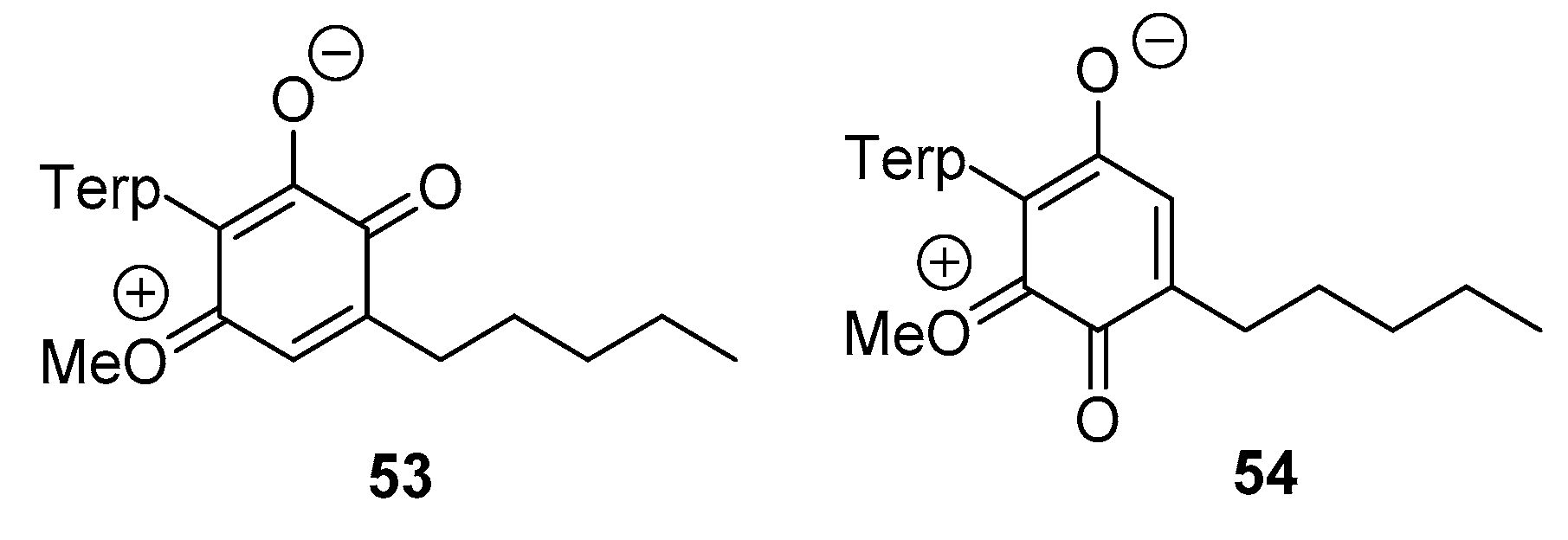
3.2.3. Thia- and Aza-Michael Addition
4. Spectroscopic Properties of Cannabinoquinones
5. Bioactivity Studies
5.1. Anticancer Activity
5.2. Modulation of Liver Microsome Activity
5.3. Neuroprotective and Immunomodulatory Activity
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Krejčí, Z.; Šantavý, F. Isolace Dalších Látek z Listí Indckého Konopi Cannabis sativa L. [Isolation of Other Substances from the Leaves of the Indian Hemp Cannabis sativa L.]. Acta Univ Palacki Olomuc 1955, 6, 59–66. [Google Scholar]
- Wood, T.B.; Spivey, W.T.N.; Easterfield, T.H. Cannabinol, Part I. J. Chem. Soc. 1899, 75, 20–36. [Google Scholar] [CrossRef] [Green Version]
- Hanuš, L.O.; Meyer, S.M.; Muñoz, E.; Taglialatela-Scafati, O.; Appendino, G. Phytocannabinoids: A Unified Critical Inventory. Nat. Prod. Rep. 2016, 33, 1357–1392. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pollastro, F.; De Petrocellis, L.; Schiano-Moriello, A.; Chianese, G.; Heyman, H.; Appendino, G.; Taglialatela-Scafati, O. Reprint of: Amorfrutin-type phytocannabinoids from Helichrysum umbraculigerum. Fitoterapia 2018, 126, 35–39. [Google Scholar] [CrossRef]
- Caprioglio, D.; Mattoteia, D.; Pollastro, F.; Negri, R.; Lopatriello, A.; Chianese, G.; Minassi, A.; Collado, J.A.; Munoz, E.; Taglialatela-Scafati, O.; et al. The Oxidation of Phytocannabinoids to Cannabinoquinoids. J. Nat. Prod. 2020, 83, 1711–1715. [Google Scholar] [CrossRef]
- Beam, W. Fourth Report Wellcome Tropical Research Lab. Chem. Sect. Rep. Sudan Gov. 1911, 25, 105. [Google Scholar]
- Appendino, G. The early history of cannabinoid research. Rend. Lincei. Sci. Fis. Nat. 2020, 31, 919–929. [Google Scholar] [CrossRef]
- del Río, C.; Navarrete, C.; Collado, J.A.; Bellido, M.L.; Gómez-Cañas, M.; Pazos, M.R.; Fernández-Ruiz, J.; Pollastro, F.; Appendino, G.; Calzado, M.A.; et al. The Cannabinoid Quinol VCE-004.8 Alleviates Bleomycin-induced Scleroderma and Exerts Potent Antifibrotic Effects Through Peroxisome Proliferator-Activated Receptor-γ and CB2 Path-ways. Sci. Rep. 2016, 6, 21703. [Google Scholar] [CrossRef] [PubMed]
- Díaz-Alonso, J.; Paraíso-Luna, J.; Navarrete, C.; Del Río, C.; Cantarero, I.; Palomares, B.; Aguareles, J.; Fernández-Ruiz, J.; Bellido, M.L.; Pollastro, F.; et al. VCE-003.2, a novel cannabigerol derivative, enhances neuronal progenitor cell survival and alleviates symptomatology in murine models of Huntington’s disease. Sci. Rep. 2016, 6, 29789. [Google Scholar] [CrossRef] [Green Version]
- Kogan, N.M.; Peters, M.; Mechoulam, R. Cannabinoid Quinones—A Review and Novel Observations. Molecules 2021, 26, 1761. [Google Scholar] [CrossRef]
- Mechoulam, R.; Ben-Zvi, Z.; Gaoni, Y. Hashsh-XIII: On the Nature of the Beam Test. Tetrahedron 1968, 24, 5615–5626. [Google Scholar] [CrossRef]
- Montgomery, C.T.; Cassels, B.K.; Shamma, M. The Rhoeadine Alkaloids. J. Nat. Prod. 1983, 46, 441–453. [Google Scholar] [CrossRef]
- Grlić, L. Recent advances in the chemical research of cannabis. Bull. Narc. 1964, 16, 29–38. [Google Scholar]
- Lewis, K.; Wagner, R.; Rodriguez-Cruz, S.E.; Weaver, M.J.; Dumke, J.C. Validation of the 4-Aminophenol Color Test for the Differentiation of Marijuana-Type and Hemp-Type Cannabis. J. Forensic Sci. 2021, 66, 285–294. [Google Scholar] [CrossRef]
- Hodjat-Kashani, H.; Lambert, G.; Duffley, R.; Razdan, R. Hashish: Oxidation of Δ8-Tetrahydrocannabinol (THC); Synthesis of Δ8-THC-1,2-dione and 2-Hydroxy-Δ8-THC. Heterocycles 1986, 24, 1973–1976. [Google Scholar]
- Kogan, N.M.; Rabinowitz, R.; Levi, P.; Gibson, D.; Sandor, P.; Schlesinger, M.; Mechoulam, R. Synthesis and Antitumor Activity of Quinonoid Derivatives of Cannabinoids. J. Med. Chem. 2004, 47, 3800–3806. [Google Scholar] [CrossRef] [PubMed]
- Peters, M.; Kogan, N.M. HU-331: A cannabinoid quinone, with uncommon cytotoxic properties and low toxicity. Expert Opin. Investig. Drugs 2007, 16, 1405–1413. [Google Scholar] [CrossRef]
- Gutzwiller, J.; Uskokovic, M.R. Total Synthesis of Cinchona Alkaloids. Stereoselective Total Syntheses of Quinine and Quinidine. J. Am. Chem. Soc. 1978, 100, 576–581. [Google Scholar] [CrossRef]
- Waugh, T.M.; Masters, J.; Aliev, A.E.; Marson, C.M. Monocyclic Quinone Structure-Activity patterns: Synthesis of Catalytic Inhibitors of Topoisomerase II with ntiproliferative Activity. Chem. Med. Chem. 2020, 15, 114–124. [Google Scholar] [CrossRef] [Green Version]
- Darzi, E.R.; Garg, N.K. Electrochemical Oxidation of Δ9-Tetrahydrocannabinol: A Simple Strategy for Marijuana Detection. Org. Lett. 2020, 22, 3951–3955. [Google Scholar] [CrossRef]
- Mattoteia, D.; Taglialatela-Scafati, O.; Muñoz, E.; de la Vega, L.; Caprioglio, D.; Appendino, G. Regiodivergent Synthesis of ortho- and para-Cannabinoquinones. Eur. J. Org. Chem. 2020, 2020, 7429–7434. [Google Scholar] [CrossRef]
- Caprioglio, D.; Mattoteia, D.; Minassi, A.; Pollastro, F.; Lopatriello, A.; Muňoz, E.; Taglialatela-Scafati, O.; Appendino, G. One-Pot Total Synthesis of Cannabinol via Iodine-Mediated Deconstructive Annulation. Org. Lett. 2019, 21, 6122–6125. [Google Scholar] [CrossRef] [PubMed]
- Appendino, G. Unpublished Work; Muñoz, E., Ed.; University of Eastern Piedmont: Vercelli, Italy, 2021. [Google Scholar]
- Caprioglio, D.; Minassi, A.; Avonto, C.; Taglialatela-Scafati, O.; Appendino, G. Thiol-trapping natural products under the lens of the cysteamine assay: Friends, foes, or simply alternatively reversible ligands? Phytochem. Rev. 2020, 19, 1307–1321. [Google Scholar] [CrossRef]
- Kogan, N.M.; Blázquez, C.; Álvarez, L.; Gallily, R.; Schlesinger, M.; Guzmán, M.; Mechoulam, R. A Cannabinoid Quinone Inhibits Angiogenesis by Targeting Vascular Endothelial Cells. Mol. Pharmacol. 2006, 70, 51–59. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kogan, N.M.; Schlesinger, M.; Priel, E.; Rabinowitz, R.; Berenshtein, E.; Chevion, M.; Mechoulam, R. HU-331, a novel cannabinoid-based anticancer topoisomerase II inhibitor. Mol. Cancer Ther. 2007, 6, 173–183. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wilson, J.T.; Fief, C.A.; Jackson, K.D.; Mercer, S.L.; Deweese, J.E. HU-331 and Oxidized Cannabidiol Act as Inhibitors of Human Topoisomerase IIα and β. Chem. Res. Toxicol. 2018, 31, 137–144. [Google Scholar] [CrossRef]
- Kogan, N.M.; Schlesinger, M.; Peters, M.; Marincheva, G.; Beeri, R.; Mechoulam, R. A Cannabinoid Anticancer Quinone, HU-331, Is More Potent and Less Cardiotoxic Than Doxorubicin: A Comparative In Vivo Study. J. Pharmacol. Exp. Ther. 2007, 322, 646–653. [Google Scholar] [CrossRef] [PubMed]
- Regal, K.M.; Mercer, S.L.; Deweese, J.E. HU-331 is a Catalytic Inhibitor of Topoisomerase IIα. Chem. Res. Toxicol. 2014, 27, 2044–2051. [Google Scholar] [CrossRef]
- Usami, N.; Yamamoto, I.; Watanabe, K. Generation of Reactive Oxygen Species during Mouse Hepatic Microsomal Metabolism of Cannabidiol and Cannabidiol Hydroxy-Quinone. Life Sci. 2008, 83, 717–724. [Google Scholar] [CrossRef]
- Watanabe, K.; Usami, N.; Yamamoto, I.; Yoshimura, H. Inhibitory Effect of Cannabidiol Hydroxy-quinone, an Oxidative Product of Cannabidiol, on the Hepatic Microsomal Drug-Metabolizing Enzymes of Mice. J. Pharm. Dyn. 1991, 14, 421–427. [Google Scholar] [CrossRef]
- Bornheim, L.M.; Grillo, M.P. Characterization of Cytochrome P450 3A Inactivation by cannabidiol: Possible involvement of cannabidiol-hydroxyquinone as a P450 inactivator. Chem. Res. Toxicol. 1998, 11, 1209–1216. [Google Scholar] [CrossRef]
- Ewing, L.E.; Skinner, C.M.; Quick, C.M.; Kennon-McGill, S.; McGill, M.R.; Walker, L.A.; ElSohly, M.A.; Gurley, B.J.; Koturbash, I. Hepatotoxicity of a Cannabidiol-Rich Cannabis Extract in the Mouse Model. Molecules 2019, 24, 1694. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jiang, R.; Yamaori, S.; Takeda, S.; Yamamoto, I.; Watanabe, K. Identification of cytochrome P450 enzymes responsible for metabolism of cannabidiol by human liver microsomes. Life Sci. 2011, 89, 165–170. [Google Scholar] [CrossRef] [PubMed]
- Nelson, K.M.; Bisson, J.; Singh, G.; Graham, J.G.; Chen, S.-N.; Friesen, J.B.; Dahlin, J.L.; Niemitz, M.; Walters, M.A.; Pauli, G.F. The Essential Medicinal Chemistry of Cannabidiol (CBD). J. Med. Chem. 2020, 63, 12137–12155. [Google Scholar] [CrossRef]
- Garcia, C.; Gómez-Cañas, M.; Burgaz, S.; Palomares, B.; Gómez-Gálvez, Y.; Palomo-Garo, C.; Campo, S.; Ferrer-Hernández, J.; Pavicic, C.; Navarrete, C.; et al. Benefits of VCE-003.2, a cannabigerol quinone derivative, against inflammation-driven neuronal deterioration in experimental Parkinson’s disease: Possible involvement of different binding sites at the PPARγ receptor. J. Neuroinflammation 2018, 15, 19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aguareles, J.; Paraíso-Luna, J.; Palomares, B.; Bajo-Grañeras, R.; Navarrete, C.M.; Ruiz-Calvo, A.; García-Rincón, D.; García-Taboada, E.; Guzmán, M.; Muñoz, E.; et al. Oral administration of the cannabigerol derivative VCE-003.2 promotes subventricular zone neurogenesis and protects against mutant huntingtin-induced neurodegeneration. Transl. Neurodegener. 2019, 8, 9. [Google Scholar] [CrossRef]
- Burgaz, S.; García, C.; Gómez-Cañas, M.; Muñoz, E.; Fernández-Ruiz, J. Development of An Oral Treatment with the PPAR-γ-Acting Cannabinoid VCE-003.2 Against the Inflammation-Driven Neuronal Deterioration in Experimental Parkinson’s Disease. Molecules 2019, 24, 2702. [Google Scholar] [CrossRef] [Green Version]
- Cueto, C.R.; Santos-García, I.; García-Toscano, L.; Espejo-Porras, F.; Bellido, M.; Fernández-Ruiz, J.; Muñoz, E.; de Lago, E. Neuroprotective effects of the cannabigerol quinone derivative VCE-003.2 in SOD1G93A transgenic mice, an experimental model of amyotrophic lateral sclerosis. Biochem. Pharmacol. 2018, 157, 217–226. [Google Scholar] [CrossRef]
- Available online: https://emeraldpharma.life/pipeline/ (accessed on 22 May 2021).
- Navarrete, C.; Carrillo-Salinas, F.; Palomares, B.; Mecha, M.; Jiménez-Jiménez, C.; Mestre, L.; Feliú, A.; Bellido, M.L.; Fiebich, B.L.; Appendino, G.; et al. Hypoxia mimetic activity of VCE-004.8, a cannabidiol quinone derivative: Implications for multiple sclerosis therapy. J. Neuroinflammation 2018, 15, 64. [Google Scholar] [CrossRef]
- Palomares, B.; Ruiz-Pino, F.; Navarrete, C.M.; Velasco, I.; Sánchez-Garrido, M.A.; Jimenez-Jimenez, C.; Pavicic, C.; Vazquez, M.J.; Appendino, G.; Bellido, M.L.; et al. VCE-004.8, A Multitarget Cannabinoquinone, Attenuates Adipogenesis and Prevents Diet-Induced Obesity. Sci. Rep. 2018, 8, 16092. [Google Scholar] [CrossRef] [Green Version]
- Navarrete, C.; García-Martin, A.; Garrido-Rodriguez, M.; Mestre, L.; Feliú, A.; Guaza, C.; Calzado, M.A.; Muñoz, E. Effects of EHP-101 on inflammation and remyelination in murine models of Multiple sclerosis. Neurobiol. Dis. 2020, 143, 104994. [Google Scholar] [CrossRef] [PubMed]
- Available online: https://emeraldpharma.life/ehp-101/ (accessed on 30 June 2021).



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Caprioglio, D.; Mattoteia, D.; Taglialatela-Scafati, O.; Muñoz, E.; Appendino, G. Cannabinoquinones: Synthesis and Biological Profile. Biomolecules 2021, 11, 991. https://doi.org/10.3390/biom11070991
Caprioglio D, Mattoteia D, Taglialatela-Scafati O, Muñoz E, Appendino G. Cannabinoquinones: Synthesis and Biological Profile. Biomolecules. 2021; 11(7):991. https://doi.org/10.3390/biom11070991
Chicago/Turabian StyleCaprioglio, Diego, Daiana Mattoteia, Orazio Taglialatela-Scafati, Eduardo Muñoz, and Giovanni Appendino. 2021. "Cannabinoquinones: Synthesis and Biological Profile" Biomolecules 11, no. 7: 991. https://doi.org/10.3390/biom11070991
APA StyleCaprioglio, D., Mattoteia, D., Taglialatela-Scafati, O., Muñoz, E., & Appendino, G. (2021). Cannabinoquinones: Synthesis and Biological Profile. Biomolecules, 11(7), 991. https://doi.org/10.3390/biom11070991








