Growth under Different Trophic Regimes and Synchronization of the Red Microalga Galdieria sulphuraria
Abstract
1. Introduction
2. Materials and Methods
2.1. Organism and Culturing
2.2. The Synchronization Procedure
2.3. Measurement of Light Irradiance
2.4. Dry Matter Determination
2.5. Commitment Points, Nuclear and Cellular Division Evaluation
2.6. Cryo-Scanning Electron Microscopy (Cryo-SEM)
2.7. RNA, DNA, Proteins Analyses
2.8. Glycogen Analysis
3. Results
3.1. Optimization of Growth Conditions
3.1.1. Effect of Light Intensity and Temperature
3.1.2. Effect of pH under Different Trophic Regimes
3.1.3. Synchronized Cultures
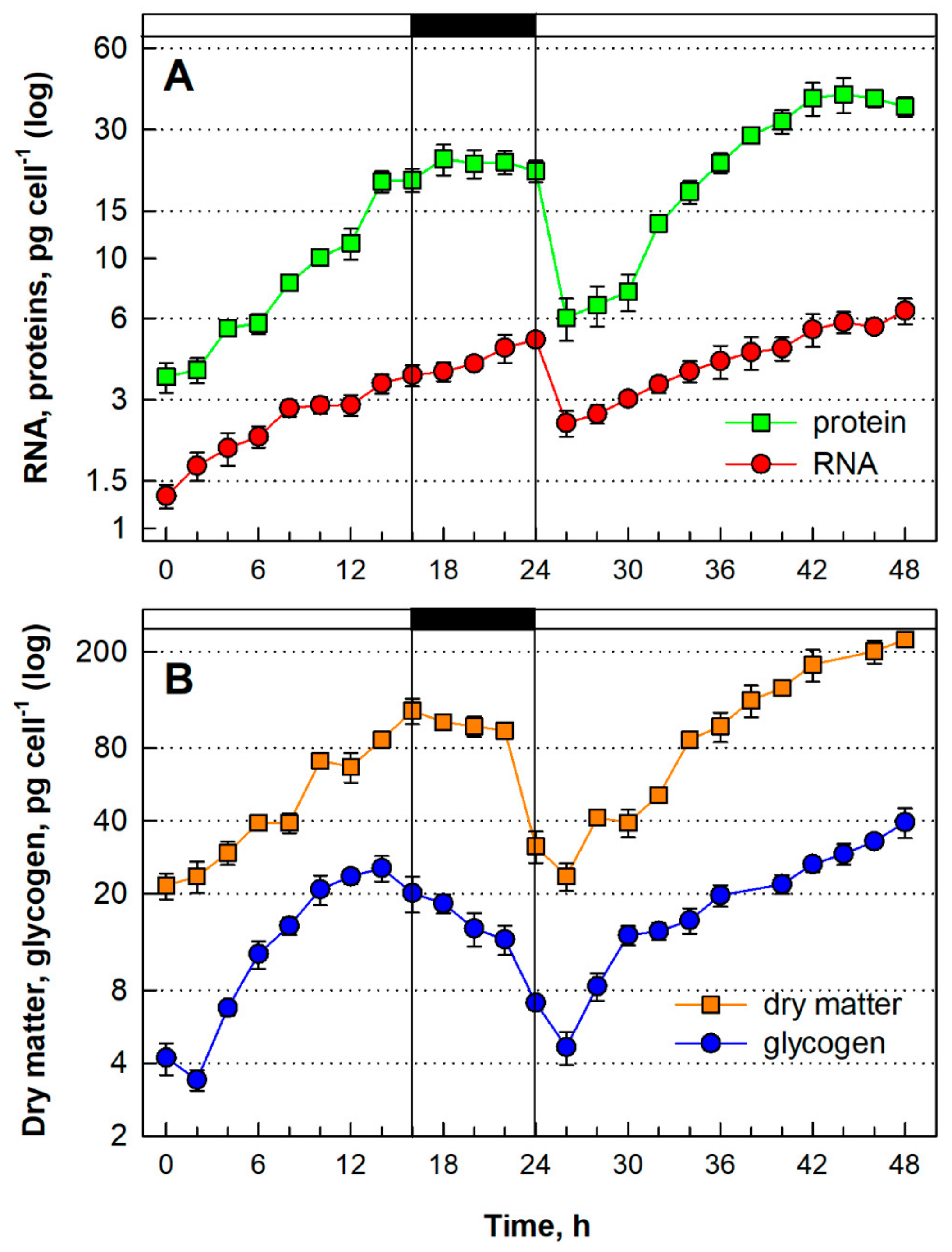
3.1.4. Growth and Energy Reserves Accumulation
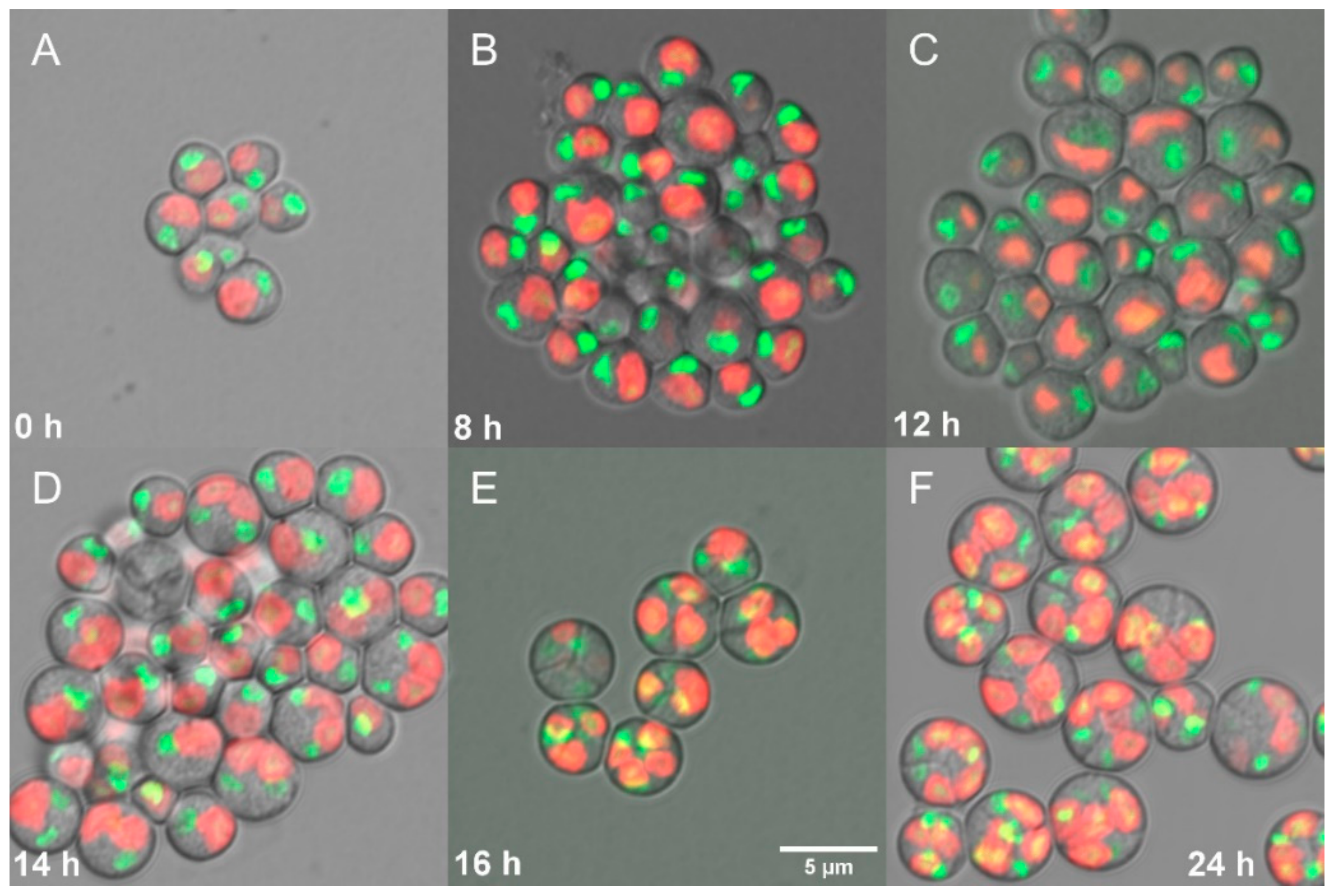
3.1.5. Cell Cycle Progression
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Merola, A.; Castaldo, R.; De Luca, P.; Gambardella, R.; Musacchio, A.; Taddei, R. Revision of Cyanidium caldarium. Three species of acidophilic algae. Plant Biosyst. 1981, 115, 189–195. [Google Scholar] [CrossRef]
- Matsuzaki, M.; Misumi, O.; Shin-i, T.; Maruyama, S.; Takahara, M.; Miyagishima, S.; Mori, T.; Nishida, K.; Yagisawa, F.; Nishida, K.; et al. Genome sequence of the ultrasmall unicellular red alga Cyanidioschyzon merolae 10D. Nature 2004, 428, 653–657. [Google Scholar] [CrossRef] [PubMed]
- Schonknecht, G.; Chen, W.H.; Ternes, C.M.; Barbier, G.G.; Shrestha, R.P.; Stanke, M.; Brautigam, A.; Baker, B.J.; Banfield, J.F.; Garavito, R.M.; et al. Gene transfer from bacteria and archaea facilitated evolution of an extremophilic eukaryote. Science 2013, 339, 1207–1210. [Google Scholar] [CrossRef] [PubMed]
- Qiu, H.; Price, D.C.; Weber, A.P.; Reeb, V.; Yang, E.C.; Lee, J.M.; Kim, S.Y.; Yoon, H.S.; Bhattacharya, D. Adaptation through horizontal gene transfer in the cryptoendolithic red alga Galdieria phlegrea. Curr. Biol. 2013, 23, 865–866. [Google Scholar] [CrossRef] [PubMed]
- Rossoni, A.W.; Price, D.C.; Seger, M.; Lyska, D.; Lammers, P.; Bhattacharya, D.; Weber, A.P.M. The genomes of polyextremophilic cyanidiales contain 1% horizontally transferred genes with diverse adaptive functions. eLife 2019, 8. [Google Scholar] [CrossRef]
- De Luca, P.; Taddei, R.; Varano, L. Cyanidioschyzon merolae: A new alga of thermal acidic environments. Webbia 1978, 33, 37–44. [Google Scholar] [CrossRef]
- Seckbach, J. Evolutionary Pathways and Enigmatic Algae: Cyanidium Caldarium (Rhodophyta) and Related Cells; Kluwer Academic Publisher: Dordrecht, The Netherlands, 1994. [Google Scholar]
- Gross, W.; Küver, J.; Tischendorf, G.; Bouchaala, N.; Büsch, W. Cryptoendolithic growth of the red alga Galdieria sulphuraria in volcanic areas. Eur. J. Phycol. 1998, 33, 25–31. [Google Scholar] [CrossRef]
- Ciniglia, C.; Yoon, H.S.; Pollio, A.; Pinto, G.; Bhattacharya, D. Hidden biodiversity of the extremophilic Cyanidiales red algae. Mol. Ecol. 2004, 13, 1827–1838. [Google Scholar] [CrossRef]
- Reeb, V.; Bhattacharya, D. The Thermo-Acidophilic Cyanidiophyceae (Cyanidiales). In Red Algae in the Genomic Age; Cellular Origin, Life in Extreme Habitats and Astrobiology; Springer: Dordrecht, The Netherlands, 2010; Volume 13, pp. 409–426. [Google Scholar] [CrossRef]
- Vítová, M.; Goecke, F.; Sigler, K.; Řezanka, T. Lipidomic analysis of the extremophilic red alga Galdieria sulphuraria in response to changes in pH. Algal Res. 2016, 13, 218–226. [Google Scholar] [CrossRef]
- Hirooka, S.; Tomita, R.; Fujiwara, T.; Ohnuma, M.; Kuroiwa, H.; Kuroiwa, T.; Miyagishima, S. Efficient open cultivation of cyanidialean red algae in acidified seawater. Sci. Rep. 2020, 10, 1–12. [Google Scholar] [CrossRef]
- Yoshimura, E.; Nagasaka, S.; Sato, Y.; Satake, K.; Mori, S. Extraordinary high aluminium tolerance of the acidophilic thermophilic alga, Cyanidium caldarium. Soil Sci. Plant. Nutr. 1999, 45, 721–724. [Google Scholar] [CrossRef][Green Version]
- Nagasaka, S.; Nishizawa, N.K.; Watanabe, T.; Mori, S.; Yoshimura, E. Evidence that electron-dense bodies in Cyanidium caldarium have an iron-storage role. Biometals 2003, 16, 465–470. [Google Scholar] [CrossRef]
- Minoda, A.; Sawada, H.; Suzuki, S.; Miyashita, S.; Inagaki, K.; Yamamoto, T.; Tsuzuki, M. Recovery of rare earth elements from the sulfothermophilic red alga Galdieria sulphuraria using aqueous acid. Appl. Microbiol. Biotechnol. 2015, 99, 1513–1519. [Google Scholar] [CrossRef]
- Cizkova, M.; Mezricky, P.; Mezricky, D.; Rucki, M.; Zachleder, V.; Vitova, M. Bioaccumulation of rare earth elements from waste luminophores in the red algae, Galdieria phlegrea. Waste Biomass Valor. 2020. [Google Scholar] [CrossRef]
- Čížková, M.; Vítová, M.; Zachleder, V. The Red Microalga Galdieria as a Promising Organisms for Applications in Biotechnolgy. In Microalgae. From Physiology to Application; Vítová, M., Ed.; IntechOpen: London, UK, 2020; pp. 1–17. [Google Scholar]
- Varshney, P.; Mikulic, P.; Vonshak, A.; Beardall, J.; Wangikar, P.P. Extremophilic micro-algae and their potential contribution in biotechnology. Biores. Technol. 2015, 184, 363–372. [Google Scholar] [CrossRef]
- Ju, X.; Igarashi, K.; Miyashita, S.; Mitsuhashi, H.; Inagaki, K.; Fujii, S.; Sawada, H.; Kuwabara, T.; Minoda, A. Effective and selective recovery of gold and palladium ions from metal wastewater using a sulfothermophilic red alga, Galdieria sulphuraria. Biores. Technol. 2016, 211, 759–764. [Google Scholar] [CrossRef]
- Gross, W.; Schnarrenberger, C. Heterotrophic growth of two strains of the acido-thermophilic red alga Galdieria sulphuraria. Plant Cell Physiol. 1995, 36, 633–638. [Google Scholar]
- Oesterhelt, C.; Schnarrenberger, C.; Gross, W. Characterization of a sugar/polyol uptake system in the red alga Galdieria sulphuraria. Eur. J. Phycol. 1999, 34, 271–277. [Google Scholar] [CrossRef]
- Oesterhelt, C.; Schmalzlin, E.; Schmitt, J.M.; Lokstein, H. Regulation of photosynthesis in the unicellular acidophilic red alga Galdieria sulphuraria. Plant J. 2007, 51, 500–511. [Google Scholar] [CrossRef]
- Lopez, G.; Yate, C.; Ramos, F.A.; Cala, M.P.; Restrepo, S.; Baena, S. Production of polyunsaturated fatty acids and lipids from autotrophic, mixotrophic and heterotrophic cultivation of Galdieria sp. strain USBA-GBX-832. Sci. Rep. 2019, 9. [Google Scholar] [CrossRef]
- Li, C.; Lesnik, K.L.; Liu, H. Conversion of waste glycerol from biodiesel production into value-added products. Energies 2013, 6, 4739–4768. [Google Scholar] [CrossRef]
- Xu, S.; Elsayed, M.; Ismail, G.A.; Li, C.; Wang, S.; El-Fatah Abomohra, A. Evaluation of bioethanol and biodiesel production from Scenedesmus obliquus grown in biodiesel waste glycerol: A sequential integrated route for enhanced energy recovery. Energy Convers. Manag. 2019, 197, 111907. [Google Scholar] [CrossRef]
- Martinez-Garcia, M.; Kormpa, A.; van der Maarel, M.J.E.C. The glycogen of Galdieria sulphuraria as alternative to starch for the production of slowly digestible and resistant glucose polymers. Carbohydr. Polym. 2017, 169, 75–82. [Google Scholar] [CrossRef]
- Martinez-Garcia, M.; Stuart, M.C.A.; van der Maarel, M.J.E. Characterization of the highly branched glycogen from the thermoacidophilic red microalga Galdieria sulphuraria and comparison with other glycogens. Int. J. Biol. Macromol. 2016, 89, 12–18. [Google Scholar] [CrossRef]
- John, P.C.L. Control of the cell division cycle in Chlamydomonas. Microbiol. Sci. 1984, 1, 96–101. [Google Scholar]
- Zachleder, V.; Šetlík, I. Timing of events in overlapping cell reproductive sequences and their mutual interactions in the alga Scenedesmus quadricauda. J. Cell Sci. 1990, 97, 631–638. [Google Scholar] [CrossRef]
- Vítová, M.; Bišová, K.; Umysová, D.; Hlavová, M.; Kawano, S.; Zachleder, V.; Čížková, M. Chlamydomonas reinhardtii: Duration of its cell cycle and phases at growth rates affected by light intensity. Planta 2011, 233, 75–86. [Google Scholar] [CrossRef]
- Šetlík, I.; Zachleder, V.; Doucha, J.; Berková, E.; Bartoš, J. The nature of temperature block in the sequence of reproductive processes in Chlorella vulgaris BEIJERINCK. Arch. Hydrobiol. 1975, 14, 70–104. [Google Scholar]
- Donnan, L.; Carvill, E.P.; Gilliland, T.J.; John, P.C.L. The cell-cycles of Chlamydomonas and Chlorella. N. Phytol. 1985, 99, 1–40. [Google Scholar] [CrossRef]
- Vítová, M.; Bišová, K.; Hlavová, M.; Kawano, S.; Zachleder, V.; Čížková, M. Chlamydomonas reinhardtii: Duration of its cell cycle and phases at growth rates affected by temperature. Planta 2011, 234, 599–608. [Google Scholar] [CrossRef]
- Vítová, M.; Zachleder, V. Points of commitment to reproductive events as a tool for analysis of the cell cycle in synchronous cultures of algae. Folia Microbiol. 2005, 50, 141–149. [Google Scholar] [CrossRef] [PubMed]
- Calhoun, S.; Bell, T.A.S.; Dahlin, L.R.; Kunde, Y.; LaButti, K.; Louie, K.B.; Kuftin, A.; Treen, D.; Dilworth, D.; Mihaltcheva, S.; et al. A multi-omic characterization of temperature stress in a halotolerant Scenedesmus strain for algal biotechnology. Commun. Biol. 2021, 4. [Google Scholar] [CrossRef] [PubMed]
- Zachleder, V.; van den Ende, H. Cell-cycle events in the green alga Chlamydomonas eugametos and their control by environmental factors. J. Cell Sci. 1992, 102, 469–474. [Google Scholar] [CrossRef]
- Zachleder, V.; Ivanov, I.; Vítová, M.; Bišová, K. Effects of cyclin-dependent kinase activity on the coordination of growth and the cell cycle in green algae at different temperatures. J. Exp. Bot. 2019, 70, 845–858. [Google Scholar] [CrossRef]
- Zachleder, V.; Ivanov, I.; Vitova, M.; Bisova, K. Cell cycle arrest by supraoptimal temperature in the alga Chlamydomonas reinhardtii. Cells 2019, 8, 1237. [Google Scholar] [CrossRef]
- Semenenko, V.E.; Vladimirova, M.G.; Orleanskaya, O.B. Physiological characteristics of Chlorella sp. K under conditions of high extremal temperatures I. Uncoupling effect of extreme temperatures on the cellular functions of Chlorella. Physiol. Plants 1967, 14, 612–625. [Google Scholar]
- Hemme, D.; Veyel, D.; Muhlhaus, T.; Sommer, F.; Juppner, J.; Unger, A.K.; Sandmann, M.; Fehrle, I.; Schonfelder, S.; Steup, M.; et al. Systems-wide analysis of acclimation responses to long-term heat stress and recovery in the photosynthetic model organism Chlamydomonas reinhardtii. Plant Cell 2014, 26, 4270–4297. [Google Scholar] [CrossRef]
- Selvaratnam, T.; Pegallapati, A.K.; Montelya, F.; Rodriguez, G.; Nirmalakhandan, N.; Van Voorhies, W.; Lammers, P.J. Evaluation of a thermo-tolerant acidophilic alga, Galdieria sulphuraria, for nutrient removal from urban wastewaters. Biores. Technol. 2014, 156, 395–399. [Google Scholar] [CrossRef]
- Albertano, P.; Ciniglia, C.; Pinto, G.; Pollio, A. The taxonomic position of Cyanidium, Cyanidioschyzon and Galdieria: An update. Hydrobiologia 2000, 433, 137–143. [Google Scholar] [CrossRef]
- Jong, L.W.; Fujiwara, T.; Hirooka, S.; Miyagishima, S.Y. Cell size for commitment to cell division and number of successive cell divisions in cyanidialean red algae. Protoplasma 2021. [Google Scholar] [CrossRef]
- Bišová, K.; Zachleder, V. Cell cycle regulation in green algae dividing by multiple fission. J. Exp. Bot. 2014, 65, 2585–2602. [Google Scholar] [CrossRef]
- Zachleder, V.; Bišová, K.; Vítová, M. The Cell Cycle of Microalgae. In The Physiology of Microalgae, 6th ed.; Borowitzka, M.A., Raven, J.A., Eds.; Springer International Publishing Switzerland: Cham, Switzerland, 2016; pp. 3–46. [Google Scholar]
- Reed, S.I. The selection of S. cerevisiae mutants defective in the start event of cell division. Genetics 1980, 95, 561–577. [Google Scholar] [CrossRef]
- Pardee, A.B. A restriction point for control of normal animal cell proliferation. Proc. Nat. Acad. Sci. USA 1974, 71, 1286–1290. [Google Scholar] [CrossRef]
- Cross, F.R.; Umen, J.G. The Chlamydomonas cell cycle. Plant J. 2015. [Google Scholar] [CrossRef]
- Reinecke, D.L.; Castillo-Flores, A.; Boussiba, S.; Zarka, A. Polyploid polynuclear consecutive cell-cycle enables large genome-size in Haematococcus pluvialis. Algal Res. Biomass Biofuels Bioprod. 2018, 33, 456–461. [Google Scholar] [CrossRef]
- Zachleder, V.; Ivanov, I.N.; Kselíková, V.; Bialevich, V.; Vítová, M.; Ota, S.; Takeshita, T.; Kawano, S.; Bišová, K. Characterization of growth and cell cycle events as affected by light intensity in the green alga Parachlorella kessleri, as a new model for cell cycle research. Biomolecules 2021, 11, 891. [Google Scholar] [CrossRef]
- Hlavová, M.; Vítová, M.; Bišová, K. Synchronization of Green Algae by Light and Dark Regimes for Cell Cycle and Cell Division Studies. In Plant Cell Division; Caillaud, M.-C., Walker, J.M., Eds.; Methods in Molecular Biology; Springer Science: New York, NY, USA, 2016; pp. 3–16. [Google Scholar]
- Miyagishima, S.Y.; Fujiwara, T.; Sumiya, N.; Hirooka, S.; Nakano, A.; Kabeya, Y.; Nakamura, M. Translation-independent circadian control of the cell cycle in a unicellular photosynthetic eukaryote. Nat. Commun. 2014, 5, 3807. [Google Scholar] [CrossRef]
- Imoto, Y.; Fujiwara, T.; Yoshida, Y.; Kuroiwa, H.; Maruyama, S.; Kuroiwa, T. Division of cell nuclei, mitochondria, plastids, and microbodies mediated by mitotic spindle poles in the primitive red alga Cyanidioschyzon merolae. Protoplasma 2010, 241, 63–74. [Google Scholar] [CrossRef]
- Moriyama, T.; Terasawa, K.; Sekine, K.; Toyoshima, M.; Koike, M.; Fujiwara, M.; Sato, N. Characterization of cell-cycle-driven and light-driven gene expression in a synchronous culture system in the unicellular rhodophyte Cyanidioschyzon merolae. Microbiol. Sgm. 2010, 156, 1730–1737. [Google Scholar] [CrossRef]
- Kanesaki, Y.; Imamura, S.; Minoda, A.; Tanaka, K. External light conditions and internal cell cycle phases coordinate accumulation of chloroplast and mitochondrial transcripts in the red alga Cyanidioschyzon merolae. DNA Res. 2012, 19, 289–303. [Google Scholar] [CrossRef]
- Sumiya, N.; Fujiwara, T.; Era, A.; Miyagishima, S. Chloroplast division checkpoint in eukaryotic algae. Proc. NatL. Acad. Sci. USA 2016, 113, 7629–7638. [Google Scholar] [CrossRef]
- Fujiwara, T.; Hirooka, S.; Ohbayashi, R.; Onuma, R.; Miyagishima, S.Y. Relationship between cell cycle and diel transcriptomic changes in metabolism in a unicellular red alga. Plant Physiol. 2020, 183, 1484–1501. [Google Scholar] [CrossRef]
- Ichinose, T.M.; Iwane, A.H. Long-term live cell cycle imaging of single Cyanidioschyzon merolae cells. Protoplasma 2021, 258, 651–660. [Google Scholar] [CrossRef]
- Sheath, R.G.; Hellebust, J.A.; Sawa, T. Floridean starch metabolism of Porphyridium purpureum (Rhodphyta) II Changes during the cell cycle. Phycologia 1979, 18, 185–190. [Google Scholar] [CrossRef]
- Simon-Bercovitch, B.; Bar-Zvi, D.; Arad, S.M. Cell-wall formation during the cell cycle of Porphyridium sp. (Rhodophyta). J. Phycol. 1999, 35, 78–83. [Google Scholar] [CrossRef]
- Knappe, J. Synchronkulturen von Porphyridium cruentum und ihr photosynthetisches Verhalten. Plant Biol. 1972, 85, 425–434. [Google Scholar] [CrossRef]
- Kopecky, J.; Lukavska, A.; Verbovikova, E.; Pfuendel, E. Changes in the photosynthetic pigment patterns during the synchronous life cycle of Porphyridium purpureum. Arch. Hydrobiol. Suppl. 2004, 154, 121–132. [Google Scholar] [CrossRef]
- Rebolloso Fuentes, M.M.; García Sánchez, J.L.; Fernández Sevilla, J.M.; Acién Fernández, F.G.; Sánchez Pérez, J.A.; Molina Grima, E. Outdoor continuous culture of Porphyridium cruentum in a tubular photobioreactor: Quantitative analysis of the daily cyclic variation of culture parameters. J. Biotechnol. 1999, 70, 271–288. [Google Scholar] [CrossRef]
- Brányiková, I.; Maršálková, B.; Doucha, J.; Brányik, T.; Bišová, K.; Zachleder, V.; Vítová, M. Microalgae-novel highly efficient starch producers. Biotechnol. Bioeng. 2011, 108, 766–776. [Google Scholar] [CrossRef]
- Šetlík, I.; Zachleder, V. The Multiple Fission Cell Reproductive Patterns in Algae. In The Microbial Cell Cycle; Nurse, P., Streiblová, E., Eds.; CRC Press Inc.: Boca Raton, FL, USA, 1984; pp. 253–279. [Google Scholar]
- Zachleder, V.; Cepák, V. Visualization of DNA containing structures by fluorochrome DAPI in those algal cells which are not freely permeable to the dye. Arch. Hydrobiol. Algol. Stud. 1987, 47, 157–168. [Google Scholar]
- Vítová, M.; Hendrychová, J.; Cepák, V.; Zachleder, V. Visualization of DNA-containing structures in vivo in various species of Chlorophyta, Rhodophyta and Cyanophyta using SYBR Green I dye. Folia Microbiol. 2005, 50, 333–340. [Google Scholar] [CrossRef] [PubMed]
- Wanka, F. Die Bestimmung der Nucleinsäuren in Chlorella pyrenoidosa. Planta 1962, 58, 594–619. [Google Scholar] [CrossRef]
- Lukavský, J.; Tetík, K.; Vendlová, J. Extraction of nucleic acid from the alga Scenedesmus quadricauda. Arch. Hydrobiol. Algol. Stud. 1973, 9, 416–426. [Google Scholar]
- Decallonne, J.R.; Weyns, C.J. A shortened procedure of the diphenylamine reaction for measurement of deoxyribonucleic acid by using light activation. Anal. Biochem. 1976, 74, 448–456. [Google Scholar] [CrossRef]
- Zachleder, V. Optimization of nucleic acids assay in green and blue-green algae: Extraction procedures and the light-activated reaction for DNA. Arch. Hydrobiol. 1984, 67, 313–328. [Google Scholar] [CrossRef]
- Lowry, O.H.; Rosenbrough, N.S.; Farr, A.L.; Randall, R.J. Protein measurement with the folin-phenol reagent. J. Biol. Chem. 1951, 193, 265–275. [Google Scholar] [CrossRef]
- Pinto, G.; Ciniglia, C.; Cascone, C.; Pollio, A. Species Composition of Cyanidiales Assemblages in Pisciarelli (Campi Flegrei, Italy) and Description of Galdieria Phlegrea sp. NOV. In Algae and Cyanobacteria in Extreme Environments; Cellular Origin, Life in Extreme Habitats and Astrobiology; Springer: Dordrecht, The Netherlands, 2007; Volume 11, pp. 489–502. [Google Scholar]
- Eren, A.; Iovinella, M.; Yoon, H.S.; Cennamo, P.; de Stefano, M.; de Castro, O.; Ciniglia, C. Genetic structure of Galdieria populations from Iceland. Polar Biol. 2018, 41, 1681–1691. [Google Scholar] [CrossRef]
- Ciniglia, C.; Yang, E.C.; Pollio, A.; Pinto, G.; Iovinella, M.; Vitale, L.; Yoon, H.S. Cyanidiophyceae in Iceland: Plastid rbcL gene elucidates origin and dispersal of extremophilic Galdieria sulphuraria and G. maxima (Galdieriaceae, Rhodophyta). Phycologia 2014, 53, 542–551. [Google Scholar] [CrossRef]
- Gross, W.; Oesterhelt, C.; Tischendorf, G.; Lederer, F. Characterization of a non-thermophilic strain of the red algal genus Galdieria isolated from SOOS (Czech Republic). Eur. J. Phycol. 2002, 37, 477–482. [Google Scholar] [CrossRef]
- Thangaraj, B.; Jolley, C.C.; Sarrou, I.; Bultema, J.B.; Greyslak, J.; Whitelegge, J.P.; Lin, S.; Kouril, R.; Subramanyam, R.; Boekema, E.J.; et al. Efficient Light Harvesting in a Dark, Hot, Acidic environment: The structure and function of PSI-LHCI from Galdieria sulphuraria. Biophys. J. 2011, 100, 135–143. [Google Scholar] [CrossRef][Green Version]
- Liu, L.; Sanchez-Arcos, C.; Pohnert, G.; Wei, D. Untargeted metabolomics unveil changes in autotrophic and mixotrophic Galdieria sulphuraria exposed to high-light intensity. Int. J. Mol. Sci. 2021, 22, 1247. [Google Scholar] [CrossRef]
- Curien, G.; Lyska, D.; Guglielmino, E.; Westhoff, P.; Janetzko, J.; Tardif, M.; Hallopeau, C.; Brugiere, S.; Dal Bo, D.; Decelle, J.; et al. Mixotrophic growth of the extremophile Galdieria sulphuraria reveals the flexibility of its carbon assimilation metabolism. N. Phytol. 2021, 231, 326–338. [Google Scholar] [CrossRef]
- Kuroiwa, T.; Nagashima, H.; Fukuda, I. Chloroplast division without DNA synthesis during the life cycle of the unicellular alga Cyanidium caldarium M-8 as revealed by quantitative fluorescence microscopy. Protoplasma 1989, 149, 120–129. [Google Scholar] [CrossRef]
- Zachleder, V.; Schläfli, O.; Boschetti, A. Growth-controlled oscillation in activity of histone H1 kinase during the cell cycle of Chlamydomonas reinhardtii (Chlorophyta). J. Phycol. 1997, 33, 673–681. [Google Scholar] [CrossRef]
- Ball, S.; Colleoni, C.; Cenci, U.; Raj, J.N.; Tirtiaux, C. The evolution of glycogen and starch metabolism in eukaryotes gives molecular clues to understand the establishment of plastid endosymbiosis. J. Exp. Bot. 2011, 62, 1775–1801. [Google Scholar] [CrossRef]
- Sakurai, T.; Aoki, M.; Ju, X.; Ueda, T.; Nakamura, Y.; Fujiwara, S.; Umemura, T.; Tsuzuki, M.; Minoda, A. Profiling of lipid and glycogen accumulations under different growth conditions in the sulfothermophilic red alga Galdieria sulphuraria. Biores. Technol. 2016, 200, 861–866. [Google Scholar] [CrossRef]
- Salbitani, G.; Cipolletta, S.; Vona, V.; Di Martino, C.; Carfagna, S. Heterotrophic cultures of Galdieria phlegrea shift to autotrophy in the presence or absence of glycerol. J. Plant Growth Regul. 2021, 40, 371–378. [Google Scholar] [CrossRef]
- Carbone, D.A.; Olivieri, G.; Pollio, A.; Melkonian, M. Comparison of Galdieria growth and photosynthetic activity in different culture systems. AMB Express 2020, 10, 170. [Google Scholar] [CrossRef]
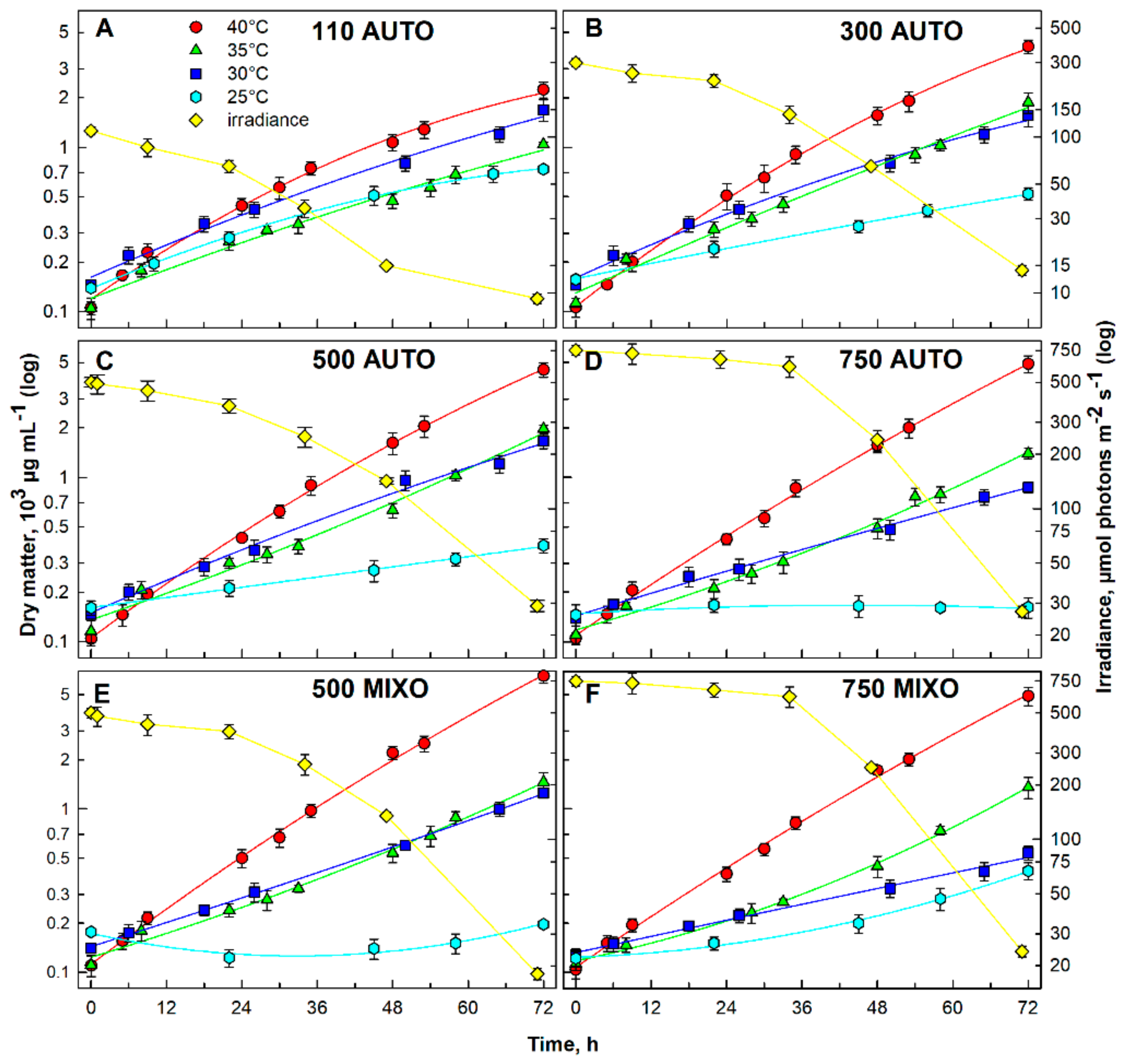



| Irradiance (µmol photons m−2 s−1) | Autotrophy | Mixotrophy | |||||
|---|---|---|---|---|---|---|---|
| Temperature (°C) | 110 | 300 | 500 | 750 | 500 | 750 | |
| 25 | 0.73 ± 0.022 | 0.52 ± 0.045 | 0.38 ± 0.036 | 0.16 ± 0.022 | 0.19 ± 0.007 | 0.41 ± 0.050 | |
| 30 | 1.69 ± 0.253 | 1.56 ± 0.23 | 1.66 ± 0.166 | 0.87 ± 0.069 | 1.25 ± 0.05 | 0.54 ± 0.054 | |
| 35 | 1.04 ± 0.031 | 1.87 ± 0.262 | 1.97 ± 0.098 | 1.40 ± 0.098 | 1.46 ± 0.204 | 1.35 ± 0.203 | |
| 40 | 2.24 ± 0.246 | 4.10 ± 0.387 | 4.52 ± 0.452 | 4.90 ± 0.588 | 6.50 ± 0.65 | 4.90 ± 0.637 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Náhlík, V.; Zachleder, V.; Čížková, M.; Bišová, K.; Singh, A.; Mezricky, D.; Řezanka, T.; Vítová, M. Growth under Different Trophic Regimes and Synchronization of the Red Microalga Galdieria sulphuraria. Biomolecules 2021, 11, 939. https://doi.org/10.3390/biom11070939
Náhlík V, Zachleder V, Čížková M, Bišová K, Singh A, Mezricky D, Řezanka T, Vítová M. Growth under Different Trophic Regimes and Synchronization of the Red Microalga Galdieria sulphuraria. Biomolecules. 2021; 11(7):939. https://doi.org/10.3390/biom11070939
Chicago/Turabian StyleNáhlík, Vít, Vilém Zachleder, Mária Čížková, Kateřina Bišová, Anjali Singh, Dana Mezricky, Tomáš Řezanka, and Milada Vítová. 2021. "Growth under Different Trophic Regimes and Synchronization of the Red Microalga Galdieria sulphuraria" Biomolecules 11, no. 7: 939. https://doi.org/10.3390/biom11070939
APA StyleNáhlík, V., Zachleder, V., Čížková, M., Bišová, K., Singh, A., Mezricky, D., Řezanka, T., & Vítová, M. (2021). Growth under Different Trophic Regimes and Synchronization of the Red Microalga Galdieria sulphuraria. Biomolecules, 11(7), 939. https://doi.org/10.3390/biom11070939







