Assessment of the Anti-Allodynic and Anti-Hyperalgesic Efficacy of a Glycine Transporter 2 Inhibitor Relative to Pregabalin, Duloxetine and Indomethacin in a Rat Model of Cisplatin-Induced Peripheral Neuropathy
Abstract
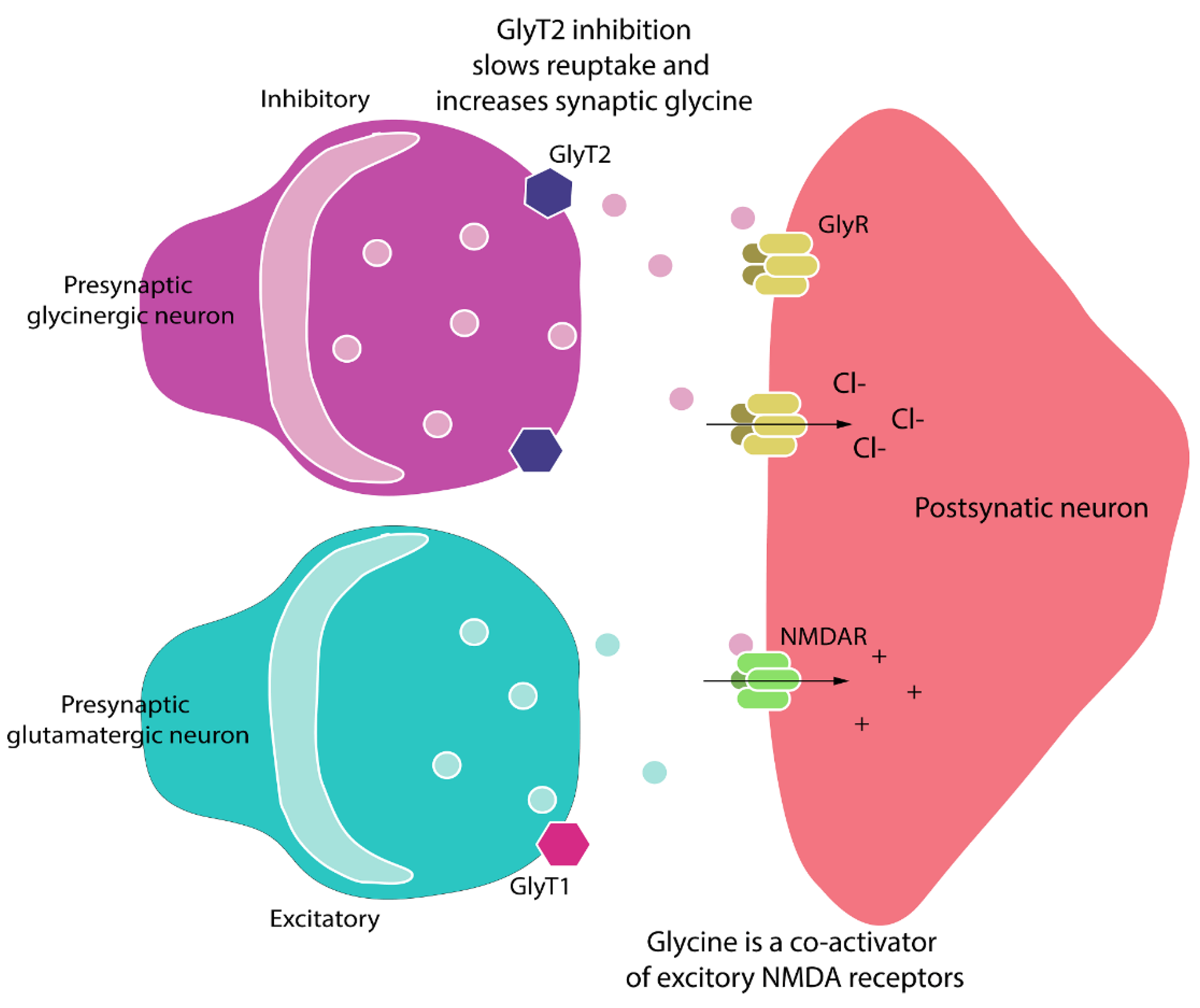
1. Introduction
2. Materials and Methods
2.1. Experimental Animals
2.2. Drugs and Reagents
2.3. Induction of Cisplatin-Induced Peripheral Neuropathy (CIPN) in Rats
2.4. Test Compound Administration
2.5. Animal Health Assessments
2.6. Assessment of Mechanical Allodynia in the Bilateral Hindpaws of CIPN-Rats
2.7. Assessment of Mechanical Hyperalgesia in the Bilateral Hindpaws of CIPN-Rats
2.8. Data and Statistical Analyses
3. Results
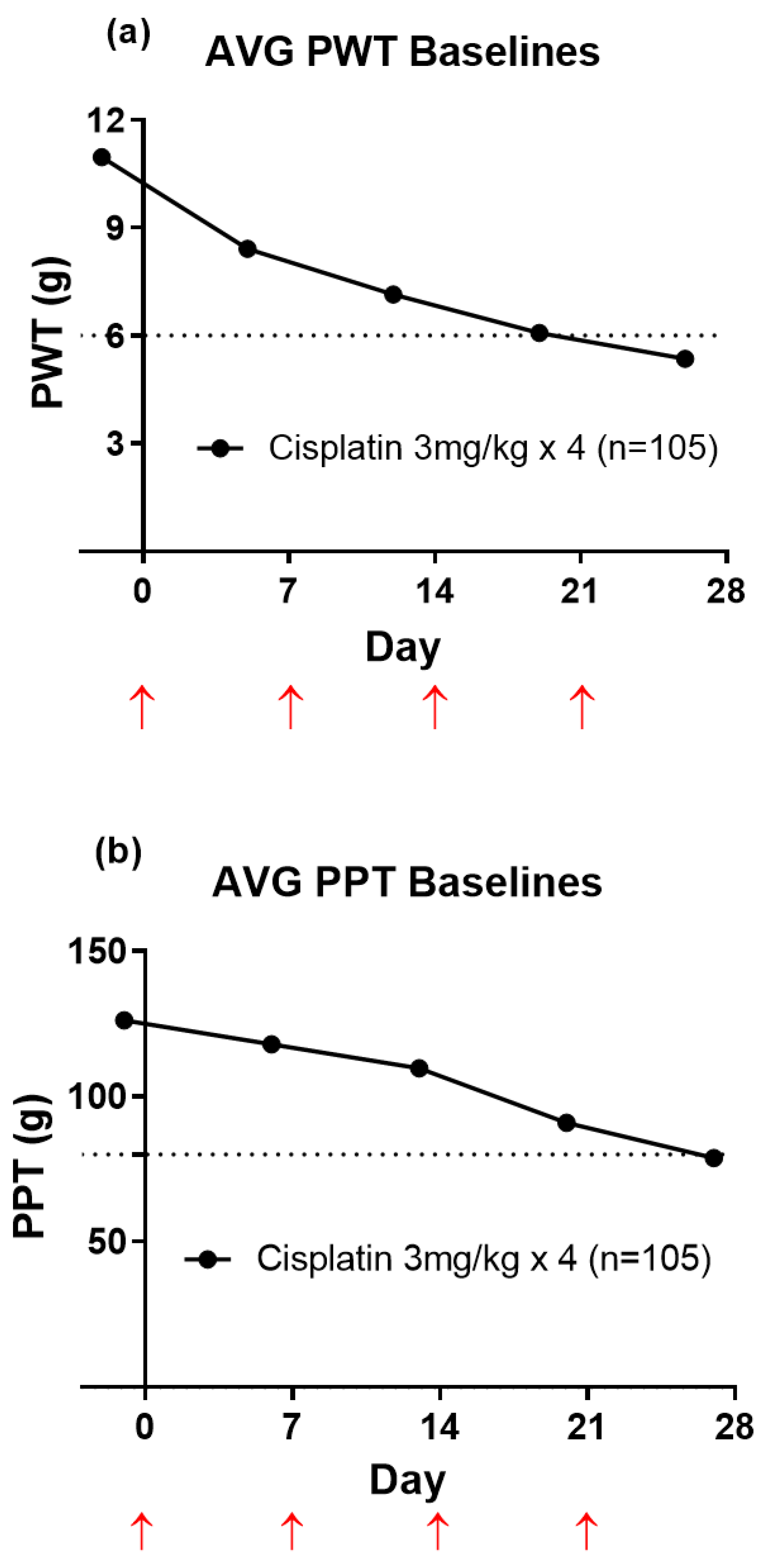
3.1. Temporal Development of Hindpaw Hypersensitivity in CIPN-Rats
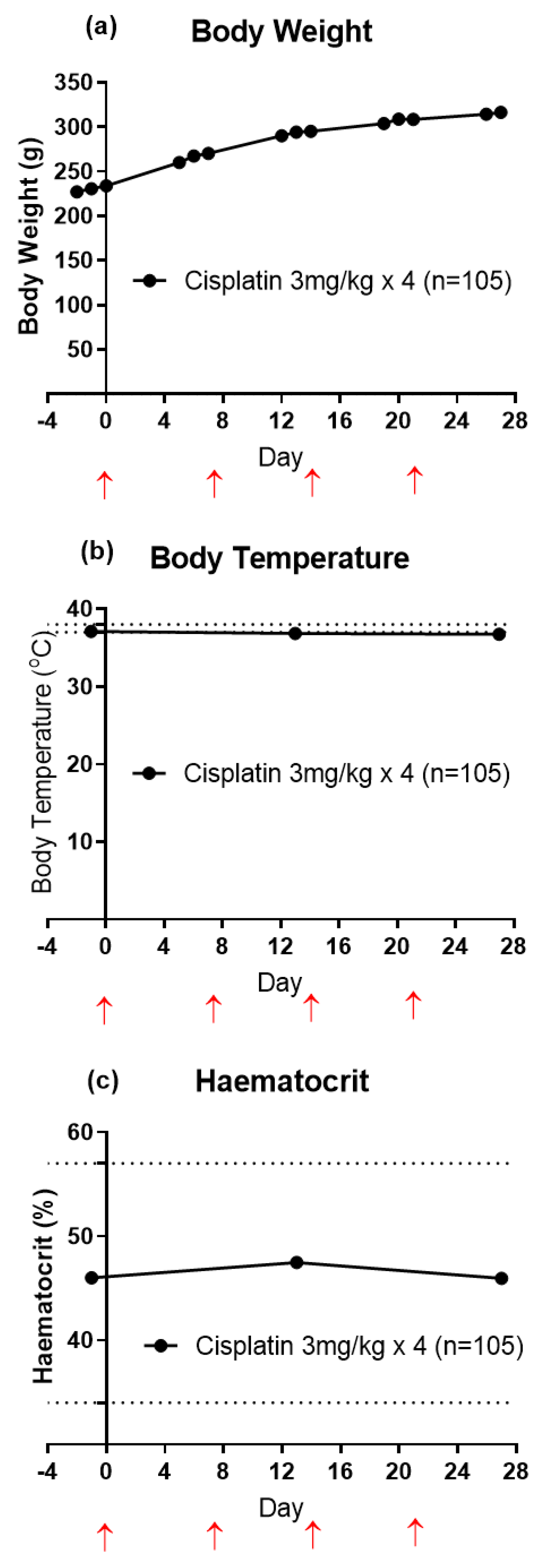
3.2. Animal Health
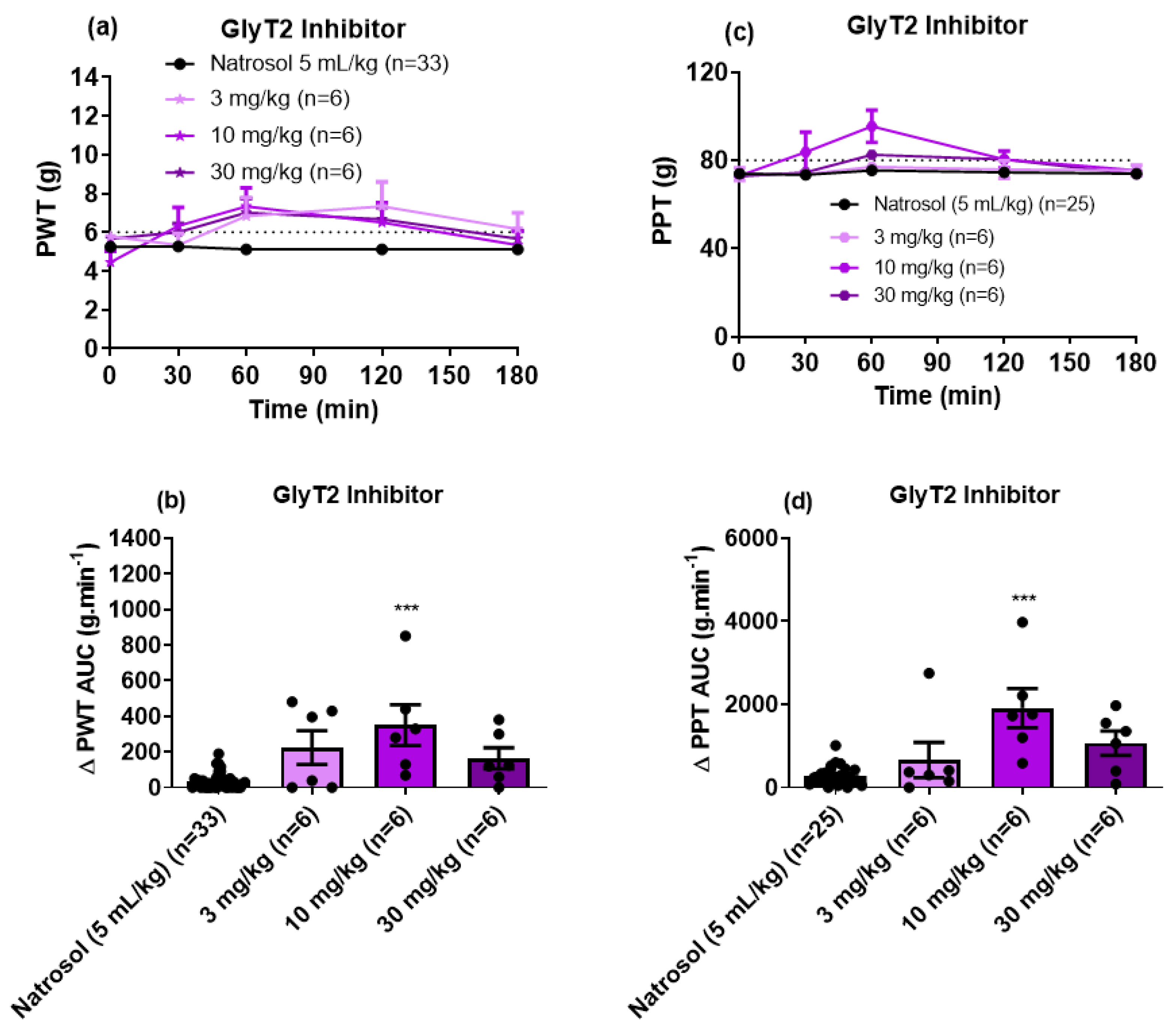
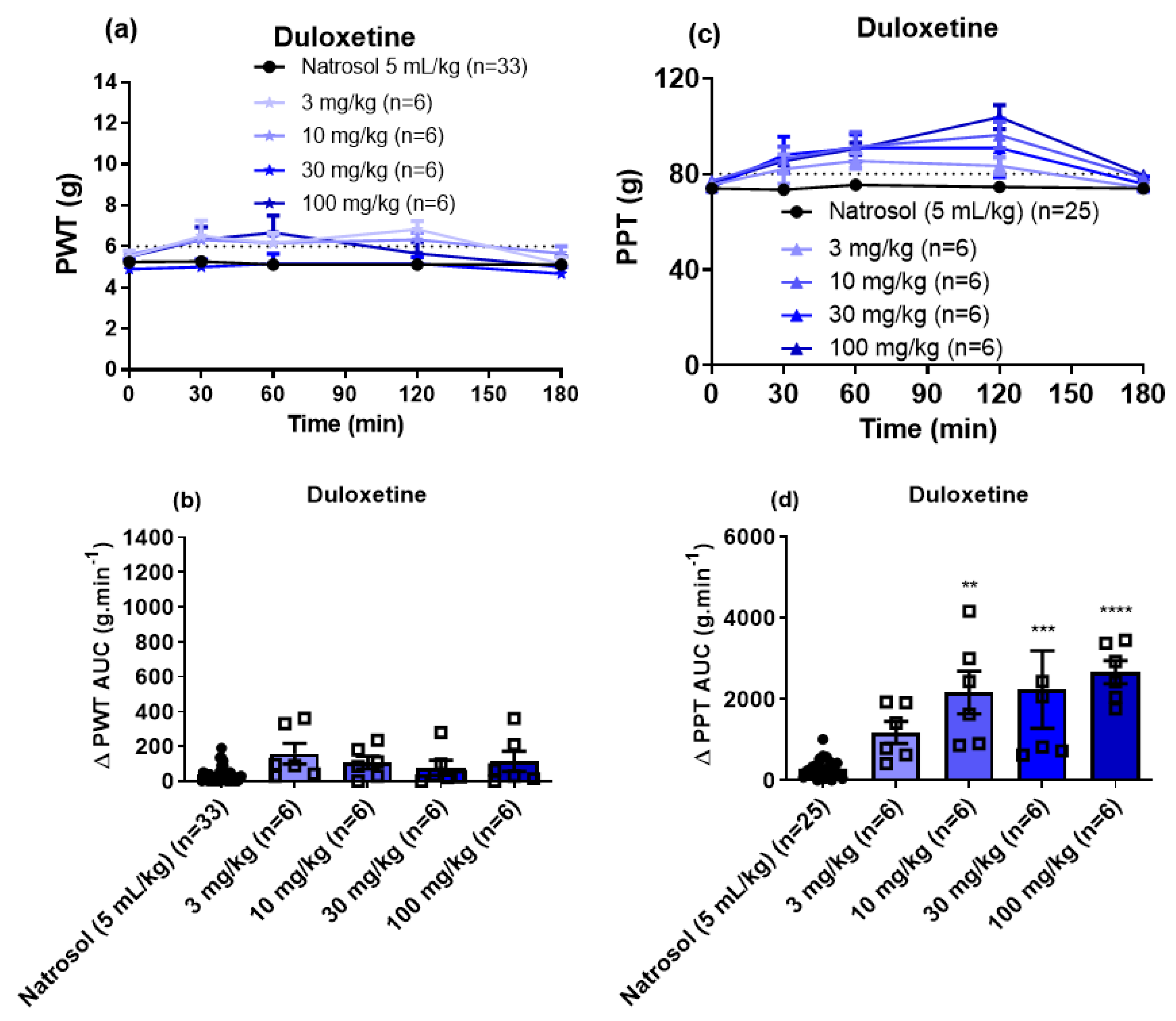
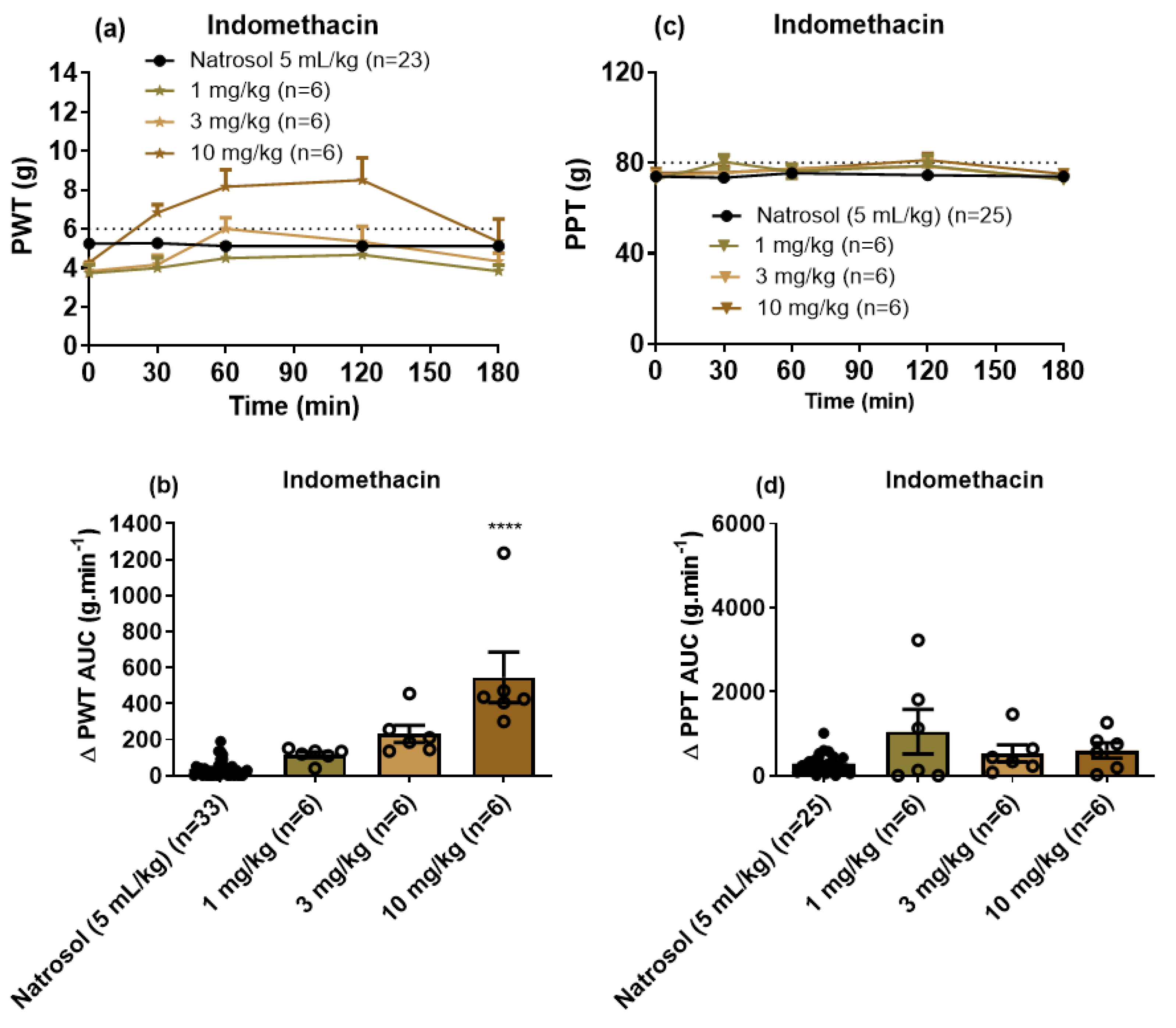
3.3. Pharmacological Assessments
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wild, C.P.; Weiderpass, E.; Stewart, B.W. World Cancer Report: Cancer Research for Cancer Prevention; International Agency for Research on Cancer: Lyon, France, 2020. [Google Scholar]
- Cancer. Available online: https://www.who.int/news-room/fact-sheets/detail/cancer (accessed on 23 February 2021).
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef]
- Gavhane, Y.N.; Shete, A.S.; Bhagat, A.K.; Shinde, V.R.; Bhong, K.K.; Khairnar, G.A.; Yadav, A.V. Solid Tumors: Facts, Challenges and Solutions. Int. J. Pharma Sci. Res. 2011, 2, 1–12. [Google Scholar]
- World Health Organization. WHO Model List of Essential Medicines—20th List; WHO: Geneva, Switzerland, 2017. [Google Scholar]
- Amptoulach, S.; Tsavaris, N. Neurotoxicity Caused by the Treatment with Platinum Analogues. Chemother. Res. Pract. 2011, 2011, 1–5. [Google Scholar] [CrossRef]
- Banach, M.; Juranek, J.K.; Zygulska, A.L. Chemotherapy-induced neuropathies-a growing problem for patients and health care providers. Brain Behav. 2016, 7, e00558. [Google Scholar] [CrossRef] [PubMed]
- Brown, A.; Kumar, S.; Tchounwou, P.B. Cisplatin-Based Chemotherapy of Human Cancers. J. Cancer Sci. Ther. 2019, 11, 11. [Google Scholar]
- Staff, N.P.; Grisold, A.; Grisold, W.; Windebank, A.J. Chemotherapy-induced peripheral neuropathy: A current review. Ann. Neurol. 2017, 81, 772–781. [Google Scholar] [CrossRef] [PubMed]
- McWhinney, S.R.; Goldberg, R.M.; McLeod, H.L. Platinum neurotoxicity pharmacogenetics. Mol. Cancer Ther. 2009, 8, 10–16. [Google Scholar] [CrossRef] [PubMed]
- Van Der Hoop, R.G.; Van Der Burg, M.E.L.; Huinink, W.W.B.T.; Van Houwelingen, J.C.; Neijt, J.P. Incidence of neuropathy in 395 patients with ovarian cancer treated with or without cisplatin. Cancer 1990, 66, 1697–1702. [Google Scholar] [CrossRef]
- Seretny, M.; Currie, G.L.; Sena, E.S.; Ramnarine, S.; Grant, R.; MacLeod, M.R.; Colvin, L.A.; Fallon, M. Incidence, prevalence, and predictors of chemotherapy-induced peripheral neuropathy: A systematic review and meta-analysis. Pain 2014, 155, 2461–2470. [Google Scholar] [CrossRef] [PubMed]
- De Moor, J.S.; Mariotto, A.B.; Parry, C.; Alfano, C.M.; Padgett, L.; Kent, E.E.; Forsythe, L.; Scoppa, S.; Hachey, M.; Rowland, J.H. Cancer Survivors in the United States: Prevalence across the Survivorship Trajectory and Implications for Care. Cancer Epidemiol. Biomark. Prev. 2013, 22, 561–570. [Google Scholar] [CrossRef]
- Zajączkowska, R.; Kocot-Kępska, M.; Leppert, W.; Wrzosek, A.; Mika, J.; Wordliczek, J. Mechanisms of Chemotherapy-Induced Peripheral Neuropathy. Int. J. Mol. Sci. 2019, 20, 1451. [Google Scholar] [CrossRef]
- Brewer, J.R.; Morrison, G.; Dolan, M.E.; Fleming, G.F. Chemotherapy-induced peripheral neuropathy: Current status and progress. Gynecol. Oncol. 2016, 140, 176–183. [Google Scholar] [CrossRef]
- Velasco, R.; Bruna, J. Chemotherapy-induced peripheral neuropathy: An unresolved issue. Neurología 2010, 25, 116–131. [Google Scholar] [CrossRef]
- Kerckhove, N.; Collin, A.; Condé, S.; Chaleteix, C.; Pezet, D.; Balayssac, D. Long-Term Effects, Pathophysiological Mechanisms, and Risk Factors of Chemotherapy-Induced Peripheral Neuropathies: A Comprehensive Literature Review. Front. Pharmacol. 2017, 8, 86. [Google Scholar] [CrossRef]
- Miltenburg, N.; Boogerd, W. Chemotherapy-induced neuropathy: A comprehensive survey. Cancer Treat. Rev. 2014, 40, 872–882. [Google Scholar] [CrossRef] [PubMed]
- Quintão, N.L.M.; Santin, J.R.; Stoeberl, L.C.; Corrêa, T.P.; Melato, J.; Costa, R. Pharmacological Treatment of Chemotherapy-Induced Neuropathic Pain: PPARγ Agonists as a Promising Tool. Front. Neurosci. 2019, 13, 907. [Google Scholar] [CrossRef] [PubMed]
- Sasane, M.; Tencer, T.; Beusterien, K. PCN63 review of the economic impact of chemotherapy induced peripheral neuropathy. Value Health 2009, 12, A268. [Google Scholar] [CrossRef][Green Version]
- Pike, C.T.; Birnbaum, H.G.; Muehlenbein, C.E.; Pohl, G.M.; Natale, R.B. Healthcare Costs and Workloss Burden of Patients with Chemotherapy-Associated Peripheral Neuropathy in Breast, Ovarian, Head and Neck, and Nonsmall Cell Lung Cancer. Chemother. Res. Pract. 2012, 2012, 1–10. [Google Scholar] [CrossRef]
- Hershman, D.L.; Lacchetti, C.; Dworkin, R.H.; Smith, E.M.L.; Bleeker, J.; Cavaletti, G.; Chauhan, C.; Gavin, P.; Lavino, A.; Lustberg, M.B.; et al. Prevention and Management of Chemotherapy-Induced Peripheral Neuropathy in Survivors of Adult Cancers: American Society of Clinical Oncology Clinical Practice Guideline. J. Clin. Oncol. 2014, 32, 1941–1967. [Google Scholar] [CrossRef]
- Hu, L.-Y.; Mi, W.-L.; Wu, G.-C.; Wang, Y.-Q.; Mao-Ying, Q.-L. Prevention and Treatment for Chemotherapy-Induced Peripheral Neuropathy: Therapies Based on CIPN Mechanisms. Curr. Neuropharmacol. 2019, 17, 184–196. [Google Scholar] [CrossRef] [PubMed]
- Boyette-Davis, J.A.; Cata, J.P.; Driver, L.C.; Novy, D.M.; Bruel, B.M.; Mooring, D.L.; Wendelschafer-Crabb, G.; Kennedy, W.R.; Dougherty, P.M. Persistent chemoneuropathy in patients receiving the plant alkaloids paclitaxel and vincristine. Cancer Chemother. Pharmacol. 2013, 71, 619–626. [Google Scholar] [CrossRef] [PubMed]
- Boyette-Davis, J.A.; Walters, E.T.; Dougherty, P.M. Mechanisms involved in the development of chemotherapy-induced neuropathy. Pain Manag. 2015, 5, 285–296. [Google Scholar] [CrossRef]
- Marques, B.L.; Oliveira-Lima, O.C.; Carvalho, G.A.; Chiarelli, R.D.A.; Ribeiro, R.I.; Parreira, R.C.; Freitas, E.M.D.M.; Resende, R.R.; Klempin, F.; Ulrich, H.; et al. Neurobiology of glycine transporters: From molecules to behavior. Neurosci. Biobehav. Rev. 2020, 118, 97–110. [Google Scholar] [CrossRef]
- Imam, M.Z.; Kuo, A.; Nicholson, J.R.; Corradini, L.; Smith, M.T. Assessment of the anti-allodynic efficacy of a glycine transporter 2 inhibitor relative to pregabalin and duloxetine in a rat model of prostate cancer-induced bone pain. Pharmacol. Rep. 2020, 72, 1418–1425. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, Y.; Hara, K.; Haranishi, Y.; Terada, T.; Obara, G.; Sata, T. Antinociceptive effect of intracerebroventricular administration of glycine transporter-2 inhibitor ALX1393 in rat models of inflammatory and neuropathic pain. Pharmacol. Biochem. Behav. 2015, 130, 46–52. [Google Scholar] [CrossRef]
- Nagasue, H.; Nishida, H.; Saitoh, F.; Yumiya, Y.; Ohkouchi, M.; Egusa, T.; Akiyama, E.; Terada, Y.; Sakazaki, H.; Hirabayashi, T.; et al. 4-Phenoxy-benzamide Derivatives as Novel Glycine Transporter Type 2 Inhibitors. Part 2: 3-Pyridyl Amide Derivatives; A New Class of Potent and Orally Active GlyT-2 Inhibitors. In Proceedings of the 8th AFMC International Medicinal Chemistry Symposium, Tokyo, Japan, 29 November–2 December 2011. [Google Scholar]
- Ohkouchi, M.; Nishida, H.; Saitoh, F.; Yumiya, Y.; Nagasue, H.; Egusa, T.; Akiyama, E.; Terada, Y.; Sakazaki, H.; Hirabayashi, T.; et al. 4-Phenoxybenzamide Derivatives as Novel Glycine Transporter Type 2 inhibitors. Part 1: Design, Synthesis and in vivo Evaluation of Ethylenediamine Class Compounds. In Proceedings of the 8th AFMC International Medicinal Chemistry Symposium, Tokyo, Japan, 29 November–2 December 2011. [Google Scholar]
- Meur, A.M.-L.; Ghisdal, P.; Mullier, B.; De Ron, P.; Downey, P.; Van Der Perren, C.; Declercq, V.; Cornelis, S.; Famelart, M.; Van Asperen, J.; et al. Reversible inhibition of the glycine transporter GlyT2 circumvents acute toxicity while preserving efficacy in the treatment of pain. Br. J. Pharmacol. 2013, 170, 1053–1063. [Google Scholar] [CrossRef]
- Mohammadzadeh, A.; Lakatos, P.; Balogh, M.; Zádor, F.; Karádi, D.; Zádori, Z.; Király, K.; Galambos, A.; Barsi, S.; Riba, P.; et al. Pharmacological Evidence on Augmented Antiallodynia Following Systemic Co-Treatment with GlyT-1 and GlyT-2 Inhibitors in Rat Neuropathic Pain Model. Int. J. Mol. Sci. 2021, 22, 2479. [Google Scholar] [CrossRef]
- National Health and Medical Research Council. Australian Code for the Care and Use of Animals for Scientific Purposes, 8th ed.; HMRC: Canberra, Australia, 2013. [Google Scholar]
- Han, F.Y.; Wyse, B.D.; Smith, M.T. Optimization and pharmacological characterization of a refined cisplatin-induced rat model of peripheral neuropathic pain. Behav. Pharmacol. 2014, 25, 732–740. [Google Scholar] [CrossRef]
- Authier, N.; Gillet, J.P.; Fialip, J.; Eschalier, A.; Coudore, F. An animal model of nociceptive peripheral neuropathy following repeated cisplatin injections. Exp. Neurol. 2003, 182, 12–20. [Google Scholar] [CrossRef]
- Shenoy, P.; Kuo, A.; Vetter, I.; Smith, M.T. Optimization and In Vivo Profiling of a Refined Rat Model of Walker 256 Breast Cancer Cell-Induced Bone Pain Using Behavioral, Radiological, Histological, Immunohistochemical and Pharmacological Methods. Front. Pharmacol. 2017, 8, 442. [Google Scholar] [CrossRef]
- Morita, K.; Motoyama, N.; Kitayama, T.; Morioka, N.; Kifune, K.; Dohi, T. Spinal Antiallodynia Action of Glycine Transporter Inhibitors in Neuropathic Pain Models in Mice. J. Pharmacol. Exp. Ther. 2008, 326, 633–645. [Google Scholar] [CrossRef] [PubMed]
- Umbricht, D.; Alberati, D.; Martin-Facklam, M.; Borroni, E.; Youssef, E.A.; Ostland, M.; Wallace, T.L.; Knoflach, F.; Dorflinger, E.; Wettstein, J.G.; et al. Effect of bitopertin, a glycine reuptake inhibitor, on negative symptoms of schizophrenia: A randomized, double-blind, proof-of-concept study. JAMA Psychiatry 2014, 71, 637–646. [Google Scholar] [CrossRef] [PubMed]
- Armbruster, A.; Neumann, E.; Kötter, V.; Hermanns, H.; Werdehausen, R.; Eulenburg, V. The GlyT1 Inhibitor Bitopertin Ameliorates Allodynia and Hyperalgesia in Animal Models of Neuropathic and Inflammatory Pain. Front. Mol. Neurosci. 2018, 10, 438. [Google Scholar] [CrossRef] [PubMed]
- Bugarski-Kirola, D.; Blaettler, T.; Arango, C.; Fleischhacker, W.W.; Garibaldi, G.; Wang, A.; Dixon, M.; Bressan, R.; Nasrallah, H.; Lawrie, S.; et al. Bitopertin in Negative Symptoms of Schizophrenia—Results from the Phase III FlashLyte and DayLyte Studies. Biol. Psychiatry 2017, 82, 8–16. [Google Scholar] [CrossRef] [PubMed]
- Zeilhofer, H.U.; Benke, D.; Yevenes, G.E. Chronic Pain States: Pharmacological Strategies to Restore Diminished Inhibitory Spinal Pain Control. Annu. Rev. Pharmacol. Toxicol. 2012, 52, 111–133. [Google Scholar] [CrossRef] [PubMed]
- Winters, B.L.; Rawling, T.; Vandenberg, R.J.; Christie, M.; Bhola, R.F.; Imlach, W.L. Activity of novel lipid glycine transporter inhibitors on synaptic signalling in the dorsal horn of the spinal cord. Br. J. Pharmacol. 2018, 175, 2337–2347. [Google Scholar] [CrossRef]
- Koltzenburg, M.; Torebjörk, H.E.; Wahren, L.K. Nociceptor modulated central sensitization causes mechanical hyperalgesia in acute chemogenic and chronic neuropathic pain. Brain 1994, 117, 579–591. [Google Scholar] [CrossRef]
- Peirs, C.; Williams, S.-P.G.; Zhao, X.; Walsh, C.E.; Gedeon, J.Y.; Cagle, N.E.; Goldring, A.; Hioki, H.; Liu, Z.; Marell, P.S.; et al. Dorsal Horn Circuits for Persistent Mechanical Pain. Neuron 2015, 87, 797–812. [Google Scholar] [CrossRef]
- Coull, J.A.M.; Boudreau, D.; Bachand, K.; Prescott, S.A.; Nault, F.; Sík, A.; De Koninck, P.; De Koninck, Y. Trans-synaptic shift in anion gradient in spinal lamina I neurons as a mechanism of neuropathic pain. Nat. Cell Biol. 2003, 424, 938–942. [Google Scholar] [CrossRef]
- Müller, F.; Heinke, B.; Sandkühler, J. Reduction of glycine receptor-mediated miniature inhibitory postsynaptic currents in rat spinal lamina I neurons after peripheral inflammation. Neuroscience 2003, 122, 799–805. [Google Scholar] [CrossRef]
- Zeilhofer, H.U.; Acuña, M.A.; Gingras, J.; Yévenes, G.E. Glycine receptors and glycine transporters: Targets for novel analgesics? Cell. Mol. Life Sci. 2018, 75, 447–465. [Google Scholar] [CrossRef] [PubMed]
- Millan, M.J. The induction of pain: An integrative review. Prog. Neurobiol. 1999, 57, 1–164. [Google Scholar] [CrossRef]
- Vo, T.; Rice, A.S.; Dworkin, R.H. Non-steroidal anti-inflammatory drugs for neuropathic pain: How do we explain continued widespread use? Pain 2009, 143, 169–171. [Google Scholar] [CrossRef] [PubMed]
- Moore, R.A.; Chi, C.-C.; Wiffen, P.J.; Derry, S.; Rice, A.S.C. Oral nonsteroidal anti-inflammatory drugs for neuropathic pain. Cochrane Database Syst. Rev. 2015, 2015, CD010902. [Google Scholar] [CrossRef]
- Zeilhofer, H.U.; Studler, B.; Arabadzisz, D.; Schweizer, C.; Ahmadi, S.; Layh, B.; Bösl, M.R.; Fritschy, J.-M. Glycinergic neurons expressing enhanced green fluorescent protein in bacterial artificial chromosome transgenic mice. J. Comp. Neurol. 2005, 482, 123–141. [Google Scholar] [CrossRef]
- Foster, E.; Wildner, H.; Tudeau, L.; Haueter, S.; Ralvenius, W.T.; Jegen, M.; Johannssen, H.; Hösli, L.; Haenraets, K.; Ghanem, A.; et al. Targeted Ablation, Silencing, and Activation Establish Glycinergic Dorsal Horn Neurons as Key Components of a Spinal Gate for Pain and Itch. Neuron 2015, 85, 1289–1304. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kuo, A.; Corradini, L.; Nicholson, J.R.; Smith, M.T. Assessment of the Anti-Allodynic and Anti-Hyperalgesic Efficacy of a Glycine Transporter 2 Inhibitor Relative to Pregabalin, Duloxetine and Indomethacin in a Rat Model of Cisplatin-Induced Peripheral Neuropathy. Biomolecules 2021, 11, 940. https://doi.org/10.3390/biom11070940
Kuo A, Corradini L, Nicholson JR, Smith MT. Assessment of the Anti-Allodynic and Anti-Hyperalgesic Efficacy of a Glycine Transporter 2 Inhibitor Relative to Pregabalin, Duloxetine and Indomethacin in a Rat Model of Cisplatin-Induced Peripheral Neuropathy. Biomolecules. 2021; 11(7):940. https://doi.org/10.3390/biom11070940
Chicago/Turabian StyleKuo, Andy, Laura Corradini, Janet R. Nicholson, and Maree T. Smith. 2021. "Assessment of the Anti-Allodynic and Anti-Hyperalgesic Efficacy of a Glycine Transporter 2 Inhibitor Relative to Pregabalin, Duloxetine and Indomethacin in a Rat Model of Cisplatin-Induced Peripheral Neuropathy" Biomolecules 11, no. 7: 940. https://doi.org/10.3390/biom11070940
APA StyleKuo, A., Corradini, L., Nicholson, J. R., & Smith, M. T. (2021). Assessment of the Anti-Allodynic and Anti-Hyperalgesic Efficacy of a Glycine Transporter 2 Inhibitor Relative to Pregabalin, Duloxetine and Indomethacin in a Rat Model of Cisplatin-Induced Peripheral Neuropathy. Biomolecules, 11(7), 940. https://doi.org/10.3390/biom11070940







