Relationship between Serum Alkaline Phosphatase and Low Muscle Mass Index Among Korean Adults: A Nationwide Population-Based Study
Abstract
1. Introduction
2. Materials and Methods
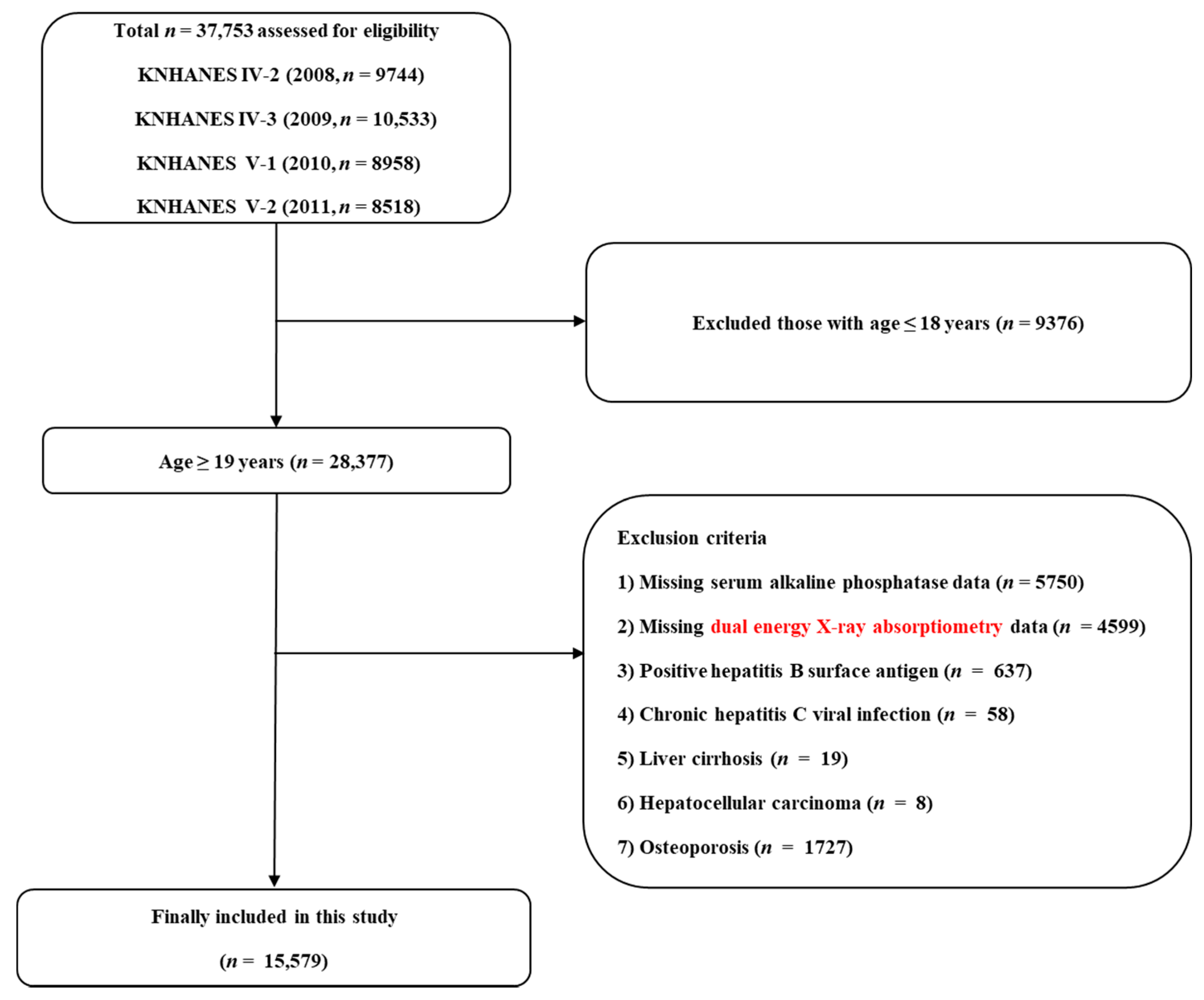
2.1. Study Population
2.2. Biochemical Measurements
2.3. Assessment of Low Skeletal Muscle Mass Index
2.4. Covariates
2.5. Statistical Analysis
3. Results
3.1. Clinical Characteristics of the Study Population
3.2. Relationship Between Serum ALP Level and LSMI
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cruz-Jentoft, A.J.; Bahat, G.; Bauer, J.; Boirie, Y.; Bruyère, O.; Cederholm, T.; Cooper, C.; Landi, F.; Rolland, Y.; Sayer, A.A.; et al. Sarcopenia: Revised European consensus on definition and diagnosis. Age Ageing 2019, 48, 16–31. [Google Scholar] [CrossRef]
- Rosenberg, I.H. Sarcopenia: Origins and Clinical Relevance. J. Nutr. 1997, 127, 990S–991S. [Google Scholar] [CrossRef]
- Anker, S.D.; Morley, J.E.; Von Haehling, S. Welcome to the ICD-10 code for sarcopenia. J. Cachex-Sarcopenia Muscle 2016, 7, 512–514. [Google Scholar] [CrossRef] [PubMed]
- Xue, Q.L.; Walston, J.D.; Fried, L.P.; Beamer, B.A. Prediction of risk of falling, physical disability, and frailty by rate of decline in grip strength: The women’s health and aging study. Arch. Intern Med. 2011, 171, 1119–1121. [Google Scholar] [CrossRef]
- E Cleasby, M.; Jamieson, P.M.; Atherton, P.J. Insulin resistance and sarcopenia: Mechanistic links between common co-morbidities. J. Endocrinol. 2016, 229, R67–R81. [Google Scholar] [CrossRef]
- Ida, S.; Kaneko, R.; Imataka, K.; Murata, K. Association between Sarcopenia and Renal Function in Patients with Diabetes: A Systematic Review and Meta-Analysis. J. Diabetes Res. 2019, 2019, 1–11. [Google Scholar] [CrossRef]
- Kim, T.N.; Choi, K.M. The Implications of Sarcopenia and Sarcopenic Obesity on Cardiometabolic Disease. J. Cell. Biochem. 2015, 116, 1171–1178. [Google Scholar] [CrossRef] [PubMed]
- Cruz-Jentoft, A.J.; Sayer, A.A. Sarcopenia. Lancet 2019, 393, 2636–2646. [Google Scholar] [CrossRef]
- Woreta, T.A.; Alqahtani, S.A. Evaluation of Abnormal Liver Tests. Med. Clin. N. Am. 2014, 98, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Haarhaus, M.L.; Brandenburg, V.; Kalantar-Zadeh, K.; Stenvinkel, P.; Magnusson, P. Alkaline phosphatase: A novel treatment target for cardiovascular disease in CKD. Nat. Rev. Nephrol. 2017, 13, 429–442. [Google Scholar] [CrossRef] [PubMed]
- Iqbal, M.P.; Mehboobali, N.; Azam, I.; Tareen, A.K. Association of alkaline phosphatase with acute myocardial infarction in a population with high prevalence of hypovitaminosis D. Clin. Chim. Acta 2013, 425, 192–195. [Google Scholar] [CrossRef]
- Lee, J.H.; Lee, J.W.; Lee, Y.J. The Relationship between Serum Alkaline Phosphatase and Arterial Stiffness in Korean Adults. J. Atheroscler. Thromb. 2019, 26, 1084–1091. [Google Scholar] [CrossRef]
- Kunutsor, S.K.; Apekey, T.A.; Khan, H. Liver enzymes and risk of cardiovascular disease in the general population: A meta-analysis of prospective cohort studies. Atherosclerosis 2014, 236, 7–17. [Google Scholar] [CrossRef]
- Schoppet, M.; Shanahan, C.M. Role for alkaline phosphatase as an inducer of vascular calcification in renal failure? Kidney Int. 2008, 73, 989–991. [Google Scholar] [CrossRef]
- Seo, M.-S.; Shim, J.-Y.; Lee, Y.-J. Relationship between serum alkaline phosphatase level, C-reactive protein and leukocyte counts in adults aged 60 years or older. Scand. J. Clin. Lab. Investig. 2019, 79, 233–237. [Google Scholar] [CrossRef]
- Kawada, T. Serum alkaline phosphatase level and metabolic syndrome. Clin. Chim. Acta 2020, 506, 187. [Google Scholar] [CrossRef] [PubMed]
- Kweon, S.; Kim, Y.; Jang, M.-J.; Kim, Y.; Kim, K.; Choi, S.; Chun, C.; Khang, Y.-H.; Oh, K. Data resource profile: The Korea National Health and Nutrition Examination Survey (KNHANES). Int. J. Epidemiol. 2014, 43, 69–77. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y. The Korea National Health and Nutrition Examination Survey (KNHANES): Current Status and Challenges. Epidemiol. Health 2014, 36, e2014002. [Google Scholar] [CrossRef] [PubMed]
- Studenski, S.A.; Peters, K.W.; Alley, D.E.; Cawthon, P.M.; McLean, R.R.; Harris, T.B.; Ferrucci, L.; Guralnik, J.M.; Fragala, M.S.; Kenny, A.M.; et al. The FNIH Sarcopenia Project: Rationale, Study Description, Conference Recommendations, and Final Estimates. J. Gerontol. Ser. A Boil. Sci. Med. Sci. 2014, 69, 547–558. [Google Scholar] [CrossRef]
- Seo, M.H.; Lee, W.-Y.; Kim, S.S.; Kang, J.-H.; Kang, J.-H.; Kim, K.K.; Kim, B.-Y.; Kim, Y.-H.; Kim, W.-J.; Kim, E.M.; et al. 2018 Korean Society for the Study of Obesity Guideline for the Management of Obesity in Korea. J. Obes. Metab. Syndr. 2019, 28, 40–45. [Google Scholar] [CrossRef]
- National Center for Health Statistics. Adult tobacco use information. Available online: https://www.cdc.gov/nchs/nhis/tobacco/tobacco_glossary.htm (accessed on 27 May 2021).
- National Institute on Alcohol Abuse and Alcoholism. Drinking levels defined. Available online: https://www.niaaa.nih.gov/alcohol-health/overview-alcohol-consumption/moderate-binge-drinking (accessed on 27 May 2021).
- E Roffman, C.; Buchanan, J.; Allison, G. Charlson Comorbidities Index. J. Physiother. 2016, 62, 171. [Google Scholar] [CrossRef]
- Meng, S.-J.; Yu, L.-J. Oxidative Stress, Molecular Inflammation and Sarcopenia. Int. J. Mol. Sci. 2010, 11, 1509–1526. [Google Scholar] [CrossRef] [PubMed]
- Dalle, S.; Rossmeislova, L.; Koppo, K. The Role of Inflammation in Age-Related Sarcopenia. Front. Physiol. 2017, 8, 1045. [Google Scholar] [CrossRef]
- Rieu, I.; Magne, H.; Savary-Auzeloux, I.; Averous, J.; Bos, C.; Peyron, M.A.; Combaret, L.; Dardevet, D. Reduction of low grade inflammation restores blunting of postprandial muscle anabolism and limits sarcopenia in old rats. J. Physiol. 2009, 587, 5483–5492. [Google Scholar] [CrossRef]
- Landi, F.; Marzetti, E.; Liperoti, R.; Pahor, M.; Russo, A.; Martone, A.M.; Colloca, G.; Capoluongo, E.; Bernabei, R. Nonsteroidal Anti-Inflammatory Drug (NSAID) Use and Sarcopenia in Older People: Results From the ilSIRENTE Study. J. Am. Med. Dir. Assoc. 2013, 14, 626.e9–626.e13. [Google Scholar] [CrossRef]
- Bano, G.; Trevisan, C.; Carraro, S.; Solmi, M.; Luchini, C.; Stubbs, B.; Manzato, E.; Sergi, G.; Veronese, N. Inflammation and sarcopenia: A systematic review and meta-analysis. Maturitas 2017, 96, 10–15. [Google Scholar] [CrossRef]
- Lee, H.S.; Koh, I.-H.; Kim, H.-S.; Kwon, Y.-J. Platelet and white blood cell count are independently associated with sarcopenia: A nationwide population-based study. Thromb. Res. 2019, 183, 36–44. [Google Scholar] [CrossRef]
- Curcio, F.; Ferro, G.; Basile, C.; Liguori, I.; Parrella, P.; Pirozzi, F.; DELLA Morte, D.; Gargiulo, G.; Testa, G.; Tocchetti, C.G.; et al. Biomarkers in sarcopenia: A multifactorial approach. Exp. Gerontol. 2016, 85, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Antonioli, L.; Pacher, P.; Vizi, E.S.; Haskó, G. CD39 and CD73 in immunity and inflammation. Trends Mol. Med. 2013, 19, 355–367. [Google Scholar] [CrossRef] [PubMed]
- Campbell, E.L.; MacManus, C.F.; Kominsky, D.J.; Keely, S.; Glover, L.E.; Bowers, B.E.; Scully, M.; Bruyninckx, W.J.; Colgan, S.P. Resolvin E1-induced intestinal alkaline phosphatase promotes resolution of inflammation through LPS detoxification. Proc. Natl. Acad. Sci. USA 2010, 107, 14298–14303. [Google Scholar] [CrossRef] [PubMed]
- Fawley, J.; Gourlay, D.M. Intestinal alkaline phosphatase: A summary of its role in clinical disease. J. Surg. Res. 2016, 202, 225–234. [Google Scholar] [CrossRef]
- Lencel, P.; Delplace, S.; Hardouin, P.; Magne, D. TNF-α stimulates alkaline phosphatase and mineralization through PPARγ inhibition in human osteoblasts. Bone 2011, 48, 242–249. [Google Scholar] [CrossRef] [PubMed]
- Cho, I.-J.; Choi, K.H.; Oh, C.H.; Hwang, Y.C.; Jeong, I.-K.; Ahn, K.J.; Chung, H.-Y. Effects of C-reactive protein on bone cells. Life Sci. 2016, 145, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Shioi, A.; Katagi, M.; Okuno, Y.; Mori, K.; Jono, S.; Koyama, H.; Nishizawa, Y. Induction of bone-type alkaline phosphatase in human vascular smooth muscle cells: Roles of tumor necrosis factor-alpha and oncostatin M derived from macrophages. Circ Res. 2002, 91, 9–16. [Google Scholar] [CrossRef] [PubMed]
- Juschten, J.; For the BASIC Study Investigators; Ingelse, S.A.; Bos, L.D.J.; Girbes, A.R.J.; Juffermans, N.P.; Van Der Poll, T.; Schultz, M.J.; Tuinman, P.R. Alkaline phosphatase in pulmonary inflammation—A translational study in ventilated critically ill patients and rats. Intensiv. Care Med. Exp. 2020, 8, 1–14. [Google Scholar] [CrossRef]


| Men | Women | |||||||
|---|---|---|---|---|---|---|---|---|
| Serum ALP level | T1 (≤200) | T2 (201–248) | T3 (≥249) | p | T1 (≤170) | T2 (171–224) | T3 (≥225) | p |
| Unweighted number, n | 2409 | 2408 | 2354 | 2804 | 2859 | 2745 | ||
| Age, years | 42.7 ± 0.4 | 42.2 ± 0.4 | 44.2 ± 0.5 | 0.001 | 37.9 ± 0.3 | 41.6 ± 0.4 | 48.5 ± 0.4 | <0.001 |
| Waist circumference, cm | 83.7 ± 0.3 | 83.9 ± 0.3 | 84.6 ± 0.3 | 0.034 | 77.0 ± 0.2 | 80.8 ± 0.2 | 83.1 ± 0.2 | <0.001 |
| BMI, kg/cm2 | 24.0 ± 0.1 | 24.1 ± 0.1 | 24.2 ± 0.1 | 0.354 | 22.3 ± 0.1 | 23.1 ± 0.1 | 24.1 ± 0.1 | <0.001 |
| Mean blood pressure, mmHg | 92.3 ± 0.4 | 93.2 ± 0.3 | 94.3 ± 0.3 | <0.001 | 83.5 ± 0.3 | 86.5 ± 0.3 | 90.7 ± 0.3 | <0.001 |
| Fasting glucose, mg/dL | 96.2 ± 0.5 | 97.0 ± 0.4 | 101.5 ± 0.7 | <0.001 | 90.7 ± 0.3 | 93.1 ± 0.4 | 98.7 ± 0.6 | <0.001 |
| Total cholesterol, mg/dL | 187.0 ± 1.0 | 187.0 ± 0.9 | 187.2 ± 1.1 | 0.980 | 179.4 ± 0.7 | 186.1 ± 0.6 | 189.6 ± 0.7 | <0.001 |
| Total calorie intake, kcal/day | 2438.1 ± 26.8 | 2398.4 ± 27.0 | 2368.7 ± 27.0 | 0.170 | 1723.1 ± 17.7 | 1672.6 ± 15.9 | 1662.8 ± 16.7 | 0.030 |
| Smoking status, % (SE) | <0.001 | 0.078 | ||||||
| Current smoker | 48.1 (1.5) | 52.7 (1.5) | 57.6 (1.5) | 7.2 (0.7) | 5.4 (0.6) | 5.5 (0.6) | ||
| Past smoker | 23.9 (1.2) | 20.4 (1.1) | 20.7 (1.1) | 2.8 (0.4) | 2.8 (0.4) | 2.1 (0.4) | ||
| Never smoker | 28.1 (1.4) | 27.0 (1.3) | 21.7 (1.2) | 89.9 (0.8) | 91.8 (0.7) | 92.4 (0.7) | ||
| Heavy alcohol drinker, % (SE) | 62.4 (1.3) | 62.5 (1.4) | 57.8 (1.3) | 0.014 | 45.3 (1.2) | 37.0 (1.2) | 26.0 (1.2) | <0.001 |
| Regular exercise, % (SE) | 27.1 (1.4) | 26.5 (1.3) | 28.0 (1.3) | 0.692 | 23.0 (1.0) | 21.9 (1.0) | 23.5 (1.1) | 0.506 |
| Number of chronic diseases, % (SE) | <0.001 | <0.001 | ||||||
| 0 | 87.2 (0.9) | 85.9 (0.9) | 80.0 (1.1) | 93.3 (0.6) | 89.8 (0.7) | 81.2 (1.0) | ||
| 1 | 10.7 (0.9) | 12.3 (0.8) | 16.4 (1.0) | 5.6 (0.6) | 9.0 (0.7) | 16.4 (0.9) | ||
| ≥2 | 2.2 (0.4) | 1.8 (0.3) | 3.6 (0.4) | 1.1 (0.3) | 1.2 (0.3) | 2.4 (0.4) | ||
| ASM, kg | 23.0 ± 0.1 | 22.8 ± 0.1 | 22.5 ± 0.1 | <0.001 | 14.6 ± 0.1 | 14.7 ± 0.1 | 14.7 ± 0.1 | 0.286 |
| BMI-adjusted ASM | 0.964 ± 0.004 | 0.952 ± 0.003 | 0.933 ± 0.004 | <0.001 | 0.662 ± 0.002 | 0.643 ± 0.002 | 0.617 ± 0.003 | <0.001 |
| Serum ALP Levels | T1 | T2 | T3 | |||
|---|---|---|---|---|---|---|
| OR (95% CI) | p | OR (95% CI) | p | Overall p | ||
| Men | ||||||
| Unadjusted | 1 (reference) | 1.05 (0.81–1.36) | 0.736 | 1.72 (1.35–2.20) | <0.001 | <0.001 |
| Model 1 | 1 (reference) | 1.06 (0.80–1.40) | 0.684 | 1.60 (1.23–2.07) | 0.001 | <0.001 |
| Model 2 | 1 (reference) | 0.98 (0.70–1.37) | 0.894 | 1.41 (1.04–1.91) | 0.027 | 0.025 |
| Women | ||||||
| Unadjusted | 1 (reference) | 1.88 (1.35–2.60) | <0.001 | 3.84 (2.81–5.24) | <0.001 | <0.001 |
| Model 1 | 1 (reference) | 1.18 (0.84–1.67) | 0.341 | 1.42 (1.00–2.02) | 0.049 | 0.123 |
| Model 2 | 1 (reference) | 1.16 (0.80–1.67) | 0.441 | 1.45 (1.00–2.10) | 0.049 | 0.107 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, J.-H.; Cho, A.-R.; Lee, Y.-J. Relationship between Serum Alkaline Phosphatase and Low Muscle Mass Index Among Korean Adults: A Nationwide Population-Based Study. Biomolecules 2021, 11, 842. https://doi.org/10.3390/biom11060842
Lee J-H, Cho A-R, Lee Y-J. Relationship between Serum Alkaline Phosphatase and Low Muscle Mass Index Among Korean Adults: A Nationwide Population-Based Study. Biomolecules. 2021; 11(6):842. https://doi.org/10.3390/biom11060842
Chicago/Turabian StyleLee, Jun-Hyuk, A-Ra Cho, and Yong-Jae Lee. 2021. "Relationship between Serum Alkaline Phosphatase and Low Muscle Mass Index Among Korean Adults: A Nationwide Population-Based Study" Biomolecules 11, no. 6: 842. https://doi.org/10.3390/biom11060842
APA StyleLee, J.-H., Cho, A.-R., & Lee, Y.-J. (2021). Relationship between Serum Alkaline Phosphatase and Low Muscle Mass Index Among Korean Adults: A Nationwide Population-Based Study. Biomolecules, 11(6), 842. https://doi.org/10.3390/biom11060842






