Effects of Anthocyanins on Vascular Health
Abstract
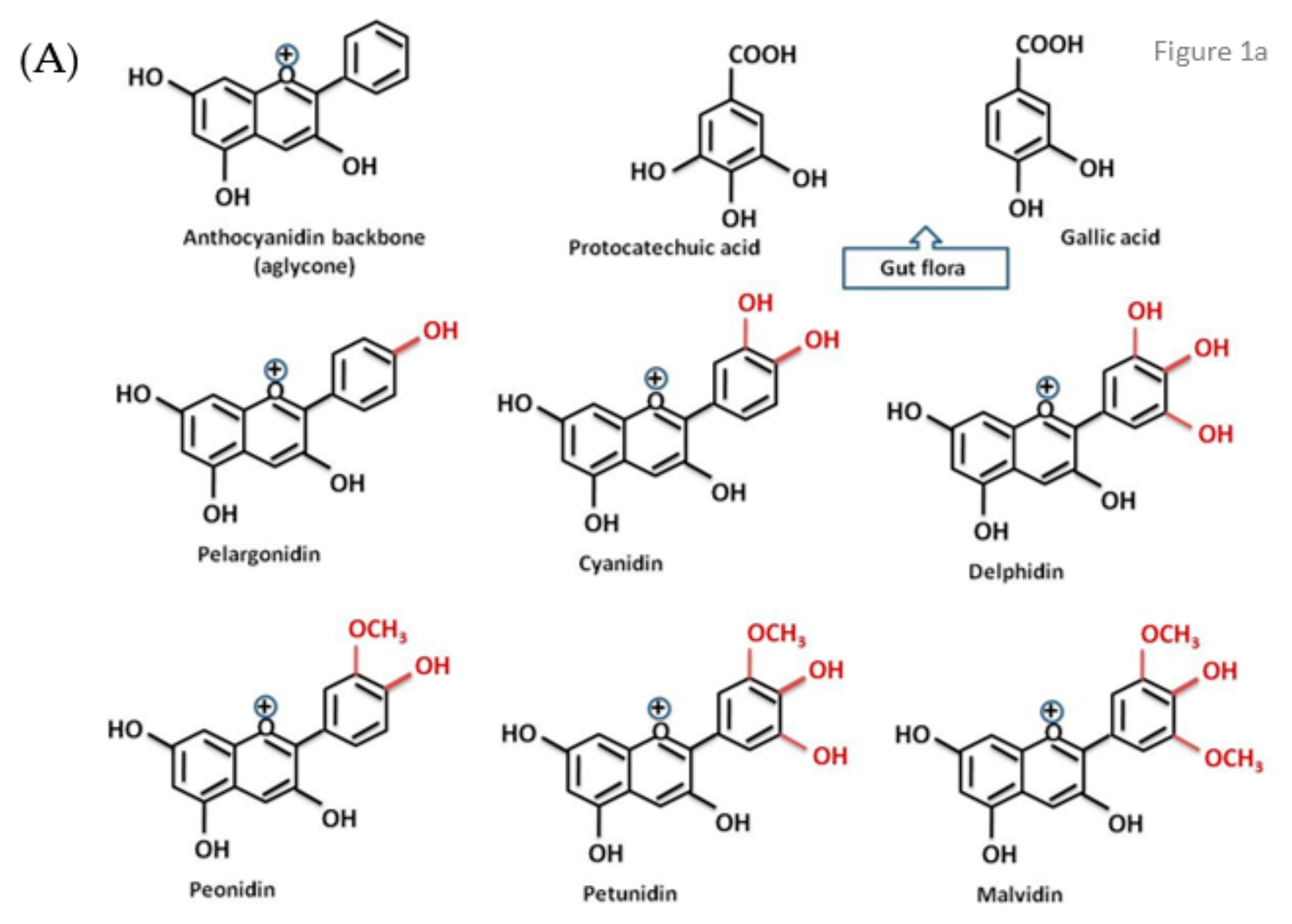
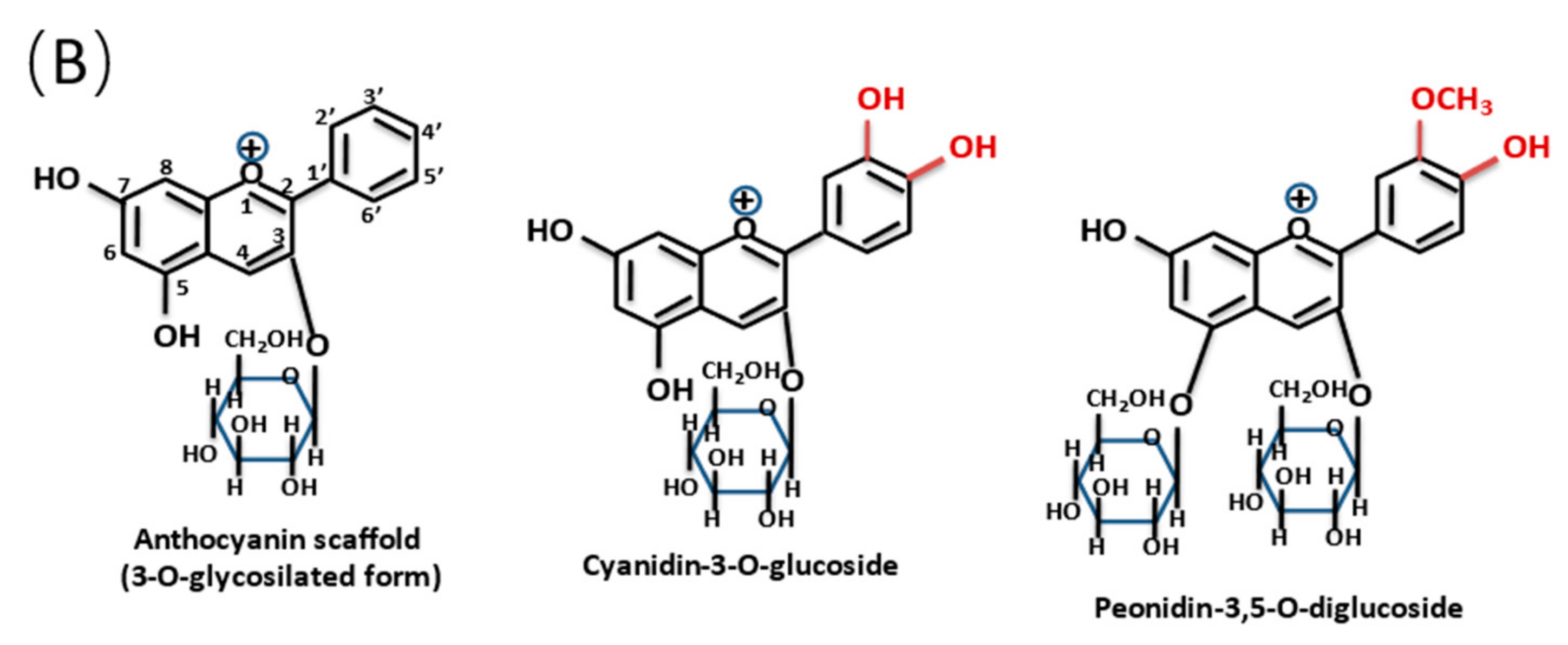
1. Introduction
| Fruits and Vegetables | Anthocyanin Content | Administrated as | References |
|---|---|---|---|
| Blackberry (Rubus fruticosus) | 820–1800 mg/kg | Fresh fruit | [39] |
| Black mulberry (Morus nigra) | 42.4 mg/100 g | Fresh fruit | [40] |
| Bilberry (Vaccinium myrtillus) | 1610–5963 mg/L | Juice 100% | [23] |
| Black carrots (Daucus carota ssp. sativus var. atrorubens) | 1750 mg/kg | Fresh vegetable | [41] |
| Black chokeberries (Aronia melanocarpa) | 1480 mg/100 g | Fresh fruit | [42] |
| Black soybean (Glycine max) | 0.1–23.04 mg/g | Seed coat | [43] |
| Black currant (Ribes nigrum) | 176–1298 mg/L | Juice 100% | [23] |
| Blood orange (Citrus sinensis) | 4.6 ± 0.7; 72.4 ± 0.6 mg/L | Fresh fruit | [44] |
| Blueberry (Vaccinium virgatum and Vaccinium corymbosum) | 134 mg/kg | Fresh fruit | [45] |
| Cherry (Prunus cerasus) | 22 mg/100 g | Fresh fruit | [23] |
| Cornelian cherry (Cornus mas) | 128.45 ± 5.14 mg/L C3G 226.78 ± 8.61 mg/L | [46] | |
| Cowpea (Vigna unguiculata) | 1.7–3.9 mg/g | Seeds | [43] |
| Cranberry (Vaccinium macrocarpon) | 460–2000 mg/kg | Fresh fruit | [39] |
| Eggplant (Solanum melongena L.) | 11.53 g/100 g DW delphinidin, 0.55 g/100 g DW of petunidin | Fruit | [25] |
| Grape (Vitis vinifera) | 300–7500 mg/kg | Fresh fruit | [39] |
| Kiwi (Actinidia melanandra) | 478 μg/g in skin, 81 μg/g in flesh | Fresh fruit | [47] |
| Mahaleb cherries (Prunus mahaleb) (g/kg DW) | 7.80 ± 1.10; 15.60 ± 3.10; 17.70 ± 3.50; 18.90 ± 0.90 | Fresh fruit | [48] |
| Pepper (Capsicum annuum L.) | 0.96 mg anthocyanin/100 g fresh weight | [26] | |
| Pomegranate (Punica granatum) | 43 mg/L | Juice | [23] |
| Purple maize (Zea mays indurate) | 4.3 to 117-mg C3G/g | dark-colored purple corncob | [49] |
| Purple sweet potato (Ipomoea batatas L.) | 0.94- 1.75 g/kg | Fresh weight | [24] |
| Strawberry (Fragaria × ananassa) | 232 mg/100 g | Fresh fruit | [23] |
2. Preclinical and Clinical Research and Molecular Mechanisms
2.1. Metabolic Effects of Anthocyanins
2.1.1. Clinical Research
2.1.2. Preclinical Research
2.2. Effects of Anthocyanins on Endothelial Function
2.3. Anti-Inflammatory Effects of Anthocyanins
2.4. Anthocyanins as Antioxidants
2.5. Other Vascular Effects of Anthocyanins
2.6. Anthocyanins and Gene Expression
3. Population-Based Studies
3.1. Observational and Intervention Studies
3.2. Randomized Controlled Trials (RCT)
4. Study Limitations
5. Future Research Directions
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Vlachopoulos, C.; Aznaoridis, K.; Stefanidis, C. Prediction of cardiovascular events and all-cause mortality with arterial stiffness. A systematic review and meta-analysis. J. Am. Coll. Cardiol. 2010, 55, 1318–1327. [Google Scholar] [CrossRef]
- Mozos, I.; Malainer, C.; Horbańczuk, J.; Gug, C.; Stoian, D.; Luca, C.T.; Atanasov, A.G. Inflammatory markers for arterial stiffness in cardiovascular diseases. Front. Immunol. 2017, 8, 1058. [Google Scholar] [CrossRef]
- Mozos, I.; Luca, C.T. Crosstalk between Oxidative and Nitrosative Stress and Arterial Stiffness. Curr. Vasc. Pharmacol. 2017, 15, 446–456. [Google Scholar] [CrossRef]
- Iurciuc, S.; Cimpean, A.M.; Mitu, F.; Heredea, R.; Iurciuc, M. Vascular aging and subclinical atherosclerosis: Why such a never ending and challenging story in cardiology? Clin. Interv. Aging. 2017, 12, 1339–1345. [Google Scholar] [CrossRef]
- Böhm, V. Lycopene and heart health. Mol. Nutr. Food. Res. 2012, 56, 296–303. [Google Scholar] [CrossRef] [PubMed]
- Piepoli, M.F.; Hoes, A.W.; Agewall, S.; Albus, C.; Brotons, C.; Catapano, A.L.; Cooney, M.T.; Corrà, U.; Cosyns, B.; Deaton, C.; et al. 2016 European Guidelines on cardiovascular disease prevention in clinical practice. The Sixth Joint Task Force of the Eu-ropean Society of Cardiology and Other Societies on Cardiovascular Disease Prevention in Clinical Practice (constituted by representatives of 10 societies and by invited experts). Developed with the special contribution of the European Association for Cardiovascular Prevention and Rehabilitation (EACPR). Eur. Hearth J. 2016, 37, 2315–2381. [Google Scholar]
- Sozanski, T.; Kucharska, A.Z.; Rapak, A.; Szumny, D.; Trocha, M.; Merwid-Ląd, A.; Dzimira, S.; Piasecki, T.; Piórecki, N.; Magdalan, J.; et al. Iridoid-loganic acid versus anthocyanins from the Cornusmas fruits (cornelian cherry): Common and different effects on diet-induced atherosclerosis, PPARs expression and inflammation. Atherosclerosis 2016, 254, 151–160. [Google Scholar] [CrossRef]
- Wang, D.; Özen, C.; Abu-Reidah, I.M.; Chigurupati, S.; Patra, J.K.; Horbanczuk, J.O.; Jóźwik, A.; Tzvetkov, N.T.; Uhrin, P.; Atanasov, A.G. Vasculoprotective Effects of Pomegranate (Punica granatum L.). Front. Pharmacol. 2018, 9, 544. [Google Scholar] [CrossRef]
- Arnett, D.K.; Blumenthal, R.S.; Albert, M.A.; Buroker, A.B.; Goldberger, Z.D.; Hahn, E.J.; Himmelfarb, C.D.; Khera, A.; Lloyd-Jones, D.; McEvoy, J.W.; et al. 2019 ACC/AHA Guideline on the Primary Prevention of Cardiovascular Disease: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation 2019, 140, e596–e646. [Google Scholar] [CrossRef] [PubMed]
- Yeung, A.W.K.; Aggarwal, B.B.; Orhan, I.E.; Horbanczuk, O.K.; Barreca, D.; Battino, M.; Belwal, T.; Bishayee, A.; Daglia, M.; Devkota, H.P.; et al. Resveratrol, a popular dietary supplement for human and animal health: Quantitative research literature analysis-A review. Anim. Sci. Pap. Rep. 2019, 37, 103–118. [Google Scholar]
- Yeung, A.W.K.; Orhan, I.E.; Aggarwal, B.B.; Battino, M.; Belwal, T.; Bishayee, A.; Daglia, M.; Devkota, H.P.; El-Demerdash, A.; Balacheva, A.A.; et al. Berberine, a popular dietary supplement for human and animal health: Quantitative research literature analysis—A review. Anim. Sci. Pap. Rep. 2020, 38, 5–19. [Google Scholar]
- Yeung, A.W.K.; Aggarwal, B.B.; Barreca, D.; Battino, M.; Belwal, T.; Horbańczuk, O.K.; Berindan-Neagoe, I.; Bishayee, A.; Daglia, M.; Devkota, H.P.; et al. Dietary natural products and their potential to influence health and disease including animal model studies. Anim. Sci. Pap. Rep. 2018, 36, 345–358. [Google Scholar]
- Liu, Y.; Tikunov, Y.; Schouten, R.; Marcelis, L.F.M.; Visser, R.G.F.; Bovy, A. Anthocyanin biosynthesis and degradation mechanisms in Solanaceous Vegetables: A review. Front. Chem. 2018, 6, 52. [Google Scholar] [CrossRef]
- Oliveira, H.; Correia, P.; Pereira, A.R.; Araújo, P.; Mateus, N.; de Freitas, V.; Oliveira, J.; Fernandes, I. Exploring the Applica-tions of the Photoprotective Properties of Anthocyanins in Biological Systems. Int. J. Mol. Sci. 2020, 21, 7464. [Google Scholar] [CrossRef] [PubMed]
- Khoo, H.E.; Azlan, A.; Tang, S.T.; Lim, S.M. Anthocyanidins and anthocyanins: Colored pigments as food, pharmaceutical ingredients, and the potential health benefits. Food Nutr. Res. 2017, 61, 1361779. [Google Scholar] [CrossRef] [PubMed]
- Cassidy, A.; Mukamal, K.J.; Liu, L.; Franz, M.; Eliassen, A.H.; Rimm, E.B. High anthocyanin intake is associated with a reduced risk of myocardial infarction in young and middle-aged women. Circulation 2013, 127, 188–196. [Google Scholar] [CrossRef]
- Lynn, A.; Mathew, S.; Moore, C.T.; Russell, J.; Robinson, E.; Soumpasi, V.; Barker, M.E. Effect of tart cherry juice supplement on arterial stiffness and inflammation in healthy adults: A randomised controlled trial. Plant Foods Hum. Nutr. 2014, 69, 122–127. [Google Scholar] [CrossRef]
- Fang, J. Classification of fruits based on anthocyanin types and relevance to their health effects. Nutrition 2015, 1301–1306. [Google Scholar] [CrossRef] [PubMed]
- Cassidy, A. Berry anthocyanin intake and cardiovascular health. Mol. Asp. Med. 2017. [Google Scholar] [CrossRef]
- Jennings, A.; MacGregor, A.; Spector, T.; Cassidy, A. Higher dietary flavonoid intakes are associated with lower objectively measured body composition in women: Evidence from discordant monozygotic twins. Am. J. Clin. Nutr. 2017, 105, 626–634. [Google Scholar] [CrossRef]
- Cook, M.D.; Myers, S.D.; Gault, M.L.; Willems, M.E.T. Blackcurrant Alters Physiological Responses and Femoral Artery Diameter during Sustained Isometric Contraction. Nutrients 2017, 9, 556. [Google Scholar] [CrossRef]
- Tsang, C.; Smail, N.F.; Almoosawi, S.; McDougall, G.J.M.; Al-Dujaili, E.A.S. Antioxidant Rich Potato Improves Arterial Stiffness in Healthy Adults. Plant Foods Hum. Nutr. 2018, 73, 203–208. [Google Scholar] [CrossRef]
- Wallace, T.C.; Giusti, M.M. Anthocyanins in Health and Disease; CRC Press Taylor & Francis Group: Boca Raton, FL, USA, 2014; pp. 49–52. [Google Scholar]
- Wegener, C.B.; Jansen, G.; Jürgens, H.U.; Schütze, W. Special quality traits of coloured potato breeding clones: Anthocyanins, soluble phenols and antioxidant capacity. J. Sci. Food Agric. 2009, 89, 206–215. [Google Scholar] [CrossRef]
- Li, H.; Deng, Z.; Zhu, H.; Hu, C.; Liu, R.; Young, J.C.; Tsao, R. Highly pigmented vegetables: Anthocyanin compositions and their role in antioxidant activities. Food Res. Int. 2012, 46, 250–259. [Google Scholar] [CrossRef]
- Wang, G.; Chen, B.; Du, H.; Zhang, F.; Zhang, H.; Wang, Y.; He, H.; Geng, S.; Zhang, X. Genetic mapping of anthocyanin ac-cumulation-related genes in pepper fruits using a combination of SLAF-seq and BSA. PLoS ONE 2018, 13, e0204690. [Google Scholar] [CrossRef]
- Kalt, W.; Cassidy, A.; Howard, L.R.; Krikorian, R.; Stull, A.J.; Tremblay, F.; Zamora-Ros, R. Recent Research on the Health Benefits of Blueberries and Their Anthocyanins. Adv. Nutr. 2020, 11, 224–236. [Google Scholar] [CrossRef] [PubMed]
- Awika, J.M.; Duodu, K.G. Bioactive polyphenols and peptides in cowpea (Vigna unguiculata) and their health promoting properties: A review. J. Funct. Foods 2017, 38, 686–697. [Google Scholar] [CrossRef]
- Wang, Z.; Zhao, Y. Gut microbiota derived metabolites in cardiovascular health and disease. Protein Cell. 2018, 9, 416–431. [Google Scholar] [CrossRef] [PubMed]
- Tena, N.; Martín, J.; Asuero, A.G. State of the Art of Anthocyanins: Antioxidant Activity, Sources, Bioavailability, and Therapeutic Effect in Human Health. Antioxidants 2020, 9, 451. [Google Scholar] [CrossRef]
- Manach, C.; Williamson, G.; Morand, C.; Scalbert, A.; Rémésy, C. Bioavailability and bioefficacy of polyphenols in humans. I. Review of 97 bioavailability studies. Am. J. Clin. Nutr. 2005, 81 (Suppl. S1), 230S–242S. [Google Scholar] [CrossRef]
- Carkeet, C.; Clevidence, B.A.; Novotny, J.A. Anthocyanin excretion by humans increases linearly with increasing strawberry dose. J. Nutr. 2008, 138, 897–902. [Google Scholar] [CrossRef]
- De Ferrars, R.M.; Czank, C.; Zhang, Q.; Botting, N.P.; Kroon, P.A.; Cassidy, A.; Kay, C.D. The pharmacokinetics of anthocyanins and their metabolites in humans. Br. J. Pharmacol. 2014, 171, 3268–3282. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Sun, H.; He, S.; Lou, Q.; Yu, M.; Tang, M.; Tu, L. Metabolism and prebiotics activity of anthocyanins from black rice (Oryza sativa L.) in vitro. PLoS ONE 2018, 13, e0195754. [Google Scholar] [CrossRef] [PubMed]
- Vitaglione, P.; Donnarumma, G.; Napolitano, A.; Galvano, F.; Gallo, A.; Scalfi, L.; Fogliano, V. Protocatechuic acid is the major human metabolite of cyanidin-glucosides. J. Nutr. 2007, 137, 204–206. [Google Scholar] [CrossRef]
- Bhaswant, M.; Raihanah, S.; Mathai, M.L.; Mouatt, P.; Brown, L. Anthocyanins in chockeberry and purple maize attenuate diet-induced metabolic syndrome in rats. Nutrition 2017, 41, 24–31. [Google Scholar] [CrossRef] [PubMed]
- Kruger, M.J.; Davies, N.; Myburgh, K.H.; Lecour, S. Proanthocyanidins, anthocyanins and cardiovascular diseases. Food Res. Int. 2014, 59, 41–52. [Google Scholar] [CrossRef]
- Ponzo, V.; Goitre, I.; Fadda, M.; Gambino, R.; De Francesco, A.; Soldati, L.; Gentile, L.; Magistroni, P.; Cassander, M.; Bo, S. Dietary flavonoid intake and cardiovascular risk: A population-based cohort study. J. Transl. Med 2015, 13, 218. [Google Scholar] [CrossRef]
- Horbowicz, M.; Kosson, R.; Grzesiuk, A.; Debski, H. Anthocyanins of fruits and vegetables their occurrence, analysis, and role in human nutrition. Veg. Crop. Res. Bull. 2018, 68, 5–22. [Google Scholar] [CrossRef]
- Jiang, Y.; Dai, M.; Nie, W.J.; Yang, X.R.; Zeng, X.C. Effects of the ethanol extract of black mulberry (Morus nigra L.) fruit on experimental atherosclerosis in rats. J. Ethnopharmacol. 2017, 200, 228–235. [Google Scholar] [CrossRef] [PubMed]
- Goyal, M.R.; Suleria, H.A.R. Human Health Benefits of Plant Bioactive Compounds. Potentials and Prospects; Apple Academic Press: Oakville, ON, Canada, 2020; p. 75. [Google Scholar]
- Wu, X.; Beecher, G.R.; Holden, J.M.; Haytowitz, D.B.; Gebhardt, S.E.; Prior, R.L. Concentrations of anthocyanins in common foods in the United States and estimation of normal consumption. J. Agric. Food Chem. 2006, 54, 4069–4075. [Google Scholar] [CrossRef]
- Watson, R.R.; Preedy, V.R.; Zibadi, S. Polyphenols: Mechanisms of Action in Human Health and Disease, 2nd ed.; Elsevier Academic Press: London, UK, 2018; pp. 84–86. [Google Scholar]
- Carmona, L.; Alquézar, B.; Tárraga, S.; Peña, L. Protein analysis of moro blood orange pulp during storage at low temperatures. Food Chem. 2019, 277, 75–83. [Google Scholar] [CrossRef]
- Miller, K.; Feucht, W.; Schmid, M. Bioactive Compounds of Strawberry and Blueberry and Their Potential Health Effects Based on Human Intervention Studies: A Brief Overview. Nutrients 2019, 11, 1510. [Google Scholar] [CrossRef]
- David, L.; Danciu, V.; Moldovan, B.; Filip, A. Effects of In Vitro Gastrointestinal Digestion on the Antioxidant Capacity and Anthocyanin Content of Cornelian Cherry Fruit Extract. Antioxidants 2019, 8, 114. [Google Scholar] [CrossRef]
- Peng, Y.; Lin-Wang, K.; Cooney, J.M.; Wang, T.; Espley, R.V.; Allan, A.C. Differential regulation of the anthocyanin profile in purple kiwifruit (Actinidia species). Hortic. Res. 2019, 6, 3. [Google Scholar] [CrossRef] [PubMed]
- Blando, F.; Albano, C.; Liu, Y.; Nicoletti, I.; Corradini, D.; Tommasi, N.; Gerardi, C.; Mita, G.; Kitts, D.D. Polyphenolic com-position and antioxidant activity of the under-utilised Prunus mahaleb L. fruit. J. Sci. Food Agric. 2016, 96, 2641–2649. [Google Scholar] [CrossRef] [PubMed]
- Lao, F.; Giusti, M.M. Quantification of Purple Corn (Zea mays L.) Anthocyanins Using Spectrophotometric and HPLC Ap-proaches: Method Comparison and Correlation. Quantification of Purple Corn (Zea mays L.) Anthocyanins Using Spectro-photometric and HPLC Approaches: Method Comparison and Correlation. Food Anal. Methods 2016, 9, 1367–1380. [Google Scholar]
- Kwon, S.H.; Ahn, I.S.; Kim, S.O.; Kong, C.S.; Chung, H.Y.; Do, M.S.; Park, K.Y. Anti-obesity and hypolipidemic effects of black soybean anthocyanins. J. Med. Food. 2007, 10, 552–556. [Google Scholar] [CrossRef]
- Liu, C.; Sun, J.; Lu, Y.; Bo, Y. Effects of Anthocyanin on Serum Lipids in Dyslipidemia Patients: A Systematic Review and Meta-Analysis. PLoS ONE 2016, 11, e0162089. [Google Scholar] [CrossRef]
- Akhtar, S.; Rauf, A.; Imran, M.; Qamar, M.; Riaz, M.; Mubarak, M.S. Black carrot (Daucus corota L.), dietary and health promoting perspectives of its polyphenols: A review. Trends. Food Sci. Technol. 2017, 66, 36–47. [Google Scholar] [CrossRef]
- Olivas-Aguirre, F.J.; Rodrigo-Garcia, J.; Martinez-Ruiz, N.D.; Cárdenas-Robles, A.I.; Mendoza-Díaz, S.O.; Álvarez-Parrilla, E.; González-Aguilar, G.A.; de la Rosa, L.A.; Ramos-Jiménez, A.; Wall-Medrano, A.; et al. Cyanidin-3-O-glucoside: Physi-cal-Chemistry, Foodomics and Health Effects. Molecules 2016, 21, 1264. [Google Scholar] [CrossRef]
- Kato, M.; Tani, T.; Terahara, N.; Tsuda, T. The Anthocyanin Delphinidin 3-Rutinoside Stimulates Glucagon-Like Peptide-1 Secretion in Murine GLUTag Cell Line via the Ca2+/Calmodulin-Dependent Kinase II Pathway. PLoS ONE 2015, 10, e0126157. [Google Scholar] [CrossRef]
- Ruel, G.; Pomerleau, S.; Couture, P.; Lemieux, S.; Lamarche, B.; Couillard, C. Favourable impact of low-calorie cranberry juice consumption on plasma HDL-cholesterol concentrations in men. Br. J. Nutr. 2006, 96, 357–364. [Google Scholar] [CrossRef] [PubMed]
- Dohadwala, M.M.; Holbrook, M.; Hamburg, N.M.; Shenouda, S.M.; Chung, W.B.; Titas, M.; Kluge, M.A.; Wang, N.; Palmisano, J.; Milbury, P.E.; et al. Effects of cranberry juice consumption on vascular function in patients with coronary artery disease. Am. J. Clin. Nutr. 2011, 93, 934–940. [Google Scholar] [CrossRef] [PubMed]
- Alvarez-Suarez, J.M.; Giampieri, F.; Tulipani, S.; Casoli, T.; Di Stefano, G.; González-Paramás, A.M.; Santos-Buelga, C.; Busco, F.; Quiles, J.L.; Cordero, M.D.; et al. One-month strawberry-rich anthocyanin supplementation ameliorates cardiovascular risk, oxidative stress markers and platelet activation in humans. J. Nutr. Biochem. 2014, 25, 289–294. [Google Scholar] [CrossRef] [PubMed]
- Millar, C.L.; Duclos, Q.; Blesso, C.N. Effects of Dietary Flavonoids on Reverse Cholesterol Transport, HDL Metabolism, and HDL Function. Adv. Nutr. 2017, 8, 226–239. [Google Scholar] [CrossRef] [PubMed]
- Bertoia, M.L.; Rimm, E.B.; Mukamal, K.J.; Hu, F.B.; Willett, W.C.; Cassidy, A. Dietary flavonoid intake and weight maintenance: Three prospective cohorts of 124,086 US men and women followed for up to 24 years. BMJ 2016, 352, 17. [Google Scholar] [CrossRef]
- Park, C.H.; Kim, J.H.; Lee, E.B.; Hur, W.; Kwon, O.J.; Yoon, S.K.; Park, H.J. Aronia melanocarpa Extract Ameliorates Hepatic Lipid Metabolism through PPARγ2 Downregulation. PLoS ONE 2017, 12, e0169685. [Google Scholar] [CrossRef] [PubMed]
- Tsuda, T.; Ueno, Y.; Kojo, H.; Yoshikawa, T.; Osawa, T. Gene expression profile of isolated rat adipocytes treated with an-thocyanins. BiochimBiophys Acta 2005, 1733, 137–147. [Google Scholar]
- Al-Awwadi, N.A.; Araiz, C.; Bornet, A.; Delbosc, S.; Cristol, J.P.; Linck, N.; Azay, J.; Teissedre, P.L.; Cros, G. Extracts enriched in different polyphenolic families normalize increased cardiac NADPH oxidase expression while having differential effects on insulin resistance, hypertension, and cardiac hypertrophy in high-fructose-fed rats. J. Agric. Food Chem. 2005, 53, 151–157. [Google Scholar] [CrossRef]
- Akkarachiyasit, S.; Charoenlertkul, P.; Yibchok-anun, S.; Adisakwattana, S. Inhibitory activities of cyanidin and its glycosides and synergistic effect with acarbose against intestinal α-glucosidase and pancreatic α-amylase. Int. J. Mol. Sci. 2010, 11, 3387–3396. [Google Scholar] [CrossRef] [PubMed]
- Badshah, H.; Ullah, I.; Kim, S.E.; Kim, T.H.; Lee, H.Y.; Kim, M.O. Anthocyanins attenuate body weight gain via modulating neuropeptide Y and GABAB1 receptor in rats hypothalamus. Neuropeptides 2013, 47, 347–353. [Google Scholar] [CrossRef] [PubMed]
- Ghattamaneni, N.K.; Sharma, A.; Panchal, S.K.; Brown, L. Pelargonidin 3-glucoside-enriched strawberry attenuates symptoms of DSS-induced inflammatory bowel disease and diet-induced metabolic syndrome in rats. Eur. J. Nutr. 2020, 59, 2905–2918. [Google Scholar] [CrossRef]
- Molonia, M.S.; Occhiuto, C.; Muscarà, C.; Speciale, A.; Bashllari, R.; Villarroya, F.; Saija, A.; Cimino, F.; Cristani, M. Cya-nidin-3-O-glucoside restores insulinsignaling and reduces inflammation in hypertrophic adipocytes. Arch. Biochem. Biophys. 2020, 691, 108488. [Google Scholar] [CrossRef] [PubMed]
- Tsuda, T.; Ueno, Y.; Yoshikawa, T.; Kojo, H.; Osawa, T. Micoarray profiling of gene expression in human adipocytes in response to anthocyanins. Biochem. Pharmacol. 2006, 71, 1184–1197. [Google Scholar] [CrossRef]
- Zhu, Y.; Huang, X.; Zhang, Y.; Wang, Y.; Liu, Y.; Sun, R.; Xia, M. Anthocyanin supplementation improves HDL-associated paraoxonase 1 activity and enhances cholesterol efflux capacity in subjects with hypercholesterolemia. J. Clin. Endocrinol. Metab. 2014, 99, 561–569. [Google Scholar] [CrossRef]
- Fallah, A.A.; Sarmast, E.; Fatehi, P.; Jafari, T. Impact of dietary anthocyanins on systemic and vascular inflammation: Sys-tematic review and meta-analysis on randomised clinical trials. Food Chem. Toxicol. 2020, 135, 110922. [Google Scholar] [CrossRef]
- Youdim, K.A.; Martin, A.; Joseph, J.A. Incorporation of the elderberry anthocyanins by endothelial cells increases protection against oxidative stress. Free Radic. Biol. Med. 2000, 29, 51–60. [Google Scholar] [CrossRef]
- Iwasaki-Kurashige, K.; Loyaga-Rendon, R.Y.; Matsumoto, H.; Tokunaga, T.; Azuma, H. Possible mediators involved in de-creasing peripheral vascular resistance with blackcurrant concentrate (BC) in hind-limb perfusion model of the rat. Vascul. Pharmacol. 2006, 44, 215–223. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Xia, M.; Yang, Y.; Liu, F.; Li, Z.; Hao, Y.; Mi, M.; Jinm, T.; Ling, W. Purified anthocyanin supplementation improves endothelial function via NO-cGMP activation in hypercholesterolemic individuals. Clin. Chem. 2011, 57, 1524–1533. [Google Scholar] [CrossRef] [PubMed]
- Watson, R.R.; Schönlau, F. Nutraceutical and antioxidant effects of a delphinidin-rich maqui berry extract Delphinol®: A review. Minerva Cardioangiol. 2015, 63 (Suppl. S1), 1–12. [Google Scholar]
- Ziberna, L.; Tramer, F.; Moze, S.; Vrhovsek, U.; Mattivi, F.; Passamonti, S. Transport and bioactivity of cyanidin 3-glucoside into the vascular endothelium. Free Radic. Biol. Med. 2012, 52, 1750–1759. [Google Scholar] [CrossRef]
- Aboonabi, A.; Singh, I. Chemopreventive role of anthocyanins in atherosclerosis via activation of Nrf2-ARE as an indicator and modulator of redox. Biomed. Pharmacother. 2015, 72, 30–36. [Google Scholar] [CrossRef]
- Karlsen, A.; Retterstøl, L.; Laake, P.; Paur, I.; Bøhn, S.K.; Sandvik, L.; Blomhoff, R. Anthocyanins inhibit nuclear factor-kappaB activation in monocytes and reduce plasma concentrations of pro-inflammatory mediators in healthy adults. J. Nutr. 2007, 137, 1951–1954. [Google Scholar] [CrossRef] [PubMed]
- Del Bo’, C.; Marino, M.; Riso, P.; Møller, P.; Porrini, M. Anthocyanins and metabolites resolve TNF-α-mediated production of E-selectin and adhesion of monocytes to endothelial cells. Chem. Biol. Interact. 2019, 300, 49–55. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Alonso, M.; Minihane, A.M.; Rimbach, G.; Rivas-Gonzalo, J.C.; de Pascual-Teresa, S. Red wine anthocyanins are rapidly absorbed in humans and affect monocyte chemoattractant protein 1 levels and antioxidant capacity of plasma. J. Nutr. Biochem. 2009, 20, 521–529. [Google Scholar] [CrossRef] [PubMed]
- Adhikari, D.P.; Francis, J.A.; Schutzki, R.E.; Chandra, A.; Nair, M.G. Quantification and characterisation of cyclo-oxygenase and lipid peroxidation inhibitory anthocyanins in fruits of Amelanchier. Phytochem. Anal. 2005, 16, 175–180. [Google Scholar] [CrossRef] [PubMed]
- Valenza, A.; Bonfanti, C.; Pasini, M.E.; Bellosta, P. Anthocyanins Function as Anti-Inflammatory Agents in a Drosophila Model for Adipose Tissue Macrophage Infiltration. Biomed. Res. Int. 2018, 2018, 6413172. [Google Scholar] [CrossRef] [PubMed]
- Migliorini, A.A.; Piroski, C.S.; Daniel, T.G.; Cruz, T.M.; Escher, G.B.; Vieira do Carmo, M.A.; Azevedo, L.; Marques, M.B.; Granato, D.; Rosso, N.D. Red Chicory (Cichorium intybus) Extract Rich in Anthocyanins: Chemical Stability, Antioxidant Activity, and Antiproliferative Activity In Vitro. J. Food Sci. 2019, 84, 990–1001. [Google Scholar] [CrossRef]
- Chiang, A.; Wu, H.; Yeh, H.; Chu, C.S.; Lin, H.C.; Lee, W.C. Antioxidant effects of black rice extract through the induction of superoxide dismutase and catalase activities. Lipids 2006, 41, 797–803. [Google Scholar] [CrossRef]
- Aybastier, O.; Dawbaa, S.; Demir, C. Investigation of antioxidant ability of grape seeds extract to prevent oxidatively induced DNA damage by gas cromatography-tandem mass spectrometry. J. Chroamtogr. B Anal. Technol. Biomed. Life. Sci. 2018, 1072, 328–335. [Google Scholar] [CrossRef]
- Pantan, R.; Tocharus, J.; Suksamrarn, A.; Tocharus, C. Synergistic effect of atorvastatin and Cyanidin-3-glucoside on angio-tensin II-induced inflammation in vascular smooth muscle cells. Exp. Cell Res. 2016, 342, 104–112. [Google Scholar] [CrossRef]
- Bachmair, E.M.; Ostertag, L.M.; Zhang, X.; de Roos, B. Dietary manipulation of platelet function. Pharmacol. Ther. 2014, 144, 97–113. [Google Scholar] [CrossRef]
- Ojeda, D.; Jiménez-Ferrer, E.; Zamilpa, A.; Herrera-Arellano, A.; Tortoriello, J.; Alvarez, L. Inhibition of angiotensin con-vertin enzyme (ACE) activity by the anthocyanins delphinidin- and cyanidin-3-O-sambubiosides from Hibiscus sabdariffa. J. Ethnopharmacol. 2010, 127, 7–10. [Google Scholar] [CrossRef] [PubMed]
- Boslett, J.; Hermann, C.; Zhao, Y.J.; Lee, H.C.; Zweier, J.L. Luteolinidin protects the postischemic heart through CD38 inhibition with preservation of NAD(P)(H). J. Pharmacol. Exp. Ther. 2017, 361, 99–108. [Google Scholar] [CrossRef]
- Caraba, A.; Crişan, V.; Romoşan, I.; Mozoş, I.; Murariu, M. Vitamin D Status, Disease Activity, and Endothelial Dysfunction in Early Rheumatoid Arthritis Patients. Dis. Markers 2017, 2017, 5241012. [Google Scholar] [CrossRef] [PubMed]
- Tibaut, M.; Caprnda, M.; Kubatka, P.; Sinkovič, A.; Valentova, V.; Filipova, S.; Gazdikova, K.; Gaspar, L.; Mozos, I.; Egom, E.E.; et al. Markers of Atherosclerosis: Part 2—Genetic and Imaging Markers. Heart Lung Circ. 2019, 28, 678–689. [Google Scholar] [CrossRef] [PubMed]
- Johnson, S.A.; Figueroa, A.; Navaei, N.; Wong, A.; Kalfon, R.; Ormsbee, L.T.; Feresin, R.G.; Elam, M.L.; Hooshmand, S.; Payton, M.E.; et al. Daily blueberry consumption improves blood pressure and arterial stiffness in postmenopausal women with pre- and stage 1-hypertension: A randomized, double-blind, placebo-controlled clinical trial. J. Acad. Nutr. Diet. 2015, 115, 369–377. [Google Scholar] [CrossRef] [PubMed]
- Tabart, J.; Auger, C.; Kevers, C.; Dommes, J.; Pollet, B.; Defraigne, J.O.; Schini-Kerth, V.B.; Pincemail, J. The potency of commercial blackcurrant juices to induce relaxation in porcine coronary artery rings is not correlated to their antioxidant capacity but to their anthocyanin content. Nutrition 2018, 51–52, 53–59. [Google Scholar] [CrossRef]
- Horie, K.; Nanashima, N.; Maeda, H. Phytoestrogenic Effects of Blackcurrant Anthocyanins Increased Endothelial Nitric Oxide Synthase (eNOS) Expression in Human Endothelial Cells and Ovariectomized Rats. Molecules 2019, 24, 1259. [Google Scholar] [CrossRef]
- Bharat, D.; Cavalcanti, R.R.M.; Petersen, C.; Begaye, N.; Cutler, B.R.; Costa, M.M.A.; Ramos, R.K.L.G.; Ferreira, M.R.; Li, Y.; Bharath, L.P.; et al. Blueberry metabolites attenuate lipotoxicity-induced endothelial dysfunction. Mol. Nutr. Food Res. 2018, 62. [Google Scholar] [CrossRef]
- Thandapilly, S.J.; LeMaistre, J.L.; Louis, X.L.; Anderson, C.M.; Netticadan, T.; Anderson, H.D. Vascular and cardiac effects of grape powder in the spontaneously hypertensive rat. Am. J. Hypertens. 2012, 25, 1070–1076. [Google Scholar] [CrossRef] [PubMed]
- Milbury, P.E.; Vita, J.A.; Blumberg, J.B. Anthocyanins are bioavailable in humans following an acute dose of cranberry juice. J. Nutr. 2010, 140, 1099–1104. [Google Scholar] [CrossRef] [PubMed]
- Fairlie-Jones, L.; Davison, K.; Fromentin, E.; Hill, A.M. The Effect of Anthocyanin-Rich Foods or Extracts on Vascular Function in Adults: A Systematic Review and Meta-Analysis of Randomised Controlled Trials. Nutrients 2017, 9, 908. [Google Scholar] [CrossRef]
- Nanashima, N.; Horie, K.; Maeda, H.; Tomisawa, T.; Kitajima, M.; Nakamura, T. Blackcurrant Anthocyanins Increase the Levels of Collagen, Elastin, and Hyaluronic Acid in Human Skin Fibroblasts and Ovariectomized Rats. Nutrients 2018, 10, 495. [Google Scholar] [CrossRef]
- Rodriguez-Mateos, A.; Istas, G.; Boschek, L.; Feliciano, R.P.; Mills, C.E.; Boby, C.; Gomez-Alonso, S.; Milenkovic, D.; Heiss, C. Circulating Anthocyanin Metabolites Mediate Vascular Benefits of Blueberries: Insights from Randomized Controlled Trials, Metabolomics, and Nutrigenomics. J. Gerontol. A Biol. Sci. Med. Sci. 2019, 74, 967–976. [Google Scholar] [CrossRef]
- Zhu, Y.; Ling, W.; Guo, H.; Song, F.; Ye, Q.; Zou, T.; Li, D.; Zhang, Y.; Li, G.; Xiao, Y.; et al. Anti-inflammatory effect of purified dietary anthocyanin in adults with hypercholesterolemia: A randomized controlled trial. Nutr. Metab. Cardiovasc. Dis. 2013, 23, 843–849. [Google Scholar] [CrossRef]
- Min, H.K.; Kim, S.M.; Baek, S.Y.; Woo, J.W.; Park, J.S.; Cho, M.L.; Lee, J.; Kwok, S.K.; Kim, S.W.; Park, S.H.; et al. Anthocyanin extracted from black soybean seedcoats prevents autoimmune arthritis by suppressing the development of Th17 cells and synthesis of proinflammatory cytokines by such cells, via inhibition of NF-kB. PLoS ONE 2015, 10, e0138201. [Google Scholar] [CrossRef] [PubMed]
- Kuntz, S.; Asseburg, H.; Dold, S.; Rompp, A.; Frohling, B.; Kunz, C.; Rudloff, S. Inhibition of low-grade inflammation by anthocyanins from grape extract in an in vitro epithelial-endothelial co-culture model. Food Funct. 2015, 6, 1136–1149. [Google Scholar] [CrossRef] [PubMed]
- Del Bo, C.; Roursgaard, M.; Porrini, M.; Loft, S.; Moller, P.; Riso, P. Different effects of anthocyanins and phenolic acids from wild blueberry (Vaccinium angustifolium) on monocytes adhesion to endothelial cells in a TNF-alpha stimulated pro-inflammatory environment. Mol. Nutr. Food Res. 2016, 60, 2355–2366. [Google Scholar] [CrossRef]
- Blando, F.; Calabriso, N.; Berland, H.; Maiorano, G.; Gerardi, C.; Carluccio, M.A.; Andersen, Ø.M. Radical Scavenging and Anti-Inflammatory Activities of Representative Anthocyanin Groupings from Pigment-Rich Fruits and Vegetables. Int. J. Mol. Sci. 2018, 19, 169. [Google Scholar] [CrossRef] [PubMed]
- Aboonabi, A.; Meyer, R.R.; Gaiz, A.; Singh, I. Anthocyanins in berries exhibited anti-atherogenicity and antiplatelet activities in a metabolic syndrome population. Nutr. Res. 2020, 76, 82–93. [Google Scholar] [CrossRef] [PubMed]
- Broncel, M.; Kozirog, M.; Duchnowicz, P.; Koter-Michalak, M.; Sikora, J.; Chojnowska-Jezierska, J. Aronia melanocarpa extract reduces blood pressure, serum endothelin, lipid, and oxidative stress marker levels in patients with metabolic syndrome. Med. Sci. Monit. 2010, 16, CR28–CR34. [Google Scholar] [PubMed]
- Tibaut, M.; Caprnda, M.; Kubatka, P.; Sinkovič, A.; Valentova, V.; Filipova, S.; Gazdikova, K.; Gaspar, L.; Mozos, I.; Egom, E.E.; et al. Markers of Atherosclerosis: Part 1—Serological Markers. Hearth Lung Circ. 2019, 28, 667–677. [Google Scholar] [CrossRef]
- Farhangi, A.M.; Najafi, M. Empirically developed dietary inflammatory potential (EDIP) in patients candidate for coronary artery bypass grafting surgery (CABG): Association with metabolic parameters, dietary antioxidant quality score and dietary phytochemical index. PLoS ONE 2018, 13, e0208711. [Google Scholar] [CrossRef]
- Dumitrescu, C.; Biondi, R.; Xia, Y.; Cardounel, A.J.; Druhan, L.J.; Ambrosio, G.; Zweier, J.L. Myocardial ischemia results in tetrahydrobiopterin (BH4) oxidation with impaired endothelial function ameliorated by BHP. Proc. Natl. Acad. Sci. USA 2007, 104, 15081–15086. [Google Scholar] [CrossRef]
- Safaeian, L.; Emami, R.; Hajhashemi, V.; Haghighatian, Z. Antihypertensive and antioxidant effects of protocatechuic acid in deoxycorticosterone acetate-salt hypertensive rats. Biomed. Pharmacother. 2018, 100, 147–155. [Google Scholar] [CrossRef]
- Barajas, B.; Che, N.; Yin, F.; Rowshanrad, A.; Orozco, L.D.; Gong, K.W.; Wang, X.; Castellani, L.W.; Reue, K.; Lusis, A.J.; et al. NF-E2-related factor 2 promotes atherosclerosis by effects on plasma lipoproteins and cholesterol transport that overshadow antioxidant protection. Arterioscler. Thromb. Vasc. Biol. 2011, 31, 58–66. [Google Scholar] [CrossRef]
- McAnulty, L.S.; Collier, S.R.; Landram, M.J.; Whittaker, D.S.; Isaacs, S.E.; Klemka, J.M.; Cheek, S.L.; Arms, J.C.; McAnulty, S.R. Six weeks ingestion of whole blueberry powder increases natural killer cell counts and reduces arterial stiffness in sedentary males and females. Nutr. Res. 2014, 34, 577–584. [Google Scholar] [CrossRef]
- Faria, A.; Fernandes, I.; Norberto, S.; Mateus, N.; Calhau, C. Interplay between anthocyanins and gut microbiota. J. Agric. Food. Chem. 2014, 62, 6898–6902. [Google Scholar] [CrossRef] [PubMed]
- Basile, T.; Alba, V.; Gentilesco, G.; Savino, M.; Tarricone, L. Anthocyanins pattern variation in relation to thinning and girdling in commercial Sugrathirteen® table grape. Sci. Hortic. 2018, 227, 202–206. [Google Scholar] [CrossRef]
- Li, Y.; Fang, J.; Qi, X.; Lin, M.; Zhong, Y.; Sun, L. A key structural gene, AaLDOX, is involved in anthocyanin biosynthesis in all red-fleshed kiwifruit (Actinidia arguta) based on transcriptome analysis. Gene 2018, 648, 31–41. [Google Scholar] [CrossRef]
- Mauray, A.; Felgines, C.; Morand, C.; Mazur, A.; Scalbert, A.; Milenkovic, D. Bilberry anthocyanin-rich extract alters expression of genes related to atherosclerosis development in aorta of apo E-deficient mice. Nutr. Metab. Cardiovasc Dis. 2012, 22, 72–80. [Google Scholar] [CrossRef]
- Jennings, A.; Welch, A.A.; Fairweather-Tait, S.J.; Kay, C.; Minihane, A.M.; Chowienczyk, P.; Jiang, B.; Cecelja, M.; Spector, T.; Macgregor, A.; et al. Higher anthocyanin intake is associated with lower arterial stiffness and central blood pressure in women. Am. J. Clin. Nutr. 2012, 96, 781–788. [Google Scholar] [CrossRef] [PubMed]
- Aviram, M.; Rosenblat, M.; Gaitini, D.; Nitecki, S.; Hoffman, A.; Dornfeld, L.; Volkova, N.; Presser, D.; Attias, J.; Liker, H.; et al. Pomegranate juice consumption for 3 years by patients with carotid artery stenosis reduces common carotid intima-media thickness, blood pressure and LDL oxidation. Clin. Nutr. 2004, 23, 423–433. [Google Scholar] [CrossRef]
- Poreba, R.; Skoczynska, A.; Gac, P.; Poreba, M.; Jedrychowska, I.; Affelska-Jercha, A.; Turczyn, B.; Wojakowska, A.; Oszmianski, J.; Andrzejak, R. Drinking of chockeberryjuice from the ecological farm Dzieciolowo and distensibility of brahial artery in men with mild hypercholesterolemia. Ann. Agric. Environ. Med. 2009, 16, 305–308. [Google Scholar] [PubMed]
- Matsumoto, H.; Takenami, E.; Iwasaki-Kurashige, K.; Osada, T.; Katsumura, T.; Hamaoka, T. Effects of blackcurrant antho-cyanin intake on peripheral muscle circulation during typing work in humans. Eur. J. Appl. Physiol. 2005, 94, 36–45. [Google Scholar] [CrossRef]
- Li, L.; Lyall, G.K.; Martinez-Blazquez, J.A.; Vallejo, F.A.; Tomas-Barberan, F.; Birch, K.M.; Boesch, C. Blood Orange Juice Consumption Increases Flow-Mediated Dilation in Adults with Overweight and Obesity: A Randomized Controlled Trial. J. Nutr. 2020, 150, 2287–2294. [Google Scholar] [CrossRef] [PubMed]
- Curtis, P.J.; van der Velpen, V.; Berends, L.; Jennings, A.; Feelisch, M.; Umpleby, A.M.; Evans, M.; Fernandez, B.O.; Meiss, M.S.; Minnion, M.; et al. Blueberries improve biomarkers of cardiometabolic function in participants with metabolic syn-drome-results from a 6-month, double-blind, randomized controlled trial. Am. J. Clin. Nutr. 2019, 109, 1535–1545. [Google Scholar] [CrossRef]
- Mozos, I.; Maidana, J.P.; Stoian, D.; Stehlik, M. Gender Differences of Arterial Stiffness and Arterial Age in Smokers. Int. J. Environ. Res. Public Health 2017, 14, 565. [Google Scholar] [CrossRef]
- Tomisawa, T.; Nanashima, N.; Kitajima, M.; Mikami, K.; Takamagi, S.; Maeda, H.; Horie, K.; Lai, F.C.; Osanai, T. Effects of Blackcurrant Anthocyanin on Endothelial Function and Peripheral Temperature in Young Smokers. Molecules 2019, 24, 4295. [Google Scholar] [CrossRef]
- Balestra, C.; Cimino, F.; Theunissen, S.; Snoeck, T.; Provyn, S.; Canali, R.; Bonina, A.; Virgili, F. A red orange extract modulates the vascular response to a recreational dive: A pilot study on the effect of anthocyanins on the physiological consequences of scuba diving. Nat. Prod. Res. 2016, 30, 2101–2106. [Google Scholar] [CrossRef]
- Pekas, E.J.; Shin, J.; Headid, R.J.; Son, W.M.; Layec, G.; Yadav, S.K.; Scott, S.D.; Park, S.Y. Combined anthocyanins and bromelain supplement improves endothelial function and skeletal muscle oxygenation status in adults: A double-blind place-bo-controlled randomised crossover clinical trial. Br. J. Nutr. 2021, 125, 161–171. [Google Scholar] [CrossRef] [PubMed]
- Okamoto, T.; Hashimoto, Y.; Kobayashi, R.; Nakazato, K.; Willems, M.E.T. Effects of blackcurrant extract on arterial functions in older adults: A randomized, double-blind, placebo-controlled, crossover trial. Clin. Exp. Hypertens. 2020, 42, 640–647. [Google Scholar] [CrossRef]
- Johnson, S.A.; Navaei, N.; Pourafshar, S.; Jaime, S.J.; Akhavan, N.S.; Alvarez-Alvarado, S.; Proaño, G.V.; Litwin, N.S.; Clark, E.A.; Foley, E.M.; et al. Effects of Montmorency Tart Cherry Juice Consumption on Cardiometabolic Biomarkers in Adults with Metabolic Syndrome: A Randomized Controlled Pilot Trial. J. Med. Food 2020, 23, 1238–1247. [Google Scholar] [CrossRef] [PubMed]
- Hollands, W.J.; Armah, C.N.; Doleman, J.F.; Perez-Moral, N.; Winterbone, M.S.; Kroon, P.A. 4-Week consumption of anthocyanin-rich blood orange juice does not affect LDL-cholesterol or other biomarkers of CVD risk and glycaemia compared with standard orange juice: A randomised con-trolled trial. Br. J. Nutr. 2018, 119, 415–421. [Google Scholar] [CrossRef] [PubMed]
- Feresin, R.G.; Johnson, S.A.; Pourafshar, S.; Campbell, J.C.; Jaime, S.J.; Navaei, N.; Elam, M.L.; Akhavan, N.S.; Alva-rez-Alvarado, S.; Tenenbaum, G.; et al. Impact of daily strawberry consumption on blood pressure and arterial stiffness in pre- and stage 1-hypertensive postmenopausal women: A randomized controlled trial. Food Funct. 2017, 8, 4139–4149. [Google Scholar] [CrossRef]
- Rodriguez-Mateos, A.; Rendeiro, C.; Bergillos-Meca, T.; Tabatabaee, S.; George, T.W.; Heiss, C.; Spencer, J.P. Intake and time dependence of blueberry flavonoid-induced improvements in vascular function: A randomized, controlled, double-blind, crossover intervention study with mechanistic insights into biological activity. Am. J. Clin. Nutr. 2013, 98, 1179–1191. [Google Scholar] [CrossRef] [PubMed]
- Davidson, M.H.; Maki, K.C.; Dicklin, M.R.; Feinstein, S.B.; Witchger, M.; Bell, M.; McGuire, D.K.; Provost, J.-C.; Liker, H.; Aviram, M. Effects of consumption of pomegranate juice on carotid intima-media thickness in men and women at moderate risk for coronary heart disease. Am. J. Cardiol. 2009, 104, 936–942. [Google Scholar] [CrossRef]
- Oboh, H.A.; Agu, K. The effects of various traditional processing methods on the glycemic index and glycemic load of cowpeas (Vigna Unguiculata). J. Food Biochem. 2008, 32, 576–596. [Google Scholar] [CrossRef]
- Gerardi, C.; Albano, C.; Calabriso, N.; Carluccio, M.A.; Durante, M.; Mita, G.; Renna, M.; Serio, F.; Blando, F. Techno-functional properties of tomato puree fortified with anthocyanin pigments. Food Chem. 2018, 240, 1184–1192. [Google Scholar] [CrossRef]
- Rodriguez-Mateos, A.; Heiss, C.; Borges, G.; Crozier, A. Berry (poly) phenols and cardiovascular health. J. Agric. Food Chem. 2014, 62, 3842–3851. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, H.; Ito, K.; Yonekura, K.; Tsuda, T.; Ichiyanagi, T.; Hirayama, M.; Konishi, T. Enhanced absorption of anthocyaninsafter oral administration of phyticacid in rats and humans. J. Agric. Food Chem. 2007, 55, 2489–2496. [Google Scholar] [CrossRef] [PubMed]
- Burgos-Edwards, A.; Jiimenez-Aspee, F.; Thomas-Valdes, S.; Schmeda-Hirschmann, G.; Theoduloz, C. Qualitative and quan-titative changes in polyphenol composition and bioactivity of Ribes magellanicum and R. punctatum after in vitro gastro-intestinal digestion. Food Chem. 2017, 237, 1073–1082. [Google Scholar] [CrossRef] [PubMed]
| Effects | Mechanisms of Action of ACYs |
|---|---|
Lipid metabolism:
|
|
| |
Carbohydrate metabolism:
|
|
| Anti-obesity: lower body weight, adipose tissue size, central adiposity, and food intake |
|
| Endothelial function | |
| Vessel wall |
|
| Anti-inflammatory | |
| Antioxidant (73.53 ± 0.13 mg per 100 g fresh weight basis sample: Cichorium intybus) [81] |
|
| Anti-atherosclerotic |
|
| Platelet function |
|
| Antihypertensive |
|
| Study Population | Anthocyanin Source | Methods | Findings, Conclusions | Ref. |
|---|---|---|---|---|
| 1898 women, 18–75 years old, from the TwinsUK registry | Validated food-frequency questionnaire | PWV, AI, central blood pressure, MAP, IMT | Consumption of 1–2 portions of berries daily reduced arterial stiffness and cardiovascular disease risk | [116] |
| 35 men with mild hypercholesterolemia | Chockeberry juice, 6 weeks regular drinking | NO, FMD, serum lipids | Regular drinking of chockeberry juice improves endothelial function and serum lipids (total and LDL cholesterol and triglycerides) in men with hypercholesterolemia. | [118] |
| 10 patients with carotid atherosclerosis | Pomegranate juice up to 3 years/control group | Common carotid IMT, blood samples | Significant IMT and SBP reduction, serum paraoxonase activity increased, LDL oxidation impaired, decreased antibodies against oxidized LDL, serum antioxidant status increased | [117] |
| Study Population | ACY/ Placebo | Methods | Findings, Conclusions | Reference |
|---|---|---|---|---|
| 18 healthy adults | combined ACYs and bromelain supplement (BE) | randomised crossover design; FMD, BP, TAC, resting heart rate, oxygen utility capacity and fatigability measured pre- and post-BE and placebo intake | BE intake is effective for improving endothelial function, BP, TAC and oxygen utility capacity | [125] |
| 14 older adults | 7-days 2X 300 mg capsule with 35% blackcurrant extract/ placebo | double-blind, placebo-controlled, crossover design study with a washout period of 28 days | ACY intake reduces carotid femoral PWV and central BP in older adults; no effects on blood lipids | [126] |
| 19 patients, 20 to 60 years old, with metabolic syndrome (MetS) | 240 mL of tart cherry juice (rich in ACYs) or an isocaloric placebo-control drink, twice daily for 12 weeks | single-blind, placebo-controlled, parallel-arm pilot clinical trial PWV, brachial and aortic BP, AI, and biomarkers of cardiovascular and metabolic health, assessed at baseline and 6 and 12 weeks | no significant changes in hemodynamics and arterial stiffness lower oxidized low-density lipoprotein, soluble vascular cell adhesion molecule-1 and total cholesterol after tart cherry juice than control | [127] |
| 15 healthy overweight and obese men and women | 200 mL blood orange juice twice daily) for 2 weeks with a washout period of 1 week | primary outcome: FMD | favorable effects on endothelial function | [120] |
| 115 participants, age 63 ± 7 years; 68% male | daily intake of 1 cup (150 g) of blueberries for 6 months | double-blind, parallel RCT; insulin resistance, FMD, AI, lipoprotein status, and NO | improvements in vascular function, lipid status, and NO bioactivity | [121] |
| 41 participants, aged 25–84 years | 500 mL blood orange juice providing 50 mg ACYs/ 500 mL blonde orange juice without ACYs for 28 days | open label, two-arm cross-over trial; total, HDL- and LDL-cholesterol, glucose, fructosamine, NO, CRP, aortic SBP and DBP or carotid-femoral and brachial-ankle PWV | No significant differences were observed between the variables measured at the start and end of each treatment period. The lack of effect may be due to the modest concentration of ACYs in the blood orange juice | [128] |
| 14 healthy male and female adults | Participants consumed 200 g/day of cooked purple potato containing 288 mg ACYs, or a white potato containing negligible ACYs for 14 days, separated by a 7-day washout period. | PWV, SBP, DBP, HDL, LDL, TG, glucose, insulin, and CRP. | PWV was significantly reduced following purple potato consumption for 14-days | [22] |
| 60 postmenopausal women with pre- and stage 1-hypertension | 8 weeks, 25 g or 50 g freeze-dried strawberry powder (FDSP) | double-blind, placebo-controlled, parallel arm clinical trial BP, arterial stiffness, superoxide dismutase (SOD) at baseline, 4 and 8 weeks | BP and arterial stiffness improved in the 25 g FDSP group | [129] |
| 13 healthy men, age: 25 ± 4 years | New Zealand blackcurrant (NZBC) extract (600 mg/day)/ placebo for 7-days separated by 14-days washout | double-blind, crossover design, Participants produced isometric maximal voluntary contractions (iMVC) and a 120-s 30%iMVC of the quadriceps: electromyography, near-infrared spectroscopy, hemodynamic and ultrasound recordings | Intake of NZBC extract impaired cardiovascular responses, muscle oxygen saturation, muscle activity and femoral artery diameter of the quadriceps and may increase exercise performance | [21] |
| 16 volunteers performing a single standard dive | 2 groups: one of them received 2x 200 mg of an ACYs-rich extract from red oranges, 12 and 4 h before diving | FMD | ACYs administration reduces the harmful endothelial effects of a recreational single dive | [124] |
| 48 postmenopausal women with pre- and stage 1 hypertension | 8-week, 22 g freeze-dried blueberry (BB) powder/control daily | double-blind, placebo-controlled clinical trial BP, PWV, CRP, NO and SOD at baseline, 4 and 8 weeks | Daily BB reduces BP and arterial stiffness, related to increase of NO production | [90] |
| 25 men and postmenopausal women, 18–50 years old | 6 weeks, 250 g BB powder/ placebo daily | BP, vascular performance testing, blood samples at baseline and after 6 weeks | BB ingestion for 6 weeks increases natural killer cells and reduces AI, SBP, DBP in sedentary males and females | [111] |
| 47 healthy adults, 30–50 years | 6 weeks, 30 mL tart cherry juice concentrate diluted with water/energy matched control drink | BP, arterial stiffness, CRP, total cholesterol, LDL, ferric reducing ability of plasma at baseline and after 6 weeks | Tart cherry juice concentrate has no effect on arterial stiffness, CRP, and cardiovascular risk markers, but increases antioxidant status | [17] |
| 21 healthy men | 766, 1278 and 1791 mg blueberry polyphenols (BBPP)/Control 319, 637, 766, 1278, 1791 mg total blueberry/ control | Double-blind, controlled, crossover trial; FMD Intake-dependence study, from baseline to 1 h | FMD increased significantly at 1–2 and 6 h after consumption of BBPP. At 1 h after consumption, FMD increased dose-dependently to up to 766 mg BBPP. The vascular benefits are linked to the circulating phenolic metabolites and activity of the neutrophil NADPH oxidase | [130] |
| 11 young, healthy male nonsmokers and 13 smokers | supplement A (50 mg of blackcurrant ACY) and supplement B (50 mg of blackcurrant anthocyanin plus vitamin E | Double-blind trial; FMD and skin temperature | Oral ACYs and Vitamin E supplementation can attenuate the smoking-induced acute endothelial dysfunction and peripheral blood flow in smokers | [123] |
| 44 patients with coronary artery disease | 480 mL of cranberry juice/placebo for 4 weeks | BP, PWV, brachial artery flow-mediated dilation, digital pulse amplitude | Chronic cranberry juice consumption reduced arterial stiffness, with only an acute benefit on endothelial vasodilator function | [56] |
| 12 patients with hypercholesterolemia 150 hypercholesterol-emic individuals | 320 mg ACYs/ placebo 320 mg ACYs/ placebo | FMD before and after the intervention FMD, cGMP | ACYs supplementation improves endothelium-dependent vasodilation in patients with hypercholesterolemia, related to activation of the NO-cGMP signaling pathway, improvement of serum lipids and an anti-inflammatory effect | [72] |
| Subjects at moderate risk for coronary heart disease | 240 mL pomegranate juice/day (n- = 146)/control bevarage (n = 143) up to 18 months | IMT | No significant effect of pomegranate juice was noticed on IMT progression rate. A slowed IMT progression was noticed in patients with increased oxidative stress and impaired TG/HDL profile | [131] |
| 9 healthy men | 17 mg kg(-1) BCA or placebo | double-blind, placebo-controlled, crossover study NIRS, improvement in shoulder stiffness plasma ACYs measured prior to ingestion and 1, 2, and 4 h later | FBF increased significantly after BCA ingestion | [119] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mozos, I.; Flangea, C.; Vlad, D.C.; Gug, C.; Mozos, C.; Stoian, D.; Luca, C.T.; Horbańczuk, J.O.; Horbańczuk, O.K.; Atanasov, A.G. Effects of Anthocyanins on Vascular Health. Biomolecules 2021, 11, 811. https://doi.org/10.3390/biom11060811
Mozos I, Flangea C, Vlad DC, Gug C, Mozos C, Stoian D, Luca CT, Horbańczuk JO, Horbańczuk OK, Atanasov AG. Effects of Anthocyanins on Vascular Health. Biomolecules. 2021; 11(6):811. https://doi.org/10.3390/biom11060811
Chicago/Turabian StyleMozos, Ioana, Corina Flangea, Daliborca C. Vlad, Cristina Gug, Costin Mozos, Dana Stoian, Constantin T. Luca, Jarosław O. Horbańczuk, Olaf K. Horbańczuk, and Atanas G. Atanasov. 2021. "Effects of Anthocyanins on Vascular Health" Biomolecules 11, no. 6: 811. https://doi.org/10.3390/biom11060811
APA StyleMozos, I., Flangea, C., Vlad, D. C., Gug, C., Mozos, C., Stoian, D., Luca, C. T., Horbańczuk, J. O., Horbańczuk, O. K., & Atanasov, A. G. (2021). Effects of Anthocyanins on Vascular Health. Biomolecules, 11(6), 811. https://doi.org/10.3390/biom11060811










