Magnetic Field Alignment, a Perspective in the Engineering of Collagen-Silica Composite Biomaterials
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of Functionalized SiNPs
2.2.1. Synthesis of nf-SiNPs
2.2.2. Synthesis of SiNP-SO3−
2.2.3. Synthesis of Amine-Modified Particles SiNP-NH2 (for Peptide Conjugation)
2.2.4. Peptide Coupling between Amine Groups on SiNPs and Peptides
2.3. Particle Characterization
2.3.1. Zeta-Potential Measurements
2.3.2. Transmission Electron Microscopy
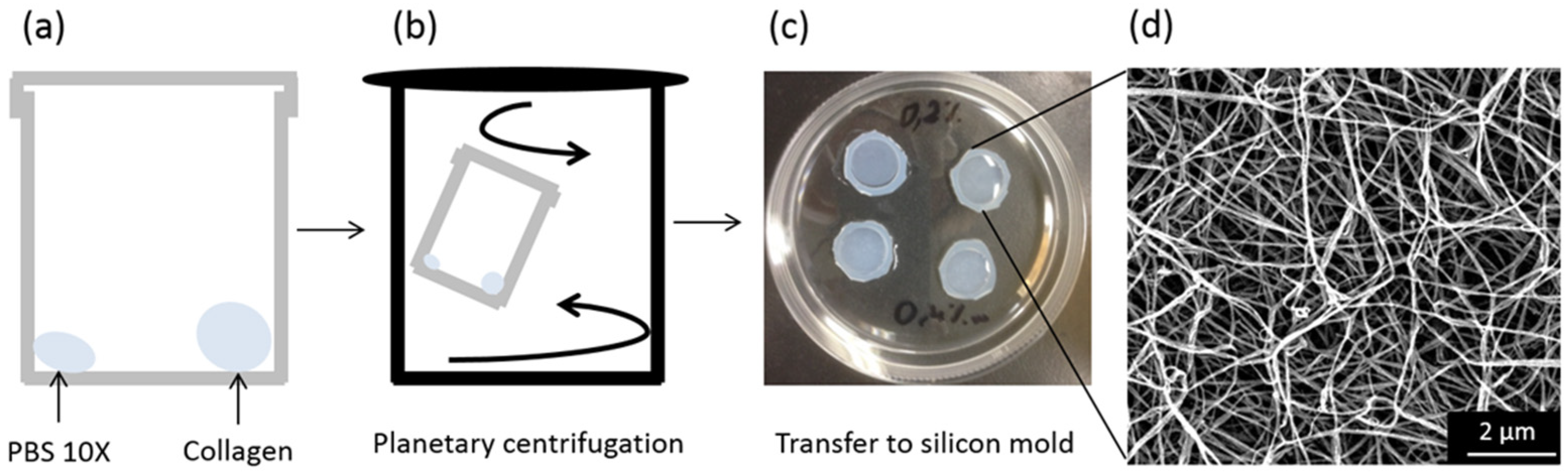
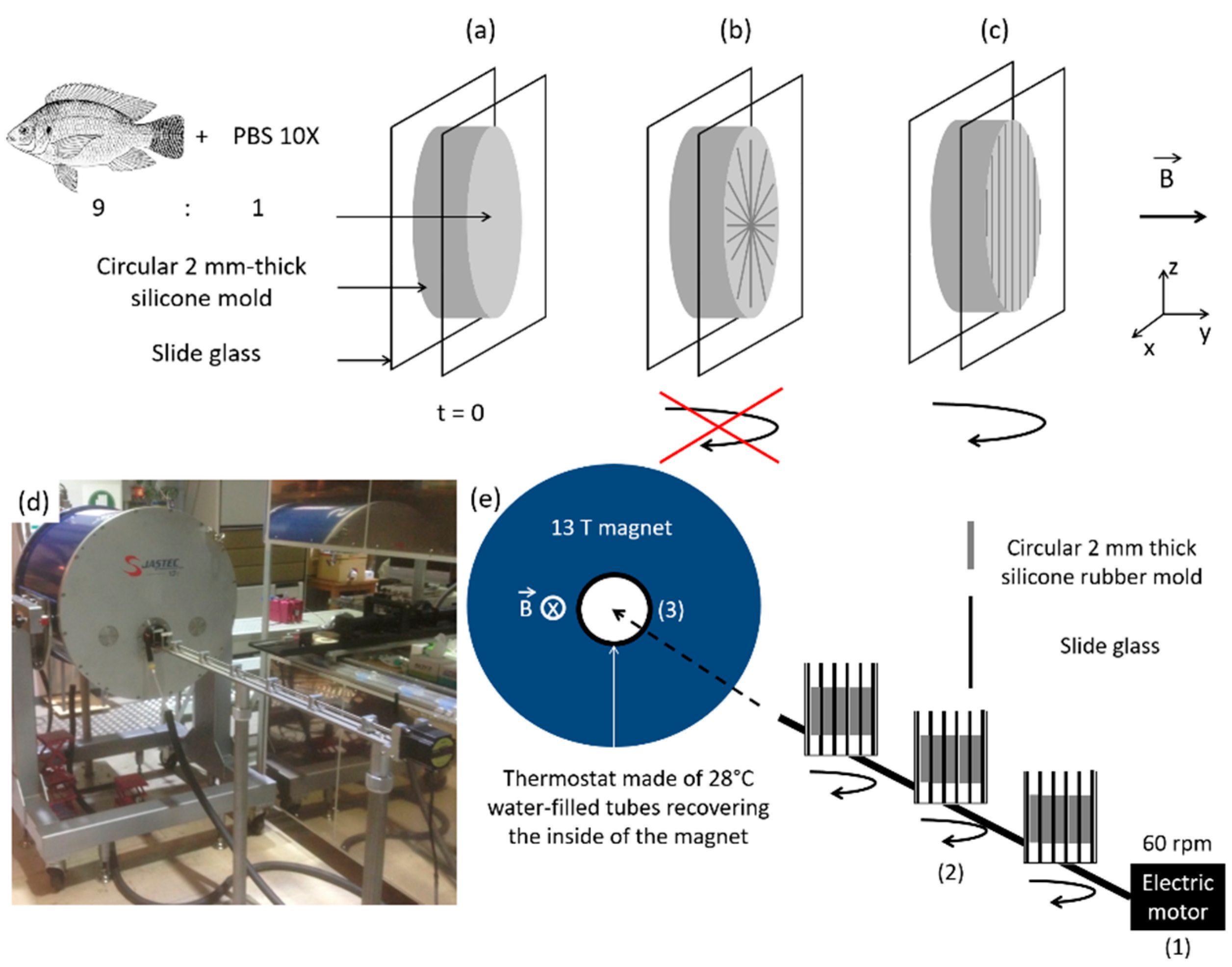
2.4. Preparation of the Bionanocomposites
2.5. Characterization of the Structural and Biological Properties of the Bionanocomposites
2.5.1. Scanning Electron Microscopy (SEM)
2.5.2. Polarized Light Microscopy (PLM)
2.5.3. Cell Culture
2.5.4. Fluorescence Microscopy
2.5.5. Statistical Analysis
3. Results
3.1. Biocomposite Engineering
3.1.1. Obtaining Self-Supported Collagen Films
3.1.2. Surface-Engineered SiNPs
3.2. Birefringence of the Composites and Collagen Alignment
3.2.1. Collagen and Composite Films in Absence of Peptides
3.2.2. Collagen: Peptide Composites
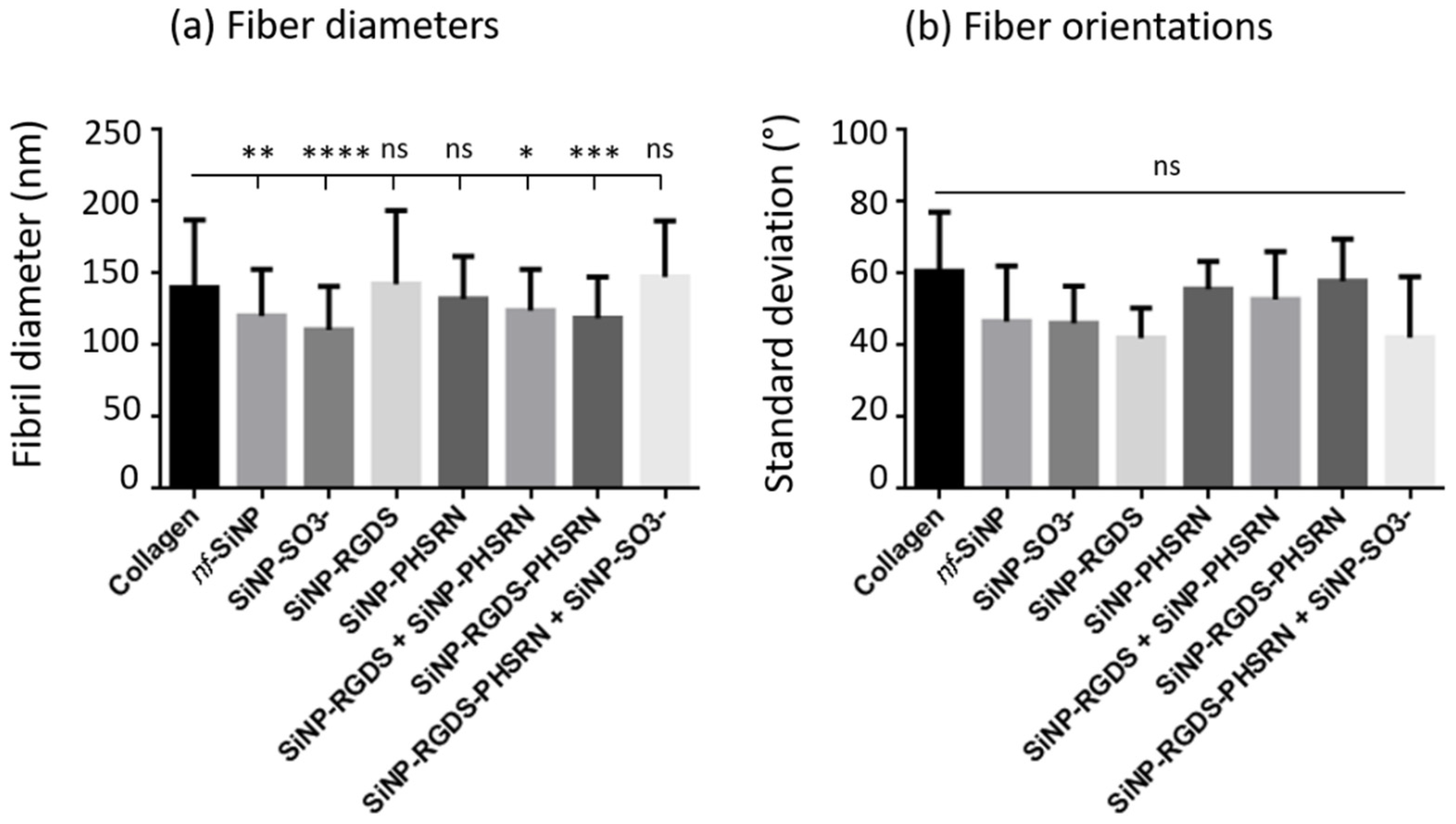
3.3. Microstructure of the Different Bionanocomposites
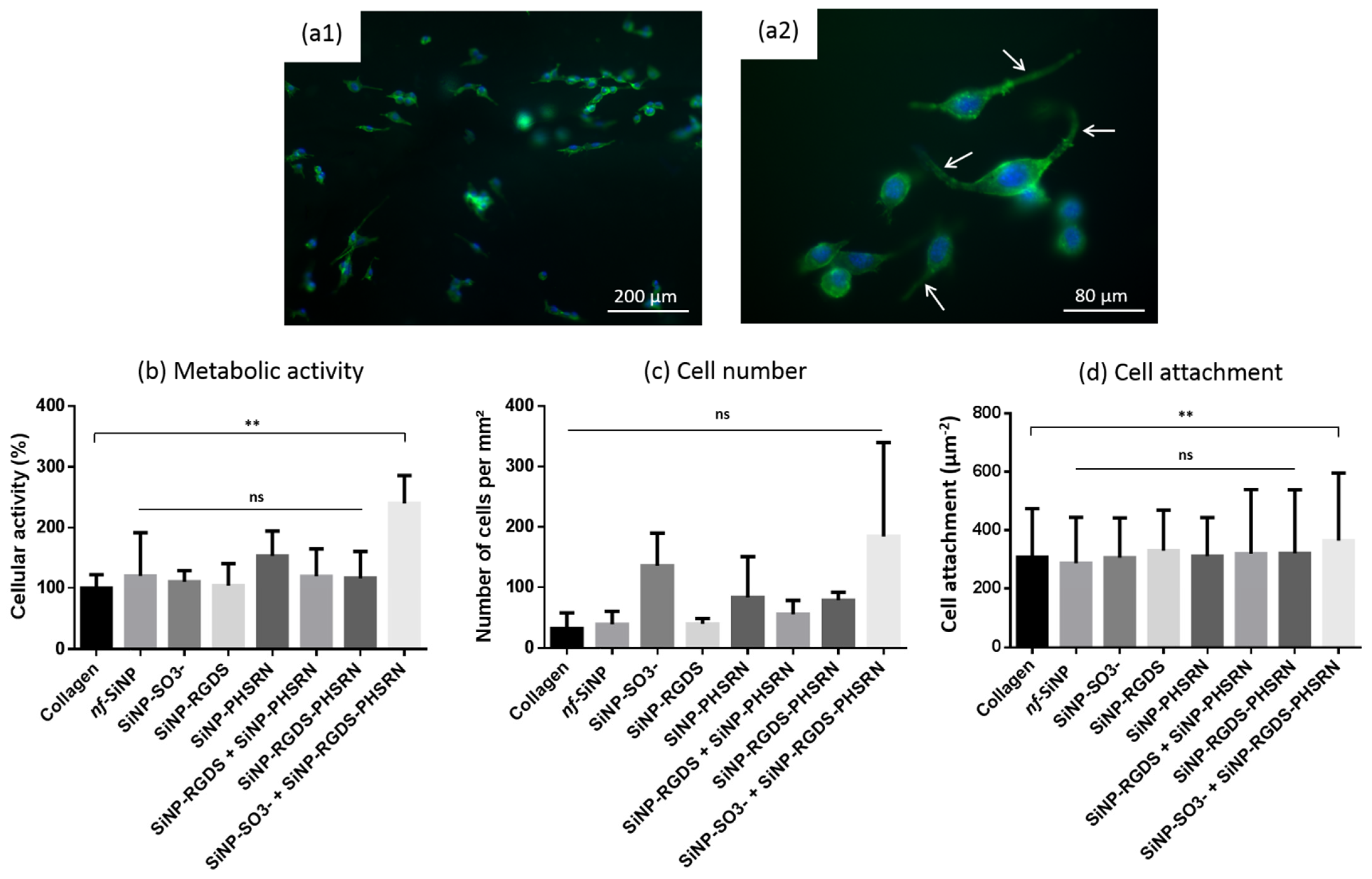
3.4. Biological Behavior
4. Discussion
- (1)
- High magnetic field can successfully be implemented on collagen-based bionanocomposites to induce a structural anisotropy at the micron and millimeter scale after addition of silica nanoparticles. Indeed, birefringence and fiber alignment were observed for most bionanocomposites investigated in this study.
- (2)
- From a biological perspective, and very importantly, all magnetically-exposed bionanocomposites were suitable for cell adhesion and spreading as no decrease in terms of metabolic activity, cell number and attachment were observed after fibroblast seeding with neither of the composites investigated compared to the pure collagen membrane.
- (3)
- From a structural point of view, the loss of birefringence of the composites can be to some extent related to the formation of SiNP clusters, as partially observed with the formation of nf-SiNP clusters, and strongly evidenced in presence of SiNP-SO3−. In the latter case, the strong interaction between sulfonate groups and collagen triple helices may prevent fiber from alignment as shown by PLM.
- (4)
- Conversely, the loss of birefringence was also observed in absence of clusters (see (collagen + SiNP-RGDS + SiNP-PHSRN) and (collagen + SiNP-RGDS-PHSRN) composites). In this case, it may be attributed to the formation of large bundles of collagen fibers evidenced by PLM, which are believed to hamper alignment at the macroscale. However, local alignment of the fibers at the micrometer scale would still be possible, explaining why no difference could be evidenced for this parameter by analysis of the SEM images.
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Aimé, C.; Coradin, T. Bionanocomposites: Integrating Biological Processes for Bioinspired Nanotechnologies, 1st ed.; Aimé, C., Coradin, T., Eds.; Wiley-VCH: Hoboken, NJ, USA, 2017. [Google Scholar]
- Aimé, C.; Coradin, T. Nanocomposites from biopolymer hydrogels: Blueprints for white biotechnology and green materials chemistry. J. Polym. Sci. Part B Polym. Phys. 2012, 50, 669–680. [Google Scholar] [CrossRef]
- Tibbitt, M.W.; Rodell, C.B.; Burdick, J.A.; Anseth, K.S. Progress in material design for biomedical applications. Proc. Natl. Acad. Sci. USA 2015, 112, 14444–14451. [Google Scholar] [CrossRef] [PubMed]
- Sorushanova, A.; Delgado, L.M.; Wu, Z.; Shologu, N.; Kshirsagar, A.; Raghunath, R.; Mullen, A.M.; Bayon, Y.; Pandit, A.; Raghunath, M.; et al. The Collagen Suprafamily: From Biosynthesis to Advanced Biomaterial Development. Adv. Mater. 2019, 31, e1801651. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Zheng, C.; Luo, X.; Wang, X.; Jiang, H. Recent advances of collagen-based biomaterials: Multi-hierarchical structure, modification and biomedical applications. Mater. Sci. Eng. C Mater. Biol. Appl. 2019, 99, 1509–1522. [Google Scholar] [CrossRef] [PubMed]
- Meyer, M. Processing of collagen based biomaterials and the resulting materials properties. Biomed. Eng. Online 2019, 18, 24. [Google Scholar] [CrossRef]
- Picaut, L.; Trichet, L.; Ronsin, O.; Haye, B.; Génois, I.; Baumberger, T.; Mosser, G. Pure dense collagen threads from extrusion to fibrillogenesis stability. Biomed. Phys. Eng. Express 2018, 4, 035008. [Google Scholar] [CrossRef]
- Lama, M.; Fernandes, F.M.; Marcellan, A.; Peltzer, J.; Trouillas, M.; Banzet, S.; Grosbot, M.; Sanchez, C.; Giraud-Guille, M.-M.; Lataillade, J.-J.; et al. Self-Assembled Collagen Microparticles by Aerosol as a Versatile Platform for Injectable Anisotropic Materials. Small 2020, 16, e1902224. [Google Scholar] [CrossRef]
- Dems, D.; Rodrigues Da Silva, J.; Hélary, C.; Wien, F.; Marchand, M.; Debons, N.; Muller, L.; Chen, Y.; Schanne-Klein, M.-C.; Laberty-Robert, C.; et al. Native collagen: Electrospinning of pure, cross-linker free self-supported membrane. ACS Appl. Bio Mater. 2020, 3, 2948–2957. [Google Scholar] [CrossRef]
- Rieu, C.; Parisi, C.; Mosser, G.; Haye, B.; Coradin, T.; Fernandes, F.M.; Trichet, L. Topotactic Fibrillogenesis of Freeze-Cast Microridged Collagen Scaffolds for 3D Cell Culture. ACS Appl. Mater. Interfaces 2019, 11, 14672–14683. [Google Scholar] [CrossRef]
- Torbet, J.; Ronziere, M.C. Magnetic alignment of collagen during self-assembly. Biochem. J. 1984, 219, 1057–1059. [Google Scholar] [CrossRef]
- Murthy, N.S. Liquid crystallinity in collagen solutions and magnetic orientation of collagen fibrils. Biopolymers 1984, 23, 1261–1267. [Google Scholar] [CrossRef]
- Chen, S.; Hirota, N.; Okuda, M.; Takeguchi, M.; Kobayashi, H.; Hanagata, N.; Ikoma, T. Microstructures and rheological properties of tilapia fish-scale collagen hydrogels with aligned fibrils fabricated under magnetic fields. Acta Biomater. 2011, 7, 644–652. [Google Scholar] [CrossRef] [PubMed]
- Novak, T.; Voytik-Harbin, S.L.; Neu, C.P. Cell encapsulation in a magnetically aligned collagen–GAG copolymer microenvironment. Acta Biomater. 2015, 11, 274–282. [Google Scholar] [CrossRef]
- Shannon, G.S.; Novak, T.; Mousoulis, C.; Voytik-Harbin, S.L.; Neu, C.P. Temperature and concentration dependent fibrillogenesis for improved magnetic alignment of collagen gels. RSC Adv. 2015, 5, 2113–2121. [Google Scholar] [CrossRef]
- Guido, S.; Tranquillo, R.T. A methodology for the systematic and quantitative study of cell contact guidance in oriented collagen gels. Correlation of fibroblast orientation and gel birefringence. J. Cell Sci. 1993, 105, 317–331. [Google Scholar] [CrossRef] [PubMed]
- Torbet, J.; Malbouyres, M.; Builles, N.; Justin, V.; Roulet, M.; Damour, O.; Oldberg, A.; Ruggiero, F.; Hulmes, D.J. Orthogonal scaffold of magnetically aligned collagen lamellae for corneal stroma reconstruction. Biomaterials 2007, 28, 4268–4276. [Google Scholar] [CrossRef]
- Kotani, H.; Iwasaka, M.; Ueno, S.; Curtis, A. Magnetic orientation of collagen and bone mixture. J. Appl. Phys. 2000, 87, 6191–6193. [Google Scholar] [CrossRef]
- Dickinson, R.B.; Guido, S.; Tranquillo, R.T. Biased cell migration of fibroblasts exhibiting contact guidance in oriented collagen gels. Ann. Biomed. Eng. 1994, 22, 342–356. [Google Scholar] [CrossRef] [PubMed]
- Kew, S.J.; Gwynne, J.H.; Enea, D.; Abu-Rub, M.; Pandit, A.; Zeugolis, D.; Brooks, R.A.; Rushton, N.; Best, S.M.; Cameron, R.E. Regeneration and repair of tendon and ligament tissue using collagen fibre biomaterials. Acta Biomater. 2011, 7, 3237–3247. [Google Scholar] [CrossRef]
- Antman-Passig, M.; Shefi, O. Remote magnetic orientation of 3D collagen hydrogels for directed neuronal regeneration. Nano Lett. 2016, 16, 2567–2573. [Google Scholar] [CrossRef]
- Han, W.; Chen, S.; Yuan, W.; Fan, Q.; Tian, J.; Wang, X.; Chen, L.; Zhang, X.; Wei, W.; Liu, R.; et al. Oriented collagen fibrils direct tumor cell intravasation. Proc. Natl. Acad. Sci. USA 2016, 113, 11208–11213. [Google Scholar] [CrossRef]
- Taufalele, P.V.; VanderBurgh, J.A.; Munoz, A.; Zanotelli, M.R.; Reinhart-King, C.A. Fibril alignment drives changes in architectural and mechanical features in collagen matrices. PLoS ONE 2019, 14, e0216537. [Google Scholar] [CrossRef] [PubMed]
- Tampieri, A.; Iafisco, M.; Sandri, M.; Panseri, S.; Cunha, C.; Sprio, S.; Savini, E.; Uhlarz, M.; Herrmannsdörfer, T. Magnetic bioinspired hybrid nanostructured collagen–hydroxyapatite scaffolds supporting cell proliferation and tuning regenerative process. ACS Appl. Mater. Interfaces 2014, 6, 15697–15707. [Google Scholar] [CrossRef] [PubMed]
- Aimé, C.; Mosser, G.; Pembouong, G.; Bouteiller, L.; Coradin, T. Controlling the nano-bio interface to build collagen/silica self-assembled networks. Nanoscale 2012, 4, 7127–7134. [Google Scholar] [CrossRef] [PubMed]
- Debons, N.; Dems, D.; Hélary, C.; Le Grill, S.; Picaut, L.; Renaud, F.; Delsuc, N.; Schanne-Klein, M.-C.; Coradin, T.; Aimé, C. Differentiation of neural-type cells on multi-scale ordered collagen-silica bionanocomposites. Biomat. Sci. 2020, 8, 569–576. [Google Scholar] [CrossRef] [PubMed]
- Aota, S.-i.; Nomizu, M.; Yamada, K.M. The short amino acid sequence Pro-His-Ser-Arg-Asn in human fibronectin enhances cell-adhesive function. J. Biol. Chem. 1994, 269, 24756–24761. [Google Scholar] [CrossRef]
- Mardon, H.J.; Grant, K.E. The role of the ninth and tenth type III domains of human fibronectin in cell adhesion. FEBS Lett. 1994, 340, 197–201. [Google Scholar] [CrossRef]
- Dems, D.; Freeman, R.; Riker, K.D.; Coradin, T.; Stupp, S.I.; Aimé, C. Multivalent Clustering of Adhesion Ligands in Nanofiber-Nanoparticle Composites. Acta Biomater. 2021, 119, 303–311. [Google Scholar] [CrossRef] [PubMed]
- Stöber, W.; Fink, A.; Bohn, E. Controlled growth of monodisperse silica spheres in the micron size range. J. Colloid Interface Sci. 1968, 26, 62–69. [Google Scholar] [CrossRef]
- Marschall, R.; Bannat, I.; Caro, J.; Wark, M. Proton Conductivity of Sulfonic Acid Functionalised Mesoporous Materials. Microporous Mesoporous Mater. 2007, 99, 190–196. [Google Scholar] [CrossRef]
- Ikoma, T.; Kobayashi, H.; Tanaka, J.; Walsh, D.; Mann, S. Physical properties of type I collagen extracted from fish scales of Pagrus major and Oreochromis niloticas. Int. J. Biol. Macromol. 2003, 32, 199–204. [Google Scholar] [CrossRef]
- Meek, K.M.; Chapman, J.A.; Hardcastle, R.A. The staining pattern of collagen fibrils. Improved correlation with sequence data. J. Biol. Chem. 1979, 254, 10710–10714. [Google Scholar] [CrossRef]
- Leikina, E.; Mertts, M.V.; Kuznetsova, N.; Leikin, S. Type I collagen is thermally unstable at body temperature. Proc. Natl. Acad. Sci. USA 2002, 99, 1314–1318. [Google Scholar] [CrossRef] [PubMed]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Debons, N.; Matsumoto, K.; Hirota, N.; Coradin, T.; Ikoma, T.; Aimé, C. Magnetic Field Alignment, a Perspective in the Engineering of Collagen-Silica Composite Biomaterials. Biomolecules 2021, 11, 749. https://doi.org/10.3390/biom11050749
Debons N, Matsumoto K, Hirota N, Coradin T, Ikoma T, Aimé C. Magnetic Field Alignment, a Perspective in the Engineering of Collagen-Silica Composite Biomaterials. Biomolecules. 2021; 11(5):749. https://doi.org/10.3390/biom11050749
Chicago/Turabian StyleDebons, Nicolas, Kenta Matsumoto, Noriyuki Hirota, Thibaud Coradin, Toshiyuki Ikoma, and Carole Aimé. 2021. "Magnetic Field Alignment, a Perspective in the Engineering of Collagen-Silica Composite Biomaterials" Biomolecules 11, no. 5: 749. https://doi.org/10.3390/biom11050749
APA StyleDebons, N., Matsumoto, K., Hirota, N., Coradin, T., Ikoma, T., & Aimé, C. (2021). Magnetic Field Alignment, a Perspective in the Engineering of Collagen-Silica Composite Biomaterials. Biomolecules, 11(5), 749. https://doi.org/10.3390/biom11050749







