Flexibility of Oxidized and Reduced States of the Chloroplast Regulatory Protein CP12 in Isolation and in Cell Extracts
Abstract
1. Introduction
2. Materials and Methods
2.1. Amide-Water Proton Exchange Kinetic Measurements by NMR
2.2. Gibbs Free Energy Derived from Protection Factors
2.3. Amide–Water Proton Exchange Kinetic Measurements by MS
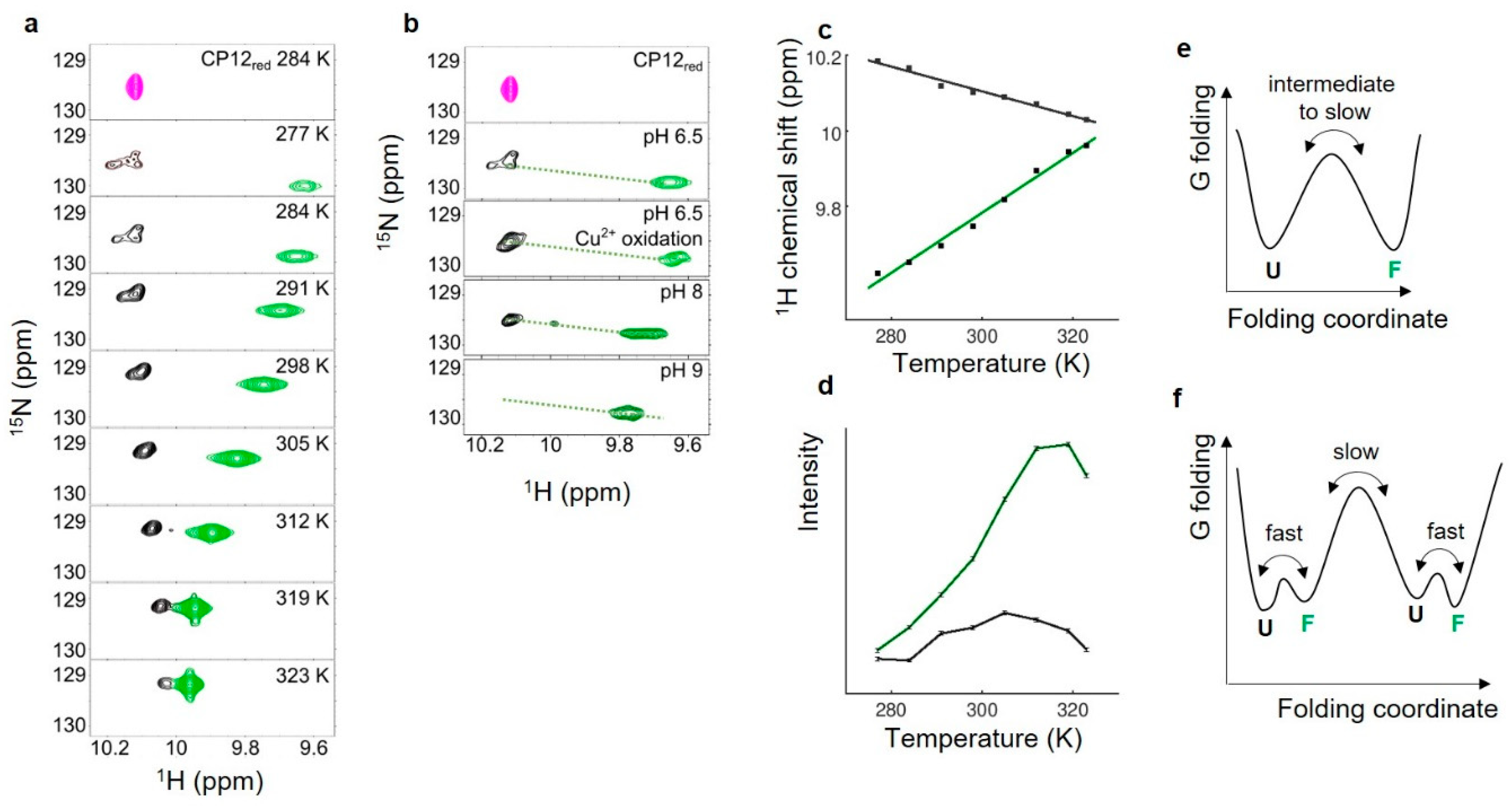
2.4. Temperature Dependence of the NMR Chemical Shift and Signal Intensity
2.5. Thermodynamic of the Redox Transition
2.6. Monitoring the Kinetic of Oxidation
2.7. Monitoring of the Kinetic of Reduction
2.8. Cell Extract Preparation
2.9. Diffusion Coefficient Determination
2.10. Determination of ΔG of Binding
3. Results
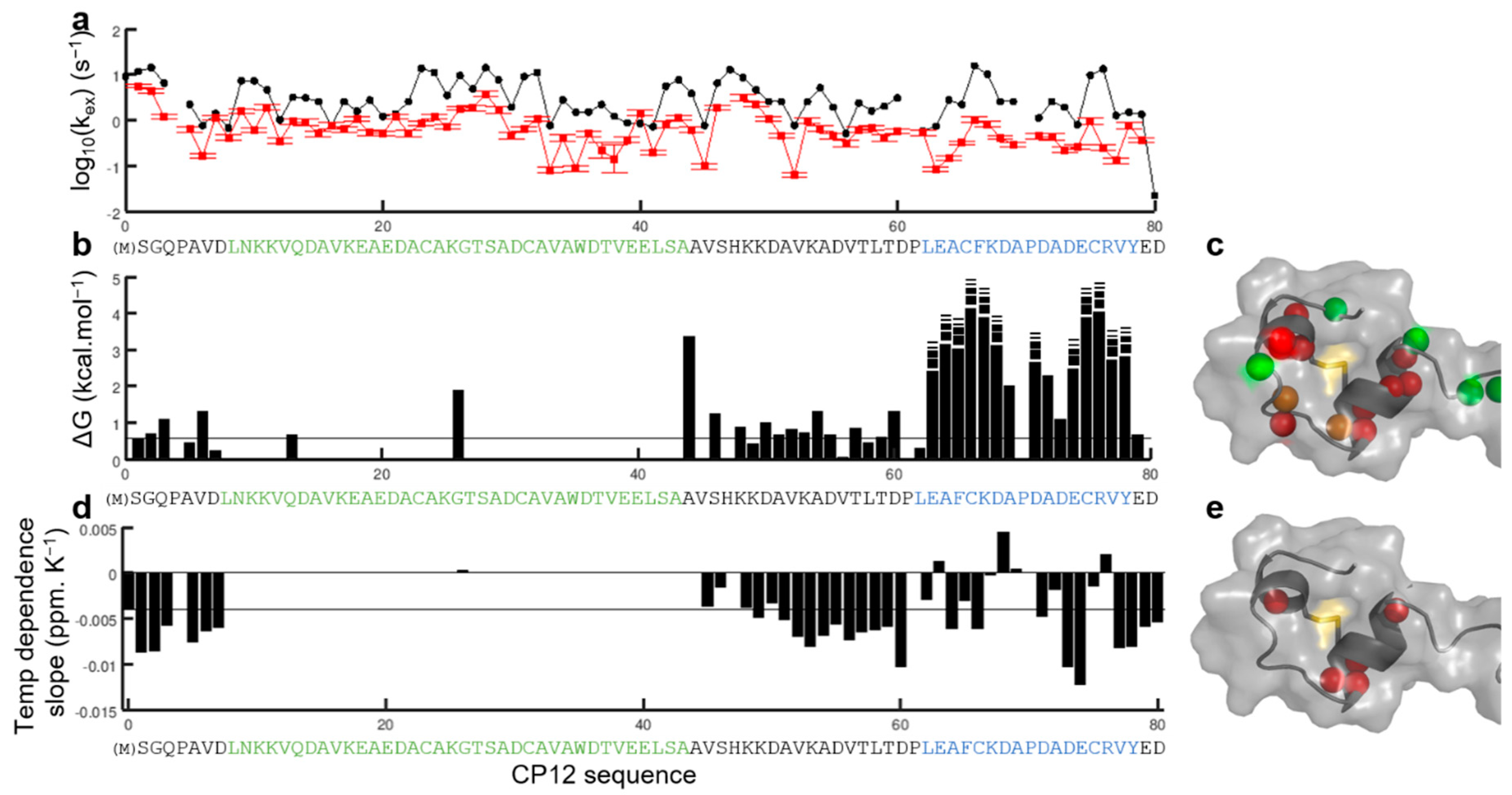
3.1. Structural Transition of the Region Encompassing the C66–C75 Disulfide Bridge upon Oxidation of the Isolated Protein
3.2. Structural Transition of the Region Encompassing the C23–C31 Disulfide Bridge upon Oxidation of the Isolated Protein
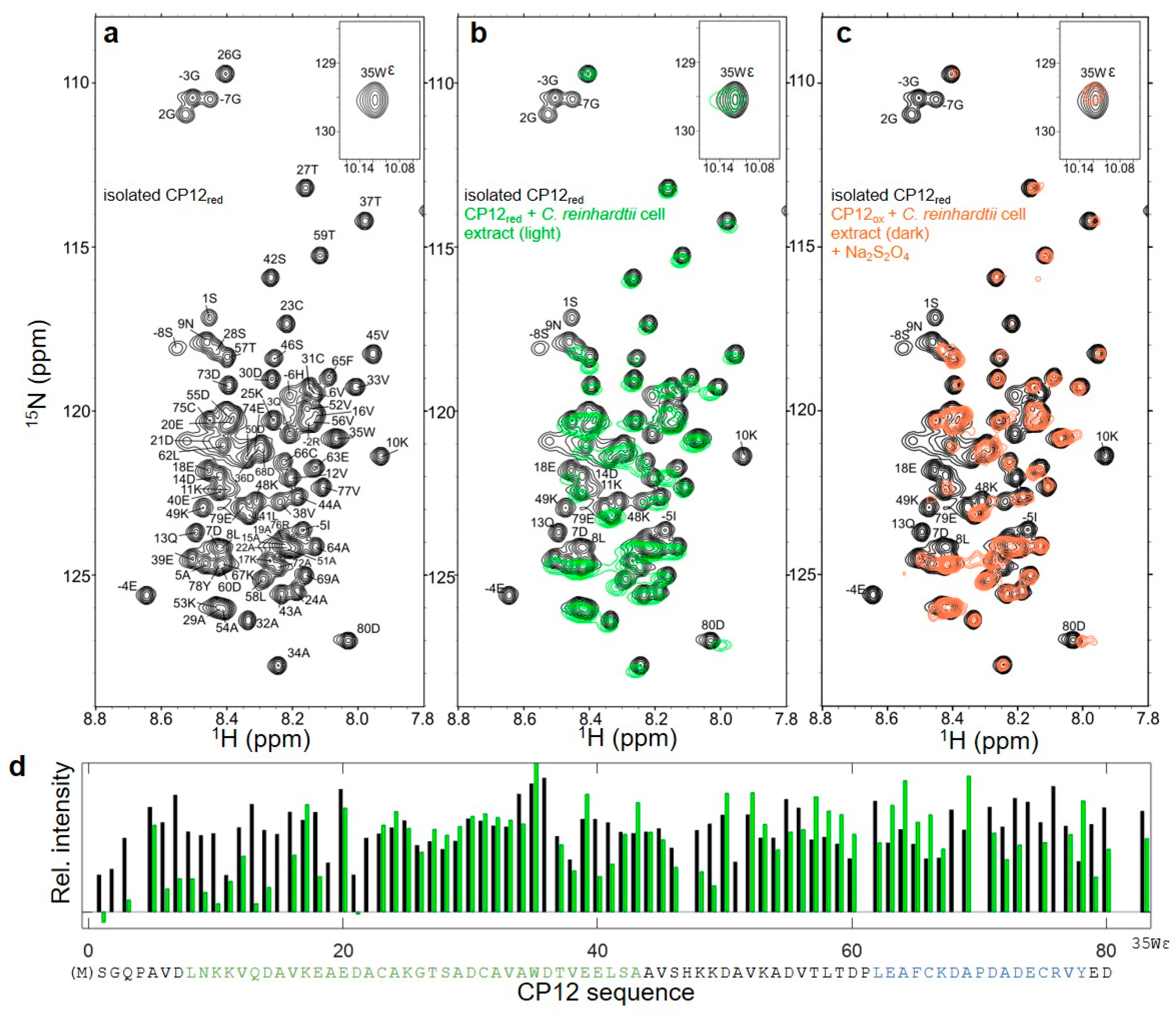
3.3. Structural Properties of CP12red in the Presence of C. reinhardtii Cell Extract
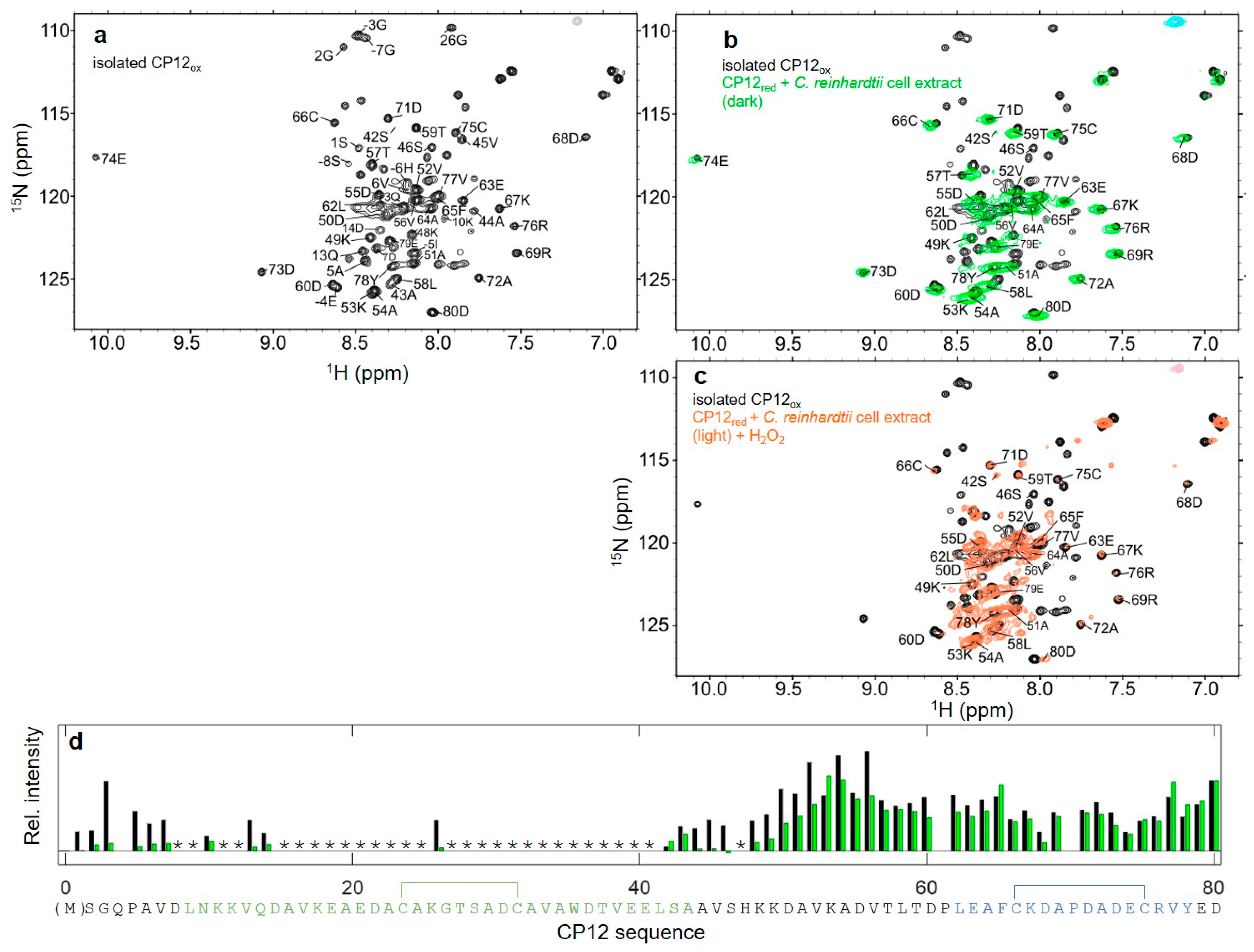
3.4. Structural Properties of the C66–C75 Disulfide Bridge in CP12ox in the Presence of C. reinhardtii Cell Extract
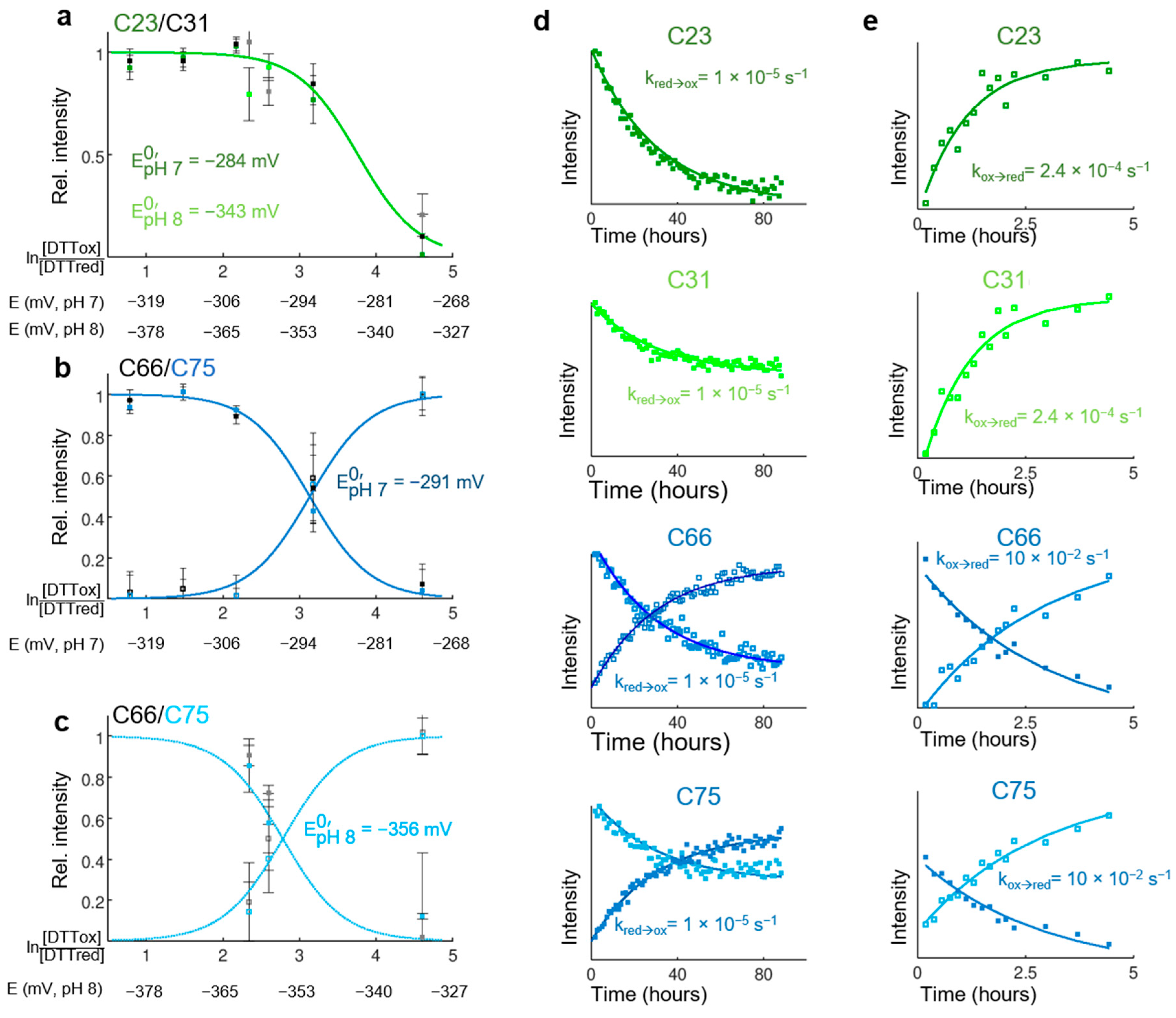
3.5. Thermodynamical Properties of the Redox Transition of Both Disulfide Bridges in Isolated CP12
3.6. Rate of Oxidation and Reduction of Both Disulfide Bridges in Isolated CP12
3.7. Reversible Redox Transition of CP12 in the Presence of C. reinhardtii Cell Extract
4. Discussion
4.1. Isolated CP12red Is Intrinsically Disordered
4.2. The Two Disulfide Bridges Are in Regions with Distinct Structural Properties in Isolated CP12ox
4.3. The Distinct Regions of CP12ox Differ in Structural Dynamics and in Affinity for Their Interacting Partners
4.4. Thermodynamically Independent and Reversible Redox Transition of Both Disulfide Bridges in CP12
4.5. Asynchrony of the Reduction of the Two Disulfide Bridges, Synchrony of Their Formation
4.6. Effect of Cell Extract on Nter-CP12 and Cter-CP12
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jakob, U.; Kriwacki, R.; Uversky, V.N. Conditionally and Transiently Disordered Proteins: Awakening Cryptic Disorder to Regulate Protein Function. Chem. Rev. 2014, 114, 6779–6805. [Google Scholar] [CrossRef] [PubMed]
- Smith, L.M.; The Consortium for Top Down Proteomics; Kelleher, N.L. Proteoform: A single term describing protein complexity. Nat. Methods 2013, 10, 186–187. [Google Scholar] [CrossRef] [PubMed]
- Reichmann, D.; Jakob, U. The roles of conditional disorder in redox proteins. Curr. Opin. Struct. Biol. 2013, 23, 436–442. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ilbert, M.; Horst, J.; Ahrens, S.; Winter, J.; Graf, P.C.F.; Lilie, H.; Jakob, U. The redox-switch domain of Hsp33 functions as dual stress sensor. Nat. Struct. Mol. Biol. 2007, 14, 556–563. [Google Scholar] [CrossRef] [PubMed]
- Winter, J.; Ilbert, M.; Graf, P.; Özcelik, D.; Jakob, U. Bleach Activates a Redox-Regulated Chaperone by Oxidative Protein Unfolding. Cell 2008, 135, 691–701. [Google Scholar] [CrossRef]
- Banci, L.; Bertini, I.; Ciofi-Baffoni, S.; Tokatlidis, K. The coiled coil-helix-coiled coil-helix proteins may be redox proteins. FEBS Lett. 2009, 583, 1699–1702. [Google Scholar] [CrossRef]
- Fraga, H.; Pujols, J.; Gil-Garcia, M.; Roque, A.; Bernardo-Seisdedos, G.; Santambrogio, C.; Bech-Serra, J.-J.; Canals, F.; Bernadó, P.; Grandori, R.; et al. Disulfide driven folding for a conditionally disordered protein. Sci. Rep. 2017, 7, 16994. [Google Scholar] [CrossRef] [PubMed]
- Holmgren, A. Thioredoxin. Annu. Rev. Biochem. 1985, 54, 237–271. [Google Scholar] [CrossRef]
- Michelet, L.; Zaffagnini, M.; Morisse, S.; Sparla, F.; Pérez-Pérez, M.E.; Francia, F.; Danon, A.; Marchand, C.H.; Fermani, S.; Trost, P.; et al. Redox regulation of the Calvin–Benson cycle: Something old, something new. Front. Plant Sci. 2013, 4, 470. [Google Scholar] [CrossRef]
- Zaffagnini, M.; Fermani, S.; Marchand, C.H.; Costa, A.; Sparla, F.; Rouhier, N.; Geigenberger, P.; Lemaire, S.D.; Trost, P. Redox Homeostasis in Photosynthetic Organisms: Novel and Established Thiol-Based Molecular Mechanisms. Antioxid. Redox Signal. 2019, 31, 155–210. [Google Scholar] [CrossRef]
- Scheibe, R.; Anderson, L.E. Dark modulation of NADP-dependent malate dehydrogenase and glucose-6-phosphate dehydrogenase in the chloroplast. Biochim. Biophys. Acta (BBA) Bioenergy 1981, 636, 58–64. [Google Scholar] [CrossRef]
- Scheibe, R.; Geissler, A.; Fickenscher, K. Chloroplast glucose-6-phosphate dehydrogenase: Km shift upon light modulation and reduction. Arch. Biochem. Biophys. 1989, 274, 290–297. [Google Scholar] [CrossRef]
- Kachru, R.B.; Anderson, L.E. Inactivation of Pea Leaf Phosphofructokinase by Light and Dithiothreitol. Plant Physiol. 1975, 55, 199–202. [Google Scholar] [CrossRef][Green Version]
- Yoshida, K.; Hisabori, T. Biochemical Basis for Redox Regulation of Chloroplast-Localized Phosphofructokinase from Arabidopsis thaliana. Plant Cell Physiol. 2021. [Google Scholar] [CrossRef] [PubMed]
- Cejudo, F.J.; Ojeda, V.; Delgado-Requerey, V.; González, M.; Pérez-Ruiz, J.M. Chloroplast Redox Regulatory Mechanisms in Plant Adaptation to Light and Darkness. Front. Plant Sci. 2019, 10, 380. [Google Scholar] [CrossRef]
- Avilan, L.; Lebreton, S.; Gontero, B. Thioredoxin Activation of Phosphoribulokinase in a Bi-enzyme Complex from Chlamydomonas reinhardtii Chloroplasts. J. Biol. Chem. 2000, 275, 9447–9451. [Google Scholar] [CrossRef] [PubMed]
- Knuesting, J.; Scheibe, R. Small Molecules Govern Thiol Redox Switches. Trends Plant Sci. 2018, 23, 769–782. [Google Scholar] [CrossRef] [PubMed]
- Leegood, R.; Walker, D. Regulation of fructose-1,6-bisphosphatase activity in intact chloroplasts. Studies of the mechanism of inactivation. Biochim. Biophys. Acta (BBA) Bioenergy 1980, 593, 362–370. [Google Scholar] [CrossRef]
- Vaseghi, M.-J.; Chibani, K.; Telman, W.; Liebthal, M.F.; Gerken, M.; Schnitzer, H.; Mueller, S.M.; Dietz, K.-J. The chloroplast 2-cysteine peroxiredoxin functions as thioredoxin oxidase in redox regulation of chloroplast metabolism. eLife 2018, 7. [Google Scholar] [CrossRef] [PubMed]
- Buchanan, B.B. The Ferredoxin/Thioredoxin System: A Key Element in the Regulatory Function of Light in Photosynthesis. Bioscience 1984, 34, 378–383. [Google Scholar] [CrossRef]
- Gontero, B.; Avilan, L.; Lebreton, S. Control of carbon fixation in chloroplasts. In Control of Primary Metabolism in Plants; Plaxton, W.C., McManus, M.T., Eds.; Blackwell Publishing Ltd.: Oxford, UK, 2006; pp. 187–218. [Google Scholar]
- Gontero, B.; Maberly, S.C. An intrinsically disordered protein, CP12: Jack of all trades and master of the Calvin cycle. Biochem. Soc. Trans. 2012, 40, 995–999. [Google Scholar] [CrossRef]
- Graciet, E.; Gans, P.; Wedel, N.; Lebreton, S.; Camadro, J.-M.; Gontero, B. The Small Protein CP12: A Protein Linker for Supramolecular Complex Assembly. Biochemistry 2003, 42, 8163–8170. [Google Scholar] [CrossRef] [PubMed]
- Avilan, L.; Puppo, C.; Erales, J.; Woudstra, M.; Lebrun, R.; Gontero, B. CP12 residues involved in the formation and regulation of the glyceraldehyde-3-phosphate dehydrogenase–CP12–phosphoribulokinase complex in Chlamydomonas reinhardtii. Mol. BioSyst. 2012, 8, 2994–3002. [Google Scholar] [CrossRef]
- Mcfarlane, C.; Shah, N.; Kabasakal, B.V.; Cotton, C.A.R.; Bubeck, D.; Murray, J.W. Structural Basis of Light-Induced Redox Regulation in the Calvin Cycle. Proc. Natl. Acad. Sci. USA 2019, 116, 20984–20990. [Google Scholar] [CrossRef] [PubMed]
- Yu, A.; Xie, Y.; Pan, X.; Zhang, H.; Cao, P.; Su, X.; Chang, W.; Li, M. Photosynthetic Phosphoribulokinase Structures: Enzymatic Mechanisms and the Redox Regulation of the Calvin-Benson-Bassham Cycle. Plant Cell 2020, 32, 1556–1573. [Google Scholar] [CrossRef] [PubMed]
- Groben, R.; Kaloudas, D.; Raines, C.A.; Offmann, B.; Maberly, S.C.; Gontero, B. Comparative sequence analysis of CP12, a small protein involved in the formation of a Calvin cycle complex in photosynthetic organisms. Photosynth. Res. 2010, 103, 183–194. [Google Scholar] [CrossRef] [PubMed]
- Stanley, D.N.; Raines, C.A.; Kerfeld, C.A. Comparative Analysis of 126 Cyanobacterial Genomes Reveals Evidence of Functional Diversity Among Homologs of the Redox-Regulated CP12 Protein. Plant Physiol. 2013, 161, 824–835. [Google Scholar] [CrossRef]
- Fermani, S.; Trivelli, X.; Sparla, F.; Thumiger, A.; Calvaresi, M.; Marri, L.; Falini, G.; Zerbetto, F.; Trost, P. Conformational Selection and Folding-upon-binding of Intrinsically Disordered Protein CP12 Regulate Photosynthetic Enzymes Assembly. J. Biol. Chem. 2012, 287, 21372–21383. [Google Scholar] [CrossRef]
- Launay, H.; Barré, P.; Puppo, C.; Manneville, S.; Gontero, B.; Receveur-Bréchot, V. Absence of residual structure in the intrinsically disordered regulatory protein CP12 in its reduced state. Biochem. Biophys. Res. Commun. 2016, 477, 20–26. [Google Scholar] [CrossRef] [PubMed]
- Launay, H.; Barré, P.; Puppo, C.; Zhang, Y.; Manneville, S.; Gontero, B.; Receveur-Bréchot, V. Cryptic Disorder Out of Disorder: Encounter between Conditionally Disordered CP12 and Glyceraldehyde-3-Phosphate Dehydrogenase. J. Mol. Biol. 2018, 430, 1218–1234. [Google Scholar] [CrossRef]
- Shao, H.; Huang, W.; Avilan, L.; Receveur-Bréchot, V.; Puppo, C.; Puppo, R.; Lebrun, R.; Gontero, B.; Launay, H. A new type of flexible CP12 protein in the marine diatom Thalassiosira pseudonana. Cell Commun. Signal. 2021, 19, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Adamski, W.; Salvi, N.; Maurin, D.; Magnat, J.; Milles, S.; Jensen, M.R.; Abyzov, A.; Moreau, C.J.; Blackledge, M. A Unified Description of Intrinsically Disordered Protein Dynamics under Physiological Conditions Using NMR Spectroscopy. J. Am. Chem. Soc. 2019, 141, 17817–17829. [Google Scholar] [CrossRef] [PubMed]
- Pauwels, K.; Lebrun, P.; Tompa, P. To be disordered or not to be disordered: Is that still a question for proteins in the cell? Cell. Mol. Life Sci. 2017, 74, 3185–3204. [Google Scholar] [CrossRef]
- Bodart, J.-F.; Wieruszeski, J.-M.; Amniai, L.; Leroy, A.; Landrieu, I.; Rousseau-Lescuyer, A.; Vilain, J.-P.; Lippens, G. NMR observation of Tau in Xenopus oocytes. J. Magn. Reson. 2008, 192, 252–257. [Google Scholar] [CrossRef] [PubMed]
- Theillet, F.-X.; Binolfi, A.; Bekei, B.; Martorana, A.; Rose, H.M.; Stuiver, M.; Verzini, S.; Lorenz, D.; Van Rossum, M.; Goldfarb, D.; et al. Structural disorder of monomeric α-synuclein persists in mammalian cells. Nat. Cell Biol. 2016, 530, 45–50. [Google Scholar] [CrossRef]
- Banci, L.; Barbieri, L.; Luchinat, E.; Secci, E. Visualization of Redox-Controlled Protein Fold in Living Cells. Chem. Biol. 2013, 20, 747–752. [Google Scholar] [CrossRef]
- Mercatelli, E.; Barbieri, L.; Luchinat, E.; Banci, L. Direct structural evidence of protein redox regulation obtained by in-cell NMR. Biochim. Biophys. Acta (BBA) Bioenergy 2016, 1863, 198–204. [Google Scholar] [CrossRef]
- Hwang, T.-L.; Van Zijl, P.C.; Mori, S. Accurate Quantitation of Water-amide Proton Exchange Rates Using the Phase-Modulated CLEAN Chemical EXchange (CLEANEX-PM) Approach with a Fast-HSQC (FHSQC) Detection Scheme. J. Biomol. NMR 1998, 11, 221–226. [Google Scholar] [CrossRef]
- Delaglio, F.; Grzesiek, S.; Vuister, G.W.; Zhu, G.; Pfeifer, J.; Bax, A. NMRPipe: A multidimensional spectral processing system based on UNIX pipes. J. Biomol. NMR 1995, 6, 277–293. [Google Scholar] [CrossRef] [PubMed]
- Lee, W.; Tonelli, M.; Markley, J.L. NMRFAM-SPARKY: Enhanced software for biomolecular NMR spectroscopy. Bioinformatics 2015, 31, 1325–1327. [Google Scholar] [CrossRef]
- Eaton, J.; Bateman, D.; Haubert, S.; Wehbring, R. GNU Octave Version 4.2.1. Available online: https://www.gnu.org/software/octave/index (accessed on 20 June 2020).
- Xu, S.; Ni, S.; Kennedy, M.A. NMR Analysis of Amide Hydrogen Exchange Rates in a Pentapeptide-Repeat Protein from A. thaliana. Biophys. J. 2017, 112, 2075–2088. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.-Z. SPHERE. Available online: https://protocol.fccc.edu/research/labs/roder/sphere (accessed on 20 June 2020).
- Piotukh, K.; Kosslick, D.; Zimmermann, J.; Krause, E.; Freund, C. Reversible disulfide bond formation of intracellular proteins probed by NMR spectroscopy. Free. Radic. Biol. Med. 2007, 43, 1263–1270. [Google Scholar] [CrossRef]
- Mochizuki, A.; Saso, A.; Zhao, Q.; Kubo, S.; Nishida, N.; Shimada, I. Balanced Regulation of Redox Status of Intracellular Thioredoxin Revealed by in-Cell NMR. J. Am. Chem. Soc. 2018, 140, 3784–3790. [Google Scholar] [CrossRef] [PubMed]
- Kruger, N.J. The Bradford Method for Protein Quantitation. Basic Protein Pept. Protoc. 1994, 32, 9–16. [Google Scholar] [CrossRef]
- Ferrage, F.; Zoonens, M.; Warschawski, D.E.; Popot, J.-L.; Bodenhausen, G. Slow Diffusion of Macromolecular Assemblies by a New Pulsed Field Gradient NMR Method†. J. Am. Chem. Soc. 2003, 125, 2541–2545. [Google Scholar] [CrossRef] [PubMed]
- Ferraro, D.M.; Lazo, N.D.; Robertson, A.D. EX1 Hydrogen Exchange and Protein Folding. Biochemistry 2004, 43, 587–594. [Google Scholar] [CrossRef] [PubMed]
- Cierpicki, T.; Otlewski, J. Amide proton temperature coefficients as hydrogen bond indicators in proteins. J. Biomol. NMR 2001, 21, 249–261. [Google Scholar] [CrossRef]
- Trainor, K.; Palumbo, J.A.; MacKenzie, D.W.S.; Meiering, E.M. Temperature dependence of NMR chemical shifts: Tracking and statistical analysis. Protein Sci. 2020, 29, 306–314. [Google Scholar] [CrossRef]
- Dujardin, M.; Madan, V.; Gandhi, N.S.; Cantrelle, F.-X.; Launay, H.; Huvent, I.; Bartenschlager, R.; Lippens, G.; Hanoulle, X. Cyclophilin A allows the allosteric regulation of a structural motif in the disordered domain 2 of NS5A and thereby fine-tunes HCV RNA replication. J. Biol. Chem. 2019, 294, 13171–13185. [Google Scholar] [CrossRef]
- Selvaratnam, R.; Chowdhury, S.; Van Schouwen, B.; Melacini, G. Mapping allostery through the covariance analysis of NMR chemical shifts. Proc. Natl. Acad. Sci. USA 2011, 108, 6133–6138. [Google Scholar] [CrossRef]
- Cavanagh, J.; Fairbrother, W.; Palmer, A.G.; Rance, M.; Skelton, N. Principles and Practice: Protein NMR Spectroscopy, 2nd ed.; Elsevier Academic Press: Burlington, MA, USA, 2006; ISBN 978-0-12-164491-8. [Google Scholar]
- Marri, L.; Trost, P.; Pupillo, P.; Sparla, F. Reconstitution and Properties of the Recombinant Glyceraldehyde-3-Phosphate Dehydrogenase/CP12/Phosphoribulokinase Supramolecular Complex of Arabidopsis. Plant Physiol. 2005, 139, 1433–1443. [Google Scholar] [CrossRef] [PubMed]
- Clement, R.; Lignon, S.; Mansuelle, P.; Jensen, E.; Pophillat, M.; Lebrun, R.; Denis, Y.; Puppo, C.; Maberly, S.C.; Gontero, B. Responses of the marine diatom Thalassiosira pseudonana to changes in CO2 concentration: A proteomic approach. Sci. Rep. 2017, 7, 42333. [Google Scholar] [CrossRef]
- López-Calcagno, P.E.; Abuzaid, A.O.; Lawson, T.; Raines, C.A. Arabidopsis CP12 mutants have reduced levels of phosphoribulokinase and impaired function of the Calvin–Benson cycle. J. Exp. Bot. 2017, 68, 2285–2298. [Google Scholar] [CrossRef] [PubMed]
- Howard, T.P.; Fryer, M.J.; Singh, P.; Metodiev, M.; Lytovchenko, A.; Obata, T.; Fernie, A.R.; Kruger, N.J.; Quick, W.P.; Lloyd, J.C.; et al. Antisense Suppression of the Small Chloroplast Protein CP12 in Tobacco Alters Carbon Partitioning and Severely Restricts Growth. Plant Physiol. 2011, 157, 620–631. [Google Scholar] [CrossRef]
- Li, K.; Qiu, H.; Zhou, M.; Lin, Y.; Guo, Z.; Lu, S. Chloroplast Protein 12 Expression Alters Growth and Chilling Tolerance in Tropical Forage Stylosanthes guianensis (Aublet) Sw. Front. Plant Sci. 2018, 9, 1319. [Google Scholar] [CrossRef]
- Dietz, K.-J. Redox signal integration: From stimulus to networks and genes. Physiol. Plant. 2008, 133, 459–468. [Google Scholar] [CrossRef]
- Buchanan, B.B.; Balmer, Y. REDOX REGULATION: A Broadening Horizon. Annu. Rev. Plant Biol. 2005, 56, 187–220. [Google Scholar] [CrossRef] [PubMed]
- Jensen, M.R.; Ruigrok, R.W.; Blackledge, M. Describing intrinsically disordered proteins at atomic resolution by NMR. Curr. Opin. Struct. Biol. 2013, 23, 426–435. [Google Scholar] [CrossRef]
- Gurrieri, L.; Del Giudice, A.; Demitri, N.; Falini, G.; Pavel, N.V.; Zaffagnini, M.; Polentarutti, M.; Crozet, P.; Marchand, C.H.; Henri, J.; et al. Arabidopsis and Chlamydomonas phosphoribulokinase crystal structures complete the redox structural proteome of the Calvin–Benson cycle. Proc. Natl. Acad. Sci. USA 2019, 116, 8048–8053. [Google Scholar] [CrossRef] [PubMed]
- Marri, L.; Zaffagnini, M.; Collin, V.; Issakidis-Bourguet, E.; Lemaire, S.D.; Pupillo, P.; Sparla, F.; Miginiac-Maslow, M.; Trost, P. Prompt and Easy Activation by Specific Thioredoxins of Calvin Cycle Enzymes of Arabidopsis thaliana Associated in the GAPDH/CP12/PRK Supramolecular Complex. Mol. Plant 2009, 2, 259–269. [Google Scholar] [CrossRef] [PubMed]
- Marri, L.; Pesaresi, A.; Valerio, C.; Lamba, D.; Pupillo, P.; Trost, P.; Sparla, F. In vitro characterization of Arabidopsis CP12 isoforms reveals common biochemical and molecular properties. J. Plant Physiol. 2010, 167, 939–950. [Google Scholar] [CrossRef]
- Yoshida, K.; Hara, A.; Sugiura, K.; Fukaya, Y.; Hisabori, T. Thioredoxin-like2/2-Cys peroxiredoxin redox cascade supports oxidative thiol modulation in chloroplasts. Proc. Natl. Acad. Sci. USA 2018, 115, E8296–E8304. [Google Scholar] [CrossRef]
- Faustino, A.F.; Barbosa, G.M.; Silva, M.; Castanho, M.A.R.B.; Da Poian, A.T.; Cabrita, E.J.; Santos, N.C.; Almeida, F.C.L.; Martins, I.C. Fast NMR method to probe solvent accessibility and disordered regions in proteins. Sci. Rep. 2019, 9, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Launay, H.; Receveur-Bréchot, V.; Carrière, F.; Gontero, B. Orchestration of algal metabolism by protein disorder. Arch. Biochem. Biophys. 2019, 672, 108070. [Google Scholar] [CrossRef]
- Bhowmick, A.; Brookes, D.H.; Yost, S.R.; Dyson, H.J.; Forman-Kay, J.D.; Gunter, D.; Head-Gordon, M.; Hura, G.L.; Pande, V.S.; Wemmer, D.E.; et al. Finding Our Way in the Dark Proteome. J. Am. Chem. Soc. 2016, 138, 9730–9742. [Google Scholar] [CrossRef]
- Teilum, K.; Olsen, J.G.; Kragelund, B.B. Globular and disordered—the non-identical twins in protein-protein interactions. Front. Mol. Biosci. 2015, 2, 2. [Google Scholar] [CrossRef] [PubMed]
- Baalmann, E.; Scheibe, R.; Martin, W.F. Functional studies of chloroplast glyceraldehyde-3-phosphate dehydrogenase subunits A and B expressed in Escherichia coli: Formation of highly active A4 and B4 homotetramers and evidence that aggregation of the B4 complex is mediated by the B subunit carboxy terminus. Plant Mol. Biol. 1996, 32, 505–513. [Google Scholar] [CrossRef] [PubMed]
- Ethieulin-Pardo, G.; Eavilan, L.; Ekojadinovic, M.; Egontero, B. Fairy “tails”: Flexibility and function of intrinsically disordered extensions in the photosynthetic world. Front. Mol. Biosci. 2015, 2, 23. [Google Scholar] [CrossRef]
- Zhang, Y.; Launay, H.; Liu, F.; Lebrun, R.; Gontero, B. Interaction between adenylate kinase 3 and glyceraldehyde-3-phosphate dehydrogenase from Chlamydomonas reinhardtii. FEBS J. 2018, 285, 2495–2503. [Google Scholar] [CrossRef]
- Lemaire, S.D.; Quesada, A.; Merchan, F.; Corral, J.M.; Igeno, M.I.; Keryer, E.; Issakidis-Bourguet, E.; Hirasawa, M.; Knaff, D.B.; Miginiac-Maslow, M. NADP-Malate Dehydrogenase from Unicellular Green Alga Chlamydomonas reinhardtii. A First Step toward Redox Regulation? Plant Physiol. 2005, 137, 514–521. [Google Scholar] [CrossRef] [PubMed]
- Mackinder, L.C.; Chen, C.; Leib, R.D.; Patena, W.; Blum, S.R.; Rodman, M.; Ramundo, S.; Adams, C.M.; Jonikas, M.C. A Spatial Interactome Reveals the Protein Organization of the Algal CO2-Concentrating Mechanism. Cell 2017, 171, 133–147.e14. [Google Scholar] [CrossRef]
- Wunder, T.; Cheng, S.L.H.; Lai, S.-K.; Li, H.-Y.; Mueller-Cajar, O. The phase separation underlying the pyrenoid-based microalgal Rubisco supercharger. Nat. Commun. 2018, 9, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Rosenzweig, E.S.F.; Xu, B.; Cuellar, L.K.; Martinez-Sanchez, A.; Schaffer, M.; Strauss, M.; Cartwright, H.N.; Ronceray, P.; Plitzko, J.M.; Förster, F.; et al. The Eukaryotic CO2-Concentrating Organelle Is Liquid-like and Exhibits Dynamic Reorganization. Cell 2017, 171, 148–162.e19. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.H.; van Spronsen, J.; Dai, Y.; Verberne, M.; Hollmann, F.; Arends, I.W.; Witkamp, G.-J.; Verpoorte, R. Are Natural Deep Eutectic Solvents the Missing Link in Understanding Cellular Metabolism and Physiology? Plant Physiol. 2011, 156, 1701–1705. [Google Scholar] [CrossRef] [PubMed]
- Dai, Y.; Varypataki, E.M.; Golovina, E.A.; Jiskoot, W.; Witkamp, G.-J.; Choi, Y.H.; Verpoorte, R. Natural deep eutectic solvents in plants and plant cells: In vitro evidence for their possible functions. In Advances in Botanical Research; Elsevier BV: Amsterdam, The Netherlands, 2021; Volume 97, pp. 159–184. [Google Scholar]
- Brillouet, J.-M.; Verdeil, J.-L.; Odoux, E.; Lartaud, M.; Grisoni, M.; Conéjéro, G. Phenol homeostasis is ensured in vanilla fruit by storage under solid form in a new chloroplast-derived organelle, the phenyloplast. J. Exp. Bot. 2014, 65, 2427–2435. [Google Scholar] [CrossRef] [PubMed]
- Marri, L.; Sparla, F.; Pupillo, P.; Trost, P. Co-ordinated gene expression of photosynthetic glyceraldehyde-3-phosphate dehydrogenase, phosphoribulokinase, and CP12 in Arabidopsis thaliana. J. Exp. Bot. 2004, 56, 73–80. [Google Scholar] [CrossRef]
- Rochaix, J.-D. Chlamydomonas reinhardtii as the Photosynthetic Yeast. Annu. Rev. Genet. 1995, 29, 209–230. [Google Scholar] [CrossRef]
- Kastritis, P.L.; O’Reilly, F.J.; Bock, T.; Li, Y.; Rogon, M.Z.; Buczak, K.; Romanov, N.; Betts, M.J.; Bui, K.H.; Hagen, W.J.; et al. Capturing protein communities by structural proteomics in a thermophilic eukaryote. Mol. Syst. Biol. 2017, 13, 936. [Google Scholar] [CrossRef] [PubMed]
- Ellis, R. Macromolecular crowding: An important but neglected aspect of the intracellular environment. Curr. Opin. Struct. Biol. 2001, 11, 114–119. [Google Scholar] [CrossRef]
- Ellis, R. Macromolecular crowding: Obvious but underappreciated. Trends Biochem. Sci. 2001, 26, 597–604. [Google Scholar] [CrossRef]
- Zimmerman, S.B.; Trach, S.O. Estimation of macromolecule concentrations and excluded volume effects for the cytoplasm of Escherichia coli. J. Mol. Biol. 1991, 222, 599–620. [Google Scholar] [CrossRef]
- Christiansen, A.; Wang, Q.; Cheung, M.S.; Wittung-Stafshede, P. Effects of macromolecular crowding agents on protein folding in vitro and in silico. Biophys. Rev. 2013, 5, 137–145. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Charlton, L.M.; Lakkavaram, A.; Seagle, C.; Wang, G.; Young, G.B.; Macdonald, J.M.; Pielak, G.J. Differential Dynamical Effects of Macromolecular Crowding on an Intrinsically Disordered Protein and a Globular Protein: Implications for In-Cell NMR Spectroscopy. J. Am. Chem. Soc. 2008, 130, 6310–6311. [Google Scholar] [CrossRef]
- Wang, Y.; Benton, L.A.; Singh, V.; Pielak, G.J. Disordered Protein Diffusion under Crowded Conditions. J. Phys. Chem. Lett. 2012, 3, 2703–2706. [Google Scholar] [CrossRef] [PubMed]
- Luchinat, E.; Banci, L. In-cell NMR: A topical review. IUCrJ 2017, 4, 108–118. [Google Scholar] [CrossRef] [PubMed]
- Cedeño, C.; Pauwels, K.; Tompa, P. Protein Delivery into Plant Cells: Toward In vivo Structural Biology. Front. Plant Sci. 2017, 8, 8. [Google Scholar] [CrossRef] [PubMed]
- Küken, A.; Sommer, F.; Yaneva-Roder, L.; Mackinder, L.C.M.; Höhne, M.; Geimer, S.; Jonikas, M.C.; Schroda, M.; Stitt, M.; Nikoloski, Z.; et al. Effects of microcompartmentation on flux distribution and metabolic pools in Chlamydomonas reinhardtii chloroplasts. eLife 2018, 7. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Launay, H.; Shao, H.; Bornet, O.; Cantrelle, F.-X.; Lebrun, R.; Receveur-Brechot, V.; Gontero, B. Flexibility of Oxidized and Reduced States of the Chloroplast Regulatory Protein CP12 in Isolation and in Cell Extracts. Biomolecules 2021, 11, 701. https://doi.org/10.3390/biom11050701
Launay H, Shao H, Bornet O, Cantrelle F-X, Lebrun R, Receveur-Brechot V, Gontero B. Flexibility of Oxidized and Reduced States of the Chloroplast Regulatory Protein CP12 in Isolation and in Cell Extracts. Biomolecules. 2021; 11(5):701. https://doi.org/10.3390/biom11050701
Chicago/Turabian StyleLaunay, Helene, Hui Shao, Olivier Bornet, Francois-Xavier Cantrelle, Regine Lebrun, Veronique Receveur-Brechot, and Brigitte Gontero. 2021. "Flexibility of Oxidized and Reduced States of the Chloroplast Regulatory Protein CP12 in Isolation and in Cell Extracts" Biomolecules 11, no. 5: 701. https://doi.org/10.3390/biom11050701
APA StyleLaunay, H., Shao, H., Bornet, O., Cantrelle, F.-X., Lebrun, R., Receveur-Brechot, V., & Gontero, B. (2021). Flexibility of Oxidized and Reduced States of the Chloroplast Regulatory Protein CP12 in Isolation and in Cell Extracts. Biomolecules, 11(5), 701. https://doi.org/10.3390/biom11050701





