Immunology of Acute and Chronic Wound Healing
Abstract
1. Introduction
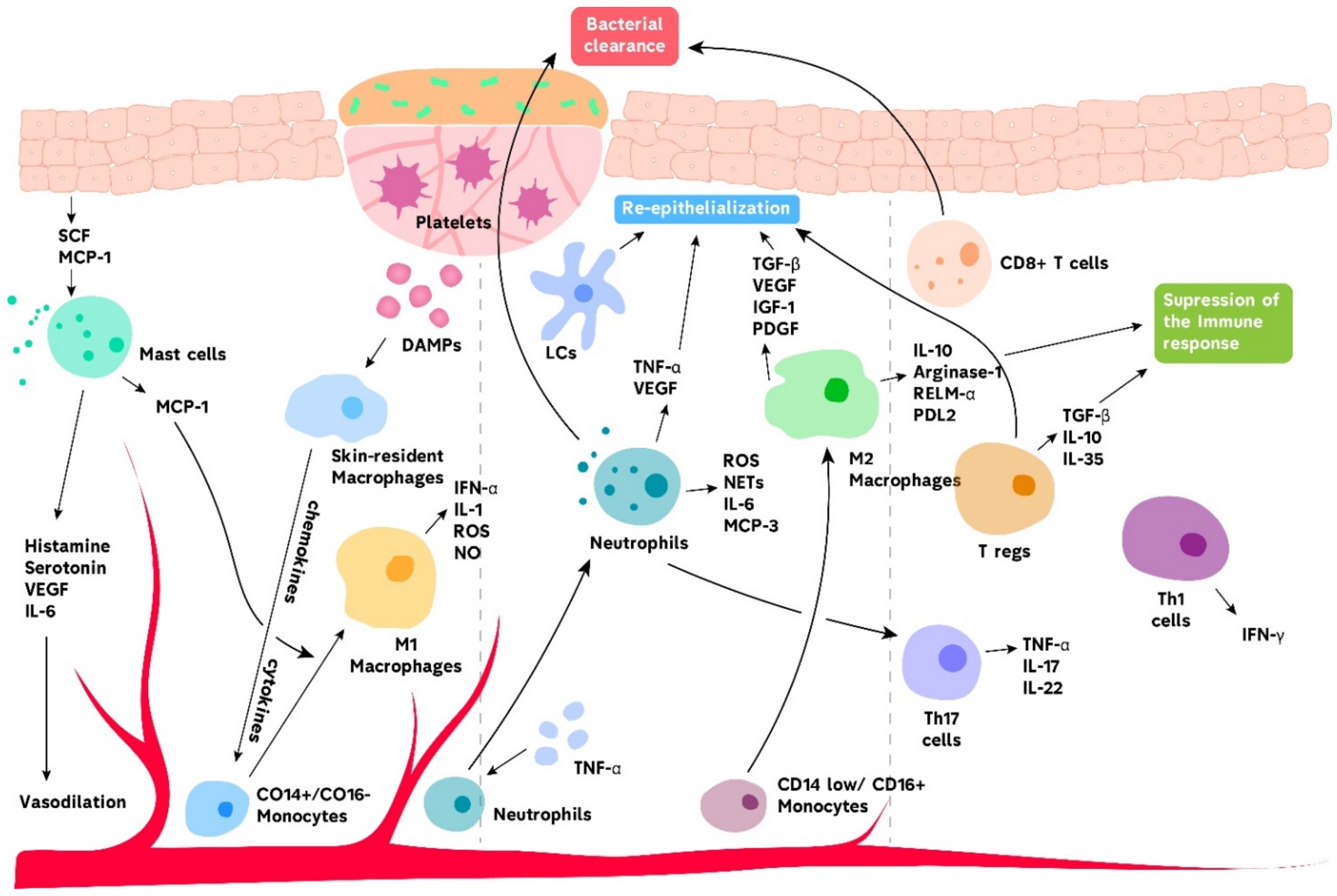
2. Acute Wound Healing
2.1. Innate Immunity in Acute Wound Healing
2.1.1. Basophils and Mast Cells
2.1.2. Neutrophils
2.1.3. Macrophages
2.1.4. Langerhans Cells and Dendritic Cells
2.2. Adaptive Immunity in Acute Wound Healing
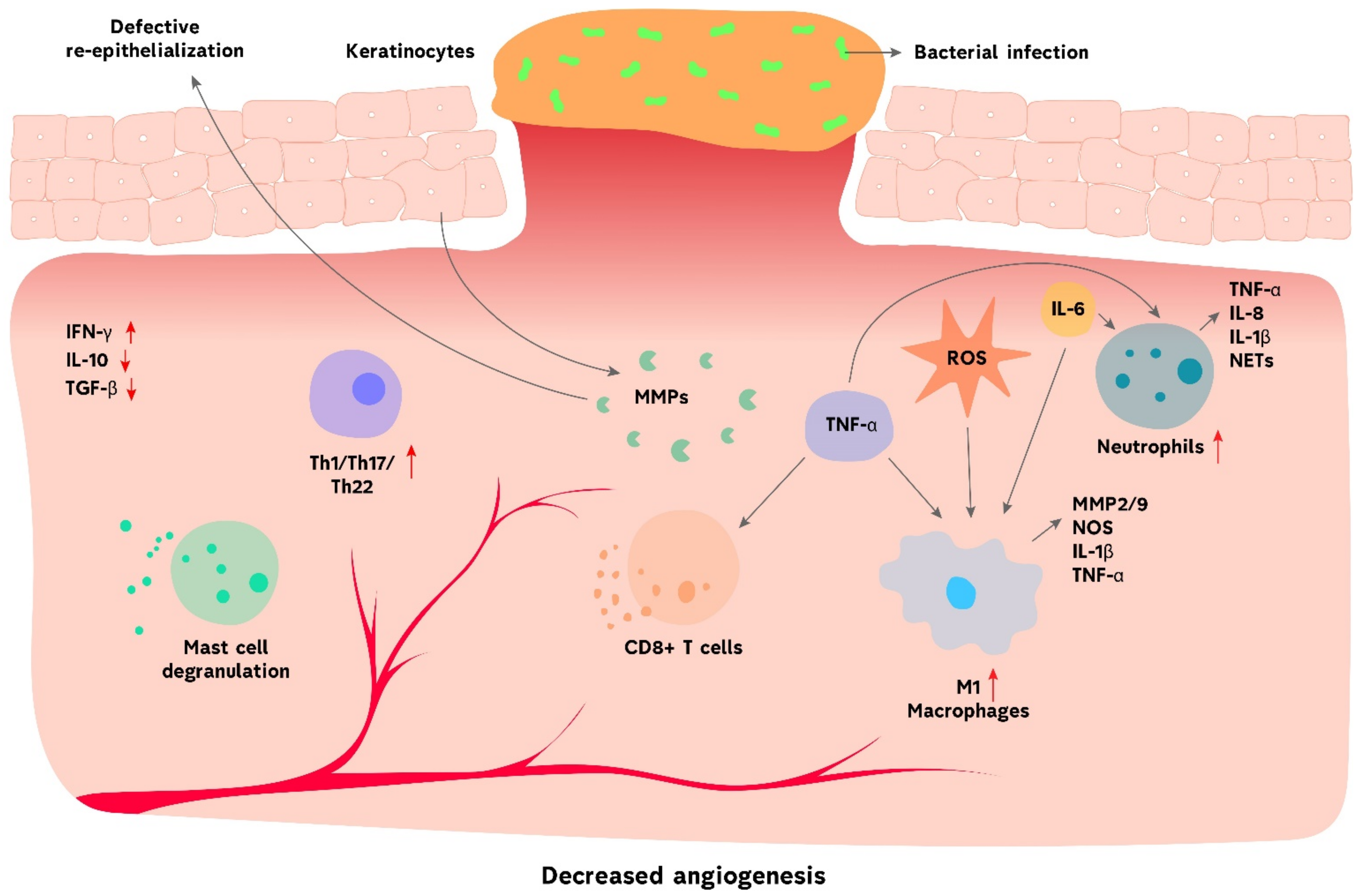
3. Chronic Wound Healing
3.1. Innate Immunity in Chronic Wound Healing
3.1.1. Neutrophils
3.1.2. Macrophages
3.1.3. Innate Lymphoid Cells
3.2. Adaptive Immunity in Chronic Wound Healing
4. Modulation of the Immune System to Improve Wound Healing
4.1. M1/M2 Polarization of Macrophages
4.2. MiRNAs
4.3. Cytokines/Growth Factors and Inhibitors
4.4. Stem Cells
4.5. Negative Pressure Wound Therapy
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Garraud, O.; Hozzein, W.N.; Badr, G. Wound healing: Time to look for intelligent, ‘natural’ immunological approaches? BMC Immunol. 2017, 18, 23. [Google Scholar] [CrossRef] [PubMed]
- Nussbaum, S.R.; Carter, M.J.; Fife, C.E.; DaVanzo, J.; Haught, R.; Nusgart, M.; Cartwright, D. An economic evaluation of the impact, cost, and medicare policy implications of chronic nonhealing wounds. Value Health 2018, 21, 27–32. [Google Scholar] [CrossRef] [PubMed]
- Olsson, M.; Järbrink, K.; Divakar, U.; Bajpai, R.; Upton, Z.; Schmidtchen, A.; Car, J. The humanistic and economic burden of chronic wounds: A systematic review. Wound Repair Regen. 2019, 27, 114–125. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, M.; Kosaric, N.; Bonham, C.A.; Gurtner, G.C. Wound healing: A cellular perspective. Physiol. Rev. 2019, 99, 665–706. [Google Scholar] [CrossRef]
- Fife, C.E.; Eckert, K.A.; Carter, M.J. Publicly reported wound healing rates: The fantasy and the reality. Adv. Wound Care 2018, 7, 77–94. [Google Scholar] [CrossRef]
- Tottoli, E.M.; Dorati, R.; Genta, I.; Chiesa, E.; Pisani, S.; Conti, B. Skin wound healing process and new emerging technologies for skin wound care and regeneration. Pharmaceutics 2020, 12, 735. [Google Scholar] [CrossRef]
- Larouche, J.; Sheoran, S.; Maruyama, K.; Martino, M.M. Immune regulation of skin wound healing: Mechanisms and novel therapeutic targets. Adv. Wound Care 2018, 7, 209–231. [Google Scholar] [CrossRef]
- Ellis, S.; Lin, E.J.; Tartar, D. Immunology of wound healing. Curr. Dermatol. Rep. 2018, 7, 350–358. [Google Scholar] [CrossRef]
- Su, L.; Zheng, J.; Wang, Y.; Zhang, W.; Hu, D. Emerging progress on the mechanism and technology in wound repair. Biomed. Pharmacother. 2019, 117, 109191. [Google Scholar] [CrossRef]
- Cañedo-Dorantes, L.; Cañedo-Ayala, M. Skin acute wound healing: A comprehensive review. Int. J. Inflamm. 2019, 2019, 3706315. [Google Scholar] [CrossRef]
- Azevedo, M.M.; Lisboa, C.; Cobrado, L.; Pina-Vaz, C.; Rodrigues, A. Hard-to-heal wounds, biofilm and wound healing: An intricate interrelationship. Br. J. Nurs. 2020, 29, S6–S13. [Google Scholar] [CrossRef]
- Wu, Y.K.; Cheng, N.C.; Cheng, C.M. Biofilms in chronic wounds: Pathogenesis and diagnosis. Trends Biotechnol. 2019, 37, 505–517. [Google Scholar] [CrossRef]
- Saleh, B.; Dhaliwal, H.K.; Portillo-Lara, R.; Shirzaei Sani, E.; Abdi, R.; Amiji, M.M.; Annabi, N. Local Immunomodulation using an adhesive hydrogel loaded with miRNA-laden nanoparticles promotes wound healing. Small 2019, 15, e1902232. [Google Scholar] [CrossRef]
- Krzyszczyk, P.; Schloss, R.; Palmer, A.; Berthiaume, F. The role of macrophages in acute and chronic wound healing and interventions to promote pro-wound healing phenotypes. Front. Physiol. 2018, 9, 419. [Google Scholar] [CrossRef]
- Omar, A.; Wright, J.B.; Schultz, G.; Burrell, R.; Nadworny, P. Microbial biofilms and chronic wounds. Microorganisms 2017, 5, 9. [Google Scholar] [CrossRef]
- Kathawala, M.H.; Ng, W.L.; Liu, D.; Naing, M.W.; Yeong, W.Y.; Spiller, K.L.; Van Dyke, M.; Ng, K.W. Healing of chronic wounds: An update of recent developments and future possibilities. Tissue Eng. Part B Rev. 2019, 25, 429–444. [Google Scholar] [CrossRef]
- Opdenakker, G.; Van Damme, J.; Vranckx, J.J. Immunomodulation as rescue for chronic atonic skin wounds. Trends Immunol. 2018, 39, 341–354. [Google Scholar] [CrossRef]
- Dehkordi, A.N.; Babaheydari, F.M.; Chehelgerdi, M.; Dehkordi, S.R. Skin tissue engineering: Wound healing based on stem-cell-based therapeutic strategies. Stem Cell Res. Ther. 2019, 10, 111. [Google Scholar] [CrossRef]
- Kim, H.S.; Sun, X.; Lee, J.H.; Kim, H.W.; Fu, X.; Leong, K.W. Advanced drug delivery systems and artificial skin grafts for skin wound healing. Adv. Drug Deliv. Rev. 2019, 146, 209–239. [Google Scholar] [CrossRef]
- Shanley, L.C.; Mahon, O.R.; Kelly, D.J.; Dunne, A. Harnessing the innate and adaptive immune system for tissue repair and regeneration: Considering more than macrophages. Acta Biomater. 2021. [Google Scholar] [CrossRef]
- Nurkesh, A.; Jaguparov, A.; Jimi, S.; Saparov, A. Recent advances in the controlled release of growth factors and cytokines for improving cutaneous wound healing. Front. Cell Dev. Biol. 2020, 8, 638. [Google Scholar] [CrossRef]
- Sultankulov, B.; Berillo, D.; Kauanova, S.; Mikhalovsky, S.; Mikhalovska, L.; Saparov, A. Composite cryogel with polyelectrolyte complexes for growth factor delivery. Pharmaceutics 2019, 11, 650. [Google Scholar] [CrossRef]
- Mansurov, N.; Chen, W.C.; Awada, H.; Huard, J.; Wang, Y.; Saparov, A. A controlled release system for simultaneous delivery of three human perivascular stem cell-derived factors for tissue repair and regeneration. J. Tissue Eng. Regen. Med. 2018, 12, e1164–e1172. [Google Scholar] [CrossRef]
- Wilkinson, H.N.; Hardman, M.J. Wound healing: Cellular mechanisms and pathological outcomes. Open Biol. 2020, 10, 200223. [Google Scholar] [CrossRef]
- Maouia, A.; Rebetz, J.; Kapur, R.; Semple, J.W. The immune nature of platelets revisited. Transfus. Med. Rev. 2020, 34, 209–220. [Google Scholar] [CrossRef]
- Etulain, J. Platelets in wound healing and regenerative medicine. Platelets 2018, 29, 556–568. [Google Scholar] [CrossRef]
- Opneja, A.; Kapoor, S.; Stavrou, E.X. Contribution of platelets, the coagulation and fibrinolytic systems to cutaneous wound healing. Thromb. Res. 2019, 179, 56–63. [Google Scholar] [CrossRef]
- Del Amo, C.; Perez-Valle, A.; Perez-Zabala, E.; Perez-Del-Pecho, K.; Larrazabal, A.; Basterretxea, A.; Bully, P.; Andia, I. Wound Dressing Selection Is Critical to Enhance Platelet-Rich Fibrin Activities in Wound Care. Int. J. Mol. Sci. 2020, 21, 624. [Google Scholar] [CrossRef]
- Sharda, A.; Flaumenhaft, R. The life cycle of platelet granules. F1000Research 2018, 7, 236. [Google Scholar] [CrossRef]
- Ren, Q.; Chan, K.W.; Huang, H.; Wang, Z.; Fang, X.; Guo, C.; Li, F.; Zhang, L.; Yao, Y.; Chen, Z.; et al. Platelet-derived alpha-granules are associated with inflammation in patients with NK/T-cell lymphoma-associated hemophagocytic syndrome. Cytokine 2020, 126, 154878. [Google Scholar] [CrossRef]
- Chicharro-Alcántara, D.; Rubio-Zaragoza, M.; Damiá-Giménez, E.; Carrillo-Poveda, J.M.; Cuervo-Serrato, B.; Peláez-Gorrea, P.; Sopena-Juncosa, J.J. Platelet rich plasma: New Insights for cutaneous wound healing management. J. Funct. Biomater. 2018, 9, 10. [Google Scholar] [CrossRef] [PubMed]
- Chong, D.L.W.; Trinder, S.; Labelle, M.; Rodriguez-Justo, M.; Hughes, S.; Holmes, A.M.; Scotton, C.J.; Porter, J.C. Platelet-derived transforming growth factor-β1 promotes keratinocyte proliferation in cutaneous wound healing. J. Tissue Eng. Regen. Med. 2020, 14, 645–649. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.W.; Chen, W.L.; Huang, S.M.; Chan, J.Y. Platelet-derived growth factor-AA is a substantial factor in the ability of adipose-derived stem cells and endothelial progenitor cells to enhance wound healing. FASEB J. 2019, 33, 2388–2395. [Google Scholar] [CrossRef] [PubMed]
- Komi, D.E.A.; Khomtchouk, K.; Santa Maria, P.L. A review of the contribution of mast cells in wound healing: Involved molecular and cellular mechanisms. Clin. Rev. Allergy Immunol. 2020, 58, 298–312. [Google Scholar] [CrossRef]
- Korkmaz, H.I.; Krijnen, P.A.J.; Ulrich, M.M.W.; de Jong, E.; van Zuijlen, P.P.M.; Niessen, H.W.M. The role of complement in the acute phase response after burns. Burns J. Int. Soc. Burn Inj. 2017, 43, 1390–1399. [Google Scholar] [CrossRef] [PubMed]
- Wilgus, T.A. Alerting the body to tissue injury: The role of alarmins and DAMPs in cutaneous wound healing. Curr. Pathobiol. Rep. 2018, 6, 55–60. [Google Scholar] [CrossRef]
- Rani, M.; Nicholson, S.E.; Zhang, Q.; Schwacha, M.G. Damage-associated molecular patterns (DAMPs) released after burn are associated with inflammation and monocyte activation. Burns J. Int. Soc. Burn Inj. 2017, 43, 297–303. [Google Scholar] [CrossRef]
- Land, W.G. Use of DAMPs and SAMPs as therapeutic targets or therapeutics: A note of caution. Mol. Diagn. Ther. 2020, 24, 251–262. [Google Scholar] [CrossRef]
- Chen, L.; DiPietro, L.A. Toll-like receptor function in acute wounds. Adv. Wound Care 2017, 6, 344–355. [Google Scholar] [CrossRef]
- Piipponen, M.; Li, D.; Landén, N.X. The immune functions of keratinocytes in skin wound healing. Int. J. Mol. Sci. 2020, 21, 8790. [Google Scholar] [CrossRef]
- Chen, L.; Guo, S.; Ranzer, M.J.; DiPietro, L.A. Toll-like receptor 4 has an essential role in early skin wound healing. J. Investig. Dermatol. 2013, 133, 258–267. [Google Scholar] [CrossRef]
- Jimi, S.; Saparov, A.; Takagi, S. Cellular and molecular mechanisms at the proliferation stage in wound healing: From scarring to tissue regeneration. Front. Cell Dev. Biol. 2021, 9, 267. [Google Scholar] [CrossRef]
- Pellefigues, C.; Mehta, P.; Chappell, S.; Yumnam, B.; Old, S.; Camberis, M.; Le Gros, G. Diverse innate stimuli activate basophils through pathways involving Syk and IκB kinases. Proc. Natl. Acad. Sci. USA 2021, 118. [Google Scholar] [CrossRef]
- Yamanishi, Y.; Mogi, K.; Takahashi, K.; Miyake, K.; Yoshikawa, S.; Karasuyama, H. Skin-infiltrating basophils promote atopic dermatitis-like inflammation via IL-4 production in mice. Allergy 2020, 75, 2613–2622. [Google Scholar] [CrossRef]
- Nguyen, J.K.; Austin, E.; Huang, A.; Mamalis, A.; Jagdeo, J. The IL-4/IL-13 axis in skin fibrosis and scarring: Mechanistic concepts and therapeutic targets. Arch. Dermatol. Res. 2020, 312, 81–92. [Google Scholar] [CrossRef]
- Zimmermann, C.; Troeltzsch, D.; Giménez-Rivera, V.A.; Galli, S.J.; Metz, M.; Maurer, M.; Siebenhaar, F. Mast cells are critical for controlling the bacterial burden and the healing of infected wounds. Proc. Natl. Acad. Sci. USA 2019, 116, 20500–20504. [Google Scholar] [CrossRef]
- Ud-Din, S.; Wilgus, T.A.; Bayat, A. Mast cells in skin scarring: A review of animal and human research. Front. Immunol. 2020, 11, 552205. [Google Scholar] [CrossRef]
- Egozi, E.I.; Ferreira, A.M.; Burns, A.L.; Gamelli, R.L.; Dipietro, L.A. Mast cells modulate the inflammatory but not the proliferative response in healing wounds. Wound Repair Regen. 2003, 11, 46–54. [Google Scholar] [CrossRef]
- Wulff, B.C.; Parent, A.E.; Meleski, M.A.; DiPietro, L.A.; Schrementi, M.E.; Wilgus, T.A. Mast cells contribute to scar formation during fetal wound healing. J. Investig. Dermatol. 2012, 132, 458–465. [Google Scholar] [CrossRef]
- Nishida, K.; Hasegawa, A.; Yamasaki, S.; Uchida, R.; Ohashi, W.; Kurashima, Y.; Kunisawa, J.; Kimura, S.; Iwanaga, T.; Watarai, H.; et al. Mast cells play role in wound healing through the ZnT2/GPR39/IL-6 axis. Sci. Rep. 2019, 9, 10842. [Google Scholar] [CrossRef]
- Nosaka, M.; Ishida, Y.; Kimura, A.; Kuninaka, Y.; Taruya, A.; Ozaki, M.; Tanaka, A.; Mukaida, N.; Kondo, T. Crucial Involvement of IL-6 in Thrombus Resolution in Mice via Macrophage Recruitment and the Induction of Proteolytic Enzymes. Front. Immunol. 2019, 10, 3150. [Google Scholar] [CrossRef] [PubMed]
- Arifuzzaman, M.; Mobley, Y.R.; Choi, H.W.; Bist, P.; Salinas, C.A.; Brown, Z.D.; Chen, S.L.; Staats, H.F.; Abraham, S.N. MRGPR-mediated activation of local mast cells clears cutaneous bacterial infection and protects against reinfection. Sci. Adv. 2019, 5, eaav0216. [Google Scholar] [CrossRef] [PubMed]
- Phillipson, M.; Kubes, P. The Healing Power of Neutrophils. Trends Immunol. 2019, 40, 635–647. [Google Scholar] [CrossRef] [PubMed]
- Kovtun, A.; Messerer, D.A.C.; Scharffetter-Kochanek, K.; Huber-Lang, M.; Ignatius, A. Neutrophils in Tissue trauma of the skin, bone, and lung: Two sides of the same coin. J. Immunol. Res. 2018, 2018, 8173983. [Google Scholar] [CrossRef] [PubMed]
- Kirchner, S.; Lei, V.; MacLeod, A.S. The cutaneous wound innate immunological microenvironment. Int. J. Mol. Sci. 2020, 21, 8748. [Google Scholar] [CrossRef] [PubMed]
- Peiseler, M.; Kubes, P. More friend than foe: The emerging role of neutrophils in tissue repair. J. Clin. Investig. 2019, 129, 2629–2639. [Google Scholar] [CrossRef]
- Vono, M.; Lin, A.; Norrby-Teglund, A.; Koup, R.A.; Liang, F.; Loré, K. Neutrophils acquire the capacity for antigen presentation to memory CD4(+) T cells in vitro and ex vivo. Blood 2017, 129, 1991–2001. [Google Scholar] [CrossRef]
- Minns, D.; Smith, K.J.; Alessandrini, V.; Hardisty, G.; Melrose, L.; Jackson-Jones, L.; MacDonald, A.S.; Davidson, D.J.; Gwyer Findlay, E. The neutrophil antimicrobial peptide cathelicidin promotes Th17 differentiation. Nat. Commun. 2021, 12, 1285. [Google Scholar] [CrossRef]
- Brockmann, L.; Giannou, A.D.; Gagliani, N.; Huber, S. Regulation of T(H)17 Cells and Associated Cytokines in Wound Healing, Tissue Regeneration, and Carcinogenesis. Int. J. Mol. Sci. 2017, 18, 1033. [Google Scholar] [CrossRef]
- Hesketh, M.; Sahin, K.B.; West, Z.E.; Murray, R.Z. Macrophage Phenotypes Regulate Scar Formation and Chronic Wound Healing. Int. J. Mol. Sci. 2017, 18, 1545. [Google Scholar] [CrossRef]
- Dovi, J.V.; He, L.K.; DiPietro, L.A. Accelerated wound closure in neutrophil-depleted mice. J. Leukoc. Biol. 2003, 73, 448–455. [Google Scholar] [CrossRef]
- Bouti, P.; Webbers, S.D.S.; Fagerholm, S.C.; Alon, R.; Moser, M.; Matlung, H.L.; Kuijpers, T.W. β2 integrin signaling cascade in neutrophils: More than a single function. Front. Immunol. 2020, 11, 619925. [Google Scholar] [CrossRef]
- Jun, J.I.; Lau, L.F. CCN1 is an opsonin for bacterial clearance and a direct activator of Toll-like receptor signaling. Nat. Commun. 2020, 11, 1242. [Google Scholar] [CrossRef]
- Kim, K.H.; Won, J.H.; Cheng, N.; Lau, L.F. The matricellular protein CCN1 in tissue injury repair. J. Cell Commun. Signal. 2018, 12, 273–279. [Google Scholar] [CrossRef]
- Minutti, C.M.; Knipper, J.A.; Allen, J.E.; Zaiss, D.M. Tissue-specific contribution of macrophages to wound healing. Semin. Cell Dev. Biol. 2017, 61, 3–11. [Google Scholar] [CrossRef]
- Boniakowski, A.E.; Kimball, A.S.; Jacobs, B.N.; Kunkel, S.L.; Gallagher, K.A. Macrophage-mediated inflammation in normal and diabetic wound healing. J. Immunol. 2017, 199, 17–24. [Google Scholar] [CrossRef]
- Kim, S.Y.; Nair, M.G. Macrophages in wound healing: Activation and plasticity. Immunol. Cell Biol. 2019, 97, 258–267. [Google Scholar] [CrossRef]
- Kim, Y.; Nurakhayev, S.; Nurkesh, A.; Zharkinbekov, Z.; Saparov, A. Macrophage polarization in cardiac tissue repair following myocardial infarction. Int. J. Mol. Sci. 2021, 22, 2715. [Google Scholar] [CrossRef]
- Brazil, J.C.; Quiros, M.; Nusrat, A.; Parkos, C.A. Innate immune cell-epithelial crosstalk during wound repair. J. Clin. Investig. 2019, 129, 2983–2993. [Google Scholar] [CrossRef]
- Xu, M.; Chen, Z.; Chen, K.; Ma, D.; Chen, L.; DiPietro, L.A. Phagocytosis of apoptotic endothelial cells reprograms macrophages in skin wounds. J. Immunol. Regen. Med. 2021, 12, 100038. [Google Scholar]
- Deckers, J.; Hammad, H.; Hoste, E. Langerhans cells: Sensing the environment in health and disease. Front. Immunol. 2018, 9, 93. [Google Scholar] [CrossRef] [PubMed]
- Hovav, A.H. Mucosal and skin langerhans cells—Nurture calls. Trends Immunol. 2018, 39, 788–800. [Google Scholar] [CrossRef] [PubMed]
- Rajesh, A.; Stuart, G.; Real, N.; Ahn, J.; Tschirley, A.; Wise, L.; Hibma, M. Depletion of langerin(+) cells enhances cutaneous wound healing. Immunology 2020, 160, 366–381. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Lamb, R.; Coles, M.C.; Bennett, C.L.; Ambler, C.A. Inducible ablation of CD11c(+) cells to determine their role in skin wound repair. Immunology 2021. [Google Scholar] [CrossRef]
- Ho, A.W.; Kupper, T.S. T cells and the skin: From protective immunity to inflammatory skin disorders. Nat. Rev. Immunol. 2019, 19, 490–502. [Google Scholar] [CrossRef]
- Matejuk, A. Skin immunity. Arch. Immunol. Ther. Exp. 2018, 66, 45–54. [Google Scholar] [CrossRef]
- Zaiss, D.M.; Minutti, C.M.; Knipper, J.A. Immune- and non-immune-mediated roles of regulatory T-cells during wound healing. Immunology 2019, 157, 190–197. [Google Scholar] [CrossRef]
- Boothby, I.C.; Cohen, J.N.; Rosenblum, M.D. Regulatory T cells in skin injury: At the crossroads of tolerance and tissue repair. Sci. Immunol. 2020, 5. [Google Scholar] [CrossRef]
- Wang, X.; Balaji, S.; Steen, E.H.; Li, H.; Rae, M.M.; Blum, A.J.; Miao, Q.; Butte, M.J.; Bollyky, P.L.; Keswani, S.G. T lymphocytes attenuate dermal scarring by regulating inflammation, neovascularization, and extracellular matrix remodeling. Adv. Wound Care 2019, 8, 527–537. [Google Scholar] [CrossRef]
- Johnson, M.D.; Witherden, D.A.; Havran, W.L. The role of tissue-resident T cells in stress surveillance and tissue maintenance. Cells 2020, 9, 686. [Google Scholar] [CrossRef]
- Munoz, L.D.; Sweeney, M.J.; Jameson, J.M. Skin Resident γδ T Cell Function and Regulation in Wound Repair. Int. J. Mol. Sci. 2020, 21, 9286. [Google Scholar] [CrossRef]
- Heath, W.R.; Carbone, F.R. The skin-resident and migratory immune system in steady state and memory: Innate lymphocytes, dendritic cells and T cells. Nat. Immunol. 2013, 14, 978–985. [Google Scholar] [CrossRef]
- Schenkel, J.M.; Fraser, K.A.; Vezys, V.; Masopust, D. Sensing and alarm function of resident memory CD8⁺ T cells. Nat. Immunol. 2013, 14, 509–513. [Google Scholar] [CrossRef]
- Weaver, C.T.; Saparov, A.; Kraus, L.A.; Rogers, W.O.; Hockett, R.D.; Bucy, R.P. Heterogeneity in the clonal T cell response. Implications for models of T cell activation and cytokine phenotype development. Immunol. Res. 1998, 17, 279–302. [Google Scholar] [CrossRef]
- Scharschmidt, T.C.; Vasquez, K.S.; Truong, H.A.; Gearty, S.V.; Pauli, M.L.; Nosbaum, A.; Gratz, I.K.; Otto, M.; Moon, J.J.; Liese, J.; et al. A wave of regulatory T cells into neonatal skin mediates tolerance to commensal microbes. Immunity 2015, 43, 1011–1021. [Google Scholar] [CrossRef]
- Hirahara, K.; Liu, L.; Clark, R.A.; Yamanaka, K.; Fuhlbrigge, R.C.; Kupper, T.S. The majority of human peripheral blood CD4+CD25highFoxp3+ regulatory T cells bear functional skin-homing receptors. J. Immunol. 2006, 177, 4488–4494. [Google Scholar] [CrossRef]
- Haertel, E.; Joshi, N.; Hiebert, P.; Kopf, M.; Werner, S. Regulatory T cells are required for normal and activin-promoted wound repair in mice. Eur. J. Immunol. 2018, 48, 1001–1013. [Google Scholar] [CrossRef]
- Romano, M.; Fanelli, G.; Albany, C.J.; Giganti, G.; Lombardi, G. Past, present, and future of regulatory T cell therapy in transplantation and autoimmunity. Front. Immunol. 2019, 10, 43. [Google Scholar] [CrossRef]
- Gieseck, R.L., 3rd; Wilson, M.S.; Wynn, T.A. Type 2 immunity in tissue repair and fibrosis. Nat. Rev. Immunol. 2018, 18, 62–76. [Google Scholar] [CrossRef]
- Farhadihosseinabadi, B.; Salimi, M.; Kazemi, B.; Samadikuchaksaraei, A.; Ghanbarian, H.; Mozafari, M.; Niknejad, H. Inducing type 2 immune response, induction of angiogenesis, and anti-bacterial and anti-inflammatory properties make Lacto-n-Neotetraose (LNnT) a therapeutic choice to accelerate the wound healing process. Med. Hypotheses 2020, 134, 109389. [Google Scholar] [CrossRef]
- DiToro, D.; Harbour, S.N.; Bando, J.K.; Benavides, G.; Witte, S.; Laufer, V.A.; Moseley, C.; Singer, J.R.; Frey, B.; Turner, H.; et al. Insulin-like growth factors are key regulators of T Helper 17 regulatory T cell balance in autoimmunity. Immunity 2020, 52, 650–667.e610. [Google Scholar] [CrossRef] [PubMed]
- Geherin, S.A.; Fintushel, S.R.; Lee, M.H.; Wilson, R.P.; Patel, R.T.; Alt, C.; Young, A.J.; Hay, J.B.; Debes, G.F. The skin, a novel niche for recirculating B cells. J. Immunol. 2012, 188, 6027–6035. [Google Scholar] [CrossRef] [PubMed]
- Sîrbulescu, R.F.; Boehm, C.K.; Soon, E.; Wilks, M.Q.; Ilieş, I.; Yuan, H.; Maxner, B.; Chronos, N.; Kaittanis, C.; Normandin, M.D.; et al. Mature B cells accelerate wound healing after acute and chronic diabetic skin lesions. Wound Repair Regen. 2017, 25, 774–791. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.Z.; Gou, M.; Da, L.C.; Zhang, W.Q.; Xie, H.Q. Mesenchymal stem cells for chronic wound healing: Current status of preclinical and clinical studies. Tissue Eng. Part B Rev. 2020, 26, 555–570. [Google Scholar] [CrossRef]
- Tomic-Canic, M.; Burgess, J.L.; O’Neill, K.E.; Strbo, N.; Pastar, I. Skin microbiota and its interplay with wound healing. Am. J. Clin. Dermatol. 2020, 21, 36–43. [Google Scholar] [CrossRef]
- Bagheri, M.; Mostafavinia, A.; Abdollahifar, M.A.; Amini, A.; Ghoreishi, S.K.; Chien, S.; Hamblin, M.R.; Bayat, S.; Bayat, M. Combined effects of metformin and photobiomodulation improve the proliferation phase of wound healing in type 2 diabetic rats. Biomed. Pharmacother. 2020, 123, 109776. [Google Scholar] [CrossRef]
- Seraphim, P.M.; Leal, E.C.; Moura, J.; Gonçalves, P.; Gonçalves, J.P.; Carvalho, E. Lack of lymphocytes impairs macrophage polarization and angiogenesis in diabetic wound healing. Life Sci. 2020, 254, 117813. [Google Scholar] [CrossRef]
- Joshi, N.; Pohlmeier, L.; Ben-Yehuda Greenwald, M.; Haertel, E.; Hiebert, P.; Kopf, M.; Werner, S. Comprehensive characterization of myeloid cells during wound healing in healthy and healing-impaired diabetic mice. Eur. J. Immunol. 2020, 50, 1335–1349. [Google Scholar] [CrossRef]
- Dong, J.; Chen, L.; Zhang, Y.; Jayaswal, N.; Mezghani, I.; Zhang, W.; Veves, A. Mast Cells in diabetes and diabetic wound healing. Adv. Ther. 2020, 37, 4519–4537. [Google Scholar] [CrossRef]
- Strang, H.; Kaul, A.; Parikh, U.; Masri, L.; Saravanan, S.; Li, H.; Miao, Q.; Balaji, S. Role of cytokines and chemokines in wound healing. In Wound Healing, Tissue Repair, and Regeneration in Diabetes; Bagchi, D., Das, A., Roy, S., Eds.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 197–235. [Google Scholar]
- Li, D.; Peng, H.; Qu, L.; Sommar, P.; Wang, A.; Chu, T.; Li, X.; Bi, X.; Liu, Q.; Gallais Sérézal, I.; et al. miR-19a/b and miR-20a promote wound healing by regulating the inflammatory response of keratinocytes. J. Investig. Dermatol. 2021, 141, 659–671. [Google Scholar] [CrossRef]
- Yuan, L.; Sun, Y.; Xu, M.; Zeng, F.; Xiong, X. miR-203 Acts as an inhibitor for epithelial-mesenchymal transition process in diabetic foot ulcers via targeting interleukin-8. Neuroimmunomodulation 2019, 26, 239–249. [Google Scholar] [CrossRef]
- Wu, J.; Li, X.; Li, D.; Ren, X.; Li, Y.; Herter, E.K.; Qian, M.; Toma, M.A.; Wintler, A.M.; Sérézal, I.G.; et al. MicroRNA-34 family enhances wound inflammation by targeting LGR4. J. Investig. Dermatol. 2020, 140, 465–476.e411. [Google Scholar] [CrossRef]
- Nie, X.; Zhao, J.; Ling, H.; Deng, Y.; Li, X.; He, Y. Exploring microRNAs in diabetic chronic cutaneous ulcers: Regulatory mechanisms and therapeutic potential. Br. J. Pharmacol. 2020, 177, 4077–4095. [Google Scholar] [CrossRef]
- Theocharidis, G.; Baltzis, D.; Roustit, M.; Tellechea, A.; Dangwal, S.; Khetani, R.S.; Shu, B.; Zhao, W.; Fu, J.; Bhasin, S.; et al. Integrated skin transcriptomics and serum multiplex assays reveal novel mechanisms of wound healing in diabetic foot ulcers. Diabetes 2020, 69, 2157–2169. [Google Scholar] [CrossRef]
- Saito, K.; Iwata, Y.; Fukushima, H.; Watanabe, S.; Tanaka, Y.; Hasegawa, Y.; Akiyama, M.; Sugiura, K. IL-36 receptor antagonist deficiency resulted in delayed wound healing due to excessive recruitment of immune cells. Sci. Rep. 2020, 10, 14772. [Google Scholar] [CrossRef]
- Barros, J.F.; Waclawiak, I.; Pecli, C.; Borges, P.A.; Georgii, J.L.; Ramos-Junior, E.S.; Canetti, C.; Courau, T.; Klatzmann, D.; Kunkel, S.L.; et al. Role of chemokine receptor CCR4 and regulatory T cells in wound healing of diabetic mice. J. Investig. Dermatol. 2019, 139, 1161–1170. [Google Scholar] [CrossRef]
- Sawaya, A.P.; Stone, R.C.; Brooks, S.R.; Pastar, I.; Jozic, I.; Hasneen, K.; O’Neill, K.; Mehdizadeh, S.; Head, C.R.; Strbo, N.; et al. Deregulated immune cell recruitment orchestrated by FOXM1 impairs human diabetic wound healing. Nat. Commun. 2020, 11, 4678. [Google Scholar] [CrossRef]
- Dissemond, J.; Augustin, M.; Dietlein, M.; Faust, U.; Keuthage, W.; Lobmann, R.; Münter, K.C.; Strohal, R.; Stücker, M.; Traber, J.; et al. Efficacy of MMP-inhibiting wound dressings in the treatment of chronic wounds: A systematic review. J. Wound Care 2020, 29, 102–118. [Google Scholar] [CrossRef]
- Goldberg, S.R.; Diegelmann, R.F. What makes wounds chronic. Surg. Clin. New Am. 2020, 100, 681–693. [Google Scholar] [CrossRef]
- Kaur, K.; Singh, A.; Attri, S.; Malhotra, D.; Verma, A.; Bedi, N.; Bedi, P.M.S. Matrix metalloproteinases (MMPs) and diabetic foot: Pathophysiological findings and recent developments in their inhibitors of natural as well as synthetic origin. In The Eye and Foot in Diabetes; Grigsby, J., Derbel, F., Eds.; IntechOpen: London, UK, 2020. [Google Scholar]
- Nguyen, T.T.; Ding, D.; Wolter, W.R.; Pérez, R.L.; Champion, M.M.; Mahasenan, K.V.; Hesek, D.; Lee, M.; Schroeder, V.A.; Jones, J.I.; et al. Validation of matrix metalloproteinase-9 (MMP-9) as a novel target for treatment of diabetic foot ulcers in humans and discovery of a potent and selective small-molecule MMP-9 inhibitor that accelerates healing. J. Med. Chem. 2018, 61, 8825–8837. [Google Scholar] [CrossRef]
- Lang, J.; Yang, C.; Liu, L.; Li, L.; Wu, L.; Liu, Y.; Luo, H.; Yan, L.; Chen, S.; Ning, J.; et al. High glucose activates ERK1/2 to stabilize AP1 and increase MMP9 expression in diabetic foot ulcers. Exp. Cell Res. 2021, 112550. [Google Scholar] [CrossRef]
- Han, G.; Havnaer, A.; Lee, H.H.; Carmichael, D.J.; Martinez, L.R. Biological depletion of neutrophils attenuates pro-inflammatory markers and the development of the psoriatic phenotype in a murine model of psoriasis. Clin. Immunol. 2020, 210, 108294. [Google Scholar] [CrossRef]
- Bi, X.; Zhou, L.; Liu, Y.; Gu, J.; Mi, Q. MicroRNA-146a deficiency delays wound healing in normal and diabetic mice. Adv. Wound Care 2021. [Google Scholar] [CrossRef]
- Umehara, T.; Mori, R.; Mace, K.A.; Murase, T.; Abe, Y.; Yamamoto, T.; Ikematsu, K. Identification of Specific miRNAs in Neutrophils of Type 2 Diabetic Mice: Overexpression of miRNA-129-2-3p Accelerates Diabetic Wound Healing. Diabetes 2019, 68, 617–630. [Google Scholar] [CrossRef]
- Yang, S.; Gu, Z.; Lu, C.; Zhang, T.; Guo, X.; Xue, G.; Zhang, L. Neutrophil Extracellular Traps Are Markers of Wound Healing Impairment in Patients with Diabetic Foot Ulcers Treated in a Multidisciplinary Setting. Adv. Wound Care 2020, 9, 16–27. [Google Scholar] [CrossRef]
- Kaur, T.; Dumoga, S.; Koul, V.; Singh, N. Modulating neutrophil extracellular traps for wound healing. Biomater. Sci. 2020, 8, 3212–3223. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.S.; Kang, S.U.; Lee, M.H.; Kim, H.J.; Han, C.H.; Won, H.R.; Park, Y.U.; Kim, C.H. GnRH impairs diabetic wound healing through enhanced NETosis. Cell. Mol. Immunol. 2020, 17, 856–864. [Google Scholar] [CrossRef]
- Huang, W.; Jiao, J.; Liu, J.; Huang, M.; Hu, Y.; Ran, W.; Yan, L.; Xiong, Y.; Li, M.; Quan, Z.; et al. MFG-E8 accelerates wound healing in diabetes by regulating “NLRP3 inflammasome-neutrophil extracellular traps” axis. Cell Death Discov. 2020, 6, 84. [Google Scholar] [CrossRef]
- Zomer, H.D.; Jeremias, T.D.S.; Ratner, B.; Trentin, A.G. Mesenchymal stromal cells from dermal and adipose tissues induce macrophage polarization to a pro-repair phenotype and improve skin wound healing. Cytotherapy 2020, 22, 247–260. [Google Scholar] [CrossRef] [PubMed]
- Kohno, K.; Koya-Miyata, S.; Harashima, A.; Tsukuda, T.; Katakami, M.; Ariyasu, T.; Ushio, S.; Iwaki, K. Inflammatory M1-like macrophages polarized by NK-4 undergo enhanced phenotypic switching to an anti-inflammatory M2-like phenotype upon co-culture with apoptotic cells. J. Inflamm. 2021, 18, 2. [Google Scholar] [CrossRef] [PubMed]
- Kimball, A.S.; Davis, F.M.; den Dekker, A.; Joshi, A.D.; Schaller, M.A.; Bermick, J.; Xing, X.; Burant, C.F.; Obi, A.T.; Nysz, D.; et al. The Histone Methyltransferase Setdb2 Modulates Macrophage Phenotype and Uric Acid Production in Diabetic Wound Repair. Immunity 2019, 51, 258–271.e255. [Google Scholar] [CrossRef]
- Liechty, C.; Hu, J.; Zhang, L.; Liechty, K.W.; Xu, J. Role of microRNA-21 and Its Underlying Mechanisms in Inflammatory Responses in Diabetic Wounds. Int. J. Mol. Sci. 2020, 21, 3328. [Google Scholar] [CrossRef]
- DiPietro, L.A.; Wilgus, T.A.; Koh, T.J. Macrophages in Healing Wounds: Paradoxes and Paradigms. Int. J. Mol. Sci. 2021, 22, 950. [Google Scholar] [CrossRef]
- Feng, Y.; Sun, Z.L.; Liu, S.Y.; Wu, J.J.; Zhao, B.H.; Lv, G.Z.; Du, Y.; Yu, S.; Yang, M.L.; Yuan, F.L.; et al. Direct and Indirect Roles of Macrophages in Hypertrophic Scar Formation. Front. Physiol. 2019, 10, 1101. [Google Scholar] [CrossRef]
- Gay, D.; Ghinatti, G.; Guerrero-Juarez, C.F.; Ferrer, R.A.; Ferri, F.; Lim, C.H.; Murakami, S.; Gault, N.; Barroca, V.; Rombeau, I.; et al. Phagocytosis of Wnt inhibitor SFRP4 by late wound macrophages drives chronic Wnt activity for fibrotic skin healing. Sci. Adv. 2020, 6, eaay3704. [Google Scholar] [CrossRef]
- Jiang, D.; Rinkevich, Y. Scars or regeneration? Dermal fibroblasts as drivers of diverse skin wound responses. Int. J. Mol. Sci. 2020, 21, 617. [Google Scholar] [CrossRef]
- Chen, Y.L.; Hardman, C.S.; Yadava, K.; Ogg, G. Innate lymphocyte mechanisms in skin diseases. Ann. Rev. Immunol. 2020, 38, 171–202. [Google Scholar] [CrossRef]
- Rak, G.D.; Osborne, L.C.; Siracusa, M.C.; Kim, B.S.; Wang, K.; Bayat, A.; Artis, D.; Volk, S.W. IL-33-Dependent group 2 innate lymphoid cells promote cutaneous wound healing. J. Investig. Dermatol. 2016, 136, 487–496. [Google Scholar] [CrossRef]
- Kanno, E.; Tanno, H.; Masaki, A.; Sasaki, A.; Sato, N.; Goto, M.; Shisai, M.; Yamaguchi, K.; Takagi, N.; Shoji, M.; et al. Defect of interferon γ leads to impaired wound healing through prolonged neutrophilic inflammatory response and enhanced MMP-2 Activation. Int. J. Mol. Sci. 2019, 20, 5657. [Google Scholar] [CrossRef]
- Victor, A.R.; Nalin, A.P.; Dong, W.; McClory, S.; Wei, M.; Mao, C.; Kladney, R.D.; Youssef, Y.; Chan, W.K.; Briercheck, E.L.; et al. IL-18 drives ILC3 proliferation and promotes IL-22 production via NF-κB. J. Immunol. 2017, 199, 2333–2342. [Google Scholar] [CrossRef]
- Li, Z.; Hodgkinson, T.; Gothard, E.J.; Boroumand, S.; Lamb, R.; Cummins, I.; Narang, P.; Sawtell, A.; Coles, J.; Leonov, G.; et al. Epidermal Notch1 recruits RORγ(+) group 3 innate lymphoid cells to orchestrate normal skin repair. Nat. Commun. 2016, 7, 11394. [Google Scholar] [CrossRef] [PubMed]
- Kalekar, L.A.; Rosenblum, M.D. Regulatory T cells in inflammatory skin disease: From mice to humans. Int. Immunol. 2019, 31, 457–463. [Google Scholar] [CrossRef] [PubMed]
- Ameri, A.H.; Moradi Tuchayi, S.; Zaalberg, A.; Park, J.H.; Ngo, K.H.; Li, T.; Lopez, E.; Colonna, M.; Lee, R.T.; Mino-Kenudson, M.; et al. IL-33/regulatory T cell axis triggers the development of a tumor-promoting immune environment in chronic inflammation. Proc. Natl. Acad. Sci. USA 2019, 116, 2646–2651. [Google Scholar] [CrossRef] [PubMed]
- Linehan, J.L.; Harrison, O.J.; Han, S.J.; Byrd, A.L.; Vujkovic-Cvijin, I.; Villarino, A.V.; Sen, S.K.; Shaik, J.; Smelkinson, M.; Tamoutounour, S.; et al. Non-classical Immunity controls microbiota impact on skin immunity and tissue repair. Cell 2018, 172, 784–796.e718. [Google Scholar] [CrossRef]
- Debes, G.F.; McGettigan, S.E. Skin-associated B cells in health and inflammation. J. Immunol. 2019, 202, 1659–1666. [Google Scholar] [CrossRef]
- Domingo, S.; Solé, C.; Moliné, T.; Ferrer, B.; Cortés-Hernández, J. MicroRNAs in several cutaneous autoimmune diseases: Psoriasis, cutaneous lupus erythematosus and atopic dermatitis. Cells 2020, 9, 2656. [Google Scholar] [CrossRef]
- Toita, R.; Shimizu, E.; Murata, M.; Kang, J.H. Protective and healing effects of apoptotic mimic-induced M2-like macrophage polarization on pressure ulcers in young and middle-aged mice. J. Control. Release 2021, 330, 705–714. [Google Scholar] [CrossRef]
- Ferreira, D.W.; Ulecia-Morón, C.; Alvarado-Vázquez, P.A.; Cunnane, K.; Moracho-Vilriales, C.; Grosick, R.L.; Cunha, T.M.; Romero-Sandoval, E.A. CD163 overexpression using a macrophage-directed gene therapy approach improves wound healing in ex vivo and in vivo human skin models. Immunobiology 2020, 225, 151862. [Google Scholar] [CrossRef]
- Kim, H.; Wang, S.Y.; Kwak, G.; Yang, Y.; Kwon, I.C.; Kim, S.H. Exosome-guided phenotypic switch of M1 to M2 macrophages for cutaneous wound healing. Adv. Sci. 2019, 6, 1900513. [Google Scholar] [CrossRef]
- Hu, J.; Zhang, L.; Liechty, C.; Zgheib, C.; Hodges, M.M.; Liechty, K.W.; Xu, J. Long noncoding RNA GAS5 regulates macrophage polarization and diabetic wound healing. J. Investig. Dermatol. 2020, 140, 1629–1638. [Google Scholar] [CrossRef]
- Nguyen, V.T.; Farman, N.; Palacios-Ramirez, R.; Sbeih, M.; Behar-Cohen, F.; Aractingi, S.; Jaisser, F. Cutaneous Wound healing in diabetic mice is improved by topical mineralocorticoid receptor blockade. J. Investig. Dermatol. 2020, 140, 223–234.e227. [Google Scholar] [CrossRef]
- Jia, Y.C.; Qiu, S.; Xu, J.; Kang, Q.L.; Chai, Y.M. Docosahexaenoic acid improves diabetic wound healing in a rat model by restoring impaired plasticity of macrophage progenitor cells. Plast. Reconstr. Surg. 2020, 145, 942e–950e. [Google Scholar] [CrossRef]
- Jia, Y.; Wei, Y. Modulators of MicroRNA function in the immune system. Int. J. Mol. Sci. 2020, 21, 2357. [Google Scholar] [CrossRef]
- Mulholland, E.J.; Dunne, N.; McCarthy, H.O. MicroRNA as therapeutic targets for chronic wound healing. Mol. Ther. Nucleic Acids 2017, 8, 46–55. [Google Scholar] [CrossRef]
- Niemiec, S.M.; Louiselle, A.E.; Liechty, K.W.; Zgheib, C. Role of microRNAs in pressure ulcer immune response, pathogenesis, and treatment. Int. J. Mol. Sci. 2020, 22, 64. [Google Scholar] [CrossRef]
- Huang, J.; Fu, J.; Liu, B.; Wang, R.; You, T. A synthetic curcuminoid analog, (2E,6E)-2,6-bis(2-(trifluoromethyl)benzylidene) cyclohexanone, ameliorates impaired wound healing in streptozotocin-induced diabetic mice by increasing miR-146a. Molecules 2020, 25, 920. [Google Scholar] [CrossRef]
- Zgheib, C.; Hilton, S.A.; Dewberry, L.C.; Hodges, M.M.; Ghatak, S.; Xu, J.; Singh, S.; Roy, S.; Sen, C.K.; Seal, S.; et al. Use of cerium oxide nanoparticles conjugated with MicroRNA-146a to correct the diabetic wound healing impairment. J. Am. Coll. Surg. 2019, 228, 107–115. [Google Scholar] [CrossRef]
- Li, Q.; Zhao, H.; Chen, W.; Huang, P.; Bi, J. Human keratinocyte-derived microvesicle miRNA-21 promotes skin wound healing in diabetic rats through facilitating fibroblast function and angiogenesis. Int. J. Biochem. Cell Biol. 2019, 114, 105570. [Google Scholar] [CrossRef]
- Wu, Y.; Zhang, K.; Liu, R.; Zhang, H.; Chen, D.; Yu, S.; Chen, W.; Wan, S.; Zhang, Y.; Jia, Z.; et al. MicroRNA-21-3p accelerates diabetic wound healing in mice by downregulating SPRY1. Aging 2020, 12, 15436–15445. [Google Scholar] [CrossRef]
- Jimi, S.; Jaguparov, A.; Nurkesh, A.; Sultankulov, B.; Saparov, A. Sequential delivery of cryogel released growth factors and cytokines accelerates wound healing and improves tissue regeneration. Front. Bioeng. Biotechnol. 2020, 8, 345. [Google Scholar] [CrossRef]
- Ritsu, M.; Kawakami, K.; Kanno, E.; Tanno, H.; Ishii, K.; Imai, Y.; Maruyama, R.; Tachi, M. Critical role of tumor necrosis factor-α in the early process of wound healing in skin. J. Dermatol. Dermatol. Surg. 2017, 21, 14–19. [Google Scholar] [CrossRef]
- Huang, S.M.; Wu, C.S.; Chiu, M.H.; Wu, C.H.; Chang, Y.T.; Chen, G.S.; Lan, C.E. High glucose environment induces M1 macrophage polarization that impairs keratinocyte migration via TNF-α: An important mechanism to delay the diabetic wound healing. J. Dermatol. Sci. 2019, 96, 159–167. [Google Scholar] [CrossRef]
- Perrault, D.P.; Bramos, A.; Xu, X.; Shi, S.; Wong, A.K. Local administration of interleukin-1 receptor antagonist improves diabetic wound healing. Ann. Plast. Surg. 2018, 80, S317–S321. [Google Scholar] [CrossRef]
- Tan, J.L.; Lash, B.; Karami, R.; Nayer, B.; Lu, Y.Z.; Piotto, C.; Julier, Z.; Martino, M.M. Restoration of the healing microenvironment in diabetic wounds with matrix-binding IL-1 receptor antagonist. Commun. Biol. 2021, 4, 422. [Google Scholar] [CrossRef]
- Zubair, M.; Ahmad, J. Role of growth factors and cytokines in diabetic foot ulcer healing: A detailed review. Rev. Endocr. Metab. Disord. 2019, 20, 207–217. [Google Scholar] [CrossRef]
- Oliveira, A.; Simões, S.; Ascenso, A.; Reis, C.P. Therapeutic advances in wound healing. J. Dermatol. Treat. 2020, 1–21. [Google Scholar] [CrossRef]
- Pan, H.; Shi, C.; Yang, R.; Xi, G.; Lu, C.; Wang, X.; Chen, J.; Chen, L.; Pan, J. Controlled release of KGF-2 for regulation of wound healing by KGF-2 complexed with “lotus seedpod surface-like” porous microsphere. J. Mater. Chem. B 2021. [Google Scholar] [CrossRef]
- Kucharzewski, M.; Rojczyk, E.; Wilemska-Kucharzewska, K.; Wilk, R.; Hudecki, J.; Los, M.J. Novel trends in application of stem cells in skin wound healing. Eur. J. Pharmacol. 2019, 843, 307–315. [Google Scholar] [CrossRef]
- Ren, S.; Chen, J.; Duscher, D.; Liu, Y.; Guo, G.; Kang, Y.; Xiong, H.; Zhan, P.; Wang, Y.; Wang, C.; et al. Microvesicles from human adipose stem cells promote wound healing by optimizing cellular functions via AKT and ERK signaling pathways. Stem Cell Res. Ther. 2019, 10, 47. [Google Scholar] [CrossRef]
- Raziyeva, K.; Smagulova, A.; Kim, Y.; Smagul, S.; Nurkesh, A.; Saparov, A. Preconditioned and genetically modified stem cells for myocardial infarction treatment. Int. J. Mol. Sci. 2020, 21, 7301. [Google Scholar] [CrossRef]
- Fernandes-Cunha, G.M.; Na, K.S.; Putra, I.; Lee, H.J.; Hull, S.; Cheng, Y.C.; Blanco, I.J.; Eslani, M.; Djalilian, A.R.; Myung, D. Corneal wound healing effects of mesenchymal stem cell secretome delivered within a viscoelastic gel carrier. Stem Cells Transl. Med. 2019, 8, 478–489. [Google Scholar] [CrossRef] [PubMed]
- Domenis, R.; Cifù, A.; Quaglia, S.; Pistis, C.; Moretti, M.; Vicario, A.; Parodi, P.C.; Fabris, M.; Niazi, K.R.; Soon-Shiong, P.; et al. Pro inflammatory stimuli enhance the immunosuppressive functions of adipose mesenchymal stem cells-derived exosomes. Sci. Rep. 2018, 8, 13325. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Xie, X.; Lian, W.; Shi, R.; Han, S.; Zhang, H.; Lu, L.; Li, M. Exosomes from adipose-derived stem cells overexpressing Nrf2 accelerate cutaneous wound healing by promoting vascularization in a diabetic foot ulcer rat model. Exp. Mol. Med. 2018, 50, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.Y.; Rao, S.S.; Ren, L.; Hu, X.K.; Tan, Y.J.; Hu, Y.; Luo, J.; Liu, Y.W.; Yin, H.; Huang, J.; et al. Exosomal DMBT1 from human urine-derived stem cells facilitates diabetic wound repair by promoting angiogenesis. Theranostics 2018, 8, 1607–1623. [Google Scholar] [CrossRef]
- Stojanović, S.; Najman, S. The Effect of Conditioned Media of Stem Cells Derived from Lipoma and Adipose Tissue on Macrophages’ Response and Wound Healing in Indirect Co-culture System In Vitro. Int. J. Mol. Sci. 2019, 20, 1671. [Google Scholar] [CrossRef]
- Omar, M.T.; Gwada, R.F.; Shaheen, A.A.; Saggini, R. Extracorporeal shockwave therapy for the treatment of chronic wound of lower extremity: Current perspective and systematic review. Int. Wound J. 2017, 14, 898–908. [Google Scholar] [CrossRef]
- Takagi, S.; Oyama, T.; Jimi, S.; Saparov, A.; Ohjimi, H. A novel autologous micrografts technology in combination with negative pressure wound therapy (NPWT) for quick granulation tissue formation in chronic/refractory ulcer. Healthcare 2020, 8, 513. [Google Scholar] [CrossRef]
- Liu, S.; He, C.Z.; Cai, Y.T.; Xing, Q.P.; Guo, Y.Z.; Chen, Z.L.; Su, J.L.; Yang, L.P. Evaluation of negative-pressure wound therapy for patients with diabetic foot ulcers: Systematic review and meta-analysis. Ther. Clin. Risk Manag. 2017, 13, 533–544. [Google Scholar] [CrossRef]
- Wynn, M.; Freeman, S. The efficacy of negative pressure wound therapy for diabetic foot ulcers: A systematised review. J. Tissue Viability 2019, 28, 152–160. [Google Scholar] [CrossRef]
- Wang, T.; He, R.; Zhao, J.; Mei, J.C.; Shao, M.Z.; Pan, Y.; Zhang, J.; Wu, H.S.; Yu, M.; Yan, W.C.; et al. Negative pressure wound therapy inhibits inflammation and upregulates activating transcription factor-3 and downregulates nuclear factor-κB in diabetic patients with foot ulcerations. Diabetes Metab. Res. Rev. 2017, 33. [Google Scholar] [CrossRef]
- Wang, T.; Li, X.; Fan, L.; Chen, B.; Liu, J.; Tao, Y.; Wang, X. Negative pressure wound therapy promoted wound healing by suppressing inflammation via down-regulating MAPK-JNK signaling pathway in diabetic foot patients. Diabetes Res. Clin. Pract. 2019, 150, 81–89. [Google Scholar] [CrossRef]
- Song, H.; Xu, Y.; Chang, W.; Zhuang, J.; Wu, X. Negative pressure wound therapy promotes wound healing by suppressing macrophage inflammation in diabetic ulcers. Regen. Med. 2020, 15, 2341–2349. [Google Scholar] [CrossRef]
| Immunomodulation Strategy | Treatment | Wound Type | Mediated Effects | Reference |
|---|---|---|---|---|
| M2 macrophage polarization | Phosphatidylserine-containing liposomes | Pressure ulcer in young and middle-aged mice | Prevented pressure ulcer formation, promoted wound healing and enhanced ECM deposition and angiogenesis | [139] |
| Exosomes derived from M2 macrophages | Acute skin wounds in a murine model | Accelerated primary as well as complete wound closure; enhanced re-epithelization, ECM formation and angiogenesis | [141] | |
| Knockdown of long non-coding RNA GAS5 | Diabetic wounds in a murine model | Accelerated wound closure | [142] | |
| Topical pharmacological blockade of the mineralocorticoid receptor | Diabetic wounds in a murine model | Accelerated wound closure and improved angiogenesis; suppressed inflammation | [143] | |
| Docosahexaenoic acid | Diabetic wounds in a murine model | Faster wound healing; increased vessel density and perfusion; alleviated inflammation | [144] | |
| microRNA overexpression/stimulation | microRNA-146a overexpression with a synthetic curcuminoid analog | Diabetic wounds in a murine model | Enhanced wound closure, faster re-epithelialization, suppressed inflammatory mediators | [148] |
| Cerium Oxide Nanoparticles Conjugated with MicroRNA-146a | Diabetic wounds in murine and porcine models | Accelerated wound closure, increased strength and elasticity in a murine model; improved wound healing, increased angiogenesis and reduced inflammation in a porcine model | [149] | |
| Human keratinocyte-derived microvesicles expressing microRNA-21 | Diabetic wounds in a rat model | Rapid wound closure, increased angiogenesis and enhanced fibroblast differentiation | [150] | |
| miR-21-3p agonist | Diabetic wounds in a murine model | Accelerated wound healing and enhanced fibroblast activity | [151] | |
| Pro-inflammatory cytokine inhibition | Etanercept, a TNF-α neutralizing peptide | Diabetic wounds in a rat model | Improved wound healing and closure | [154] |
| IL-1R antagonist | Diabetic wounds in a murine model | Reduced inflammation, decreased neutrophil and macrophage infiltration, accelerated wound closure | [155] | |
| Growth factors | PDGF-BB | Ulcers in diabetic patients | Attracted neutrophils and macrophages into the wound; stimulated fibroblast recruitment and proliferation; enhanced collagen synthesis and ECM deposition; and accelerated wound healing | [158] |
| KGF-2 | A full-layer skin cutting model in rats | Suppressed inflammation and accelerated wound healing | [159] | |
| Stem cells and microvesicles | Human adipose stem cell-derived microvesicles | Full-thickness cutaneous wound models in mice | Accelerated wound closure, enhanced re-epithelialization, collagen deposition and angiogenesis, increased cell proliferation | [161] |
| Human adipose stem cell-derived microvesicles | Diabetic skin wound models in rats | Enhanced formation of granulation tissue, increased angiogenesis, greater expression of growth factors, decreased inflammatory and oxidative factors | [165] | |
| Exosomes from human urine-derived stem cells | Full-thickness excisional skin wounds in diabetic mice | Accelerated wound healing and enhanced angiogenesis | [166] | |
| Lipoma-derived stem cells | In vitro “scratch” wound assay | Increased fibroblast migration and wound closure | [167] | |
| Negative pressure wound therapy | Vacuum-assisted closure (VAC) therapy system | Human diabetic foot ulcers | Attenuated inflammation, increased ECM formation, decreased the expression of TNF-α, IL-6 and iNOS | [172] |
| Vacuum-assisted closure (VAC) therapy system | Human diabetic foot ulcers | Suppressed wound inflammation, decreased levels of IL-6, iNOS, TNF-α and P-c-Jun N-terminal kinase | [173] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Raziyeva, K.; Kim, Y.; Zharkinbekov, Z.; Kassymbek, K.; Jimi, S.; Saparov, A. Immunology of Acute and Chronic Wound Healing. Biomolecules 2021, 11, 700. https://doi.org/10.3390/biom11050700
Raziyeva K, Kim Y, Zharkinbekov Z, Kassymbek K, Jimi S, Saparov A. Immunology of Acute and Chronic Wound Healing. Biomolecules. 2021; 11(5):700. https://doi.org/10.3390/biom11050700
Chicago/Turabian StyleRaziyeva, Kamila, Yevgeniy Kim, Zharylkasyn Zharkinbekov, Kuat Kassymbek, Shiro Jimi, and Arman Saparov. 2021. "Immunology of Acute and Chronic Wound Healing" Biomolecules 11, no. 5: 700. https://doi.org/10.3390/biom11050700
APA StyleRaziyeva, K., Kim, Y., Zharkinbekov, Z., Kassymbek, K., Jimi, S., & Saparov, A. (2021). Immunology of Acute and Chronic Wound Healing. Biomolecules, 11(5), 700. https://doi.org/10.3390/biom11050700









