Glycine-Conjugated Bile Acids Protect RPE Tight Junctions against Oxidative Stress and Inhibit Choroidal Endothelial Cell Angiogenesis In Vitro
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Culture
2.2. HRPEpiC Trans-Epithelial Electrical Resistance Assay
2.3. Immunostaining of HRPEpiC Tight Junctions
2.4. RF/6A Cell Proliferation Assay
2.5. RF/6A Transwell Cell Migration Assay
2.6. RF/6A Cell Tube Formation Assay
2.7. Statistical Analysis
3. Results
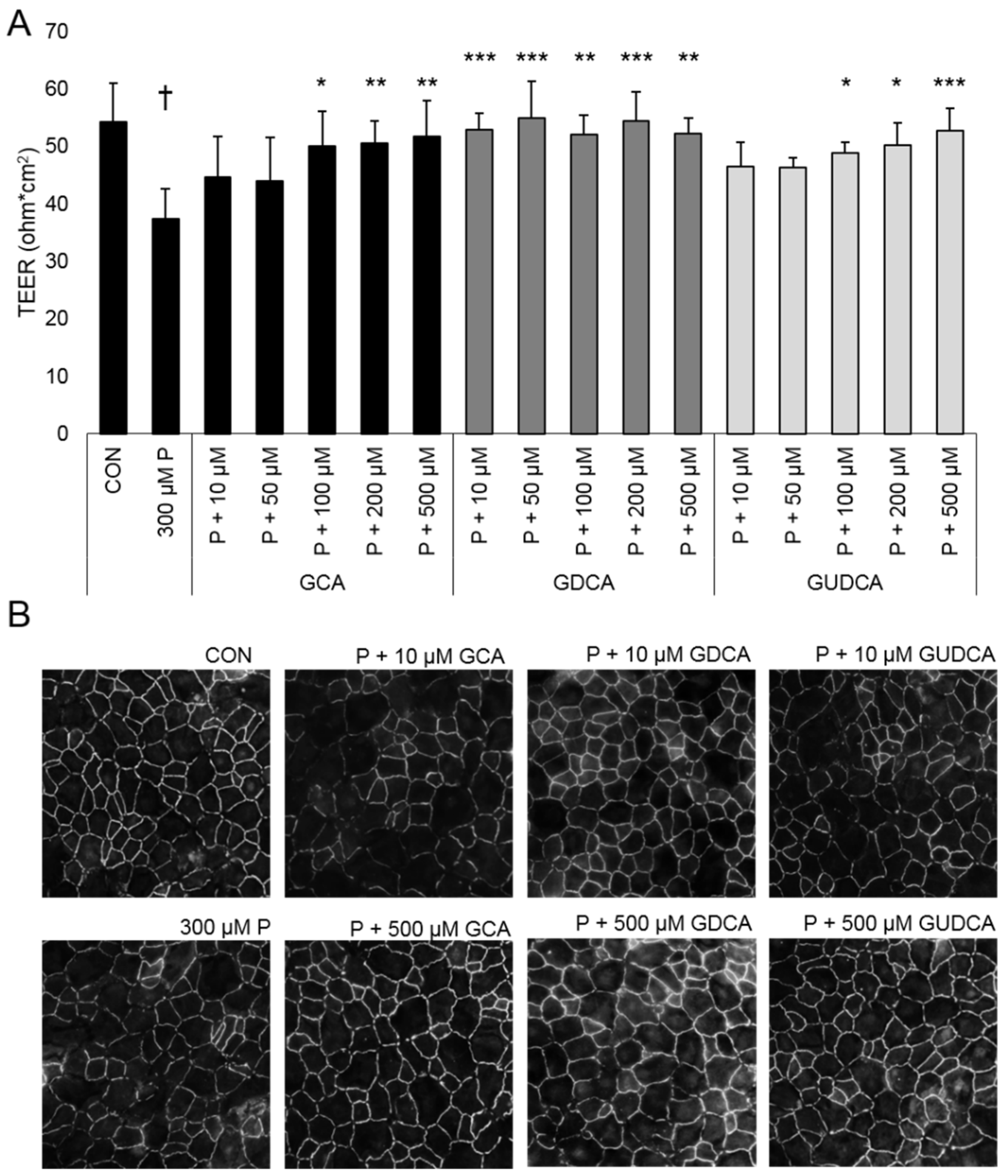
3.1. GCA, GDCA, and GUDCA Protect RPE Tight Junctions against Oxidative Stress
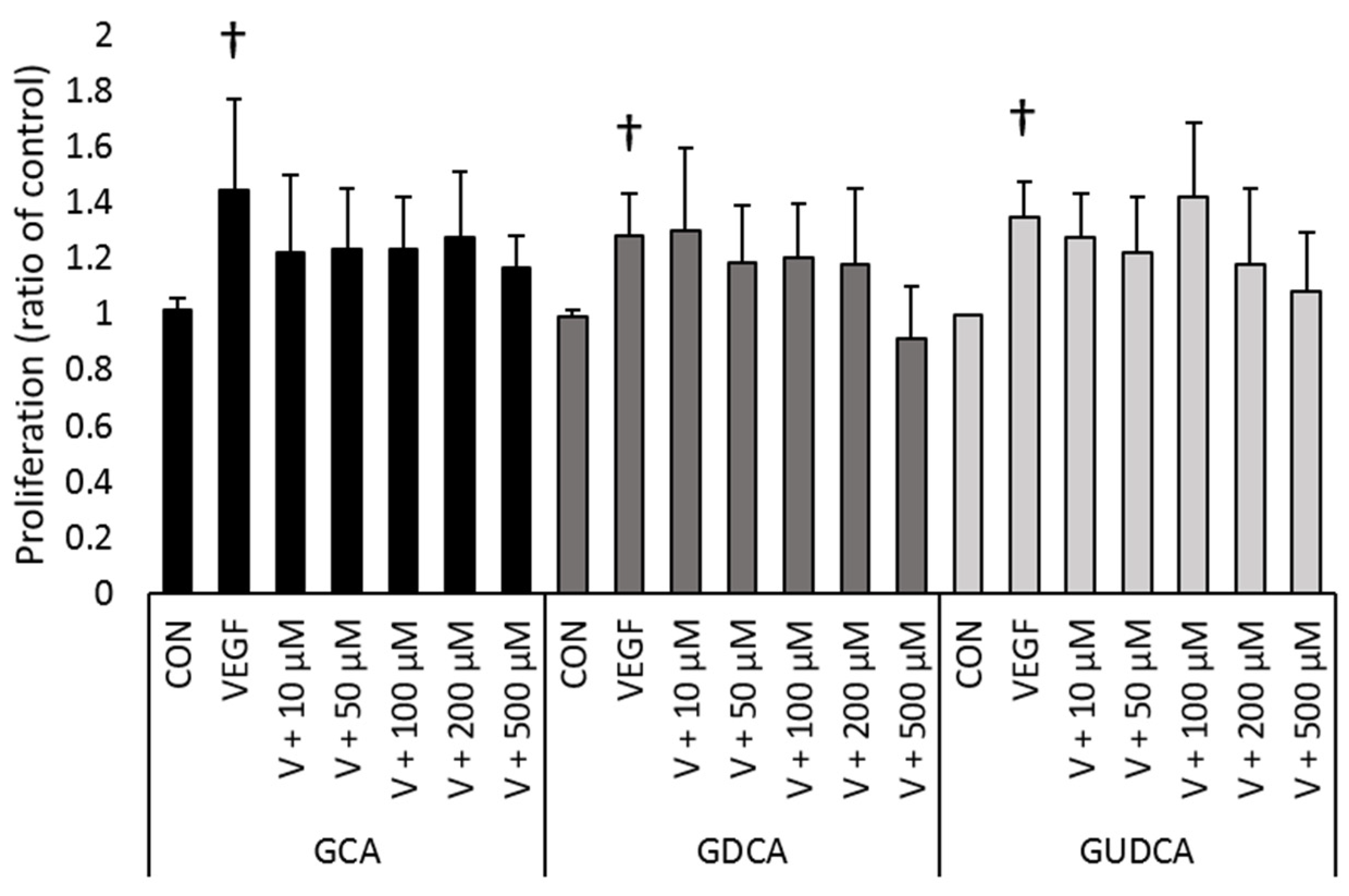
3.2. GCA, GDCA, and GUDCA Do Not Inhibit VEGF-Induced CEC Proliferation
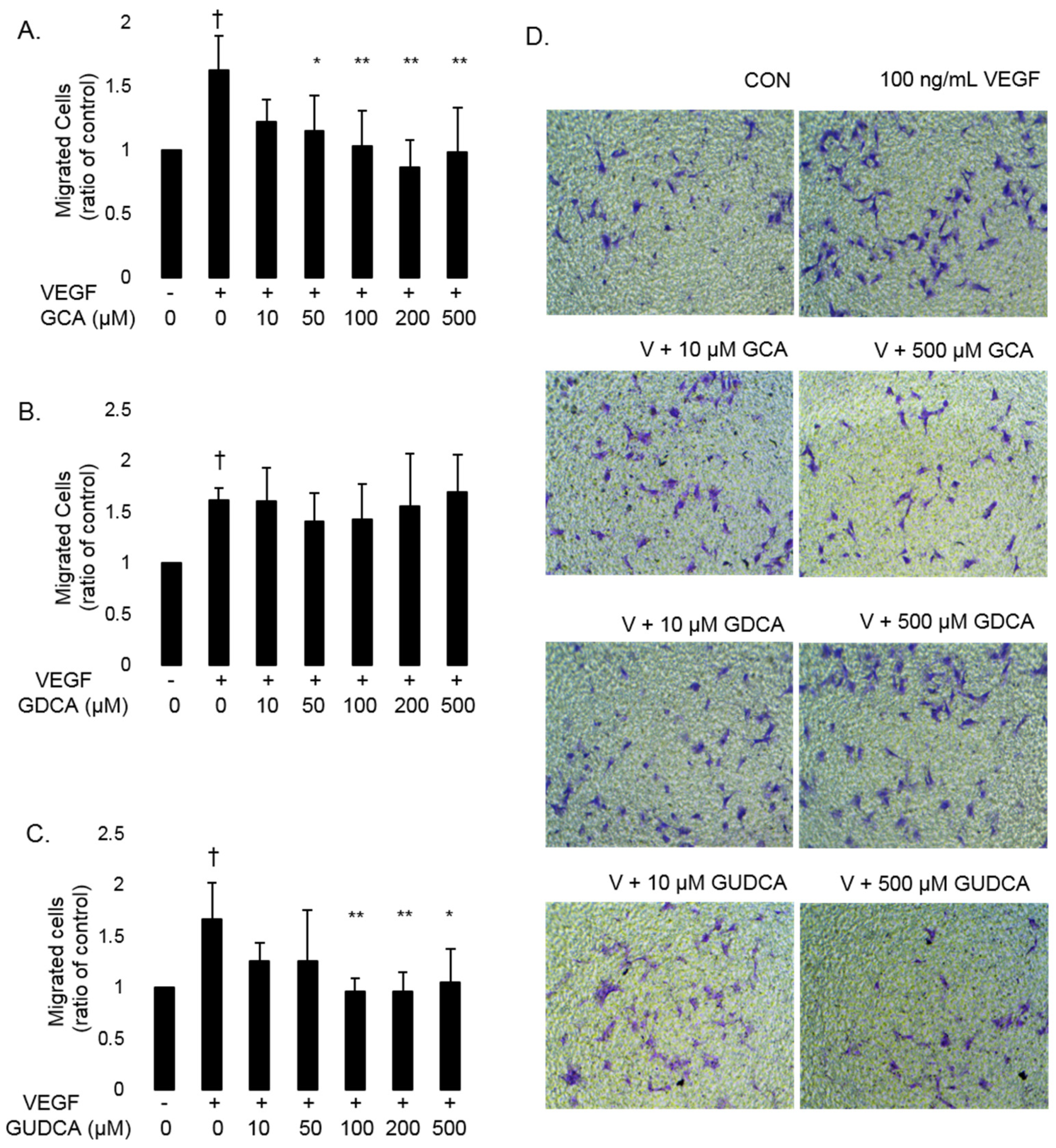
3.3. GCA and GUDCA Inhibit VEGF-Induced CEC Migration
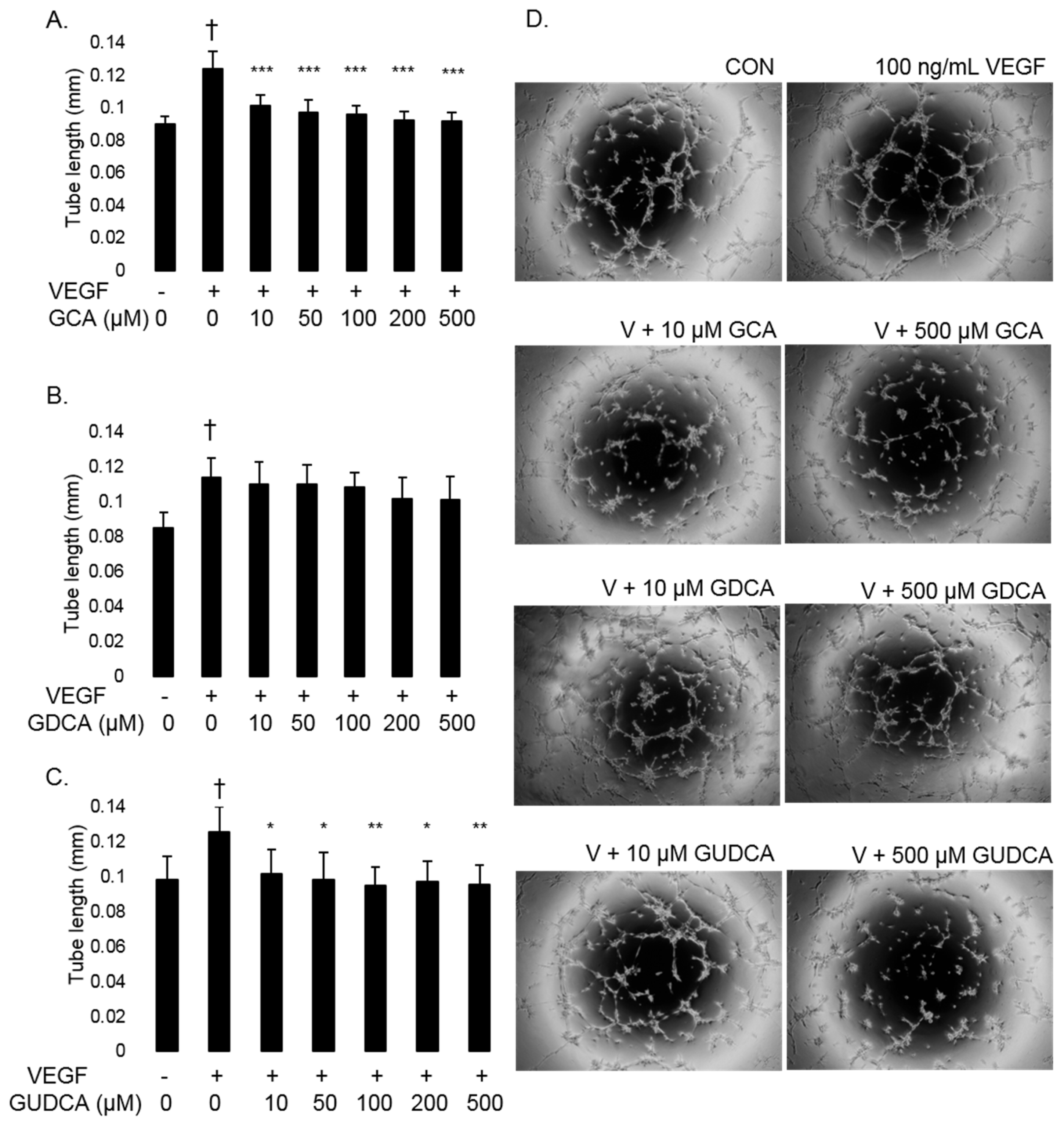
3.4. GCA and GUDCA Inhibit VEGF-Induced CEC Tube Formation
4. Discussion
Author Contributions
Funding
Conflicts of Interest
References
- Hylemon, P.B.; Zhou, H.; Pandak, W.M.; Ren, S.; Gil, G.; Dent, P. Bile Acids as Regulatory Molecules. J. Lipid Res. 2009, 50, 1509–1520. [Google Scholar] [CrossRef]
- Boatright, J.H.; Moring, A.G.; McElroy, C.; Phillips, M.J.; Do, V.T.; Chang, B.; Hawes, N.L.; Boyd, A.P.; Sidney, S.S.; Stewart, R.E.; et al. Tool from Ancient Pharmacopoeia Prevents Vision Loss. Mol. Vis. 2006, 12, 1706–1714. [Google Scholar]
- Drack, A.V.; Dumitrescu, A.V.; Bhattarai, S.; Gratie, D.; Stone, E.M.; Mullins, R.; Sheffield, V.C. TUDCA Slows Retinal Degeneration in Two Different Mouse Models of Retinitis Pigmentosa and Prevents Obesity in Bardet-Biedl Syndrome Type 1 Mice. Investig. Ophthalmol. Vis. Sci. 2012, 53, 100–106. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Sánchez, L.; Lax, P.; Pinilla, I.; Martín-Nieto, J.; Cuenca, N. Tauroursodeoxycholic Acid Prevents Retinal Degeneration in Transgenic P23H Rats. Investig. Ophthalmol. Vis. Sci. 2011, 52, 4998–5008. [Google Scholar] [CrossRef] [PubMed]
- Lawson, E.C.; Bhatia, S.K.; Han, M.K.; Aung, M.H.; Ciavatta, V.; Boatright, J.H.; Pardue, M.T. Tauroursodeoxycholic Acid Protects Retinal Function and Structure in Rd1 Mice. Adv. Exp. Med. Biol. 2016, 854, 431–436. [Google Scholar] [CrossRef] [PubMed]
- Phillips, M.J.; Walker, T.A.; Choi, H.-Y.; Faulkner, A.E.; Kim, M.K.; Sidney, S.S.; Boyd, A.P.; Nickerson, J.M.; Boatright, J.H.; Pardue, M.T. Tauroursodeoxycholic Acid Preservation of Photoreceptor Structure and Function in the Rd10 Mouse through Postnatal Day 30. Investig. Ophthalmol. Vis. Sci. 2008, 49, 2148–2155. [Google Scholar] [CrossRef] [PubMed]
- Shiraya, T.; Araki, F.; Ueta, T.; Fukunaga, H.; Totsuka, K.; Arai, T.; Uemura, A.; Moriya, K.; Kato, S. Ursodeoxycholic Acid Attenuates the Retinal Vascular Abnormalities in Anti-PDGFR-β Antibody-Induced Pericyte Depletion Mouse Models. Sci. Rep. 2020, 10, 977. [Google Scholar] [CrossRef]
- Beli, E.; Yan, Y.; Moldovan, L.; Vieira, C.P.; Gao, R.; Duan, Y.; Prasad, R.; Bhatwadekar, A.; White, F.A.; Townsend, S.D.; et al. Restructuring of the Gut Microbiome by Intermittent Fasting Prevents Retinopathy and Prolongs Survival in Db/Db Mice. Diabetes 2018, 67, 1867–1879. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Wang, W.; Xie, T.-H.; Zou, J.; Nie, X.; Wang, X.; Zhang, M.-Y.; Wang, Z.-Y.; Gu, S.; Zhuang, M.; et al. TGR5 Receptor Activation Attenuates Diabetic Retinopathy through Suppression of RhoA/ROCK Signaling. FASEB J. 2020, 34, 4189–4203. [Google Scholar] [CrossRef] [PubMed]
- Alhasani, R.H.; Almarhoun, M.; Zhou, X.; Reilly, J.; Patterson, S.; Zeng, Z.; Shu, X. Tauroursodeoxycholic Acid Protects Retinal Pigment Epithelial Cells from Oxidative Injury and Endoplasmic Reticulum Stress In Vitro. Biomedicines 2020, 8, 367. [Google Scholar] [CrossRef]
- Wang, C.-F.; Yuan, J.-R.; Qin, D.; Gu, J.-F.; Zhao, B.-J.; Zhang, L.; Zhao, D.; Chen, J.; Hou, X.-F.; Yang, N.; et al. Protection of Tauroursodeoxycholic Acid on High Glucose-Induced Human Retinal Microvascular Endothelial Cells Dysfunction and Streptozotocin-Induced Diabetic Retinopathy Rats. J. Ethnopharmacol. 2016, 185, 162–170. [Google Scholar] [CrossRef] [PubMed]
- Woo, S.J.; Kim, J.H.; Yu, H.G. Ursodeoxycholic Acid and Tauroursodeoxycholic Acid Suppress Choroidal Neovascularization in a Laser-Treated Rat Model. J. Ocul. Pharmacol. Ther. 2010, 26, 223–229. [Google Scholar] [CrossRef] [PubMed]
- Osborn, M.P.; Park, Y.; Parks, M.B.; Burgess, L.G.; Uppal, K.; Lee, K.; Jones, D.P.; Brantley, M.A. Metabolome-Wide Association Study of Neovascular Age-Related Macular Degeneration. PLoS ONE 2013, 8, e72737. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, S.L.; Uppal, K.; Williamson, S.M.; Liu, K.; Burgess, L.G.; Tran, V.; Umfress, A.C.; Jarrell, K.L.; Cooke Bailey, J.N.; Agarwal, A.; et al. The Carnitine Shuttle Pathway Is Altered in Patients With Neovascular Age-Related Macular Degeneration. Investig. Ophthalmol. Vis. Sci. 2018, 59, 4978–4985. [Google Scholar] [CrossRef]
- Warden, C.; Barnett, J.M.; Brantley, M.A. Taurocholic Acid Inhibits Features of Age-Related Macular Degeneration in Vitro. Exp. Eye Res. 2020, 193, 107974. [Google Scholar] [CrossRef]
- Garcia-Ramírez, M.; Villarroel, M.; Corraliza, L.; Hernández, C.; Simó, R. Measuring Permeability in Human Retinal Epithelial Cells (ARPE-19): Implications for the Study of Diabetic Retinopathy. Methods Mol. Biol. Clifton NJ 2011, 763, 179–194. [Google Scholar] [CrossRef]
- Justus, C.R.; Leffler, N.; Ruiz-Echevarria, M.; Yang, L.V. In Vitro Cell Migration and Invasion Assays. J. Vis. Exp. JoVE 2014. [Google Scholar] [CrossRef]
- Daruich, A.; Picard, E.; Boatright, J.H.; Behar-Cohen, F. Review: The Bile Acids Urso- and Tauroursodeoxycholic Acid as Neuroprotective Therapies in Retinal Disease. Mol. Vis. 2019, 25, 610–624. [Google Scholar]
- Perez, M.-J.; Briz, O. Bile-Acid-Induced Cell Injury and Protection. World J. Gastroenterol. 2009, 15, 1677–1689. [Google Scholar] [CrossRef] [PubMed]
- Monte, M.J.; Marin, J.J.G.; Antelo, A.; Vazquez-Tato, J. Bile Acids: Chemistry, Physiology, and Pathophysiology. World J. Gastroenterol. 2009, 15, 804–816. [Google Scholar] [CrossRef] [PubMed]
- Drechsel, D.A.; Patel, M. Differential Contribution of the Mitochondrial Respiratory Chain Complexes to Reactive Oxygen Species Production by Redox Cycling Agents Implicated in Parkinsonism. Toxicol. Sci. 2009, 112, 427–434. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.A.; Jung, J.H.; Kang, I.G.; Choi, Y.S.; Kim, S.T. ROS Is Involved in Disruption of Tight Junctions of Human Nasal Epithelial Cells Induced by HRV16. Laryngoscope 2018, 128, E393–E401. [Google Scholar] [CrossRef]
- Narimatsu, T.; Ozawa, Y.; Miyake, S.; Kubota, S.; Hirasawa, M.; Nagai, N.; Shimmura, S.; Tsubota, K. Disruption of Cell-Cell Junctions and Induction of Pathological Cytokines in the Retinal Pigment Epithelium of Light-Exposed Mice. Investig. Ophthalmol. Vis. Sci. 2013, 54, 4555–4562. [Google Scholar] [CrossRef] [PubMed]
- Xie, L.; Zhou, D.; Xiong, J.; You, J.; Zeng, Y.; Peng, L. Paraquat Induce Pulmonary Epithelial–Mesenchymal Transition through Transforming Growth Factor-Β1-Dependent Mechanism. Exp. Toxicol. Pathol. 2016, 68, 69–76. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Tan, J.; Xie, H.; Wang, J.; Meng, X.; Wang, R. HIF-1α Regulates EMT via the Snail and Β-catenin Pathways in Paraquat Poisoning-induced Early Pulmonary Fibrosis. J. Cell. Mol. Med. 2016, 20, 688–697. [Google Scholar] [CrossRef]
- Sarathy, J.; Detloff, S.J.; Ao, M.; Khan, N.; French, S.; Sirajuddin, H.; Nair, T.; Rao, M.C. The Yin and Yang of Bile Acid Action on Tight Junctions in a Model Colonic Epithelium. Physiol. Rep. 2017, 5, e13294. [Google Scholar] [CrossRef] [PubMed]
- Goldman, A.; Condon, A.; Adler, E.; Minnella, M.; Bernstein, C.; Bernstein, H.; Dvorak, K. Protective Effects of Glycoursodeoxycholic Acid in Barrett’s Esophagus Cells. Dis. Esophagus 2010, 23, 83–93. [Google Scholar] [CrossRef] [PubMed]
- Kundu, S.; Bansal, S.; Muthukumarasamy, K.M.; Sachidanandan, C.; Motiani, R.K.; Bajaj, A. Deciphering the Role of Hydrophobic and Hydrophilic Bile Acids in Angiogenesis Using in Vitro and in Vivo Model Systems. MedChemComm 2017, 8, 2248–2257. [Google Scholar] [CrossRef] [PubMed]
- Chiang, J.Y.L. Bile Acid Metabolism and Signaling. Compr. Physiol. 2013, 3, 1191–1212. [Google Scholar] [CrossRef]
- Suga, T.; Yamaguchi, H.; Sato, T.; Maekawa, M.; Goto, J.; Mano, N. Preference of Conjugated Bile Acids over Unconjugated Bile Acids as Substrates for OATP1B1 and OATP1B3. PLoS ONE 2017, 12. [Google Scholar] [CrossRef] [PubMed]
- Okabe, M.; Szakács, G.; Reimers, M.A.; Suzuki, T.; Hall, M.D.; Abe, T.; Weinstein, J.N.; Gottesman, M.M. Profiling SLCO and SLC22 Genes in the NCI-60 Cancer Cell Lines to Identify Drug Uptake Transporters. Mol. Cancer Ther. 2008, 7, 3081–3091. [Google Scholar] [CrossRef] [PubMed]
- Daruich, A.; Jaworski, T.; Henry, H.; Zola, M.; Youale, J.; Parenti, L.; Naud, M.-C.; Delaunay, K.; Bertrand, M.; Berdugo, M.; et al. Oral Ursodeoxycholic Acid Crosses the Blood Retinal Barrier in Patients with Retinal Detachment and Protects Against Retinal Degeneration in an Ex Vivo Model. Neurother. J. Am. Soc. Exp. Neurother. 2021. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Warden, C.; Brantley, M.A., Jr. Glycine-Conjugated Bile Acids Protect RPE Tight Junctions against Oxidative Stress and Inhibit Choroidal Endothelial Cell Angiogenesis In Vitro. Biomolecules 2021, 11, 626. https://doi.org/10.3390/biom11050626
Warden C, Brantley MA Jr. Glycine-Conjugated Bile Acids Protect RPE Tight Junctions against Oxidative Stress and Inhibit Choroidal Endothelial Cell Angiogenesis In Vitro. Biomolecules. 2021; 11(5):626. https://doi.org/10.3390/biom11050626
Chicago/Turabian StyleWarden, Cassandra, and Milam A. Brantley, Jr. 2021. "Glycine-Conjugated Bile Acids Protect RPE Tight Junctions against Oxidative Stress and Inhibit Choroidal Endothelial Cell Angiogenesis In Vitro" Biomolecules 11, no. 5: 626. https://doi.org/10.3390/biom11050626
APA StyleWarden, C., & Brantley, M. A., Jr. (2021). Glycine-Conjugated Bile Acids Protect RPE Tight Junctions against Oxidative Stress and Inhibit Choroidal Endothelial Cell Angiogenesis In Vitro. Biomolecules, 11(5), 626. https://doi.org/10.3390/biom11050626




