Dual Role for Astroglial Copper-Assisted Polyamine Metabolism during Intense Network Activity
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Buffers
2.3. Slice Preparation
2.4. In Vitro Electrophysiology
2.5. Data Evaluation
3. Results
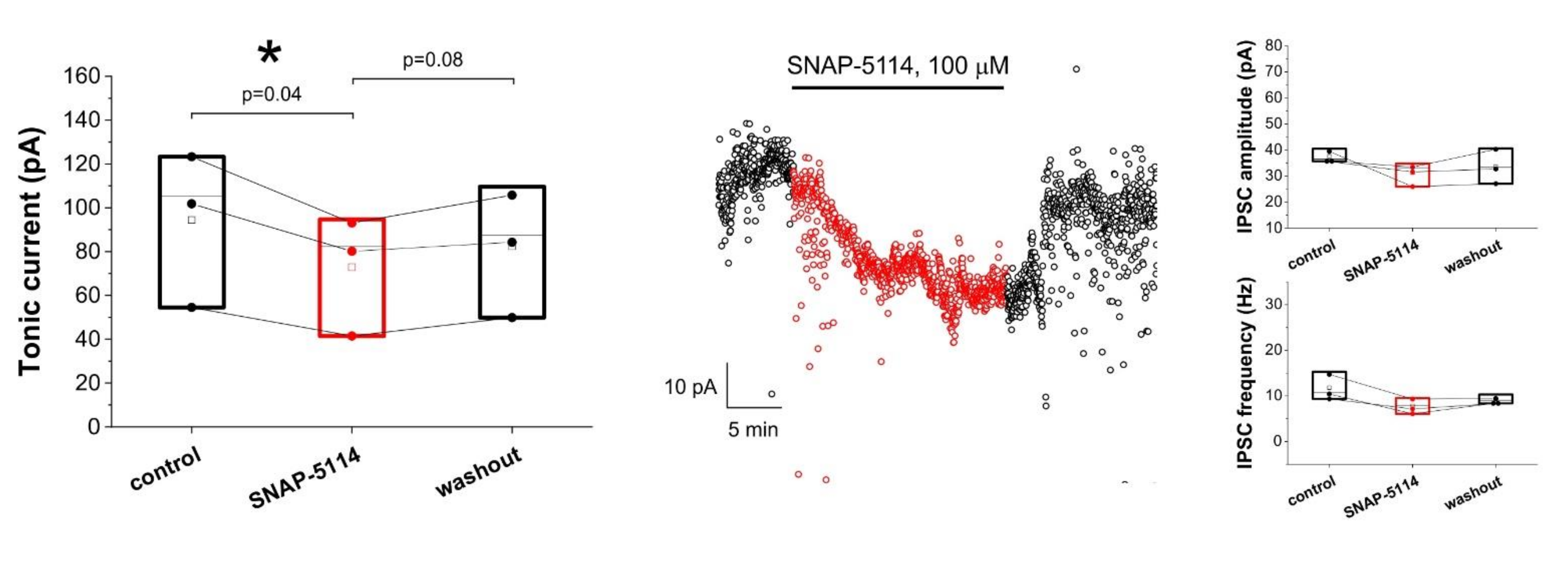
3.1. Assessing Astroglial GABA Transporter-Specific Component of Tonic Inhibitory Current in the Low-[Mg2+] Model of Experimental Epilepsy
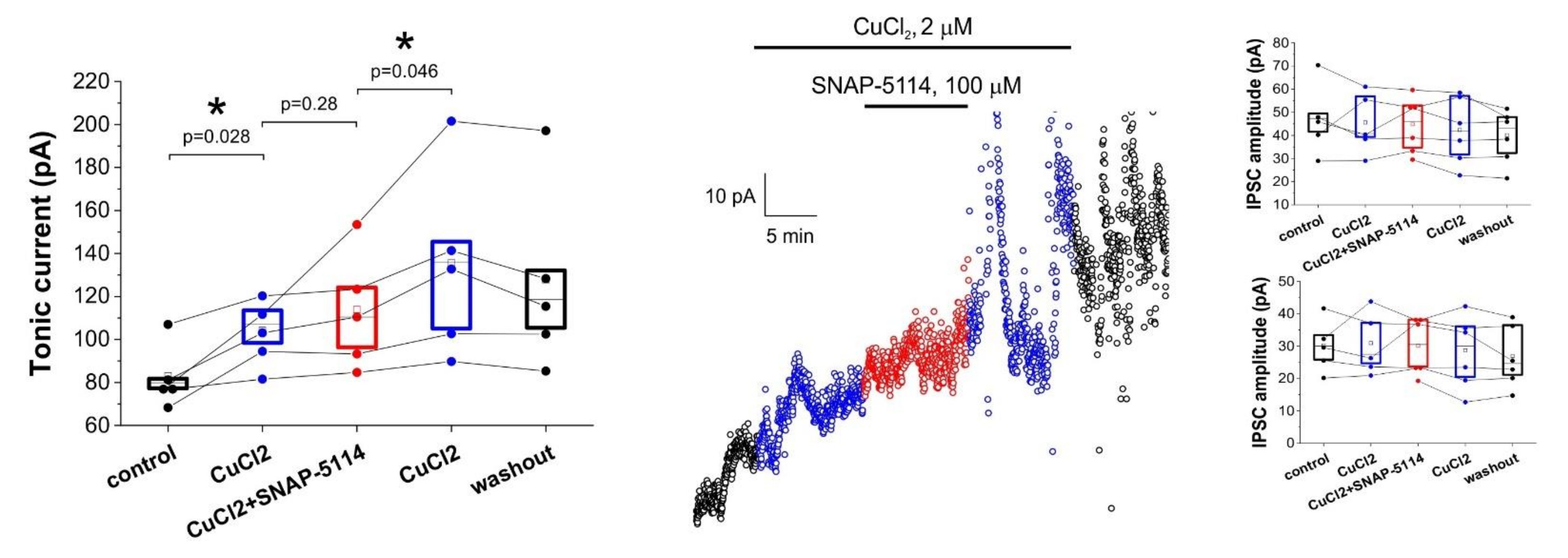
3.2. Effects of Added AgNO3 or MnCl2 on the GAT-2/3 Specific Tonic Inhibitory Component
3.3. Direct Copper Application Generates Tonic Current
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Caudal, L.C.; Gobbo, D.; Scheller, A.; Kirchhoff, F. The Paradox of Astroglial Ca2+ Signals at the Interface of Excitation and Inhibition. Front. Cell. Neurosci. 2020, 14, 399. [Google Scholar] [CrossRef] [PubMed]
- Cho, J.; Huh, Y. Astrocytic Calcium Dynamics Along the Pain Pathway. Front. Cell. Neurosci. 2020, 14, 333. [Google Scholar] [CrossRef] [PubMed]
- De Pittà, M. Neuron-Glial Interactions. In Encyclopedia of Computational Neuroscience; Springer: New York, NY, USA, 2020; pp. 1–30. [Google Scholar]
- Yoon, B.-E.; Jo, S.; Woo, J.; Lee, J.-H.; Kim, T.; Kim, D.; Lee, C.J. The amount of astrocytic GABA positively correlates with the degree of tonic inhibition in hippocampal CA1 and cerebellum. Mol. Brain 2011, 4, 42. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Lubin, H.; Ioja, E.; Kékesi, O.; Simon, Á.; Apáti, Á.; Orbán, T.I.; Héja, L.; Kardos, J.; Markó, I.E. Straightforward and effective synthesis of γ-aminobutyric acid transporter subtype 2-selective acyl-substituted azaspiro[4.5]decanes. Bioorg. Med. Chem. Lett. 2016, 26. [Google Scholar] [CrossRef]
- Semyanov, A.; Henneberger, C.; Agarwal, A. Making sense of astrocytic calcium signals—From acquisition to interpretation. Nat. Rev. Neurosci. 2020, 21, 551–564. [Google Scholar] [CrossRef]
- Zhou, Y.; Shao, A.; Yao, Y.; Tu, S.; Deng, Y.; Zhang, J. Dual roles of astrocytes in plasticity and reconstruction after traumatic brain injury. Cell Commun. Signal. 2020, 18. [Google Scholar] [CrossRef]
- Szabó, Z.; Héja, L.; Szalay, G.; Kékesi, O.; Füredi, A.; Szebényi, K.; Dobolyi, Á.; Orbán, T.I.; Kolacsek, O.; Tompa, T.; et al. Extensive astrocyte synchronization advances neuronal coupling in slow wave activity in vivo. Sci. Rep. 2017, 7, 6018. [Google Scholar] [CrossRef]
- Felix, L.; Stephan, J.; Rose, C.R. Astrocytes of the early postnatal brain. Eur. J. Neurosci. 2020. [Google Scholar] [CrossRef]
- Héja, L.; Simon, Á.; Szabó, Z.; Kardos, J. Feedback adaptation of synaptic excitability via Glu:Na+ symport driven astrocytic GABA and Gln release. Neuropharmacology 2019, 161. [Google Scholar] [CrossRef]
- Fiacco, T.A.; McCarthy, K.D. Multiple lines of evidence indicate that gliotransmission does not occur under physiological conditions. J. Neurosci. 2018, 38. [Google Scholar] [CrossRef]
- Savtchouk, I.; Volterra, A. Gliotransmission: Beyond black-and-white. J. Neurosci. 2018, 38. [Google Scholar] [CrossRef]
- Braganza, O.; Bedner, P.; Hüttmann, K.; von Staden, E.; Friedman, A.; Seifert, G.; Steinhäuser, C. Albumin is taken up by hippocampal NG2 cells and astrocytes and decreases gap junction coupling. Epilepsia 2012, 53, 1898–1906. [Google Scholar] [CrossRef]
- Nagatomo, K.; Ueda, Y.; Doi, T.; Takaki, M.; Tsuru, N. Functional role of GABA transporters for kindling development in GLAST KO mice. Neurosci Res 2007, 57, 319–321. [Google Scholar] [CrossRef]
- Unichenko, P.; Dvorzhak, A.; Kirischuk, S. Transporter-mediated replacement of extracellular glutamate for GABA in the developing murine neocortex. Eur. J. Neurosci. 2013, 38, 3580–3588. [Google Scholar] [CrossRef]
- Verhoog, Q.P.; Holtman, L.; Aronica, E.; van Vliet, E.A. Astrocytes as Guardians of Neuronal Excitability: Mechanisms Underlying Epileptogenesis. Front. Neurol. 2020, 11, 591690. [Google Scholar] [CrossRef]
- Héja, L.; Szabó, Z.; Péter, M.; Kardos, J. Spontaneous Ca2+ Fluctuations Arise in Thin Astrocytic Processes with Real 3D Geometry. Front. Cell. Neurosci. 2021, 15, 617989. [Google Scholar] [CrossRef]
- Henneberger, C.; Papouin, T.; Oliet, S.H.R.; Rusakov, D.A. Long-term potentiation depends on release of D-serine from astrocytes. Nature 2010, 463, 232–236. [Google Scholar] [CrossRef]
- Henneberger, C.; Bard, L.; Panatier, A.; Reynolds, J.P.; Kopach, O.; Medvedev, N.I.; Minge, D.; Herde, M.K.; Anders, S.; Kraev, I.; et al. LTP Induction Boosts Glutamate Spillover by Driving Withdrawal of Perisynaptic Astroglia. Neuron 2020, 108, 919–936.e11. [Google Scholar] [CrossRef]
- Inyushin, M.; Kucheryavykh, L.Y.; Kucheryavykh, Y.V.; Nichols, C.G.; Buono, R.J.; Ferraro, T.N.; Skatchkov, S.N.; Eaton, M.J. Potassium channel activity and glutamate uptake are impaired in astrocytes of seizure-susceptible DBA/2 mice. Epilepsia 2010, 51. [Google Scholar] [CrossRef]
- Lee, S.; Yoon, B.-E.; Berglund, K.; Oh, S.-J.; Park, H.; Shin, H.-S.; Augustine, G.J.; Lee, C.J. Channel-mediated tonic GABA release from glia. Science 2010, 330, 790–796. [Google Scholar] [CrossRef]
- Matsui, K.; Jahr, C.E.; Rubio, M.E. High-concentration rapid transients of glutamate mediate neural-glial communication via ectopic release. J. Neurosci. 2005, 25, 7538–7547. [Google Scholar] [CrossRef]
- Pál, I.; Kardos, J.; Dobolyi, Á.; Héja, L. Appearance of fast astrocytic component in voltage-sensitive dye imaging of neural activity. Mol. Brain 2015, 8. [Google Scholar] [CrossRef]
- Mederos, S.; Sánchez-Puelles, C.; Esparza, J.; Valero, M.; Ponomarenko, A.; Perea, G. GABAergic signaling to astrocytes in the prefrontal cortex sustains goal-directed behaviors. Nat. Neurosci. 2021, 24. [Google Scholar] [CrossRef]
- Héja, L.; Barabás, P.; Nyitrai, G.; Kékesi, K.A.K.A.; Lasztóczi, B.; Toke, O.; Tárkányi, G.; Madsen, K.; Schousboe, A.; Dobolyi, A.; et al. Glutamate uptake triggers transporter-mediated GABA release from astrocytes. PLoS ONE 2009, 4, e7153. [Google Scholar] [CrossRef]
- Héja, L.; Nyitrai, G.; Kékesi, O.; Dobolyi, A.; Szabó, P.; Fiáth, R.; Ulbert, I.; Pál-Szenthe, B.; Palkovits, M.; Kardos, J. Astrocytes convert network excitation to tonic inhibition of neurons. BMC Biol. 2012, 10, 26. [Google Scholar] [CrossRef]
- Yoon, B.-E.; Woo, J.; Chun, Y.-E.; Chun, H.; Jo, S.; Bae, J.Y.; An, H.; Min, J.O.; Oh, S.-J.; Han, K.-S.; et al. Glial GABA, synthesized by monoamine oxidase B, mediates tonic inhibition. J. Physiol. 2014, 592, 4951–4968. [Google Scholar] [CrossRef]
- Jo, S.; Yarishkin, O.; Hwang, Y.J.; Chun, Y.E.; Park, M.; Woo, D.H.; Bae, J.Y.; Kim, T.; Lee, J.; Chun, H.; et al. GABA from reactive astrocytes impairs memory in mouse models of Alzheimer’s disease. Nat. Med. 2014, 20, 886–896. [Google Scholar] [CrossRef]
- Ormel, L.; Lauritzen, K.H.; Schreiber, R.; Kunzelmann, K.; Gundersen, V. GABA, but Not Bestrophin-1, Is Localized in Astroglial Processes in the Mouse Hippocampus and the Cerebellum. Front. Mol. Neurosci. 2020, 13. [Google Scholar] [CrossRef]
- Skatchkov, S.N.; Bukauskas, F.F.; Benedikt, J.; Inyushin, M.; Kucheryavykh, Y.V. Intracellular spermine prevents acid-induced uncoupling of Cx43 gap junction channels. Neuroreport 2015, 26. [Google Scholar] [CrossRef]
- Kucheryavykh, L.Y.; Benedikt, J.; Cubano, L.A.; Skatchkov, S.N.; Bukauskas, F.F.; Kucheryavykh, Y.V. Polyamines preserve connexin 43-mediated gap junctional communication during intracellular hypercalcemia and acidosis. Neuroreport 2017, 28. [Google Scholar] [CrossRef]
- Kim, J.I.; Ganesan, S.; Luo, S.X.; Wu, Y.W.; Park, E.; Huang, E.J.; Chen, L.; Ding, J.B. Aldehyde dehydrogenase 1a1 mediates a GABA synthesis pathway in midbrain dopaminergic neurons. Science 2015, 350. [Google Scholar] [CrossRef] [PubMed]
- Laschet, J.; Grisar, T.; Bureau, M.; Guillaume, D. Characteristics of putrescine uptake and subsequent GABA formation in primary cultured astrocytes from normal C57BL/6J and epileptic DBA/2J mouse brain cortices. Neuroscience 1992, 48, 151–157. [Google Scholar] [CrossRef]
- Laube, G.; Veh, R.W. Astrocytes, not neurons, show most prominent staining for spermidine/spermine-like immunoreactivity in adult rat brain. Glia 1997, 19. [Google Scholar] [CrossRef]
- Kremzner, L.T.; Hiller, J.M.; Simon, E.J. Metabolism of Polyamines in Mouse Neuroblastoma Cells in Culture: Formation of Gaba and Putreanine. J. Neurochem. 1975, 25. [Google Scholar] [CrossRef] [PubMed]
- Seiler, N.; Al-Therib, M.J.; Kataoka, K. Formation of Gaba from Putrescine in the Brain of Fish (Salmo irideus gibb.). J. Neurochem. 1973, 20. [Google Scholar] [CrossRef]
- Seiler, N. On the role of GABA in vertebrate polyamine metabolism. Physiol. Chem. Phys. 1980, 12, 411–429. [Google Scholar] [PubMed]
- Seiler, N. Polyamine Metabolism. Digestion 1990, 46, 319–330. [Google Scholar] [CrossRef]
- Lopes de Carvalho, L.; Bligt-Lindén, E.; Ramaiah, A.; Johnson, M.S.; Salminen, T.A. Evolution and functional classification of mammalian copper amine oxidases. Mol. Phylogenet. Evol. 2019, 139. [Google Scholar] [CrossRef]
- Chang, C.J. Searching for harmony in transition-metal signaling. Nat. Chem. Biol. 2015, 11, 744–747. [Google Scholar] [CrossRef]
- Kardos, J.; Héja, L.; Simon, Á.; Jablonkai, I.; Kovács, R.; Jemnitz, K. Copper signalling: Causes and consequences. Cell Commun. Signal. 2018, 16. [Google Scholar] [CrossRef]
- Kardos, J.; Kovács, I.; Hajós, F.; Kálmán, M.; Simonyi, M. Nerve endings from rat brain tissue release copper upon depolarization. A possible role in regulating neuronal excitability. Neurosci. Lett. 1989, 103. [Google Scholar] [CrossRef]
- Boyd, S.D.; Ullrich, M.S.; Skopp, A.; Winkler, D.D. Copper sources for sod1 activation. Antioxidants 2020, 9. [Google Scholar] [CrossRef]
- Liang, Z.D.; Long, Y.; Chen, H.H.W.; Savaraj, N.; Kuo, M.T. Regulation of the high-affinity copper transporter (hCtr1) expression by cisplatin and heavy metals. J. Biol. Inorg. Chem. 2014, 19. [Google Scholar] [CrossRef]
- Lee, J.; Peña, M.M.O.; Nose, Y.; Thiele, D.J. Biochemical characterization of the human copper transporter Ctr1. J. Biol. Chem. 2002, 277. [Google Scholar] [CrossRef]
- Wolff, N.A.; Garrick, M.D.; Zhao, L.; Garrick, L.M.; Ghio, A.J.; Thévenod, F. A role for divalent metal transporter (DMT1) in mitochondrial uptake of iron and manganese. Sci. Rep. 2018, 8. [Google Scholar] [CrossRef]
- Kern, A.D.; Oliveira, M.A.; Coffino, P.; Hackert, M.L. Structure of mammalian ornithine decarboxylase at 1.6 Å resolution: Stereochemical implications of PLP-dependent amino acid decarboxylases. Structure 1999, 7. [Google Scholar] [CrossRef]
- Lenis, Y.Y.; Elmetwally, M.A.; Maldonado-Estrada, J.G.; Bazer, F.W. Physiological importance of polyamines. Zygote 2017, 25. [Google Scholar] [CrossRef]
- Bae, D.H.; Lane, D.J.R.; Jansson, P.J.; Richardson, D.R. The old and new biochemistry of polyamines. Biochim. Biophys. Acta Gen. Subj. 2018, 1862, 2053–2068. [Google Scholar] [CrossRef]
- Baroli, G.; Sanchez, J.R.; Agostinelli, E.; Mariottini, P.; Cervelli, M. Polyamines: The possible missing link between mental disorders and epilepsy (Review). Int. J. Mol. Med. 2020, 45, 3–9. [Google Scholar] [CrossRef]
- Van Veen, S.; Martin, S.; Van den Haute, C.; Benoy, V.; Lyons, J.; Vanhoutte, R.; Kahler, J.P.; Decuypere, J.P.; Gelders, G.; Lambie, E.; et al. ATP13A2 deficiency disrupts lysosomal polyamine export. Nature 2020, 578. [Google Scholar] [CrossRef]
- Vrijsen, S.; Besora-Casals, L.; Van Veen, S.; Zielich, J.; Van Den Haute, C.; Hamouda, N.N.; Fischer, C.; Ghesquière, B.; Tournev, I.; Agostinis, P.; et al. ATP13A2-mediated endo-lysosomal polyamine export counters mitochondrial oxidative stress. Proc. Natl. Acad. Sci. USA 2020, 117. [Google Scholar] [CrossRef]
- Lane, D.J.R.; Bae, D.H.; Siafakas, A.R.; Suryo Rahmanto, Y.; Al-Akra, L.; Jansson, P.J.; Casero, R.A.; Richardson, D.R. Coupling of the polyamine and iron metabolism pathways in the regulation of proliferation: Mechanistic links to alterations in key polyamine biosynthetic and catabolic enzymes. Biochim. Biophys. Acta Mol. Basis Dis. 2018, 1864. [Google Scholar] [CrossRef]
- Skatchkov, S.N.; Antonov, S.M.; Eaton, M.J. Glia and glial polyamines. Role in brain function in health and disease. Biochem. Suppl. Ser. A Membr. Cell Biol. 2016, 10, 73–98. [Google Scholar] [CrossRef]
- Skatchkov, S.N.; Woodbury-Fariña, M.A.; Eaton, M. The role of glia in stress: Polyamines and brain disorders. Psychiatr. Clin. North Am. 2014, 37. [Google Scholar]
- Handa, A.K.; Fatima, T.; Mattoo, A.K. Polyamines: Bio-Molecules with Diverse Functions in Plant and Human Health and Disease. Front. Chem. 2018, 6. [Google Scholar] [CrossRef]
- Jain, V.; Raina, S.; Gheware, A.P.; Singh, R.; Rehman, R.; Negi, V.; Murray Stewart, T.; Mabalirajan, U.; Mishra, A.K.; Casero, R.A.; et al. Reduction in polyamine catabolism leads to spermine-mediated airway epithelial injury and induces asthma features. Allergy Eur. J. Allergy Clin. Immunol. 2018, 73. [Google Scholar] [CrossRef]
- Kahana, C. The antizyme family for regulating polyamines. J. Biol. Chem. 2018, 293, 18730–18735. [Google Scholar] [CrossRef]
- Mahajan, U.V.; Varma, V.R.; Griswold, M.E.; Blackshear, C.T.; An, Y.; Oommen, A.M.; Varma, S.; Troncoso, J.C.; Pletnikova, O.; O’Brien, R.; et al. Dysregulation of multiple metabolic networks related to brain transmethylation and polyamine pathways in Alzheimer disease: A targeted metabolomic and transcriptomic study. PLoS Med. 2020, 17. [Google Scholar] [CrossRef]
- Makletsova, M.G.; Syatkin, S.P.; Poleshchuk, V.V.; Urazgildeeva, G.R.; Chigaleychik, L.A.; Sungrapova, C.Y.; Illarioshkin, S.N. Polyamines in Parkinson’s Disease: Their Role in Oxidative Stress Induction and Protein Aggregation. J. Neurol. Res. 2019, 9. [Google Scholar] [CrossRef]
- Miller-Fleming, L.; Olin-Sandoval, V.; Campbell, K.; Ralser, M. Remaining Mysteries of Molecular Biology: The Role of Polyamines in the Cell. J. Mol. Biol. 2015, 427, 3389–3406. [Google Scholar] [CrossRef]
- Pegg, A.E. Functions of polyamines in mammals. J. Biol. Chem. 2016, 291, 14904–14912. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Jiménez, F.; Medina, M.Á.; Villalobos-Rueda, L.; Urdiales, J.L. Polyamines in mammalian pathophysiology. Cell. Mol. Life Sci. 2019, 76, 3987–4008. [Google Scholar] [CrossRef] [PubMed]
- Beckonert, N.M.; Opitz, T.; Pitsch, X.; da Silva, P.S.; Beck, H. Polyamine modulation of anticonvulsant drug response: A potential mechanism contributing to pharmacoresistance in chronic epilepsy. J. Neurosci. 2018, 38. [Google Scholar] [CrossRef] [PubMed]
- Derera, I.D.; Dulla, C.G. THAR SHE BLOWS! The Search for the Great Spermine Whale of Carbamazepine Resistance. Epilepsy Curr. 2019, 19, 59–61. [Google Scholar] [CrossRef]
- Kapfhamer, D.; McKenna, J.; Yoon, C.J.; Murray-Stewart, T.; Casero, R.A.; Gambello, M.J. Ornithine decarboxylase, the rate-limiting enzyme of polyamine synthesis, modifies brain pathology in a mouse model of tuberous sclerosis complex. Hum. Mol. Genet. 2020, 29. [Google Scholar] [CrossRef]
- Fredriksson, R.; Sreedharan, S.; Nordenankar, K.; Alsiö, J.; Lindberg, F.A.; Hutchinson, A.; Eriksson, A.; Roshanbin, S.; Ciuculete, D.M.; Klockars, A.; et al. The polyamine transporter Slc18b1(VPAT) is important for both short and long time memory and for regulation of polyamine content in the brain. PLoS Genet. 2019, 15. [Google Scholar] [CrossRef]
- Guerra, G.P.; Rubin, M.A.; Mello, C.F. Modulation of learning and memory by natural polyamines. Pharmacol. Res. 2016, 112, 99–118. [Google Scholar] [CrossRef]
- Anderson, J.G.; Fordahl, S.C.; Cooney, P.T.; Weaver, T.L.; Colyer, C.L.; Erikson, K.M. Manganese exposure alters extracellular GABA, GABA receptor and transporter protein and mRNA levels in the developing rat brain. Neurotoxicology 2008, 29. [Google Scholar] [CrossRef]
- Kardos, J.; Héja, L.; Jemnitz, K.; Kovács, R.; Palkovits, M. The nature of early astroglial protection—Fast activation and signaling. Prog. Neurobiol. 2017, 153. [Google Scholar] [CrossRef]
- Assaf, S.Y.; Chung, S.H. Release of endogenous Zn2+ from brain tissue during activity. Nature 1984, 308. [Google Scholar] [CrossRef]
- Krzywoszyńska, K.; Witkowska, D.; Swiatek-kozlowska, J.; Szebesczyk, A.; Kozłowski, H. General aspects of metal ions as signaling agents in health and disease. Biomolecules 2020, 10, 1417. [Google Scholar] [CrossRef]
- Frederickson, C.J.; Bush, A.I. Synaptically released zinc: Physiological functions and pathological effects. BioMetals 2001, 14, 353–366. [Google Scholar] [CrossRef]
- Cohen-Kfir, E.; Lee, W.; Eskandari, S.; Nelson, N. Zinc inhibition of γ-aminobutyric acid transporter 4 (GAT4) a link between excitatory and inhibitory neurotransmission. Proc. Natl. Acad. Sci. USA 2005, 102, 6154–6159. [Google Scholar] [CrossRef]
- Kardos, J.; Pallo, A.; Bencsura, A.; Simon, A. Assessing Structure, Function and Druggability of Major Inhibitory Neurotransmitter -Aminobutyrate Symporter Subtypes. Curr. Med. Chem. 2010, 17, 2203–2213. [Google Scholar] [CrossRef]
- Palló, A.; Simon, A.; Bencsura, A.; Héja, L.; Kardos, J. Substrate-Na+ complex formation: Coupling mechanism for gamma-aminobutyrate symporters. Biochem. Biophys. Res. Commun. 2009, 385, 210–214. [Google Scholar] [CrossRef]
- Weber, D.S.; Warren, J.J. The interaction between methionine and two aromatic amino acids is an abundant and multifunctional motif in proteins. Arch. Biochem. Biophys. 2019, 672. [Google Scholar] [CrossRef]
- Yeung, P.S.W.; Ing, C.E.; Yamashita, M.; Pomès, R.; Prakriya, M. A sulfur-aromatic gate latch is essential for opening of the orai1 channel pore. Elife 2020, 9. [Google Scholar] [CrossRef]
- Fordahl, S.C.; Anderson, J.G.; Cooney, P.T.; Weaver, T.L.; Colyer, C.L.; Erikson, K.M. Manganese exposure inhibits the clearance of extracellular GABA and influences taurine homeostasis in the striatum of developing rats. Neurotoxicology 2010, 31. [Google Scholar] [CrossRef]
- Carrillo, E.; Bhatia, N.K.; Akimzhanov, A.M.; Jayaraman, V. Activity dependent inhibition of AMPA receptors by Zn2+. J. Neurosci. 2020, 40. [Google Scholar] [CrossRef]
- Fisher, J.L.; Macdonald, R.L. The role of an α subtype M2-M3 his in regulating inhibition of GABA(A) receptor current by zinc and other divalent cations. J. Neurosci. 1998, 18, 2944–2953. [Google Scholar] [CrossRef]
- Granzotto, A.; Canzoniero, L.M.T.; Sensi, S.L. A Neurotoxic Ménage-à-trois: Glutamate, Calcium, and Zinc in the Excitotoxic Cascade. Front. Mol. Neurosci. 2020, 13, 225. [Google Scholar] [CrossRef]
- Hosie, A.M.; Dunne, E.L.; Harvey, R.J.; Smart, T.G. Zinc-mediated inhibition of GABAA receptors: Discrete binding sites underlie subtype specificity. Nat. Neurosci. 2003, 6, 362–369. [Google Scholar] [CrossRef]
- Krall, R.F.; Tzounopoulos, T.; Aizenman, E. The Function and Regulation of Zinc in the Brain. Neuroscience 2021. [Google Scholar] [CrossRef]
- Kardos, J. The GABAA receptor channel mediated chloride ion translocation through the plasma membrane: New insights from 36Cl- ion flux measurements. Synapse 1993, 13, 74–93. [Google Scholar] [CrossRef]
- Paoletti, P.; Vergnano, A.M.; Barbour, B.; Casado, M. Zinc at glutamatergic synapses. Neuroscience 2009, 158, 126–136. [Google Scholar] [CrossRef]
- Wu, Q.; Wada, M.; Shimada, A.; Yamamoto, A.; Fujita, T. Functional characterization of Zn2+-sensitive GABA transporter expressed in primary cultures of astrocytes from rat cerebral cortex. Brain Res. 2006, 1075. [Google Scholar] [CrossRef]
- Fernandes, J.; Chandler, J.D.; Liu, K.H.; Uppal, K.; Go, Y.M.; Jones, D.P. Putrescine as indicator of manganese neurotoxicity: Dose-response study in human SH-SY5Y cells. Food Chem. Toxicol. 2018, 116. [Google Scholar] [CrossRef] [PubMed]
- Browne, L.E.; Compan, V.; Bragg, L.; North, R.A. P2X7 receptor channels allow direct permeation of nanometer-sized dyes. J. Neurosci. 2013, 33. [Google Scholar] [CrossRef] [PubMed]
- Ye, Z.C.; Wyeth, M.S.; Baltan-Tekkok, S.; Ransom, B.R. Functional hemichannels in astrocytes: A novel mechanism of glutamate release. J. Neurosci. 2003, 23. [Google Scholar] [CrossRef]
- Stuerenburg, H.J. CSF copper concentrations, blood-brain barrier function, and coeruloplasmin synthesis during the treatment of Wilson’s disease. J. Neural Transm. 2000, 107. [Google Scholar] [CrossRef] [PubMed]
- Maureira, C.; Letelier, J.C.; Alvarez, O.; Delgado, R.; Vergara, C. Copper enhances cellular and network excitabilities, and improves temporal processing in the rat hippocampus. Eur. J. Neurosci. 2015, 42. [Google Scholar] [CrossRef] [PubMed]
- Adelson, C.N.; Johnston, E.M.; Hilmer, K.M.; Watts, H.; Dey, S.G.; Brown, D.E.; Broderick, J.B.; Shepard, E.M.; Dooley, D.M.; Solomon, E.I. Characterization of the Preprocessed Copper Site Equilibrium in Amine Oxidase and Assignment of the Reactive Copper Site in Topaquinone Biogenesis. J. Am. Chem. Soc. 2019, 141. [Google Scholar] [CrossRef]
- Ruggiero, C.E.; Dooley, D.M. Stoichiometry of the topa quinone biogenesis reaction in copper amine oxidases. Biochemistry 1999, 38. [Google Scholar] [CrossRef]
- Kehoe, C.A.; Faughnan, M.S.; Gilmore, W.S.; Coulter, J.S.; Howard, A.N.; Strain, J.J. Plasma diamine oxidase activity is greater in copper-adequate than copper-marginal or copper-deficient rats. J. Nutr. 2000, 130. [Google Scholar] [CrossRef]
- Disilvestro, R.A.; Jones, A.A.; Smith, D.; Wildman, R. Plasma diamine oxidase activities in renal dialysis patients, a human with spontaneous copper deficiency and marginally copper deficient rats. Clin. Biochem. 1997, 30. [Google Scholar] [CrossRef]
- Gou, D.H.; Huang, T.T.; Li, W.; Gao, X.D.; Haikal, C.; Wang, X.H.; Song, D.Y.; Liang, X.; Zhu, L.; Tang, Y.; et al. Inhibition of copper transporter 1 prevents α-synuclein pathology and alleviates nigrostriatal degeneration in AAV-based mouse model of Parkinson’s disease. Redox Biol. 2021, 38. [Google Scholar] [CrossRef]
- Wang, L.; Yin, Y.L.; Liu, X.Z.; Shen, P.; Zheng, Y.G.; Lan, X.R.; Lu, C.B.; Wang, J.Z. Current understanding of metal ions in the pathogenesis of Alzheimer’s disease. Transl. Neurodegener. 2020, 9. [Google Scholar] [CrossRef]
- Schoonover, K.E.; Farmer, C.B.; Morgan, C.J.; Sinha, V.; Odom, L.; Roberts, R.C. Abnormalities in the copper transporter CTR1 in postmortem hippocampus in schizophrenia: A subregion and laminar analysis. Schizophr. Res. 2021, 228. [Google Scholar] [CrossRef]
- Inoue, K.; Tsutsui, H.; Akatsu, H.; Hashizume, Y.; Matsukawa, N.; Yamamoto, T.; Toyo’Oka, T. Metabolic profiling of Alzheimer’s disease brains. Sci. Rep. 2013, 3. [Google Scholar] [CrossRef]
- Morrison, L.D.; Cao, X.C.; Kish, S.J. Ornithine decarboxylase in human brain: Influence of aging, regional distribution, and Alzheimer’s disease. J. Neurochem. 1998, 71. [Google Scholar] [CrossRef] [PubMed]
- Morrison, L.D.; Bergeron, C.; Kish, S.J. Brain S-adenosylmethionine decarboxylase activity is increased in Alzheimer’s disease. Neurosci. Lett. 1993, 154, 141–144. [Google Scholar] [CrossRef]
- González-Domínguez, R.; García, A.; García-Barrera, T.; Barbas, C.; Gómez-Ariza, J.L. Metabolomic profiling of serum in the progression of Alzheimer’s disease by capillary electrophoresis-mass spectrometry. Electrophoresis 2014, 35. [Google Scholar] [CrossRef] [PubMed]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Szabó, Z.; Péter, M.; Héja, L.; Kardos, J. Dual Role for Astroglial Copper-Assisted Polyamine Metabolism during Intense Network Activity. Biomolecules 2021, 11, 604. https://doi.org/10.3390/biom11040604
Szabó Z, Péter M, Héja L, Kardos J. Dual Role for Astroglial Copper-Assisted Polyamine Metabolism during Intense Network Activity. Biomolecules. 2021; 11(4):604. https://doi.org/10.3390/biom11040604
Chicago/Turabian StyleSzabó, Zsolt, Márton Péter, László Héja, and Julianna Kardos. 2021. "Dual Role for Astroglial Copper-Assisted Polyamine Metabolism during Intense Network Activity" Biomolecules 11, no. 4: 604. https://doi.org/10.3390/biom11040604
APA StyleSzabó, Z., Péter, M., Héja, L., & Kardos, J. (2021). Dual Role for Astroglial Copper-Assisted Polyamine Metabolism during Intense Network Activity. Biomolecules, 11(4), 604. https://doi.org/10.3390/biom11040604






