Chirality of Novel Bitopic Agonists Determines Unique Pharmacology at the Dopamine D3 Receptor
Abstract
1. Introduction
2. Materials and Methods
2.1. Bioluminescence Resonance Energy Transfer (BRET) Studies
2.2. Bias Factor Analysis
2.3. An In-House Program for Kinetics Analysis of Functional Assay Data
2.4. Statistics
3. Results
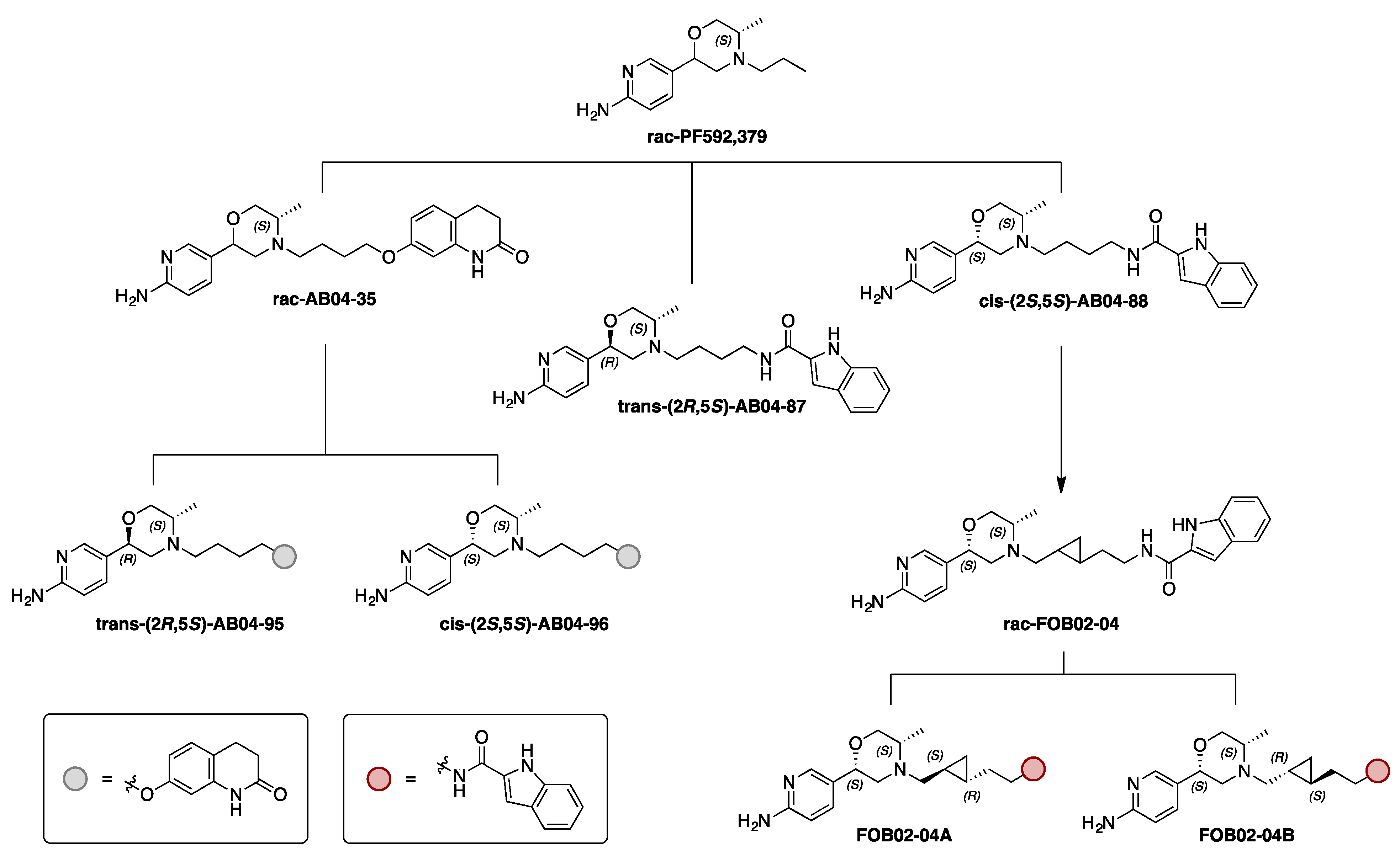
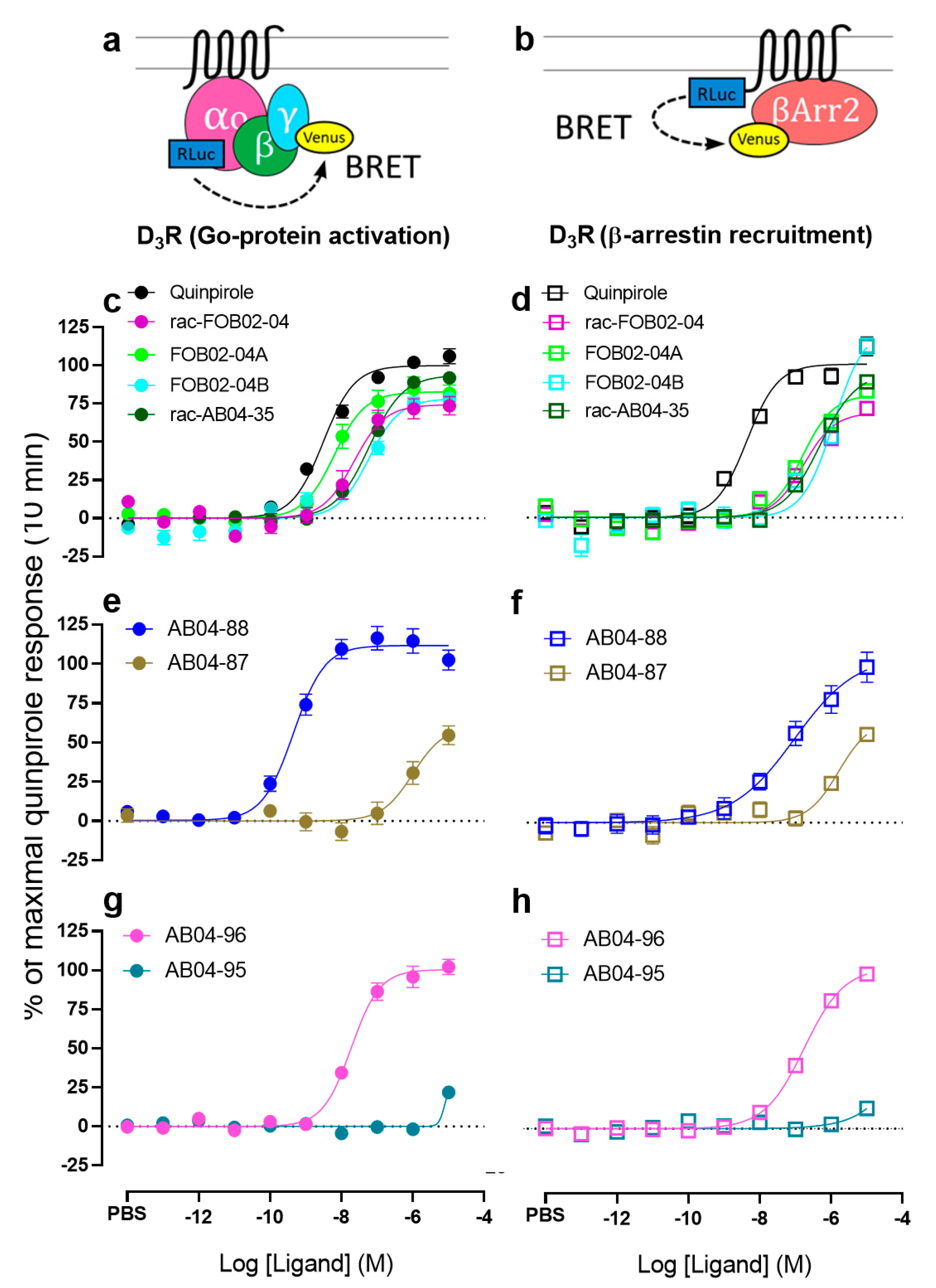
3.1. Bitopic Compounds Exhibit Varying Pharmacological Profiles at Both D3R and D2R Compared to the Reference D2R/D3R Agonist Quinpirole
3.2. The cis-AB04-88 but not the trans-AB04-87 Shows an Improved D3R Selectivity Over D2R
3.3. The cis-AB04-88 But Not the trans-AB04-87 Shows D3R Selective Go Protein Bias
3.4. Time-Dependent Pharmacological Analysis Reveals Higher Go Protein Activation Emax for AB04-88 at Later Time Points
4. Discussion
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Free, R.B.; Chun, L.S.; Moritz, A.E.; Miller, B.N.; Doyle, T.B.; Conroy, J.L.; Padron, A.; Meade, J.A.; Xiao, J.; Hu, X.; et al. Discovery and characterization of a G protein-biased agonist that inhibits beta-arrestin recruitment to the D2 dopamine receptor. Mol. Pharmacol. 2014, 86, 96–105. [Google Scholar] [CrossRef] [PubMed]
- Beaulieu, J.M.; Gainetdinov, R.R. The physiology, signaling, and pharmacology of dopamine receptors. Pharmacol. Rev. 2011, 63, 182–217. [Google Scholar] [CrossRef]
- Heidbreder, C.A.; Newman, A.H. Current perspectives on selective dopamine D (3) receptor antagonists as pharmacotherapeutics for addictions and related disorders. Ann. N. Y. Acad. Sci. 2010, 1187, 4–34. [Google Scholar] [CrossRef]
- Yang, P.; Perlmutter, J.S.; Benzinger, T.L.S.; Morris, J.C.; Xu, J. Dopamine D3 receptor: A neglected participant in Parkinson Disease pathogenesis and treatment? Ageing Res. Rev. 2020, 57, 100994. [Google Scholar] [CrossRef] [PubMed]
- Murray, A.M.; Ryoo, H.L.; Gurevich, E.; Joyce, J.N. Localization of dopamine D3 receptors to mesolimbic and D2 receptors to mesostriatal regions of human forebrain. Proc. Natl. Acad. Sci. USA 1994, 91, 11271–11275. [Google Scholar] [CrossRef] [PubMed]
- Gonsai, N.H.; Amin, V.H.; Mendpara, C.G.; Speth, R.; Hale, G.M. Effects of dopamine receptor antagonist antipsychotic therapy on blood pressure. J. Clin. Pharm. Ther. 2018, 43, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Ballon, J.S.; Pajvani, U.; Freyberg, Z.; Leibel, R.L.; Lieberman, J.A. Molecular pathophysiology of metabolic effects of antipsychotic medications. Trends Endocrinol. Metab. 2014, 25, 593–600. [Google Scholar] [CrossRef] [PubMed]
- Bezard, E.; Ferry, S.; Mach, U.; Stark, H.; Leriche, L.; Boraud, T.; Gross, C.; Sokoloff, P. Attenuation of levodopa-induced dyskinesia by normalizing dopamine D3 receptor function. Nat. Med. 2003, 9, 762–767. [Google Scholar] [CrossRef] [PubMed]
- Solis, O.; Garcia-Montes, J.R.; Gonzalez-Granillo, A.; Xu, M.; Moratalla, R. Dopamine D3 Receptor Modulates l-DOPA-Induced Dyskinesia by Targeting D1 Receptor-Mediated Striatal Signaling. Cereb. Cortex 2017, 27, 435–446. [Google Scholar] [CrossRef]
- Ferre, S.; Lluis, C.; Lanciego, J.L.; Franco, R. Prime time for G-protein-coupled receptor heteromers as therapeutic targets for CNS disorders: The dopamine D(1)-D(3) receptor heteromer. CNS Neurol. Disord. Drug Targets 2010, 9, 596–600. [Google Scholar] [CrossRef] [PubMed]
- Leggio, G.M.; Bucolo, C.; Platania, C.B.; Salomone, S.; Drago, F. Current drug treatments targeting dopamine D3 receptor. Pharmacol. Ther. 2016, 165, 164–177. [Google Scholar] [CrossRef]
- Keck, T.M.; John, W.S.; Czoty, P.W.; Nader, M.A.; Newman, A.H. Identifying Medication Targets for Psychostimulant Addiction: Unraveling the Dopamine D3 Receptor Hypothesis. J. Med. Chem. 2015, 58, 5361–5380. [Google Scholar] [CrossRef]
- Das, B.; Modi, G.; Dutta, A. Dopamine D3 agonists in the treatment of Parkinson's disease. Curr. Top. Med. Chem. 2015, 15, 908–926. [Google Scholar] [CrossRef] [PubMed]
- Lao, C.L.; Kuo, Y.H.; Hsieh, Y.T.; Chen, J.C. Intranasal and subcutaneous administration of dopamine D3 receptor agonists functionally restores nigrostriatal dopamine in MPTP-treated mice. Neurotox. Res. 2013, 24, 523–531. [Google Scholar] [CrossRef] [PubMed]
- Levant, B. The D3 dopamine receptor: Neurobiology and potential clinical relevance. Pharmacol. Rev. 1997, 49, 231–252. [Google Scholar] [PubMed]
- Sokoloff, P.; Giros, B.; Martres, M.P.; Bouthenet, M.L.; Schwartz, J.C. Molecular cloning and characterization of a novel dopamine receptor (D3) as a target for neuroleptics. Nature 1990, 347, 146–151. [Google Scholar] [CrossRef]
- Chien, E.Y.; Liu, W.; Zhao, Q.; Katritch, V.; Han, G.W.; Hanson, M.A.; Shi, L.; Newman, A.H.; Javitch, J.A.; Cherezov, V.; et al. Structure of the human dopamine D3 receptor in complex with a D2/D3 selective antagonist. Science 2010, 330, 1091–1095. [Google Scholar] [CrossRef] [PubMed]
- Pou, C.; Mannoury la Cour, C.; Stoddart, L.A.; Millan, M.J.; Milligan, G. Functional homomers and heteromers of dopamine D2L and D3 receptors co-exist at the cell surface. J. Biol. Chem. 2012, 287, 8864–8878. [Google Scholar] [CrossRef]
- Scarselli, M.; Novi, F.; Schallmach, E.; Lin, R.; Baragli, A.; Colzi, A.; Griffon, N.; Corsini, G.U.; Sokoloff, P.; Levenson, R.; et al. D2/D3 dopamine receptor heterodimers exhibit unique functional properties. J. Biol. Chem. 2001, 276, 30308–30314. [Google Scholar] [CrossRef]
- Fiorentini, C.; Busi, C.; Gorruso, E.; Gotti, C.; Spano, P.; Missale, C. Reciprocal regulation of dopamine D1 and D3 receptor function and trafficking by heterodimerization. Mol. Pharmacol. 2008, 74, 59–69. [Google Scholar] [CrossRef] [PubMed]
- Maggio, R.; Scarselli, M.; Capannolo, M.; Millan, M.J. Novel dimensions of D3 receptor function: Focus on heterodimerisation, transactivation and allosteric modulation. Eur. Neuropsychopharmacol. 2015, 25, 1470–1479. [Google Scholar] [CrossRef]
- Maggio, R.; Scarselli, M.; Novi, F.; Millan, M.J.; Corsini, G.U. Potent activation of dopamine D3/D2 heterodimers by the antiparkinsonian agents, S32504, pramipexole and ropinirole. J. Neurochem. 2003, 87, 631–641. [Google Scholar] [CrossRef] [PubMed]
- Mailman, R.B. GPCR functional selectivity has therapeutic impact. Trends Pharmacol. Sci. 2007, 28, 390–396. [Google Scholar] [CrossRef] [PubMed]
- Urban, J.D.; Clarke, W.P.; von Zastrow, M.; Nichols, D.E.; Kobilka, B.; Weinstein, H.; Javitch, J.A.; Roth, B.L.; Christopoulos, A.; Sexton, P.M.; et al. Functional selectivity and classical concepts of quantitative pharmacology. J. Pharmacol. Exp. Ther. 2007, 320, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Kenakin, T. Functional selectivity and biased receptor signaling. J. Pharmacol. Exp. Ther. 2011, 336, 296–302. [Google Scholar] [CrossRef] [PubMed]
- Weiwer, M.; Xu, Q.; Gale, J.P.; Lewis, M.; Campbell, A.J.; Schroeder, F.A.; Van de Bittner, G.C.; Walk, M.; Amaya, A.; Su, P.; et al. Functionally Biased D2R Antagonists: Targeting the beta-Arrestin Pathway to Improve Antipsychotic Treatment. ACS Chem. Biol. 2018, 13, 1038–1047. [Google Scholar] [CrossRef]
- Allen, J.A.; Yost, J.M.; Setola, V.; Chen, X.; Sassano, M.F.; Chen, M.; Peterson, S.; Yadav, P.N.; Huang, X.P.; Feng, B.; et al. Discovery of beta-arrestin-biased dopamine D2 ligands for probing signal transduction pathways essential for antipsychotic efficacy. Proc. Natl. Acad. Sci. USA 2011, 108, 18488–18493. [Google Scholar] [CrossRef] [PubMed]
- Park, S.M.; Chen, M.; Schmerberg, C.M.; Dulman, R.S.; Rodriguiz, R.M.; Caron, M.G.; Jin, J.; Wetsel, W.C. Effects of beta-Arrestin-Biased Dopamine D2 Receptor Ligands on Schizophrenia-Like Behavior in Hypoglutamatergic Mice. Neuropsychopharmacology 2016, 41, 704–715. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Wang, X.; Tocker, A.M.; Huang, P.; Reith, M.E.; Liu-Chen, L.Y.; Smith, A.B., 3rd; Kortagere, S. Functional Characterization of a Novel Series of Biased Signaling Dopamine D3 Receptor Agonists. ACS Chem. Neurosci. 2017, 8, 486–500. [Google Scholar] [CrossRef]
- Simms, S.L.; Huettner, D.P.; Kortagere, S. In vivo characterization of a novel dopamine D3 receptor agonist to treat motor symptoms of Parkinson’s disease. Neuropharmacology 2016, 100, 106–115. [Google Scholar] [CrossRef] [PubMed]
- Harris, S.S.; Urs, N.M. Targeting beta-Arrestins in the Treatment of Psychiatric and Neurological Disorders. CNS Drugs 2021, 35, 253–264. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Reith, M.E.A.; Liu-Chen, L.Y.; Kortagere, S. Biased signaling agonist of dopamine D3 receptor induces receptor internalization independent of beta-arrestin recruitment. Pharmacol. Res. 2019, 143, 48–57. [Google Scholar] [CrossRef] [PubMed]
- Zheng, M.; Zhang, X.; Sun, N.; Min, X.; Acharya, S.; Kim, K.M. A novel molecular mechanism responsible for phosphorylation-independent desensitization of G protein-coupled receptors exemplified by the dopamine D3 receptor. Biochem. Biophys. Res. Commun. 2020, 528, 432–439. [Google Scholar] [CrossRef] [PubMed]
- Michino, M.; Boateng, C.A.; Donthamsetti, P.; Yano, H.; Bakare, O.M.; Bonifazi, A.; Ellenberger, M.P.; Keck, T.M.; Kumar, V.; Zhu, C.; et al. Toward Understanding the Structural Basis of Partial Agonism at the Dopamine D3 Receptor. J. Med. Chem. 2017, 60, 580–593. [Google Scholar] [CrossRef] [PubMed]
- Michino, M.; Donthamsetti, P.; Beuming, T.; Banala, A.; Duan, L.; Roux, T.; Han, Y.; Trinquet, E.; Newman, A.H.; Javitch, J.A.; et al. A single glycine in extracellular loop 1 is the critical determinant for pharmacological specificity of dopamine D2 and D3 receptors. Mol. Pharmacol. 2013, 84, 854–864. [Google Scholar] [CrossRef] [PubMed]
- Newman, A.H.; Beuming, T.; Banala, A.K.; Donthamsetti, P.; Pongetti, K.; LaBounty, A.; Levy, B.; Cao, J.; Michino, M.; Luedtke, R.R.; et al. Molecular determinants of selectivity and efficacy at the dopamine D3 receptor. J. Med. Chem. 2012, 55, 6689–6699. [Google Scholar] [CrossRef]
- Fronik, P.; Gaiser, B.I.; Sejer Pedersen, D. Bitopic Ligands and Metastable Binding Sites: Opportunities for G Protein-Coupled Receptor (GPCR) Medicinal Chemistry. J. Med. Chem. 2017, 60, 4126–4134. [Google Scholar] [CrossRef] [PubMed]
- Newman, A.H.; Battiti, F.O.; Bonifazi, A. 2016 Philip, S. Portoghese Medicinal Chemistry Lectureship: Designing Bivalent or Bitopic Molecules for G-Protein Coupled Receptors. The Whole Is Greater Than the Sum of Its Parts. J. Med. Chem. 2020, 63, 1779–1797. [Google Scholar] [CrossRef]
- Newman, A.H.; Ku, T.; Jordan, C.J.; Bonifazi, A.; Xi, Z.X. New Drugs, Old Targets: Tweaking the Dopamine System to Treat Psychostimulant Use Disorders. Annu. Rev. Pharmacol. Toxicol. 2021, 61, 609–628. [Google Scholar] [CrossRef]
- Draper-Joyce, C.J.; Michino, M.; Verma, R.K.; Klein Herenbrink, C.; Shonberg, J.; Kopinathan, A.; Scammells, P.J.; Capuano, B.; Thal, D.M.; Javitch, J.A.; et al. The structural determinants of the bitopic binding mode of a negative allosteric modulator of the dopamine D2 receptor. Biochem. Pharmacol. 2018, 148, 315–328. [Google Scholar] [CrossRef]
- Rossi, M.; Fasciani, I.; Marampon, F.; Maggio, R.; Scarselli, M. The First Negative Allosteric Modulator for Dopamine D2 and D3 Receptors, SB269652 May Lead to a New Generation of Antipsychotic Drugs. Mol. Pharmacol. 2017, 91, 586–594. [Google Scholar] [CrossRef]
- Bonifazi, A.; Yano, H.; Ellenberger, M.P.; Muller, L.; Kumar, V.; Zou, M.F.; Cai, N.S.; Guerrero, A.M.; Woods, A.S.; Shi, L.; et al. Novel Bivalent Ligands Based on the Sumanirole Pharmacophore Reveal Dopamine D2 Receptor (D2R) Biased Agonism. J. Med. Chem. 2017, 60, 2890–2907. [Google Scholar] [CrossRef] [PubMed]
- Battiti, F.O.; Cemaj, S.L.; Guerrero, A.M.; Shaik, A.B.; Lam, J.; Rais, R.; Slusher, B.S.; Deschamps, J.R.; Imler, G.H.; Newman, A.H.; et al. The Significance of Chirality in Drug Design and Synthesis of Bitopic Ligands as D3 Receptor (D3R) Selective Agonists. J. Med. Chem. 2019, 62, 6287–6314. [Google Scholar] [CrossRef]
- Allerton, C.M.N.; Cook, A.S.; Hepworth, D.; Miller, D.C. Aminopyridine Derivatives as Selective Dopamine D3 Agonists WO 115985 Al, 12 August 2005.
- Ackley, M.A. Morpholine Dopamine Agonists for The Treatment of Pain. WO 087512 Al, 24 July 2008. [Google Scholar]
- Battiti, F.O.; Newman, A.H.; Bonifazi, A. Exception That Proves the Rule: Investigation of Privileged Stereochemistry in Designing Dopamine D3R Bitopic Agonists. ACS Med. Chem. Lett. 2020, 11, 1956–1964. [Google Scholar] [CrossRef] [PubMed]
- Moritz, A.E.; Bonifazi, A.; Guerrero, A.M.; Kumar, V.; Free, R.B.; Lane, J.R.; Verma, R.K.; Shi, L.; Newman, A.H.; Sibley, D.R. Evidence for a stereoselective mechanism for bitopic activity by extended-length antagonists of the D3 dopamine receptor. ACS Chem. Neurosci. 2020, 11, 3309–3320. [Google Scholar] [CrossRef] [PubMed]
- Virtanen, P.; Gommers, R.; Oliphant, T.E.; Haberland, M.; Reddy, T.; Cournapeau, D.; Burovski, E.; Peterson, P.; Weckesser, W.; Bright, J.; et al. SciPy 1.0: Fundamental algorithms for scientific computing in Python. Nat. Methods 2020, 17, 261–272. [Google Scholar] [CrossRef]
- Brooks, W.H.; Guida, W.C.; Daniel, K.G. The significance of chirality in drug design and development. Curr. Top Med. Chem. 2011, 11, 760–770. [Google Scholar] [CrossRef] [PubMed]
- Kumar, V.; Bonifazi, A.; Ellenberger, M.P.; Keck, T.M.; Pommier, E.; Rais, R.; Slusher, B.S.; Gardner, E.; You, Z.B.; Xi, Z.X.; et al. Highly Selective Dopamine D3 Receptor (D3R) Antagonists and Partial Agonists Based on Eticlopride and the D3R Crystal Structure: New Leads for Opioid Dependence Treatment. J. Med. Chem. 2016, 59, 7634–7650. [Google Scholar] [CrossRef]
- Kumar, V.; Moritz, A.E.; Keck, T.M.; Bonifazi, A.; Ellenberger, M.P.; Sibley, C.D.; Free, R.B.; Shi, L.; Lane, J.R.; Sibley, D.R.; et al. Synthesis and Pharmacological Characterization of Novel trans-Cyclopropylmethyl-Linked Bivalent Ligands That Exhibit Selectivity and Allosteric Pharmacology at the Dopamine D3 Receptor (D3R). J. Med. Chem. 2017, 60, 1478–1494. [Google Scholar] [CrossRef] [PubMed]
- Bonifazi, A.; Yano, H.; Guerrero, A.M.; Kumar, V.; Hoffman, A.F.; Lupica, C.R.; Shi, L.; Newman, A.H. Novel and Potent Dopamine D2 Receptor Go-Protein Biased Agonists. ACS Pharmacol. Transl. Sci. 2019, 2, 52–65. [Google Scholar] [CrossRef] [PubMed]
- Lane, J.R.; May, L.T.; Parton, R.G.; Sexton, P.M.; Christopoulos, A. A kinetic view of GPCR allostery and biased agonism. Nat. Chem. Biol. 2017, 13, 929–937. [Google Scholar] [CrossRef] [PubMed]
- Klein Herenbrink, C.; Sykes, D.A.; Donthamsetti, P.; Canals, M.; Coudrat, T.; Shonberg, J.; Scammells, P.J.; Capuano, B.; Sexton, P.M.; Charlton, S.J.; et al. The role of kinetic context in apparent biased agonism at GPCRs. Nat. Commun. 2016, 7, 10842. [Google Scholar] [CrossRef] [PubMed]
- Moritz, A.E.; Free, R.B.; Sibley, D.R. Advances and challenges in the search for D2 and D3 dopamine receptor-selective compounds. Cell. Signal. 2018, 41, 75–81. [Google Scholar] [CrossRef] [PubMed]
- Urs, N.M.; Gee, S.M.; Pack, T.F.; McCorvy, J.D.; Evron, T.; Snyder, J.C.; Yang, X.; Rodriguiz, R.M.; Borrelli, E.; Wetsel, W.C.; et al. Distinct cortical and striatal actions of a beta-arrestin-biased dopamine D2 receptor ligand reveal unique antipsychotic-like properties. Proc. Natl. Acad. Sci. USA 2016, 113, E8178–E8186. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Sassano, M.F.; Zheng, L.; Setola, V.; Chen, M.; Bai, X.; Frye, S.V.; Wetsel, W.C.; Roth, B.L.; Jin, J. Structure-functional selectivity relationship studies of beta-arrestin-biased dopamine D (2) receptor agonists. J. Med. Chem. 2012, 55, 7141–7153. [Google Scholar] [CrossRef] [PubMed]
- Zou, M.F.; Keck, T.M.; Kumar, V.; Donthamsetti, P.; Michino, M.; Burzynski, C.; Schweppe, C.; Bonifazi, A.; Free, R.B.; Sibley, D.R.; et al. Novel Analogues of (R)-5-(Methylamino)-5,6-dihydro-4H-imidazo[4,5,1-ij]quinolin-2(1H)-one (Sumanirole) Provide Clues to Dopamine D2/D3 Receptor Agonist Selectivity. J. Med. Chem. 2016, 59, 2973–2988. [Google Scholar] [CrossRef] [PubMed]


| D3R, 10 min | Go Protein Activation Assay | β-arrestin Recruitment | ||||||
|---|---|---|---|---|---|---|---|---|
| Compounds | Emax ± SEM (% of Quinpirole) | pEC50 ± SEM | Change Emax over Quinpirole | Fold Potency over Quinpirole | Emax ± SEM (% of Quinpirole) | pEC50 ± SEM | Change Emax over Quinpirole | Fold Potency over Quinpirole |
| Quinpirole | 100 ± 2.27 δδδδ | 8.53 ± 0.08 δδδδ | 0 | 1.000 | 100 ± 2.6 δδδδ | 8.36 ± 0.09 δδδδ | 0 | 1.000 |
| rac-FOB02-04 | 74.4 ± 3.4 *** | 7.67 ± 0.16 ***,δδδδ | −25.6 | 0.138 | 68.5 ± 1.8 *** | 6.78 ± 0.07 ****, δδδ | −31.5 | 0.026 |
| FOB02-04A | 82.8 ± 3.0 **,δδ | 8.22 ± 0.12 δδδδ | −17.2 | 0.490 | 80.2 ± 3.3 * | 6.80 ± 0.10 ****, δδδδ | −19.8 | 0.028 |
| FOB02-04B | 78.4 ± 3.2 ****,δδ | 7.25 ± 0.11 ****, δδδδ | −21.6 | 0.052 | 112 ± 7.0 δδδδ | 5.87 ± 0.10 **** | 33.2 | 0.003 |
| AB04-87 | 60.3 ± 8.1 **** | 6.00 ± 0.23 **** | −39.7 | 0.003 | 67.2 ± 8.2 **** | 5.80 ± 0.19 **** | −32.8 | 0.003 |
| AB04-88 | 111.6 ± 2.8 δδδδ | 9.34 ± 0.09 ***,δδδδ | 11.6 | 6.500 | 110 ± 0.9 δδδδ | 7.03 ± 0.10 ****, δδδδ | 10.0 | 0.047 |
| rac-AB04-35 | 93.9 ± 3.0 δδδδ | 7.24 ± 0.07 ****, δδδδ | −6.1 | 0.051 | 94.8 ± 3.7 δδ | 6.31 ± 0.07 ****, δ | −5.2 | 0.009 |
| AB04-95 | ND | ND | ND | ND | ND | ND | ND | ND |
| AB04-96 | 100.4 ± 2.7 δδδδ | 7.73 ± 0.08 ***, δδδδ | 0.4 | 0.158 | 104.0 ± 2.9 δδδ | 6.76 ± 0.06 ****, δδδ | 4.0 | 0.025 |
| D2R, 10 min | Go Protein Activation Assay | β-arrestin Recruitment | ||||||
|---|---|---|---|---|---|---|---|---|
| Compounds | Emax ± SEM (% of Quinpirole) | pEC50 ± SEM | Change Emax over Quinpirole | Fold Potency over Quinpirole | Emax ± SEM (% of Quinpirole) | pEC50 ± SEM | Change Emax over Quinpirole | Fold Potency over Quinpirole |
| Quinpirole | 100 ± 2.0 | 7.46 ± 0.07 | 0.00 | 1.00 | 100 ± 2.8 | 6.93 ± 0.08 | 0.0 | 1.00 |
| rac-FOB02-04 | 93.2 ± 2.2 | 7.13 ± 0.06 * | −6.80 | 0.47 | 62.9 ± 1.9 **** | 5.72 ± 0.07 **** | −37.1 | 0.06 |
| FOB02-04A | 91.2 ± 2.8 | 7.31 ± 0.08 | −8.80 | 0.71 | 60.4 ± 1.9 **** | 6.04 ± 0.07 **** | −39.6 | 0.13 |
| FOB02-04B | 81.4 ± 3.8 *** | 6.44 ± 0.13 **** | −18.60 | 0.10 | 56.9 ± 1.4 **** | 5.45 ± 0.05 **** | −43.1 | 0.03 |
| AB04-87 | ND | ND | ND | ND | ND | ND | ND | ND |
| AB04-88 | 95.2 ± 2.4 | 7.25 ± 0.07 | −4.80 | 0.62 | 72.6 ± 2.6 **** | 5.84 ± 0.06 **** | −27.4 | 0.08 |
| rac-AB04-35 | 75.9 ± 5.3 *** | 6.48 ± 0.13 **** | −24.10 | 0.10 | 102.8 ± 2.5 | 5.74 ± 0.06 **** | 2.80 | 0.06 |
| AB04-95 | ND | ND | ND | ND | ND | ND | ND | ND |
| AB04-96 | 102.7 ± 4.0 | 7.28 ± 0.11 | 2.70 | 0.66 | 121.6 ± 8.2 *** | 6.14 ± 0.13 **** | 21.6 | 0.16 |
| Selectivity (D2R/D3R) | Bias Factors | |||
|---|---|---|---|---|
| Compounds | Go Protein Activation | β-arrestin Recruitment | D3R | D2R |
| Quinpirole | 11.7 | 26.9 | ND | ND |
| rac-FOB02-04 | 3.5 | 11.5 | 0.9 | 1.6 |
| FOB02-04A | 8.1 | 5.8 | 1.4 | 1.4 |
| FOB02-04B | 6.5 | 2.6 | 1.1 | 1.1 |
| AB04-87 | ND | ND | 0.2 | ND |
| AB04-88 | 123.0 | 15.5 | 2.3 | 1.5 |
| rac-AB04-35 | 5.8 | 3.7 | 0.9 | 0.6 |
| AB04-95 | ND | ND | ND | ND |
| AB04-96 | 2.8 | 4.2 | 1.0 | 1.1 |
| Quinpirole | AB04-88 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Go Protein Activation | β-arrestin Recruitment | Bias Factors | Go Protein Activation | β-arrestin Recruitment | Bias Factors | |||||
| Time-points (min) | pEC50 ± SEM | Emax (% of quinpirole) | pEC50 ± SEM | Emax (% of quinpirole) | pEC50 ± SEM | Emax (% of quinpirole) | pEC50 ± SEM | Emax (% of quinpirole) | ||
| 2 | 8.51 ± 0.25 | 101 ± 5.6 | 8.20 ± 0.26 | 92.8 ± 3.9 | 0.4 | 9.20 ± 0.21 | 112.7 ± 5.8 | 7.64 ± 0.12 | 94.4 ± 1.4 | 1.6 |
| 4 | 8.72 ± 0.14 | 101.4 ± 2.0 | 8.23 ± 0.23 | 97.9 ± 1.9 | 0.5 | 9.27 ± 0.14 | 113.8 ± 3.3 | 7.57 ± 0.08 * | 96.4 ± 0.2 | 1.8 |
| 6 | 8.81 ± 0.13 | 102.3 ± 1.0 | 8.21 ± 0.19 | 100.3 ± 1 | 0.6 | 9.32 ± 0.12 | 114.0 ± 1.6 | 7.43 ± 0.07 ** | 99.1 ± 0.2 | 1.9 |
| 8 | 8.86 ± 0.13 | 101.4 ± 0.4 | 8.13 ± 0.14 | 100.6 ± 0.3 | 0.7 | 9.37 ± 0.12 | 112.9 ± 0.6 | 7.30 ± 0.09 *** | 101.3 ± 0.1 | 2.1 |
| 10 | 8.91 ± 0.14 | 100.0 ± 0.0 | 8.07 ± 0.12 | 100.0 ± 0 | 0.8 | 9.39 ± 0.12 | 111.7 ± 0.0 | 7.20 ± 0.13 *** | 102.2 ± 0 | 2.3 |
| 12 | 8.94 ± 0.14 | 98.9 ± 0.3 | 8.04 ± 0.10 | 99.4 ± 0.3 | 0.9 | 9.40 ± 0.11 | 110.0 ± 0.5 | 7.16 ± 0.15 *** | 101.8 ± 0.9 | 2.3 |
| 14 | 8.96 ± 0.15 | 97.3 ± 0.6 | 8.01 ± 0.11 | 98.3 ± 0.6 | 0.9 | 9.40 ± 0.10 | 108.9 ± 0.8 | 7.14 ± 0.16 *** | 100.7 ± 1.8 | 2.3 |
| 16 | 8.97 ± 0.15 | 95.6 ± 0.9 | 7.97 ± 0.11 | 97.0 ± 0.9 | 1.0 | 9.39 ± 0.10 | 107.6 ± 1.2 | 7.13 ± 0.14 *** | 100.2 ± 2.8 | 2.3 |
| 18 | 8.98 ± 0.15 | 94.2 ± 1.1 | 7.94 ± 0.12 | 95.9 ± 1.3 | 1.0 | 9.39 ± 0.10 | 106.4 ± 1.5 | 7.13 ± 0.13 *** | 99.1 ± 3.3 | 2.3 |
| 20 | 8.99 ± 0.14 | 93.5 ± 1.2 | 7.92 ± 0.14 | 95.5 ± 1.5 | 1.0 | 9.39 ± 0.08 | 105.3 ± 1.7 | 7.14 ± 0.12 ** | 98.4 ± 3.5 | 2.3 |
| 22 | 8.99 ± 0.15 | 92.6 ± 1.4 | 7.92 ± 0.14 | 94.5 ± 1.7 | 1.0 | 9.40 ± 0.08 | 104.0 ± 1.9 | 7.15 ± 0.14** | 97.1 ± 3.8 | 2.3 |
| 24 | 9.00 ± 0.15 | 91.8 ± 1.6 | 7.86 ± 0.18 | 93.9 ± 1.9 | 1.1 | 9.38 ± 0.07 | 104.6 ± 2.1 | 7.17 ± 0.14 ** | 96.2 ± 4.5 | 2.2 |
| 26 | 9.00 ± 0.14 | 90.9 ± 1.6 | 7.89 ± 0.14 | 93.1 ± 1.9 | 1.1 | 9.36 ± 0.09 | 104.2 ± 2.3 | 7.20 ± 0.13 ** | 94.6 ± 5.1 | 2.2 |
| 28 | 9.03 ± 0.13 | 91.1 ± 1.9 | 7.87 ± 0.15 | 93.4 ± 1.9 | 1.1 | 9.35 ± 0.09 | 105.3 ± 3.1 | 7.20 ± 0.11 * | 94.0 ± 6.1 | 2.2 |
| 30 | 9.06 ± 0.12 | 91.0 ± 2.3 | 7.91 ± 0.10 | 93.1 ± 2.2 | 1.1 | 9.36 ± 0.08 | 105.3 ± 4.0 | 7.21 ± 0.11 ** | 93.0 ± 6.4 | 2.2 |
| 32 | 9.03 ± 0.13 | 91.6 ± 2.6 | 7.94 ± 0.08 | 93.1 ± 2.5 | 1.1 | 9.35 ± 0.06 | 108.0 ± 4.8 | 7.24 ± 0.11 ** | 92.1 ± 7.6 | 2.2 |
| 34 | 9.03 ± 0.13 | 93.3 ± 3.5 | 7.96 ± 0.07 | 94.3 ± 3.2 | 1.1 | 9.30 ± 0.08 | 111.6 ± 5.5 | 7.25 ± 0.13 ** | 90.6 ± 6.9 | 2.1 |
| 36 | 9.00 ± 0.15 | 94.7 ± 4.3 | 7.99 ± 0.06 | 94.7 ± 3.9 | 1.0 | 9.30 ± 0.07 | 116.2 ± 6.6 | 7.23 ± 0.15 ** | 89.7 ± 6.7 | 2.2 |
| 38 | 9.03 ± 0.15 | 95.8 ± 4.1 | 7.99 ± 0.05 | 95.1 ± 3.1 | 1.0 | 9.26 ± 0.09 | 121.3 ± 7.7 * | 7.23 ± 0.15 ** | 88.5 ± 6.0 | 2.2 |
| 40 | 8.99 ± 0.16 | 98.6 ± 4.8 | 7.97 ± 0.05 | 96.2 ± 3.5 | 1.0 | 9.27 ± 0.07 | 125.3 ± 9.4 * | 7.25 ± 0.14 ** | 87.6 ± 6.8 | 2.2 |
| 42 | 9.00 ± 0.15 | 102.5 ± 6.2 | 7.96 ± 0.03 | 97.6 ± 4.3 | 1.1 | 9.24 ± 0.07 | 134.6 ± 12.5 ** | 7.32 ± 0.09 * | 85.9 ± 6.9 | 2.1 |
| 44 | 9.05 ± 0.13 | 105.2 ± 8.7 | 7.96 ± 0.04 | 98.0 ± 6.5 | 1.1 | 9.24 ± 0.06 | 145.6 ± 17.3 **** | 7.31 ± 0.08 * | 85.4 ± 6.8 | 2.2 |
| 46 | 9.19 ± 0.17 | 112.0 ± 10.9 | 7.99 ± 0.07 | 100.6 ± 5.2 | 1.3 | 9.23 ± 0.09 | 160.0 ± 23.6 **** | 7.25 ± 0.09 ** | 84.9 ± 6.6 | 2.3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Adhikari, P.; Xie, B.; Semeano, A.; Bonifazi, A.; Battiti, F.O.; Newman, A.H.; Yano, H.; Shi, L. Chirality of Novel Bitopic Agonists Determines Unique Pharmacology at the Dopamine D3 Receptor. Biomolecules 2021, 11, 570. https://doi.org/10.3390/biom11040570
Adhikari P, Xie B, Semeano A, Bonifazi A, Battiti FO, Newman AH, Yano H, Shi L. Chirality of Novel Bitopic Agonists Determines Unique Pharmacology at the Dopamine D3 Receptor. Biomolecules. 2021; 11(4):570. https://doi.org/10.3390/biom11040570
Chicago/Turabian StyleAdhikari, Pramisha, Bing Xie, Ana Semeano, Alessandro Bonifazi, Francisco O. Battiti, Amy H. Newman, Hideaki Yano, and Lei Shi. 2021. "Chirality of Novel Bitopic Agonists Determines Unique Pharmacology at the Dopamine D3 Receptor" Biomolecules 11, no. 4: 570. https://doi.org/10.3390/biom11040570
APA StyleAdhikari, P., Xie, B., Semeano, A., Bonifazi, A., Battiti, F. O., Newman, A. H., Yano, H., & Shi, L. (2021). Chirality of Novel Bitopic Agonists Determines Unique Pharmacology at the Dopamine D3 Receptor. Biomolecules, 11(4), 570. https://doi.org/10.3390/biom11040570







