Role of Thrombin in Central Nervous System Injury and Disease
Abstract
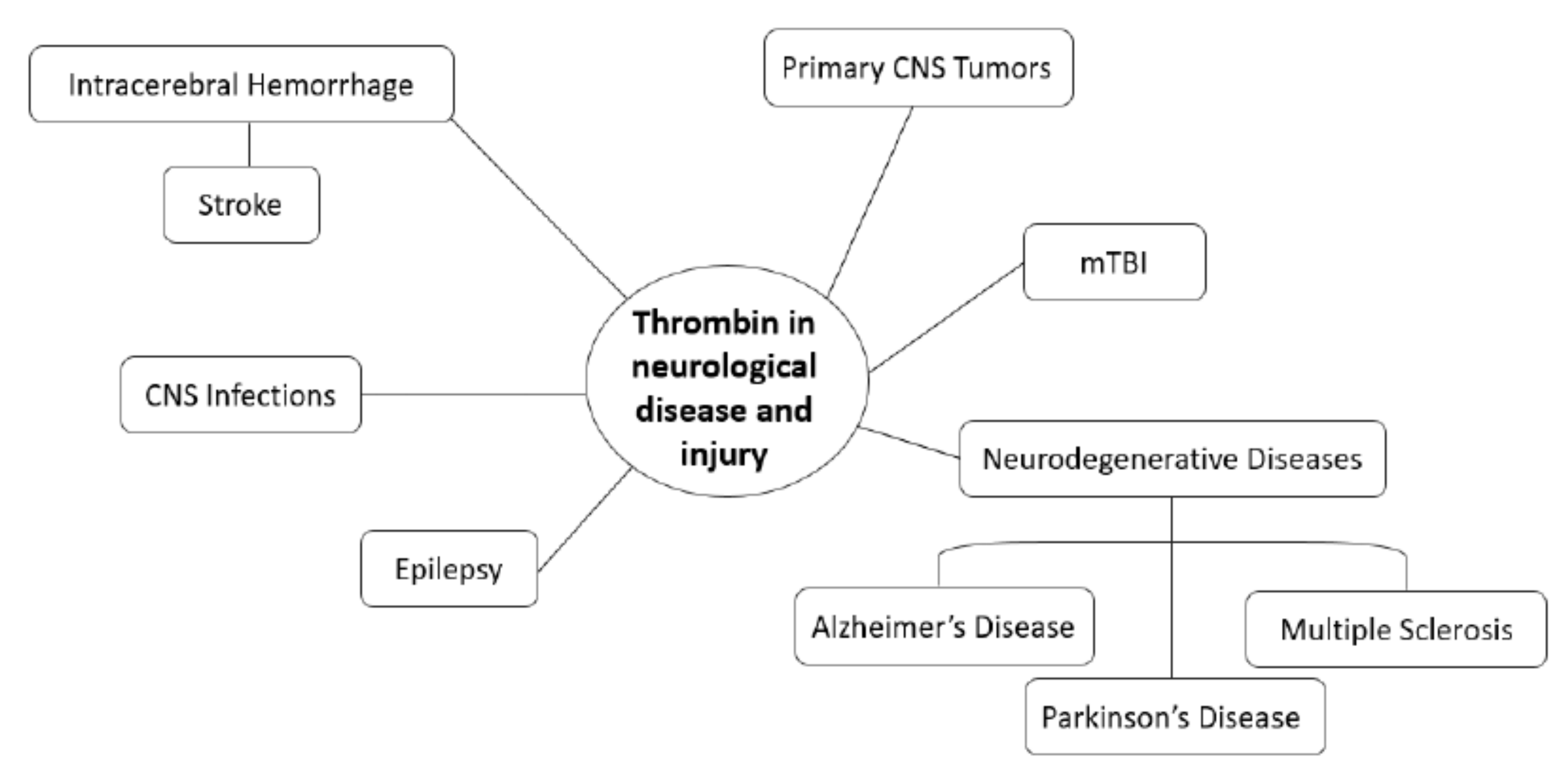
1. Introduction
2. Mechanisms of Thrombin Action
2.1. Coagulation
2.2. Thrombotic and Immune Functions
2.3. Cellular Protection and Apoptosis
3. Thrombin in the Central Nervous System
3.1. Localization in Brain
3.2. Neuro-Physiological Functions
3.3. Neuro-Pathological Functions
4. Neurodegenerative Diseases
4.1. Alzheimer’s Disease
4.2. Parkinson’s Disease
4.3. Multiple Sclerosis
5. Intracerebral Hemorrhage and Stroke
6. CNS Infections
7. Mild Traumatic Brain Injury (mTBI)
8. Epilepsy
9. Primary CNS Tumors
10. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Di Cera, E. Thrombin. Mol. Asp. Med. 2008, 29, 203–254. [Google Scholar] [CrossRef] [PubMed]
- Di Cera, E.; Page, M.J.; Bah, A.; Bush-Pelc, L.A.; Garvey, L.C. Thrombin allostery. Phys. Chem. Chem. Phys. 2007, 9, 1291–1306. [Google Scholar] [CrossRef] [PubMed]
- Wells, C.M.; Di Cera, E. Thrombin is a sodium ion activated enzyme. Biochemistry 1992, 31, 11721–11730. [Google Scholar] [CrossRef]
- Posma, J.; Posthuma, J.; Spronk, H. Coagulation and non-coagulation effects of thrombin. J. Thromb. Haemost. 2016, 14, 1908–1916. [Google Scholar] [CrossRef]
- Ma, L.; Dorling, A. The roles of thrombin and protease-activated receptors in inflammation. Semin. Immunopathol. 2012, 34, 63–72. [Google Scholar] [CrossRef] [PubMed]
- Crawley, J.; Zanardelli, S.; Chion, C.; Lane, D. The central role of thrombin in hemostasis. J. Thromb. Haemost. 2007, 5, 95–101. [Google Scholar] [CrossRef]
- Walker, C.; Royston, D. Thrombin generation and its inhibition: A review of the scientific basis and mechanism of action of anticoagulant therapies. Br. J. Anaesth. 2002, 88, 848–863. [Google Scholar] [CrossRef] [PubMed]
- Krenzlin, H.; Lorenz, V.; Danckwardt, S.; Kempski, O.; Alessandri, B. The importance of thrombin in cerebral injury and disease. Int. J. Mol. Sci. 2016, 17, 84. [Google Scholar] [CrossRef]
- Huntington, J. Molecular recognition mechanisms of thrombin. J. Thromb. Haemost. 2005, 3, 1861–1872. [Google Scholar] [CrossRef] [PubMed]
- Coughlin, S.R. How the protease thrombin talks to cells. Proc. Natl. Acad. Sci. USA 1999, 96, 11023–11027. [Google Scholar] [CrossRef]
- Donovan, F.M.; Cunningham, D.D. Signaling pathways involved in thrombin-induced cell protection. J. Biol. Chem. 1998, 273, 12746–12752. [Google Scholar] [CrossRef]
- Davie, E.W.; Fujikawa, K.; Kisiel, W. The coagulation cascade: Initiation, maintenance, and regulation. Biochemistry 1991, 30, 10363–10370. [Google Scholar] [CrossRef]
- Weitz, J.; Hudoba, M.; Massel, D.; Maraganore, J.; Hirsh, J. Clot-bound thrombin is protected from inhibition by heparin-antithrombin III but is susceptible to inactivation by antithrombin III-independent inhibitors. J. Clin. Investig. 1990, 86, 385–391. [Google Scholar] [CrossRef] [PubMed]
- Roemisch, J.; Gray, E.; Hoffmann, J.; Wiedermann, C. Antithrombin: A new look at the actions of a serine protease inhibitor. Blood Coagul. Fibrinolysis 2002, 13, 657–670. [Google Scholar] [CrossRef] [PubMed]
- Grütter, M.; Priestle, J.; Rahuel, J.; Grossenbacher, H.; Bode, W.; Hofsteenge, J.; Stone, S. Crystal structure of the thrombin-hirudin complex: A novel mode of serine protease inhibition. EMBO J. 1990, 9, 2361–2365. [Google Scholar] [CrossRef]
- Stone, S.R.; Hofsteenge, J. Kinetics of the inhibition of thrombin by hirudin. Biochemistry 1986, 25, 4622–4628. [Google Scholar] [CrossRef] [PubMed]
- Siller-Matula, J.M.; Schwameis, M.; Blann, A.; Mannhalter, C.; Jilma, B. Thrombin as a multi-functional enzyme. Thromb. Haemost. 2011, 106, 1020–1033. [Google Scholar]
- De Luca, C.; Virtuoso, A.; Maggio, N.; Papa, M. Neuro-coagulopathy: Blood coagulation factors in central nervous system diseases. Int. J. Mol. Sci. 2017, 18, 2128. [Google Scholar] [CrossRef]
- Mann, K.; Brummel, K.; Butenas, S. What is all that thrombin for? J. Thromb. Haemost. 2003, 1, 1504–1514. [Google Scholar] [CrossRef]
- Spiel, A.O.; Bartko, J.; Schwameis, M.; Firbas, C.; Siller-Matula, J.; Schuetz, M.; Weigl, M.; Jilma, B. Increased platelet aggregation and in vivo platelet activation after granulocyte colony-stimulating factor administration. A randomised controlled trial. Thromb. Haemost. 2011, 105, 655. [Google Scholar] [CrossRef]
- Wu, C.-C.; Wang, W.-Y.; Wei, C.-K.; Teng, C.-M. Combined blockade of thrombin anion binding exosite-1 and PAR4 produces synergistic antiplatelet effect in human platelets. Thromb. Haemost. 2011, 105, 88–95. [Google Scholar]
- Siller-Matula, J.M.; Krumphuber, J.; Jilma, B. Pharmacokinetic, pharmacodynamic and clinical profile of novel antiplatelet drugs targeting vascular diseases. Br. J. Pharmacol. 2010, 159, 502–517. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.H.; Garand, M.; Zagorac, B.; Schadinger, S.L.; Scipione, C.; Koschinsky, M.L.; Boffa, M.B. Identification of human thrombin-activatable fibrinolysis inhibitor in vascular and inflammatory cells. Thromb. Haemost. 2011, 105, 999–1009. [Google Scholar] [CrossRef]
- Vorjohann, S.; Fish, R.J.; Biron-Andreani, C.; Nagaswami, C.; Weisel, J.W.; Boulot, P.; Reyftmann, L.; De Moerloose, P.; Neerman-Arbez, M. Hypodysfibrinogenaemia due to production of mutant fibrinogen alpha-chains lacking fibrinopeptide A and polymerisation knob ‘A’. Thromb. Haemost. 2010, 104, 990. [Google Scholar] [CrossRef] [PubMed]
- Martorell, L.; Martínez-González, J.; Rodriguez, C.; Gentile, M.; Calvayrac, O.; Badimon, L. Thrombin and protease-activated receptors (PARs) in atherothrombosis. Thromb. Haemost. 2008, 99, 305–315. [Google Scholar] [CrossRef] [PubMed]
- Rex, S.; Beaulieu, L.M.; Perlman, D.H.; Vitseva, O.; Blair, P.S.; McComb, M.E.; Costello, C.E.; Freedman, J.E. Immune vs. Thrombotic stimulation of platelets differentially regulates signaling pathways, intracellular protein-protein interactions, and α-granule release. Thromb. Haemost. 2009, 102, 97. [Google Scholar] [CrossRef] [PubMed]
- Schuepbach, R.A.; Feistritzer, C.; Fernández, J.A.; Griffin, J.H.; Riewald, M. Protection of vascular barrier integrity by activated protein C in murine models depends on protease-activated receptor-1. Thromb. Haemost. 2009, 101, 724. [Google Scholar]
- Chen, L.B.; Buchanan, J.M. Mitogenic activity of blood components. I. Thrombin and prothrombin. Proc. Natl. Acad. Sci. USA 1975, 72, 131–135. [Google Scholar] [CrossRef]
- Bar-Shavit, R.; Benezra, M.; Eldor, A.; Hy-Am, E.; Fenton, J., 2nd; Wilner, G.D.; Vlodavsky, I. Thrombin immobilized to extracellular matrix is a potent mitogen for vascular smooth muscle cells: Nonenzymatic mode of action. Cell Regul. 1990, 1, 453–463. [Google Scholar] [CrossRef]
- Weiss, R.H.; Maduri, M. The mitogenic effect of thrombin in vascular smooth muscle cells is largely due to basic fibroblast growth factor. J. Biol. Chem. 1993, 268, 5724–5727. [Google Scholar] [CrossRef]
- Rabiet, M.-J.P.; Plantier, J.-L.; Rival, Y.; Genoux, Y.; Lampugnani, M.-G.; Dejana, E. Thrombin-induced increase in endothelial permeability is associated with changes in cell-to-cell junction organization. Arterioscler. Thromb. Vasc. Biol. 1996, 16, 488–496. [Google Scholar] [CrossRef]
- Garcia, J.; Pavalko, F.; Patterson, C. Vascular endothelial cell activation and permeability responses to thrombin. Blood Coagul. Fibrinolysis Int. J. Haemost. Thromb. 1995, 6, 609–626. [Google Scholar] [CrossRef] [PubMed]
- Derkach, D.N.; Ihara, E.; Hirano, K.; Nishimura, J.; Takahashi, S.; Kanaide, H. Thrombin causes endothelium-dependent biphasic regulation of vascular tone in the porcine renal interlobar artery. Br. J. Pharmacol. 2000, 131, 1635–1642. [Google Scholar] [CrossRef]
- Rickles, F.R.; Patierno, S.; Fernandez, P.M. Tissue factor, thrombin, and cancer. Chest 2003, 124, 58S–68S. [Google Scholar] [CrossRef]
- Kumar, P.; Shen, Q.; Pivetti, C.D.; Lee, E.S.; Wu, M.H.; Yuan, S.Y. Molecular mechanisms of endothelial hyperpermeability: Implications in inflammation. Expert Rev. Mol. Med. 2009, 11, e19. [Google Scholar] [CrossRef]
- Petäjä, J. Inflammation and coagulation. An overview. Thromb. Res. 2011, 127, S34–S37. [Google Scholar] [CrossRef]
- Rukoyatkina, N.; Begonja, A.J.; Geiger, J.; Eigenthaler, M.; Walter, U.; Gambaryan, S. Phosphatidylserine surface expression and integrin αIIbβ3 activity on thrombin/convulxin stimulated platelets/particles of different sizes. Br. J. Haematol. 2009, 144, 591–602. [Google Scholar] [CrossRef] [PubMed]
- Tsopanoglou, N.E.; Maragoudakis, M.E. Role of thrombin in angiogenesis and tumor progression. Semin. Thromb. Hemost. 2004, 30, 63–69. [Google Scholar] [PubMed]
- Zhang, P.; Ozdemir, T.; Chung, C.-Y.; Robertson, G.P.; Dong, C. Sequential binding of αVβ3 and ICAM-1 determines fibrin-mediated melanoma capture and stable adhesion to CD11b/CD18 on neutrophils. J. Immunol. 2011, 186, 242–254. [Google Scholar] [CrossRef]
- Diebold, I.; Djordjevic, T.; Hess, J.; Görlach, A. Rac-1 promotes pulmonary artery smooth muscle cell proliferation by upregulation of plasminogen activator inhibitor-1: Role of NFκB-dependent hypoxia-inducible factor-1α transcription. Thromb. Haemost. 2008, 100, 1021–1028. [Google Scholar] [CrossRef] [PubMed]
- Diebold, I.; Petry, A.; Djordjevic, T.; BelAiba, R.S.; Fineman, J.; Black, S.; Schreiber, C.; Fratz, S.; Hess, J.; Kietzmann, T. Reciprocal regulation of Rac1 and PAK-1 by HIF-1α: A positive-feedback loop promoting pulmonary vascular remodeling. Antioxid. Redox Signal. 2010, 13, 399–412. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Dorling, A. Critical roles for thrombin in acute and chronic inflammation. J. Thromb. Haemost. 2009, 7, 122–126. [Google Scholar] [CrossRef] [PubMed]
- Miyamoto, M.; Ohno, M.; Yamada, N.; Ohtake, A.; Matsushita, T. TRA-418, a thromboxane A2 receptor antagonist and prostacyclin receptor agonist, inhibits platelet-leukocyte interaction in human whole blood. Thromb. Haemost. 2010, 104, 788–795. [Google Scholar] [PubMed]
- Verkleij, C.J.; Roelofs, J.J.; Havik, S.R.; Meijers, J.C.; Marx, P.F. The role of thrombin-activatable fibrinolysis inhibitor in diabetic wound healing. Thromb. Res. 2010, 126, 442–446. [Google Scholar] [CrossRef] [PubMed]
- Naldini, A.; Carney, D.H.; Pucci, A.; Pasquali, A.; Carraro, F. Thrombin regulates the expression of proangiogenic cytokines via proteolytic activation of protease-activated receptor-1. Gen. Pharmacol. Vasc. Syst. 2000, 35, 255–259. [Google Scholar] [CrossRef]
- Bae, J.S.; Kim, Y.U.; Park, M.K.; Rezaie, A.R. Concentration dependent dual effect of thrombin in endothelial cells via Par-1 and Pi3 Kinase. J. Cell. Physiol. 2009, 219, 744–751. [Google Scholar] [CrossRef]
- Donovan, F.M.; Pike, C.J.; Cotman, C.W.; Cunningham, D.D. Thrombin induces apoptosis in cultured neurons and astrocytes via a pathway requiring tyrosine kinase and RhoA activities. J. Neurosci. 1997, 17, 5316–5326. [Google Scholar] [CrossRef]
- Zampatis, D.E.; Rutz, C.; Furkert, J.; Schmidt, A.; Wüstenhagen, D.; Kubick, S.; Tsopanoglou, N.E.; Schülein, R. The protease-activated receptor 1 possesses a functional and cleavable signal peptide which is necessary for receptor expression. FEBS Lett. 2012, 586, 2351–2359. [Google Scholar] [CrossRef]
- O’Brien, P.J.; Molino, M.; Kahn, M.; Brass, L.F. Protease activated receptors: Theme and variations. Oncogene 2001, 20, 1570–1581. [Google Scholar] [CrossRef]
- Abraham, L.A.; Mackie, E.J. Modulation of osteoblast-like cell behavior by activation of protease-activated receptor-1. J. Bone Miner. Res. 1999, 14, 1320–1329. [Google Scholar] [CrossRef]
- Lin, H.; Liu, A.P.; Smith, T.H.; Trejo, J. Cofactoring and dimerization of proteinase-activated receptors. Pharmacol. Rev. 2013, 65, 1198–1213. [Google Scholar] [CrossRef]
- Bae, J.-S.; Yang, L.; Manithody, C.; Rezaie, A.R. The ligand occupancy of endothelial protein C receptor switches the protease-activated receptor 1-dependent signaling specificity of thrombin from a permeability-enhancing to a barrier-protective response in endothelial cells. Blood J. Am. Soc. Hematol. 2007, 110, 3909–3916. [Google Scholar] [CrossRef]
- Bea, F.; Kreuzer, J.; Preusch, M.; Schaab, S.; Isermann, B.; Rosenfeld, M.E.; Katus, H.; Blessing, E. Melagatran reduces advanced atherosclerotic lesion size and may promote plaque stability in Apolipoprotein E–deficient mice. Arterioscler. Thromb. Vasc. Biol. 2006, 26, 2787–2792. [Google Scholar] [CrossRef] [PubMed]
- Griffin, J.H.; Zlokovic, B.V.; Mosnier, L.O. Activated protein C: Biased for translation. Blood 2015, 125, 2898–2907. [Google Scholar] [CrossRef]
- Soh, U.J.; Dores, M.R.; Chen, B.; Trejo, J. Signal transduction by protease-activated receptors. Br. J. Pharmacol. 2010, 160, 191–203. [Google Scholar] [CrossRef] [PubMed]
- Rezaie, A.R. The occupancy of endothelial protein C receptor by its ligand modulates the par-1 dependent signaling specificity of coagulation proteases. IUBMB Life 2011, 63, 390–396. [Google Scholar] [CrossRef] [PubMed]
- Tohgo, A.; Pierce, K.L.; Choy, E.W.; Lefkowitz, R.J.; Luttrell, L.M. β-Arrestin scaffolding of the ERK cascade enhances cytosolic ERK activity but inhibits ERK-mediated transcription following angiotensin AT1a receptor stimulation. J. Biol. Chem. 2002, 277, 9429–9436. [Google Scholar] [CrossRef]
- Tohgo, A.; Choy, E.W.; Gesty-Palmer, D.; Pierce, K.L.; Laporte, S.; Oakley, R.H.; Caron, M.G.; Lefkowitz, R.J.; Luttrell, L.M. The stability of the G protein-coupled receptor-β-arrestin interaction determines the mechanism and functional consequence of ERK activation. J. Biol. Chem. 2003, 278, 6258–6267. [Google Scholar] [CrossRef] [PubMed]
- Shenoy, S.K.; Barak, L.S.; Xiao, K.; Ahn, S.; Berthouze, M.; Shukla, A.K.; Luttrell, L.M.; Lefkowitz, R.J. Ubiquitination of β-arrestin links seven-transmembrane receptor endocytosis and ERK activation. J. Biol. Chem. 2007, 282, 29549–29562. [Google Scholar] [CrossRef]
- Steen, A.; Larsen, O.; Thiele, S.; Rosenkilde, M.M. Biased and g protein-independent signaling of chemokine receptors. Front. Immunol. 2014, 5, 277. [Google Scholar] [CrossRef]
- Sokolova, E.; Reiser, G. Prothrombin/thrombin and the thrombin receptors PAR-1 and PAR-4 in the brain: Localization, expression and participation in neurodegenerative diseases. Thromb. Haemost. 2008, 100, 576–581. [Google Scholar] [CrossRef]
- Choi, B.; Suzuki, M.; Kim, T.; Wagner, S.; Cunningham, D. Protease nexin-1. Localization in the human brain suggests a protective role against extravasated serine proteases. Am. J. Pathol. 1990, 137, 741. [Google Scholar]
- Deschepper, C.F.; Bigornia, V.; Berens, M.E.; Lapointe, M.C. Production of thrombin and antithrombin III by brain and astroglial cell cultures. Mol. Brain Res. 1991, 11, 355–358. [Google Scholar] [CrossRef]
- Niclou, S.P.; Suidan, H.S.; Pavlik, A.; Vejsada, R.; Monard, D. Changes in the expression of protease-activated receptor 1 and protease nexin-1 mRNA during rat nervous system development and after nerve lesion. Eur. J. Neurosci. 1998, 10, 1590–1607. [Google Scholar] [CrossRef] [PubMed]
- Shikamoto, Y.; Morita, T. Expression of factor X in both the rat brain and cells of the central nervous system. FEBS Lett. 1999, 463, 387–389. [Google Scholar] [CrossRef]
- Dihanich, M.; Kaser, M.; Reinhard, E.; Cunningham, D.; Monard, D. Prothrombin mRNA is expressed by cells of the nervous system. Neuron 1991, 6, 575–581. [Google Scholar] [CrossRef]
- Ben Shimon, M.; Lenz, M.; Ikenberg, B.; Becker, D.; Shavit Stein, E.; Chapman, J.; Tanne, D.; Pick, C.G.; Blatt, I.; Neufeld, M. Thrombin regulation of synaptic transmission and plasticity: Implications for health and disease. Front. Cell. Neurosci. 2015, 9, 151. [Google Scholar] [CrossRef]
- Xi, G.; Reiser, G.; Keep, R.F. The role of thrombin and thrombin receptors in ischemic, hemorrhagic and traumatic brain injury: Deleterious or protective? J. Neurochem. 2003, 84, 3–9. [Google Scholar] [CrossRef] [PubMed]
- Stein, E.S.; Itsekson-Hayosh, Z.; Aronovich, A.; Reisner, Y.; Bushi, D.; Pick, C.G.; Tanne, D.; Chapman, J.; Vlachos, A.; Maggio, N. Thrombin induces ischemic LTP (iLTP): Implications for synaptic plasticity in the acute phase of ischemic stroke. Sci. Rep. 2015, 5, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Ubl, J.; Vöhringer, C.; Reiser, G. Co-existence of two types of [Ca2+] i-inducing protease-activated receptors (PAR-1 and PAR-2) in rat astrocytes and C6 glioma cells. Neuroscience 1998, 86, 597–609. [Google Scholar] [CrossRef]
- Striggow, F.; Riek-Burchardt, M.; Kiesel, A.; Schmidt, W.; Henrich-Noack, P.; Breder, J.; Krug, M.; Reymann, K.G.; Reiser, G. Four different types of protease-activated receptors are widely expressed in the brain and are up-regulated in hippocampus by severe ischemia. Eur. J. Neurosci. 2001, 14, 595–608. [Google Scholar] [CrossRef]
- Junge, C.E.; Lee, C.J.; Hubbard, K.B.; Zhang, Z.; Olson, J.J.; Hepler, J.R.; Brat, D.J.; Traynelis, S.F. Protease-activated receptor-1 in human brain: Localization and functional expression in astrocytes. Exp. Neurol. 2004, 188, 94–103. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Ubl, J.J.; Reiser, G. Four subtypes of protease-activated receptors, co-expressed in rat astrocytes, evoke different physiological signaling. Glia 2002, 37, 53–63. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Ubl, J.J.; Stricker, R.; Reiser, G. Thrombin (PAR-1)-induced proliferation in astrocytes via MAPK involves multiple signaling pathways. Am. J. Physiol. Cell Physiol. 2002, 283, C1351–C1364. [Google Scholar] [CrossRef]
- Jiang, Y.; Wu, J.; Hua, Y.; Keep, R.F.; Xiang, J.; Hoff, J.T.; Xi, G. Thrombin-receptor activation and thrombin-induced brain tolerance. J. Cereb. Blood Flow Metab. 2002, 22, 404–410. [Google Scholar] [CrossRef]
- Jamison, C.S.; Degen, S.J.F. Prenatal and postnatal expression of mRNA coding for rat prothrombin. Biochim. Biophys. Acta Gene Struct. Expr. 1991, 1088, 208–216. [Google Scholar] [CrossRef]
- Weinstein, J.; Gold, S.J.; Cunningham, D.D.; Gall, C. Cellular localization of thrombin receptor mRNA in rat brain: Expression by mesencephalic dopaminergic neurons and codistribution with prothrombin mRNA. J. Neurosci. 1995, 15, 2906–2919. [Google Scholar] [CrossRef]
- Debeir, T.; Gueugnon, J.; Vigé, X.; Benavides, J. Transduction mechanisms involved in thrombin receptor-induced nerve growth factor secretion and cell division in primary cultures of astrocytes. J. Neurochem. 1996, 66, 2320–2328. [Google Scholar] [CrossRef]
- Suo, Z.; Wu, M.; Ameenuddin, S.; Anderson, H.E.; Zoloty, J.E.; Citron, B.A.; Andrade-Gordon, P.; Festoff, B.W. Participation of protease-activated receptor-1 in thrombin-induced microglial activation. J. Neurochem. 2002, 80, 655–666. [Google Scholar] [CrossRef] [PubMed]
- Laskowski, A.; Reiser, G.; Reymann, K.G. Protease-activated receptor-1 induces generation of new microglia in the dentate gyrus of traumatised hippocampal slice cultures. Neurosci. Lett. 2007, 415, 17–21. [Google Scholar] [CrossRef]
- Pai, K.S.; Mahajan, V.B.; Lau, A.; Cunningham, D.D. Thrombin receptor signaling to cytoskeleton requires Hsp90. J. Biol. Chem. 2001, 276, 32642–32647. [Google Scholar] [CrossRef]
- Cunningham, D.D.; Gurwitz, D. Proteolytic regulation of neurite outgrowth from neuroblastoma cells by thrombin and protease nexin-1. J. Cell. Biochem. 1989, 39, 55–64. [Google Scholar] [CrossRef]
- Grabham, P.; Cunningham, D.D. Thrombin receptor activation stimulates astrocyte proliferation and reversal of stellation by distinct pathways: Involvement of tyrosine phosphorylation. J. Neurochem. 1995, 64, 583–591. [Google Scholar] [CrossRef]
- Wang, H.; Reiser, G. Thrombin signaling in the brain: The role of protease-activated receptors. Biol. Chem. 2003, 384, 193–202. [Google Scholar] [CrossRef]
- Jalink, K.; Van Corven, E.J.; Hengeveld, T.; Morii, N.; Narumiya, S.; Moolenaar, W.H. Inhibition of lysophosphatidate-and thrombin-induced neurite retraction and neuronal cell rounding by ADP ribosylation of the small GTP-binding protein Rho. J. Cell Biol. 1994, 126, 801–810. [Google Scholar] [CrossRef] [PubMed]
- Jalink, K.; Moolenaar, W.H. Thrombin receptor activation causes rapid neural cell rounding and neurite retraction independent of classic second messengers. J. Cell Biol. 1992, 118, 411–419. [Google Scholar] [CrossRef]
- Maggio, N.; Cavaliere, C.; Papa, M.; Blatt, I.; Chapman, J.; Segal, M. Thrombin regulation of synaptic transmission: Implications for seizure onset. Neurobiol. Dis. 2013, 50, 171–178. [Google Scholar] [CrossRef] [PubMed]
- Maggio, N.; Itsekson, Z.; Dominissini, D.; Blatt, I.; Amariglio, N.; Rechavi, G.; Tanne, D.; Chapman, J. Thrombin regulation of synaptic plasticity: Implications for physiology and pathology. Exp. Neurol. 2013, 247, 595–604. [Google Scholar] [CrossRef]
- Maggio, N.; Shavit, E.; Chapman, J.; Segal, M. Thrombin induces long-term potentiation of reactivity to afferent stimulation and facilitates epileptic seizures in rat hippocampal slices: Toward understanding the functional consequences of cerebrovascular insults. J. Neurosci. 2008, 28, 732–736. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.J.; Mannaioni, G.; Yuan, H.; Woo, D.H.; Gingrich, M.B.; Traynelis, S.F. Astrocytic control of synaptic NMDA receptors. J. Physiol. 2007, 581, 1057–1081. [Google Scholar] [CrossRef] [PubMed]
- Gingrich, M.B.; Junge, C.E.; Lyuboslavsky, P.; Traynelis, S.F. Potentiation of NMDA receptor function by the serine protease thrombin. J. Neurosci. 2000, 20, 4582–4595. [Google Scholar] [CrossRef] [PubMed]
- Almonte, A.G.; Hamill, C.E.; Chhatwal, J.P.; Wingo, T.S.; Barber, J.A.; Lyuboslavsky, P.N.; Sweatt, J.D.; Ressler, K.J.; White, D.A.; Traynelis, S.F. Learning and memory deficits in mice lacking protease activated receptor-1. Neurobiol. Learn. Mem. 2007, 88, 295–304. [Google Scholar] [CrossRef] [PubMed]
- Hamill, C.E.; Mannaioni, G.; Lyuboslavsky, P.; Sastre, A.A.; Traynelis, S.F. Protease-activated receptor 1-dependent neuronal damage involves NMDA receptor function. Exp. Neurol. 2009, 217, 136–146. [Google Scholar] [CrossRef] [PubMed]
- Mannaioni, G.; Orr, A.G.; Hamill, C.E.; Yuan, H.; Pedone, K.H.; McCoy, K.L.; Palmini, R.B.; Junge, C.E.; Lee, C.J.; Yepes, M. Plasmin potentiates synaptic N-methyl-D-aspartate receptor function in hippocampal neurons through activation of protease-activated receptor-1. J. Biol. Chem. 2008, 283, 20600–20611. [Google Scholar] [CrossRef]
- Han, K.-S.; Mannaioni, G.; Hamill, C.E.; Lee, J.; Junge, C.E.; Lee, C.J.; Traynelis, S.F. Activation of protease activated receptor 1 increases the excitability of the dentate granule neurons of hippocampus. Mol. Brain 2011, 4, 1–12. [Google Scholar] [CrossRef]
- Sinnreich, M.; Meins, M.; Niclou, S.P.; Suidan, H.S.; Monard, D. Prothrombin overexpressed in post-natal neurones requires blood factors for activation in the mouse brain. J. Neurochem. 2004, 88, 1380–1388. [Google Scholar] [CrossRef]
- Brailoiu, E.; Shipsky, M.M.; Yan, G.; Abood, M.E.; Brailoiu, G.C. Mechanisms of modulation of brain microvascular endothelial cells function by thrombin. Brain Res. 2017, 1657, 167–175. [Google Scholar] [CrossRef]
- Machida, T.; Takata, F.; Matsumoto, J.; Takenoshita, H.; Kimura, I.; Yamauchi, A.; Dohgu, S.; Kataoka, Y. Brain pericytes are the most thrombin-sensitive matrix metalloproteinase-9-releasing cell type constituting the blood–brain barrier in vitro. Neurosci. Lett. 2015, 599, 109–114. [Google Scholar] [CrossRef]
- Li, L.; Tao, Y.; Tang, J.; Chen, Q.; Yang, Y.; Feng, Z.; Chen, Y.; Yang, L.; Yang, Y.; Zhu, G. A cannabinoid receptor 2 agonist prevents thrombin-induced blood–brain barrier damage via the inhibition of microglial activation and matrix metalloproteinase expression in rats. Transl. Stroke Res. 2015, 6, 467–477. [Google Scholar] [CrossRef] [PubMed]
- Machida, T.; Dohgu, S.; Takata, F.; Matsumoto, J.; Kimura, I.; Koga, M.; Nakamoto, K.; Yamauchi, A.; Kataoka, Y. Role of thrombin-PAR1-PKCθ/δ axis in brain pericytes in thrombin-induced MMP-9 production and blood–brain barrier dysfunction in vitro. Neuroscience 2017, 350, 146–157. [Google Scholar] [CrossRef]
- Nishino, A.; Suzuki, M.; Ohtani, H.; Motohashi, O.; Umezawa, K.; Nagura, H.; Yoshimoto, T. Thrombin may contribute to the pathophysiology of central nervous system injury. J. Neurotrauma 1993, 10, 167–179. [Google Scholar] [CrossRef]
- Wang, Y.; Luo, W.; Reiser, G. Activation of protease-activated receptors in astrocytes evokes a novel neuroprotective pathway through release of chemokines of the growth-regulated oncogene/cytokine-induced neutrophil chemoattractant family. Eur. J. Neurosci. 2007, 26, 3159–3168. [Google Scholar] [CrossRef]
- Ryu, J.; Pyo, H.; Jou, I.; Joe, E. Thrombin induces NO release from cultured rat microglia via protein kinase C, mitogen-activated protein kinase, and NF-κB. J. Biol. Chem. 2000, 275, 29955–29959. [Google Scholar] [CrossRef]
- Li, D.-Q.; Zhou, Y.-P.; Yang, H. Donepezil combined with natural hirudin improves the clinical symptoms of patients with mild-to-moderate Alzheimer’s disease: A 20-week open-label pilot study. Int. J. Med Sci. 2012, 9, 248. [Google Scholar] [CrossRef]
- Fujimoto, S.; Katsuki, H.; Kume, T.; Akaike, A. Thrombin-induced delayed injury involves multiple and distinct signaling pathways in the cerebral cortex and the striatum in organotypic slice cultures. Neurobiol. Dis. 2006, 22, 130–142. [Google Scholar] [CrossRef]
- Fujimoto, S.; Katsuki, H.; Ohnishi, M.; Takagi, M.; Kume, T.; Akaike, A. Thrombin induces striatal neurotoxicity depending on mitogen-activated protein kinase pathways in vivo. Neuroscience 2007, 144, 694–701. [Google Scholar] [CrossRef]
- Xue, M.; Hollenberg, M.D.; Yong, V.W. Combination of thrombin and matrix metalloproteinase-9 exacerbates neurotoxicity in cell culture and intracerebral hemorrhage in mice. J. Neurosci. 2006, 26, 10281–10291. [Google Scholar] [CrossRef] [PubMed]
- Xue, M.; Fan, Y.; Liu, S.; Zygun, D.A.; Demchuk, A.; Yong, V.W. Contributions of multiple proteases to neurotoxicity in a mouse model of intracerebral haemorrhage. Brain 2009, 132, 26–36. [Google Scholar] [CrossRef] [PubMed]
- Marques, M.A.; Tolar, M.; Harmony, J.; Crutcher, K.A. A thrombin cleavage fragment of apolipoprotein E exhibits isoform-specific neurotoxicity. Neuroreport 1996, 7, 2529–2532. [Google Scholar] [CrossRef] [PubMed]
- Tolar, M.; Marques, M.A.; Harmony, J.A.; Crutcher, K.A. Neurotoxicity of the 22 kDa thrombin-cleavage fragment of apolipoprotein E and related synthetic peptides is receptor-mediated. J. Neurosci. 1997, 17, 5678–5686. [Google Scholar] [CrossRef]
- Grammas, P.; Ottman, T.; Reimann-Philipp, U.; Larabee, J.; Weigel, P.H. Injured brain endothelial cells release neurotoxic thrombin. J. Alzheimer’s Dis. 2004, 6, 275–281. [Google Scholar] [CrossRef] [PubMed]
- Altman, K.; Shavit Stein, E.; Maggio, N. Post stroke seizures and epilepsy: From proteases to maladaptive plasticity. Front. Cell. Neurosci. 2019, 13, 397. [Google Scholar] [CrossRef]
- Lee, K.R.; Colon, G.P.; Betz, A.L.; Keep, R.F.; Kim, S.; Hoff, J.T. Edema from intracerebral hemorrhage: The role of thrombin. J. Neurosurg. 1996, 84, 91–96. [Google Scholar] [CrossRef] [PubMed]
- Akiyama, H.; Ikeda, K.; Kondo, H.; McGeer, P.L. Thrombin accumulation in brains of patients with Alzheimer’s disease. Neurosci. Lett. 1992, 146, 152–154. [Google Scholar] [CrossRef]
- Arai, T.; Miklossy, J.; Klegeris, A.; Guo, J.-P.; McGeer, P.L. Thrombin and prothrombin are expressed by neurons and glial cells and accumulate in neurofibrillary tangles in Alzheimer disease brain. J. Neuropathol. Exp. Neurol. 2006, 65, 19–25. [Google Scholar] [CrossRef] [PubMed]
- Pompili, E.; Nori, S.L.; Geloso, M.C.; Guadagni, E.; Corvino, V.; Michetti, F.; Fumagalli, L. Trimethyltin-induced differential expression of PAR subtypes in reactive astrocytes of the rat hippocampus. Mol. Brain Res. 2004, 122, 93–98. [Google Scholar] [CrossRef]
- Zamolodchikov, D.; Renné, T.; Strickland, S. The Alzheimer’s disease peptide β-amyloid promotes thrombin generation through activation of coagulation factor XII. J. Thromb. Haemost. 2016, 14, 995–1007. [Google Scholar] [CrossRef]
- Grammas, P.; Samany, P.G.; Thirumangalakudi, L. Thrombin and inflammatory proteins are elevated in Alzheimer’s disease microvessels: Implications for disease pathogenesis. J. Alzheimer’s Dis. 2006, 9, 51–58. [Google Scholar] [CrossRef]
- van der Poll, T.; Büller, H.R.; ten Cate, H.; Wortel, C.H.; Bauer, K.A.; van Deventer, S.J.; Hack, C.E.; Sauerwein, H.P.; Rosenberg, R.D.; ten Cate, J.W. Activation of coagulation after administration of tumor necrosis factor to normal subjects. New Engl. J. Med. 1990, 322, 1622–1627. [Google Scholar] [CrossRef] [PubMed]
- Iannucci, J.; Renehan, W.; Grammas, P. Thrombin, a Mediator of Coagulation, Inflammation, and Neurotoxicity at the Neurovascular Interface: Implications for Alzheimer’s Disease. Front. Neurosci. 2020, 14, 762. [Google Scholar] [CrossRef]
- Suo, Z.; Wu, M.; Citron, B.A.; Palazzo, R.E.; Festoff, B.W. Rapid tau aggregation and delayed hippocampal neuronal death induced by persistent thrombin signaling. J. Biol. Chem. 2003, 278, 37681–37689. [Google Scholar] [CrossRef]
- Suo, Z.; Wu, M.; Citron, B.A.; Gao, C.; Festoff, B.W. Persistent protease-activated receptor 4 signaling mediates thrombin-induced microglial activation. J. Biol. Chem. 2003, 278, 31177–31183. [Google Scholar] [CrossRef] [PubMed]
- Davis-Salinas, J.; Saporito-Irwin, S.M.; Donovan, F.M.; Cunningham, D.D.; Van Nostrand, W. Thrombin receptor activation induces secretion and nonamyloidogenic processing of amyloid beta-protein precursor. J. Biol. Chem. 1994, 269, 22623–22627. [Google Scholar] [CrossRef]
- Brewer, G.J. Thrombin Causes Cell Spreading and Redistribution of β-Amyloid Immunoreactivity in Cultured Hippocampal Neurons. J. Neurochem. 1996, 67, 119–130. [Google Scholar] [CrossRef] [PubMed]
- Smith-Swintosky, V.L.; Zimmer, S.; Fenton, J.W.; Mattson, M.P. Opposing Actions of Thrombin and Protease Nexin-1 on Amyloid β-Peptide Toxicity and on Accumulation of Peroxides and Calcium in Hippocampal Neurons. J. Neurochem. 1995, 65, 1415–1418. [Google Scholar] [CrossRef]
- Pike, C.J.; Vaughan, P.J.; Cunningham, D.D.; Cotman, C.W. Thrombin attenuates neuronal cell death and modulates astrocyte reactivity induced by β-amyloid in vitro. J. Neurochem. 1996, 66, 1374–1382. [Google Scholar] [CrossRef]
- Vaughan, P.J.; Su, J.; Cotman, C.W.; Cunningham, D.D. Protease nexin-1, a potent thrombin inhibitor, is reduced around cerebral blood vessels in Alzheimer’s disease. Brain Res. 1994, 668, 160–170. [Google Scholar] [CrossRef]
- Choi, S.-H.; Lee, D.Y.; Kim, S.U.; Jin, B.K. Thrombin-induced oxidative stress contributes to the death of hippocampal neurons in vivo: Role of microglial NADPH oxidase. J. Neurosci. 2005, 25, 4082–4090. [Google Scholar] [CrossRef]
- Iannucci, J.; Johnson, S.L.; Majchrzak, M.; Barlock, B.J.; Akhlaghi, F.; Seeram, N.P.; Sen, A.; Grammas, P. Short-term treatment with dabigatran alters protein expression patterns in a late-stage tau-based Alzheimer’s disease mouse model. Biochem. Biophys. Rep. 2020, 24, 100862. [Google Scholar]
- Tripathy, D.; Sanchez, A.; Yin, X.; Luo, J.; Martinez, J.; Grammas, P. Thrombin, a mediator of cerebrovascular inflammation in AD and hypoxia. Front. Aging Neurosci. 2013, 5, 19. [Google Scholar] [CrossRef]
- Rami, B.K. Direct thrombin inhibitors’ potential efficacy in Alzheimer’s disease. Am. J. Alzheimer’s Dis. Other Dement. 2012, 27, 564–567. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.-H.; Lee, D.Y.; Ryu, J.K.; Kim, J.; Joe, E.H.; Jin, B.K. Thrombin induces nigral dopaminergic neurodegeneration in vivo by altering expression of death-related proteins. Neurobiol. Dis. 2003, 14, 181–193. [Google Scholar] [CrossRef]
- Carreño-Müller, E.; Herrera, A.J.; De Pablos, R.M.; Tomás-Camardiel, M.; Venero, J.L.; Cano, J.; Machado, A. Thrombin induces in vivo degeneration of nigral dopaminergic neurones along with the activation of microglia. J. Neurochem. 2003, 84, 1201–1214. [Google Scholar] [CrossRef]
- Lee, D.Y.; Oh, Y.J.; Jin, B.K. Thrombin-activated microglia contribute to death of dopaminergic neurons in rat mesencephalic cultures: Dual roles of mitogen-activated protein kinase signaling pathways. Glia 2005, 51, 98–110. [Google Scholar] [CrossRef] [PubMed]
- Katsuki, H.; Okawara, M.; Shibata, H.; Kume, T.; Akaike, A. Nitric oxide-producing microglia mediate thrombin-induced degeneration of dopaminergic neurons in rat midbrain slice culture. J. Neurochem. 2006, 97, 1232–1242. [Google Scholar] [CrossRef]
- Choi, S.-H.; Joe, E.H.; Kim, S.U.; Jin, B.K. Thrombin-induced microglial activation produces degeneration of nigral dopaminergic neurons in vivo. J. Neurosci. 2003, 23, 5877–5886. [Google Scholar] [CrossRef] [PubMed]
- Cannon, J.R.; Hua, Y.; Richardson, R.J.; Xi, G.; Keep, R.F.; Schallert, T. The effect of thrombin on a 6-hydroxydopamine model of Parkinson’s disease depends on timing. Behav. Brain Res. 2007, 183, 161–168. [Google Scholar] [CrossRef]
- Jenner, P. Oxidative stress in Parkinson’s disease. Ann. Neurol. Off. J. Am. Neurol. Assoc. Child Neurol. Soc. 2003, 53, S26–S38. [Google Scholar] [CrossRef] [PubMed]
- Ishida, Y.; Nagai, A.; Kobayashi, S.; Kim, S.U. Upregulation of protease-activated receptor-1 in astrocytes in Parkinson disease: Astrocyte-mediated neuroprotection through increased levels of glutathione peroxidase. J. Neuropathol. Exp. Neurol. 2006, 65, 66–77. [Google Scholar] [CrossRef]
- Cannon, J.R.; Keep, R.F.; Schallert, T.; Hua, Y.; Richardson, R.J.; Xi, G. Protease-activated receptor-1 mediates protection elicited by thrombin preconditioning in a rat 6-hydroxydopamine model of Parkinson’s disease. Brain Res. 2006, 1116, 177–186. [Google Scholar] [CrossRef]
- Cannon, J.R.; Keep, R.F.; Hua, Y.; Richardson, R.J.; Schallert, T.; Xi, G. Thrombin preconditioning provides protection in a 6-hydroxydopamine Parkinson’s disease model. Neurosci. Lett. 2005, 373, 189–194. [Google Scholar] [CrossRef]
- Kandil, E.A.; Sayed, R.H.; Ahmed, L.A.; Abd El Fattah, M.A.; El-Sayeh, B.M. Modulatory role of Nurr1 activation and thrombin inhibition in the neuroprotective effects of dabigatran etexilate in rotenone-induced Parkinson’s disease in rats. Mol. Neurobiol. 2018, 55, 4078–4089. [Google Scholar] [CrossRef]
- Johnson, S.L.; Iannucci, J.; Seeram, N.P.; Grammas, P. Inhibiting thrombin improves motor function and decreases oxidative stress in the LRRK2 transgenic Drosophila melanogaster model of Parkinson’s disease. Biochem. Biophys. Res. Commun. 2020, 527, 532–538. [Google Scholar] [CrossRef]
- Pretorius, E.; Page, M.J.; Mbotwe, S.; Kell, D.B. Lipopolysaccharide-binding protein (LBP) can reverse the amyloid state of fibrin seen or induced in Parkinson’s disease. PLoS ONE 2018, 13, e0192121. [Google Scholar] [CrossRef]
- Pretorius, E.; Mbotwe, S.; Bester, J.; Robinson, C.J.; Kell, D.B. Acute induction of anomalous and amyloidogenic blood clotting by molecular amplification of highly substoichiometric levels of bacterial lipopolysaccharide. J. R. Soc. Interface 2016, 13, 20160539. [Google Scholar] [CrossRef] [PubMed]
- Paterson, P.; Koh, C.; Kwaan, H. Role of the clotting system in the pathogenesis of neuroimmunologic disease. Fed. Proc. 1987, 46, 91. [Google Scholar]
- Davalos, D.; Baeten, K.M.; Whitney, M.A.; Mullins, E.S.; Friedman, B.; Olson, E.S.; Ryu, J.K.; Smirnoff, D.S.; Petersen, M.A.; Bedard, C. Early detection of thrombin activity in neuroinflammatory disease. Ann. Neurol. 2014, 75, 303–308. [Google Scholar] [CrossRef]
- Constantinescu, C.S.; Farooqi, N.; O’Brien, K.; Gran, B. Experimental autoimmune encephalomyelitis (EAE) as a model for multiple sclerosis (MS). Br. J. Pharmacol. 2011, 164, 1079–1106. [Google Scholar] [CrossRef] [PubMed]
- Beilin, O.; Karussis, D.M.; Korczyn, A.D.; Gurwitz, D.; Aronovich, R.; Hantai, D.; Grigoriadis, N.; Mizrachi-Kol, R.; Chapman, J. Increased thrombin inhibition in experimental autoimmune encephalomyelitis. J. Neurosci. Res. 2005, 79, 351–359. [Google Scholar] [CrossRef] [PubMed]
- Inaba, Y.; Ichikawa, M.; Inoue, A.; Itoh, M.; Kyogashima, M.; Sekiguchi, Y.; Nakamura, S.; Komiyama, A.; Koh, C.-S. Plasma thrombin–antithrombin III complex is associated with the severity of experimental autoimmune encephalomyelitis. J. Neurol. Sci. 2001, 185, 89–93. [Google Scholar] [CrossRef]
- Göbel, K.; Pankratz, S.; Asaridou, C.-M.; Herrmann, A.M.; Bittner, S.; Merker, M.; Ruck, T.; Glumm, S.; Langhauser, F.; Kraft, P. Blood coagulation factor XII drives adaptive immunity during neuroinflammation via CD87-mediated modulation of dendritic cells. Nat. Commun. 2016, 7, 11626. [Google Scholar] [CrossRef] [PubMed]
- Göbel, K.; Eichler, S.; Wiendl, H.; Chavakis, T.; Kleinschnitz, C.; Meuth, S.G. The coagulation factors fibrinogen, thrombin, and factor XII in inflammatory disorders—A systematic review. Front. Immunol. 2018, 9, 1731. [Google Scholar] [CrossRef] [PubMed]
- Dziedzic, A.; Miller, E.; Bijak, M.; Przyslo, L.; Saluk-Bijak, J. Increased Pro-Thrombotic Platelet Activity Associated with Thrombin/PAR1-Dependent Pathway Disorder in Patients with Secondary Progressive Multiple Sclerosis. Int. J. Mol. Sci. 2020, 21, 7722. [Google Scholar] [CrossRef]
- Wang, Y.; Richter-Landsberg, C.; Reiser, G. Expression of protease-activated receptors (PARs) in OLN-93 oligodendroglial cells and mechanism of PAR-1-induced calcium signaling. Neuroscience 2004, 126, 69–82. [Google Scholar] [CrossRef]
- Noorbakhsh, F.; Tsutsui, S.; Vergnolle, N.; Boven, L.A.; Shariat, N.; Vodjgani, M.; Warren, K.G.; Andrade-Gordon, P.; Hollenberg, M.D.; Power, C. Proteinase-activated receptor 2 modulates neuroinflammation in experimental autoimmune encephalomyelitis and multiple sclerosis. J. Exp. Med. 2006, 203, 425–435. [Google Scholar] [CrossRef]
- Juhler, M.; Barry, D.I.; Offner, H.; Konat, G.; Klinken, L.; Paulson, O.B. Blood-brain and blood-spinal cord barrier permeability during the course of experimental allergic encephalomyelitis in the rat. Brain Res. 1984, 302, 347–355. [Google Scholar] [CrossRef]
- Kim, H.N.; Kim, Y.R.; Ahn, S.M.; Lee, S.K.; Shin, H.K.; Choi, B.T. Protease activated receptor-1 antagonist ameliorates the clinical symptoms of experimental autoimmune encephalomyelitis via inhibiting breakdown of blood–brain barrier. J. Neurochem. 2015, 135, 577–588. [Google Scholar] [CrossRef]
- Verbout, N.G.; Yu, X.; Healy, L.D.; Phillips, K.G.; Tucker, E.I.; Gruber, A.; McCarty, O.J.; Offner, H. Thrombin mutant W215A/E217A treatment improves neurological outcome and attenuates central nervous system damage in experimental autoimmune encephalomyelitis. Metab. Brain Dis. 2015, 30, 57–65. [Google Scholar] [CrossRef]
- Carcaillon, L.; Alhenc-Gelas, M.; Bejot, Y.; Spaft, C.; Ducimetière, P.; Ritchie, K.; Dartigues, J.-F.; Scarabin, P.-Y. Increased thrombin generation is associated with acute ischemic stroke but not with coronary heart disease in the elderly: The Three-City cohort study. Arterioscler. Thromb. Vasc. Biol. 2011, 31, 1445–1451. [Google Scholar] [CrossRef] [PubMed]
- Riek-Burchardt, M.; Striggow, F.; Henrich-Noack, P.; Reiser, G.; Reymann, K. Increase of prothrombin-mRNA after global cerebral ischemia in rats, with constant expression of protease nexin-1 and protease-activated receptors. Neurosci. Lett. 2002, 329, 181–184. [Google Scholar] [CrossRef]
- Thevenet, J.; Angelillo-Scherrer, A.; Price, M.; Hirt, L. Coagulation factor Xa activates thrombin in ischemic neural tissue. J. Neurochem. 2009, 111, 828–836. [Google Scholar] [CrossRef]
- Bushi, D.; Chapman, J.; Katzav, A.; Shavit-Stein, E.; Molshatzki, N.; Maggio, N.; Tanne, D. Quantitative detection of thrombin activity in an ischemic stroke model. J. Mol. Neurosci. 2013, 51, 844–850. [Google Scholar] [CrossRef] [PubMed]
- Hayosh, Z.e.I.; Bandora, E.A.; Shelestovich, N.; Nulman, M.; Bakon, M.; Yaniv, G.; Khaitovitch, B.; Balan, S.; Gerasimova, A.; Drori, T. In-thrombus thrombin secretion: A new diagnostic marker of atrial fibrillation in cryptogenic stroke. J. Neurointerv. Surg. 2020. [Google Scholar] [CrossRef]
- Vaughan, P.J.; Pike, C.J.; Cotman, C.W.; Cunningham, D.D. Thrombin receptor activation protects neurons and astrocytes from cell death produced by environmental insults. J. Neurosci. 1995, 15, 5389–5401. [Google Scholar] [CrossRef]
- Striggow, F.; Riek, M.; Breder, J.; Henrich-Noack, P.; Reymann, K.G.; Reiser, G. The protease thrombin is an endogenous mediator of hippocampal neuroprotection against ischemia at low concentrations but causes degeneration at high concentrations. Proc. Natl. Acad. Sci. USA 2000, 97, 2264–2269. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Zhang, W.; Li, J.Y.; Chai, B.X.; Peng, J.; Wang, H.; Mulholland, M.W. Induction of apoptosis by thrombin in the cultured neurons of dorsal motor nucleus of the vagus. Neurogastroenterol. Motil. 2011, 23, 279.e124. [Google Scholar] [CrossRef] [PubMed]
- Hua, Y.; Wu, J.; Keep, R.; Hoff, J.; Xi, G. Thrombin exacerbates brain edema in focal cerebral ischemia. In Brain Edema XII; Springer: Berlin/Heidelberg, Germany, 2003; pp. 163–166. [Google Scholar]
- Hua, Y.; Wu, J.; Keep, R.F.; Nakamura, T.; Hoff, J.T.; Xi, G. Tumor necrosis factor-α increases in the brain after intracerebral hemorrhage and thrombin stimulation. Neurosurgery 2006, 58, 542–550. [Google Scholar] [CrossRef]
- Bodmer, D.; Vaughan, K.A.; Zacharia, B.E.; Hickman, Z.L.; Connolly, E.S. The molecular mechanisms that promote edema after intracerebral hemorrhage. Transl. Stroke Res. 2012, 3, 52–61. [Google Scholar] [CrossRef]
- Masasda, T.; Hua, Y.; Xi, G.; Yang, G.-Y.; Hoff, J.; Keep, R.; Nagao, S. Overexpression of interleukin-1 receptor antagonist reduces brain edema induced by intracerebral hemorrhage and thrombin. In Brain Edema XII; Springer: Berlin/Heidelberg, Germany, 2003; pp. 463–467. [Google Scholar]
- Nakamura, T.; Xi, G.; Park, J.-W.; Hua, Y.; Hoff, J.T.; Keep, R.F. Holo-transferrin and thrombin can interact to cause brain damage. Stroke 2005, 36, 348–352. [Google Scholar] [CrossRef] [PubMed]
- Hua, Y.; Keep, R.F.; Hoff, J.T.; Xi, G. Brain injury after intracerebral hemorrhage: The role of thrombin and iron. Stroke 2007, 38, 759–762. [Google Scholar] [CrossRef]
- Rashidian, J.; Iyirhiaro, G.; Aleyasin, H.; Rios, M.; Vincent, I.; Callaghan, S.; Bland, R.J.; Slack, R.S.; During, M.J.; Park, D.S. Multiple cyclin-dependent kinases signals are critical mediators of ischemia/hypoxic neuronal death in vitro and in vivo. Proc. Natl. Acad. Sci. USA 2005, 102, 14080–14085. [Google Scholar] [CrossRef]
- Rao, H.V.; Thirumangalakudi, L.; Desmond, P.; Grammas, P. Cyclin D1, cdk4, and Bim are involved in thrombin-induced apoptosis in cultured cortical neurons. J. Neurochem. 2007, 101, 498–505. [Google Scholar] [CrossRef] [PubMed]
- Svedin, P.; Hagberg, H.; Sävman, K.; Zhu, C.; Mallard, C. Matrix metalloproteinase-9 gene knock-out protects the immature brain after cerebral hypoxia–ischemia. J. Neurosci. 2007, 27, 1511–1518. [Google Scholar] [CrossRef] [PubMed]
- Boven, L.A.; Vergnolle, N.; Henry, S.D.; Silva, C.; Imai, Y.; Holden, J.; Warren, K.; Hollenberg, M.D.; Power, C. Up-regulation of proteinase-activated receptor 1 expression in astrocytes during HIV encephalitis. J. Immunol. 2003, 170, 2638–2646. [Google Scholar] [CrossRef]
- Ramos-Mandujano, G.; Vázquez-Juárez, E.; Hernández-Benítez, R.; Pasantes-Morales, H. Thrombin potently enhances swelling-sensitive glutamate efflux from cultured astrocytes. Glia 2007, 55, 917–925. [Google Scholar] [CrossRef]
- Ohnishi, M.; Katsuki, H.; Fujimoto, S.; Takagi, M.; Kume, T.; Akaike, A. Involvement of thrombin and mitogen-activated protein kinase pathways in hemorrhagic brain injury. Exp. Neurol. 2007, 206, 43–52. [Google Scholar] [CrossRef]
- Xi, G.; Keep, R.F.; Hua, Y.; Xiang, J.; Hoff, J.T. Attenuation of thrombin-induced brain edema by cerebral thrombin preconditioning. Stroke 1999, 30, 1247–1254. [Google Scholar] [CrossRef]
- Xi, G.; Keep, R.; Hua, Y.; Hoff, J. Thrombin preconditioning, heat shock proteins and thrombin-induced brain edema. In Brain Edema XI; Springer: Berlin/Heidelberg, Germany, 2000; pp. 511–515. [Google Scholar]
- Henrich-Noack, P.; Striggow, F.; Reiser, G.; Reymann, K.G. Preconditioning with thrombin can be protective or worsen damage after endothelin-1-induced focal ischemia in rats. J. Neurosci. Res. 2006, 83, 469–475. [Google Scholar] [CrossRef] [PubMed]
- Karabiyikoglu, M.; Hua, Y.; Keep, R.F.; Ennis, S.R.; Xi, G. Intracerebral hirudin injection attenuates ischemic damage and neurologic deficits without altering local cerebral blood flow. J. Cereb. Blood Flow Metab. 2004, 24, 159–166. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Hua, Y.; Nakamura, T.; Keep, R.; Xi, G. Up-regulation of brain ceruloplasmin in thrombin preconditioning. In Brain Edema XIII; Springer: Berlin/Heidelberg, Germany, 2006; pp. 203–206. [Google Scholar]
- Wang, Y.; Luo, W.; Stricker, R.; Reiser, G. Protease-activated receptor-1 protects rat astrocytes from apoptotic cell death via JNK-mediated release of the chemokine GRO/CINC-1. J. Neurochem. 2006, 98, 1046–1060. [Google Scholar] [CrossRef]
- Hoffmann, M.-C.; Nitsch, C.; Scotti, A.; Reinhard, E.; Monard, D. The prolonged presence of glia-derived nexin, an endogenous protease inhibitor, in the hippocampus after ischemia-induced delayed neuronal death. Neuroscience 1992, 49, 397–408. [Google Scholar] [CrossRef]
- Cunningham, D.D.; Pulliam, L.; Vaughan, P.J. Protease nexin-1 and thrombin: Injury-related processes in the brain. Thromb. Haemost. 1993, 69, 168–171. [Google Scholar] [CrossRef]
- Executive Steering Committee on Behalf of the SPORTIF III Investigators. Stroke prevention with the oral direct thrombin inhibitor ximelagatran compared with warfarin in patients with non-valvular atrial fibrillation (SPORTIF III): Randomised controlled trial. Lancet 2003, 362, 1691–1698. [Google Scholar] [CrossRef]
- Diener, H.-C.; Sacco, R.L.; Easton, J.D.; Granger, C.B.; Bernstein, R.A.; Uchiyama, S.; Kreuzer, J.; Cronin, L.; Cotton, D.; Grauer, C. Dabigatran for prevention of stroke after embolic stroke of undetermined source. New Engl. J. Med. 2019, 380, 1906–1917. [Google Scholar] [CrossRef] [PubMed]
- Bai, Y.; Deng, H.; Shantsila, A.; Lip, G.Y. Rivaroxaban versus dabigatran or warfarin in real-world studies of stroke prevention in atrial fibrillation: Systematic review and meta-analysis. Stroke 2017, 48, 970–976. [Google Scholar] [CrossRef]
- Dans, A.L.; Connolly, S.J.; Wallentin, L.; Yang, S.; Nakamya, J.; Brueckmann, M.; Ezekowitz, M.; Oldgren, J.; Eikelboom, J.W.; Reilly, P.A. Concomitant use of antiplatelet therapy with dabigatran or warfarin in the Randomized Evaluation of Long-Term Anticoagulation Therapy (RE-LY) trial. Circulation 2013, 127, 634–640. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.S. Mechanisms of microbial traversal of the blood–brain barrier. Nat. Rev. Microbiol. 2008, 6, 625–634. [Google Scholar] [CrossRef]
- Kim, K.S. Microbial translocation of the blood–brain barrier. Int. J. Parasitol. 2006, 36, 607–614. [Google Scholar] [CrossRef]
- Mook-Kanamori, B.; Valls Seron, M.; Geldhoff, M.; Havik, S.; van der Ende, A.; Baas, F.; van der Poll, T.; Meijers, J.; Morgan, B.P.; Brouwer, M. Thrombin-activatable fibrinolysis inhibitor influences disease severity in humans and mice with pneumococcal meningitis. J. Thromb. Haemost. 2015, 13, 2076–2086. [Google Scholar] [CrossRef]
- Kremer Hovinga, J.; Franco, R.; Zago, M.; Ten Cate, H.; Westendorp, R.; Reitsma, P. A functional single nucleotide polymorphism in the thrombin-activatable fibrinolysis inhibitor (TAFI) gene associates with outcome of meningococcal disease. J. Thromb. Haemost. 2004, 2, 54–57. [Google Scholar] [CrossRef]
- Emonts, M.; De Bruijne, E.; Guimarães, A.; Declerck, P.; Leebeek, F.; De Maat, M.; Rijken, D.; Hazelzet, J.; Gils, A. Thrombin activatable fibrinolysis inhibitor is associated with severity and outcome of severe meningococcal infection in children. J. Thromb. Haemost. 2008, 6, 268–276. [Google Scholar] [CrossRef]
- Faust, S.N.; Levin, M.; Harrison, O.B.; Goldin, R.D.; Lockhart, M.S.; Kondaveeti, S.; Laszik, Z.; Esmon, C.T.; Heyderman, R.S. Dysfunction of endothelial protein C activation in severe meningococcal sepsis. New Engl. J. Med. 2001, 345, 408–416. [Google Scholar] [CrossRef] [PubMed]
- Baker, J.V.; Brummel-Ziedins, K.; Neuhaus, J.; Duprez, D.; Cummins, N.; Dalmau, D.; DeHovitz, J.; Lehmann, C.; Sullivan, A.; Woolley, I. HIV replication alters the composition of extrinsic pathway coagulation factors and increases thrombin generation. J. Am. Heart Assoc. 2013, 2, e000264. [Google Scholar] [CrossRef] [PubMed]
- Hsue, P.Y.; Scherzer, R.; Grunfeld, C.; Nordstrom, S.M.; Schnell, A.; Kohl, L.P.; Nitta, E.; Martin, J.N.; Deeks, S.G.; Weiss, E.J. HIV infection is associated with decreased thrombin generation. Clin. Infect. Dis. 2012, 54, 1196–1203. [Google Scholar] [CrossRef]
- Ivey, N.S.; MacLean, A.G.; Lackner, A.A. Acquired immunodeficiency syndrome and the blood-brain barrier. J. Neurovirol. 2009, 15, 111–122. [Google Scholar] [CrossRef]
- Hurley, A.; Smith, M.; Karpova, T.; Hasley, R.B.; Belkina, N.; Shaw, S.; Balenga, N.; Druey, K.M.; Nickel, E.; Packard, B. Enhanced effector function of CD8+ T cells from healthy controls and HIV-infected patients occurs through thrombin activation of protease-activated receptor 1. J. Infect. Dis. 2013, 207, 638–650. [Google Scholar] [CrossRef]
- Noorbakhsh, F.; Vergnolle, N.; McArthur, J.C.; Silva, C.; Vodjgani, M.; Andrade-Gordon, P.; Hollenberg, M.D.; Power, C. Proteinase-activated receptor-2 induction by neuroinflammation prevents neuronal death during HIV infection. J. Immunol. 2005, 174, 7320–7329. [Google Scholar] [CrossRef] [PubMed]
- Kim, W.; Zekas, E.; Lodge, R.; Susan-Resiga, D.; Marcinkiewicz, E.; Essalmani, R.; Mihara, K.; Ramachandran, R.; Asahchop, E.; Gelman, B. Neuroinflammation-induced interactions between protease-activated receptor 1 and proprotein convertases in HIV-associated neurocognitive disorder. Mol. Cell. Biol. 2015, 35, 3684–3700. [Google Scholar] [CrossRef]
- Avril, M.; Benjamin, M.; Dols, M.-M.; Smith, J.D. Interplay of Plasmodium falciparum and thrombin in brain endothelial barrier disruption. Sci. Rep. 2019, 9, 1–13. [Google Scholar] [CrossRef]
- Zeng, Y.; Liang, J.; Weng, C.; Lu, Z.; Zhou, Y. β-Arrestin 2 protects against neurological function defects in HSV-1-induced encephalitis mice. J. Med Virol. 2020, 92, 78–85. [Google Scholar] [CrossRef]
- Amlie-Lefond, C.; Kleinschmidt-Demasters, B.K.; Mahalingam, R.; Davis, L.E.; Gilden, D.H. The vasculopathy of varicella-zoster virus encephalitis. Ann. Neurol. Off. J. Am. Neurol. Assoc. Child Neurol. Soc. 1995, 37, 784–790. [Google Scholar] [CrossRef] [PubMed]
- Itsekson-Hayosh, Z.; Shavit-Stein, E.; Katzav, A.; Rubovitch, V.; Maggio, N.; Chapman, J.; Harnof, S.; Pick, C.G. Minimal traumatic brain injury in mice: Protease-activated receptor 1 and thrombin-related changes. J. Neurotrauma 2016, 33, 1848–1854. [Google Scholar] [CrossRef]
- Ben Shimon, M.; Zeimer, T.; Shavit Stein, E.; Artan-Furman, A.; Harnof, S.; Chapman, J.; Eisenkraft, A.; Pick, C.G.; Maggio, N. Recovery from trauma induced amnesia correlates with normalization of thrombin activity in the mouse hippocampus. PLoS ONE 2017, 12, e0188524. [Google Scholar] [CrossRef] [PubMed]
- Itzekson, Z.; Maggio, N.; Milman, A.; Shavit, E.; Pick, C.G.; Chapman, J. Reversal of trauma-induced amnesia in mice by a thrombin receptor antagonist. J. Mol. Neurosci. 2014, 53, 87–95. [Google Scholar] [CrossRef]
- Piao, C.-S.; Holloway, A.L.; Hong-Routson, S.; Wainwright, M.S. Depression following traumatic brain injury in mice is associated with down-regulation of hippocampal astrocyte glutamate transporters by thrombin. J. Cereb. Blood Flow Metab. 2019, 39, 58–73. [Google Scholar] [CrossRef] [PubMed]
- Tomkins, O.; Kaufer, D.; Korn, A.; Shelef, I.; Golan, H.; Reichenthal, E.; Soreq, H.; Friedman, A. Frequent blood–brain barrier disruption in the human cerebral cortex. Cell. Mol. Neurobiol. 2001, 21, 675–691. [Google Scholar] [CrossRef]
- Ballabh, P.; Braun, A.; Nedergaard, M. The blood–brain barrier: An overview: Structure, regulation, and clinical implications. Neurobiol. Dis. 2004, 16, 1–13. [Google Scholar] [CrossRef]
- Lee, K.R.; Drury, I.; Vitarbo, E.; Hoff, J.T. Seizures induced by intracerebral injection of thrombin: A model of intracerebral hemorrhage. J. Neurosurg. 1997, 87, 73–78. [Google Scholar] [CrossRef]
- Kelly, K.M. Thrombin: Is it on a par with seizures and epilepsy? Epilepsy Curr. 2008, 8, 110–112. [Google Scholar] [CrossRef] [PubMed]
- Chodobski, A.; Zink, B.J.; Szmydynger-Chodobska, J. Blood–brain barrier pathophysiology in traumatic brain injury. Transl. Stroke Res. 2011, 2, 492–516. [Google Scholar] [CrossRef] [PubMed]
- Pisapia, J.M.; Xu, X.; Kelly, J.; Yeung, J.; Carrion, G.; Tong, H.; Meghan, S.; El-Falaky, O.M.; Grady, M.S.; Smith, D.H. Microthrombosis after experimental subarachnoid hemorrhage: Time course and effect of red blood cell-bound thrombin-activated pro-urokinase and clazosentan. Exp. Neurol. 2012, 233, 357–363. [Google Scholar] [CrossRef]
- Stein, S.C.; Chen, X.-H.; Sinson, G.P.; Smith, D.H. Intravascular coagulation: A major secondary insult in nonfatal traumatic brain injury. J. Neurosurg. 2002, 97, 1373–1377. [Google Scholar] [CrossRef] [PubMed]
- Maggio, N.; Blatt, I.; Vlachos, A.; Tanne, D.; Chapman, J.; Segal, M. Treating seizures and epilepsy with anticoagulants? Front. Cell. Neurosci. 2013, 7, 19. [Google Scholar] [CrossRef]
- Isaeva, E.; Hernan, A.; Isaev, D.; Holmes, G.L. Thrombin facilitates seizures through activation of persistent sodium current. Ann. Neurol. 2012, 72, 192–198. [Google Scholar] [CrossRef]
- Lenz, M.; Shimon, M.B.; Benninger, F.; Neufeld, M.Y.; Shavit-Stein, E.; Vlachos, A.; Maggio, N. Systemic thrombin inhibition ameliorates seizures in a mouse model of pilocarpine-induced status epilepticus. J. Mol. Med. 2019, 97, 1567–1574. [Google Scholar] [CrossRef] [PubMed]
- Nierodzik, M.L.; Karpatkin, S. Thrombin induces tumor growth, metastasis, and angiogenesis: Evidence for a thrombin-regulated dormant tumor phenotype. Cancer Cell 2006, 10, 355–362. [Google Scholar] [CrossRef]
- Hu, L.; Lee, M.; Campbell, W.; Perez-Soler, R.; Karpatkin, S. Role of endogenous thrombin in tumor implantation, seeding, and spontaneous metastasis. Blood 2004, 104, 2746–2751. [Google Scholar] [CrossRef]
- Krenzlin, H.; Lorenz, V.; Alessandri, B. The involvement of thrombin in the pathogenesis of glioblastoma. J. Neurosci. Res. 2017, 95, 2080–2085. [Google Scholar] [CrossRef]
- Itsekson-Hayosh, Z.E.; Shavit-Stein, E.; Last, D.; Goez, D.; Daniels, D.; Bushi, D.; Gera, O.; Zibly, Z.; Mardor, Y.; Chapman, J. Thrombin activity and thrombin receptor in rat glioblastoma model: Possible markers and targets for intervention? J. Mol. Neurosci. 2015, 56, 644–651. [Google Scholar] [CrossRef] [PubMed]
- Kaufmann, R.; Zieger, M.; Tausch, S.; Henklein, P.; Nowak, G. Meizothrombin, an intermediate of prothrombin activation, stimulates human glioblastoma cells by interaction with PAR-1-type thrombin receptors. J. Neurosci. Res. 2000, 59, 643–648. [Google Scholar] [CrossRef]
- Auvergne, R.; Wu, C.; Connell, A.; Au, S.; Cornwell, A.; Osipovitch, M.; Benraiss, A.; Dangelmajer, S.; Guerrero-Cazares, H.; Quinones-Hinojosa, A. PAR1 inhibition suppresses the self-renewal and growth of A2B5-defined glioma progenitor cells and their derived gliomas in vivo. Oncogene 2016, 35, 3817–3828. [Google Scholar] [CrossRef]
- Kuhn, S.A.; Martin, M.; Brodhun, M.; Kratzsch, T.; Hanisch, U.-K.; Haberl, H. Overexpression of protease-activated receptor type 1 (PAR-1) in glioblastoma multiforme WHO IV cells and blood vessels revealed by NCAM-assisted glioblastoma border labeling. Neurol. Res. 2014, 36, 709–721. [Google Scholar] [CrossRef] [PubMed]
- de Almeida, V.H.; Monteiro, R.Q. Protease-activated receptor 1 (PAR1): A promising target for the treatment of glioblastoma. Transl. Cancer Res. 2016, 5, S1274–S1280. [Google Scholar] [CrossRef]
- Kaufmann, R.; Patt, S.; Kraft, R.; Zieger, M.; Henklein, P.; Neupert, G.; Nowak, G. PAR 1-type thrombin receptors are involved in thrombin-induced calcium signaling in human meningioma cells. J. Neuro Oncol. 1999, 42, 131–136. [Google Scholar] [CrossRef]
- Zieger, M.; Tausch, S.; Henklein, P.; Nowak, G.; Kaufmann, R. A novel PAR-1-type thrombin receptor signaling pathway: Cyclic AMP-independent activation of PKA in SNB-19 glioblastoma cells. Biochem. Biophys. Res. Commun. 2001, 282, 952–957. [Google Scholar] [CrossRef]
- Hayakawa, Y.; Kurimoto, M.; Nagai, S.; Kurosaki, K.; Tsuboi, Y.; Hamada, H.; Hayashi, N.; Endo, S. Thrombin-induced cell proliferation and platelet-derived growth factor-AB release from A172 human glioblastoma cells. J. Thromb. Haemost. 2007, 5, 2219–2226. [Google Scholar] [CrossRef]
- da Gama Fischer, J.D.S.; Carvalho, P.C.; da Costa Neves-Ferreira, A.G.; da Fonseca, C.O.; Perales, J.; da Costa Carvalho, M.D.G.; Domont, G.B. Anti-thrombin as a prognostic biomarker candidate for patients with recurrent glioblastoma multiform under treatment with perillyl alcohol. J. Exp. Oncol. 2008, 7, 285–290. [Google Scholar]
- Shavit-Stein, E.; Sheinberg, E.; Golderman, V.; Sharabi, S.; Wohl, A.; Gofrit, S.G.; Zivli, Z.; Shelestovich, N.; Last, D.; Guez, D. A novel compound targeting protease receptor 1 activators for the treatment of glioblastoma. Front. Neurol. 2018, 9, 1087. [Google Scholar] [CrossRef]
- Vianello, F.; Sambado, L.; Goss, A.; Fabris, F.; Prandoni, P. Dabigatran antagonizes growth, cell-cycle progression, migration, and endothelial tube formation induced by thrombin in breast and glioblastoma cell lines. Cancer Med. 2016, 5, 2886–2898. [Google Scholar] [CrossRef]
- Hua, Y.; Tang, L.; Keep, R.; Schallert, T.; Fewel, M.; Muraszko, K.; Hoff, J.; Xi, G. The role of thrombin in gliomas. J. Thromb. Haemost. 2005, 3, 1917–1923. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shlobin, N.A.; Har-Even, M.; Itsekson-Hayosh, Z.; Harnof, S.; Pick, C.G. Role of Thrombin in Central Nervous System Injury and Disease. Biomolecules 2021, 11, 562. https://doi.org/10.3390/biom11040562
Shlobin NA, Har-Even M, Itsekson-Hayosh Z, Harnof S, Pick CG. Role of Thrombin in Central Nervous System Injury and Disease. Biomolecules. 2021; 11(4):562. https://doi.org/10.3390/biom11040562
Chicago/Turabian StyleShlobin, Nathan A., Meirav Har-Even, Ze’ev Itsekson-Hayosh, Sagi Harnof, and Chaim G. Pick. 2021. "Role of Thrombin in Central Nervous System Injury and Disease" Biomolecules 11, no. 4: 562. https://doi.org/10.3390/biom11040562
APA StyleShlobin, N. A., Har-Even, M., Itsekson-Hayosh, Z., Harnof, S., & Pick, C. G. (2021). Role of Thrombin in Central Nervous System Injury and Disease. Biomolecules, 11(4), 562. https://doi.org/10.3390/biom11040562





