Lipid Peroxidation in Subretinal Fluid: Some Light on the Prognosis Factors
Abstract
1. Introduction
2. Materials and Methods
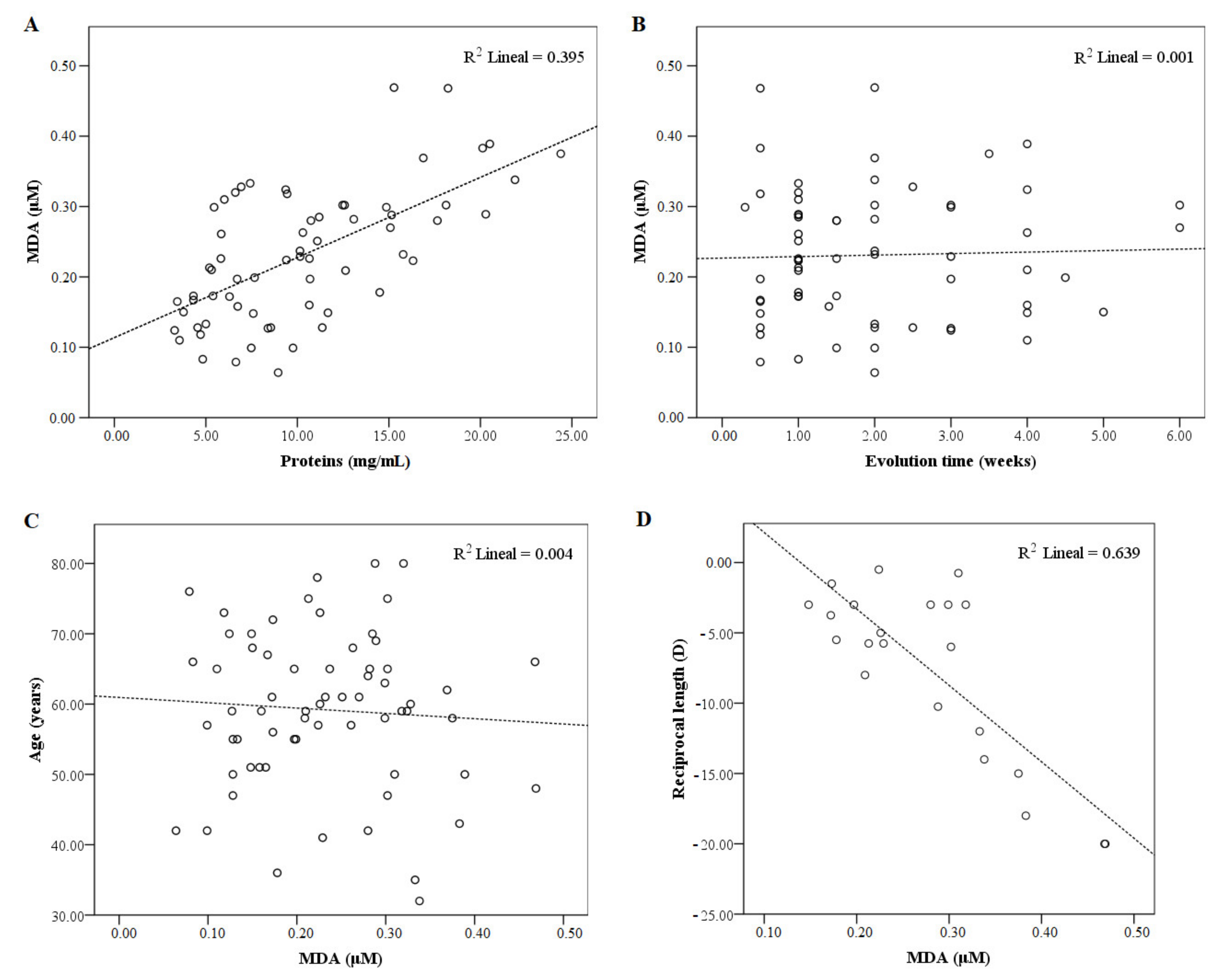
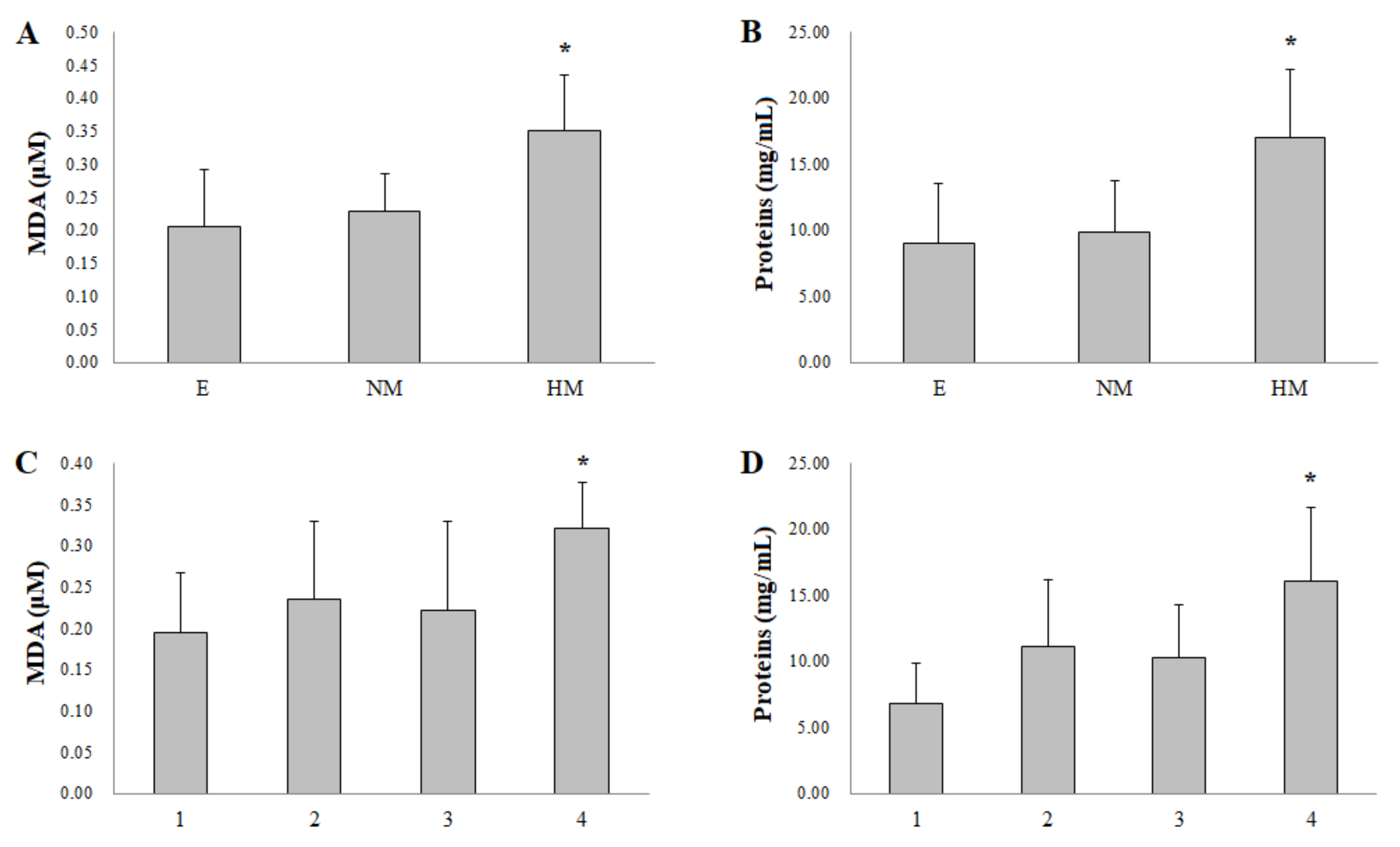
3. Results
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Holden, B.A.; Fricke, T.R.; Wilson, D.A.; Jong, M.; Naidoo, K.S.; Sankaridurg, P.; Wong, T.Y.; Naduvilath, T.J.; Resnikoff, S. Global Prevalence of Myopia and High Myopia and Temporal Trends from 2000 through 2050. Ophthalmology 2016, 123, 1036–1042. [Google Scholar] [CrossRef]
- Mitry, D.; Singh, J.; Yorston, D.; Siddiqui, M.A.R.; Wright, A.; Fleck, B.W.; Campbell, H.; Charteris, D.G. The Predisposing Pathology and Clinical Characteristics in the Scottish Retinal Detachment Study. Ophthalmology 2011, 118, 1429–1434. [Google Scholar] [CrossRef]
- Jackson, T.L.; Donachie, P.H.; Sallam, A.; Sparrow, J.M.; Johnston, R.L. United Kingdom National Ophthalmology Database Study of Vitreoretinal Surgery. Ophthalmology 2014, 121, 643–648. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.-J.; Lai, W.W.; Edward, D.P.; Tso, M.O.M. Apoptotic photoreceptor cell death after traumatic retinal detachment in humans. Arch. Ophthalmol. 1995, 113, 880–886. [Google Scholar] [CrossRef]
- Carpineto, P.; Aharrh-Gnama, A.; Ciciarelli, V.; Borrelli, E.; Petti, F.; Aloia, R.; Lamolinara, A.; Di Nicola, M.; Mastropasqua, L. Subretinal Fluid Levels of Signal-Transduction Proteins and Apoptosis Molecules in Macula-Off Retinal Detachment Undergoing Scleral Buckle Surgery. Investig. Ophthalmol. Vis. Sci. 2016, 57, 6895. [Google Scholar] [CrossRef] [PubMed]
- Tangvarasittichai, O.; Tangvarasittichai, S. Oxidative Stress, Ocular Disease and Diabetes Retinopathy. Curr. Pharm. Des. 2019, 24, 4726–4741. [Google Scholar] [CrossRef] [PubMed]
- Maeno, A.; Suzuki, Y.; Adachi, K.; Takahashi, S.; Yokoi, Y.; Nakazawa, M. Characterization of the biological antioxidant potential in the vitreous fluid from patients with rhegmatogenous retinal detachment. Acta Ophthalmol. 2016, 94, e515–e516. [Google Scholar] [CrossRef]
- Cederlund, M.; Ghosh, F.; Arnér, K.; Andréasson, S.; Åkerström, B. Vitreous levels of oxidative stress biomarkers and the radical-scavenger α1-microglobulin/A1M in human rhegmatogenous retinal detachment. Graefe’s Arch. Clin. Exp. Ophthalmol. 2012, 251, 725–732. [Google Scholar] [CrossRef] [PubMed]
- Ayala, A.; Muñoz, M.F.; Argüelles, S. Lipid Peroxidation: Production, Metabolism, and Signaling Mechanisms of Malondialdehyde and 4-Hydroxy-2-Nonenal. Oxidative Med. Cell. Longev. 2014, 2014, 1–31. [Google Scholar] [CrossRef]
- Gaschler, M.M.; Stockwell, B.R. Lipid peroxidation in cell death. Biochem. Biophys. Res. Commun. 2017, 482, 419–425. [Google Scholar] [CrossRef]
- Sultan, Z.N.; Agorogiannis, E.I.; Iannetta, D.; Steel, D.; Sandinha, T. Rhegmatogenous retinal detachment:A review of current practice in diagnosis and management. BMJ Open Ophthalmol. 2020, 5, e000474. [Google Scholar] [CrossRef]
- Esterbauer, H.; Schaur, R.J.; Zollner, H. Chemistry and biochemistry of 4-hydroxynonenal, malonaldehyde and related aldehydes. Free Radic. Biol. Med. 1991, 11, 81–128. [Google Scholar] [CrossRef]
- Lazzarino, G.; Di Pierro, D.; Tavazzi, B.; Cerroni, L.; Giardina, B. Simultaneous separation of malondialdehyde, ascorbic acid, and adenine nucleotide derivatives from biological samples by ion-pairing high-performance liquid chromatography. Anal. Biochem. 1991, 197, 191–196. [Google Scholar] [CrossRef]
- Romero, F.J.; Bosch-Morell, F.; Jareño, E.J.; Romero, B.; Marín, N.; Romá, J. Lipid peroxidation products and antioxidants in human disease. Environ. Health Perspect. 1998, 106, 1229–1234. [Google Scholar] [CrossRef]
- Lowry, O.H.; Rosebrough, N.J.; Farr, A.L.; Randall, R.J. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 1951, 193, 265–275. [Google Scholar] [CrossRef]
- Elejalde Guerra, J.I. Oxidative stress, diseases and antioxidant treatment. An. Med. Interna 2001, 18, 326–335. (In Spanish) [Google Scholar]
- Grotto, D.; Maria, L.S.; Valentini, J.; Paniz, C.; Schmitt, G.; Garcia, S.C.; Pomblum, V.J.; Rocha, J.B.T.; Farina, M. Importance of the lipid peroxidation biomarkers and methodological aspects FOR malondialdehyde quantification. Química Nova 2009, 32, 169–174. [Google Scholar] [CrossRef]
- Moore, K.; Roberts, L.J. Measurement of Lipid Peroxidation. Free Radic. Res. 1998, 28, 659–671. [Google Scholar] [CrossRef]
- Su, L.-J.; Zhang, J.-H.; Gomez, H.; Murugan, R.; Hong, X.; Xu, D.; Jiang, F.; Peng, Z.-Y. Reactive Oxygen Species-Induced Lipid Peroxidation in Apoptosis, Autophagy, and Ferroptosis. Oxidative Med. Cell. Longev. 2019, 2019, 1–13. [Google Scholar] [CrossRef]
- Gęgotek, A.; Skrzydlewska, E. Biological effect of protein modifications by lipid peroxidation products. Chem. Phys. Lipids 2019, 221, 46–52. [Google Scholar] [CrossRef] [PubMed]
- Weismann, D.; Hartvigsen, K.; Lauer, N.; Bennett, K.L.; Scholl, H.P.N.; Issa, P.C.; Cano, M.; Brandstätter, H.; Tsimikas, S.; Skerka, C.; et al. Complement factor H binds malondialdehyde epitopes and protects from oxidative stress. Nat. Cell Biol. 2011, 478, 76–81. [Google Scholar] [CrossRef]
- Veneskoski, M.; Turunen, S.P.; Kummu, O.; Nissinen, A.; Rannikko, S.; Levonen, A.-L.; Hörkkö, S. Specific recognition of malondialdehyde and malondialdehyde acetaldehyde adducts on oxidized LDL and apoptotic cells by complement anaphylatoxin C3a. Free Radic. Biol. Med. 2011, 51, 834–843. [Google Scholar] [CrossRef] [PubMed]
- Kharbanda, K.K.; Shubert, K.A.; Wyatt, T.A.; Sorrell, M.F.; Tuma, D.J. Effect of malondialdehyde–acetaldehyde–protein adducts on the protein kinase C-dependent secretion of urokinase-type plasminogen activator in hepatic stellate cells. Biochem. Pharmacol. 2002, 63, 553–562. [Google Scholar] [CrossRef][Green Version]
- Argüelles-Castilla, S.; Camandola, S.; Hutchison, E.R.; Cutler, R.G.; Ayala, A.; Mattson, M.P. Molecular control of the amount, subcellular location, and activity state of translation elongation factor 2 in neurons experiencing stress. Free Radic. Biol. Med. 2013, 61, 61–71. [Google Scholar] [CrossRef]
- Gentile, F.; Arcaro, A.; Pizzimenti, S.; Daga, M.; Cetrangolo, G.P.; Dianzani, C.; Lepore, A.; Graf, M.; Ames, P.R.J.; Barrera, G. DNA damage by lipid peroxidation products: Implications in cancer, inflammation and autoimmunity. AIMS Genet. 2017, 4, 103–137. [Google Scholar] [CrossRef]
- Cai, J.; Nelson, K.C.; Wu, M.; Sternberg, P.; Jones, D.P. Oxidative damage and protection of the RPE. Prog. Retin. Eye Res. 2000, 19, 205–221. [Google Scholar] [CrossRef]
- Bosch-Morell, F.; Sanz, A.; Díaz-Llopis, M.; Romero, F.J. Lipid peroxidation products in human subretinal fluid. Free Radic. Biol. Med. 1996, 20, 899–903. [Google Scholar] [CrossRef]
- Francisco, B.-M.; Salvador, M.; Amparo, N. Oxidative Stress in Myopia. Oxidative Med. Cell. Longev. 2015, 2015, 1–12. [Google Scholar] [CrossRef]
- Forman, H.J.; Augusto, O.; Brigelius-Flohe, R.; Dennery, P.A.; Kalyanaraman, B.; Ischiropoulos, H.; Mann, G.E.; Radi, R.; Roberts, L.J.; Vina, J.; et al. Even free radicals should follow some rules: A Guide to free radical research terminology and methodology. Free Radic. Biol. Med. 2015, 78, 233–235. [Google Scholar] [CrossRef]
- McNeil, J.D.; Wiebkin, O.W.; Betts, W.H.; Cleland, L.G. Depolymerisation products of hyaluronic acid after exposure to oxygen-derived free radicals. Ann. Rheum. Dis. 1985, 44, 780–789. [Google Scholar] [CrossRef] [PubMed]
- Sickel, W. Electrical and Metabolic Manifestations of Receptor and Higher-Order Neuron Activity in Vertebrate Retina. Chem. Biol. Pteridines Folates 1972, 24, 101–118. [Google Scholar] [CrossRef]
- Kreissig, I. Prognosis of return of macular function after retinal reattachment. Mod. Probl. Ophthalmol. 1977, 18, 415–429. [Google Scholar] [PubMed]
- Mohamed, Y.H.; Ono, K.; Kinoshita, H.; Uematsu, M.; Tsuiki, E.; Fujikawa, A.; Kitaoka, T. Success Rates of Vitrectomy in Treatment of Rhegmatogenous Retinal Detachment. J. Ophthalmol. 2016, 2016, 1–9. [Google Scholar] [CrossRef]
- Daruich, A.; Sauvain, J.-J.; Matet, A.; Eperon, S.; Schweizer, C.; Berthet, A.; Danuser, B.; Behar-Cohen, F. Levels of the oxidative stress biomarker malondialdehyde in tears of patients with central serous chorioretinopathy relate to disease activity. Mol. Vis. 2020, 26, 722–730. [Google Scholar] [PubMed]
- Gao, S.; Li, N.; Wang, Y.; Zhong, Y.; Shen, X. Blockade of Adenosine A2A Receptor Protects Photoreceptors after Retinal Detachment by Inhibiting Inflammation and Oxidative Stress. Oxidative Med. Cell. Longev. 2020, 2020, 1–12. [Google Scholar] [CrossRef]
- Huang, W.; Li, G.; Qiu, J.; Gonzalez, P.; Challa, P. Protective Effects of Resveratrol in Experimental Retinal Detachment. PLoS ONE 2013, 8, e75735. [Google Scholar] [CrossRef] [PubMed]
- Ding, J.; Yang, N.; Yan, Y.; Wang, Y.; Wang, X.; Lu, L.; Dong, K. Rapamycin Inhibited Photoreceptor Necroptosis and Protected the Retina by Activation of Autophagy in Experimental Retinal Detachment. Curr. Eye Res. 2019, 44, 739–745. [Google Scholar] [CrossRef]
- Pardue, M.T.; Allen, R.S. Neuroprotective strategies for retinal disease. Prog. Retin. Eye Res. 2018, 65, 50–76. [Google Scholar] [CrossRef]
- She, X.; Lü, X.; Li, T.; Sun, J.; Liang, J.; Zhai, Y.; Yang, S.; Gu, Q.; Wei, F.; Zhu, H.; et al. Inhibition of Mitochondrial Fission Preserves Photoreceptors after Retinal Detachment. Am. J. Pathol. 2018, 188, 1713–1722. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.B.; Kim, H.K.; Hyon, J.Y.; Wee, W.R.; Shin, Y.J. Oxidative Stress Levels in Aqueous Humor from High Myopic Patients. Korean J. Ophthalmol. 2016, 30, 172–179. [Google Scholar] [CrossRef]
- Du, B.; Jin, N.; Zhu, X.; Lu, D.; Jin, C.; Li, Z.; Han, C.; Zhang, Y.; Lai, D.; Liu, K.; et al. A prospective study of serum metabolomic and lipidomic changes in myopic children and adolescents. Exp. Eye Res. 2020, 199, 108182. [Google Scholar] [CrossRef] [PubMed]
- Wei, Q.; Zhang, T.; Fan, J.; Jiang, R.; Chang, Q.; Hong, J.; Xu, G. Pathological myopia-induced antioxidative proteins in the vitreous humor. Ann. Transl. Med. 2020, 8, 193. [Google Scholar] [CrossRef] [PubMed]
| Age (Years) | 59.2 ± 11.1 * | 30–80 ** | ||
| Evolution time (weeks) | 2.0 ± 1.4 * | 0.3–6 ** | ||
| Extension retinal detachment (RD) (quadrants) | Q1 19 | Q2 25 | Q3 13 | Q4 8 |
| Refractive classification | E 43 | LM 13 | HM 9 |
| Mean Value | Range | |
|---|---|---|
| Malondialdehyde (MDA) (µM) | 0.23 ± 0.10 | 0.06 to 0.47 |
| Protein (mg/mL) | 10.30 ± 5.18 | 3.29 to 24.39 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bosch-Morell, F.; García-Gen, E.; Mérida, S.; Penadés, M.; Desco, C.; Navea, A. Lipid Peroxidation in Subretinal Fluid: Some Light on the Prognosis Factors. Biomolecules 2021, 11, 514. https://doi.org/10.3390/biom11040514
Bosch-Morell F, García-Gen E, Mérida S, Penadés M, Desco C, Navea A. Lipid Peroxidation in Subretinal Fluid: Some Light on the Prognosis Factors. Biomolecules. 2021; 11(4):514. https://doi.org/10.3390/biom11040514
Chicago/Turabian StyleBosch-Morell, Francisco, Enrique García-Gen, Salvador Mérida, Mariola Penadés, Carmen Desco, and Amparo Navea. 2021. "Lipid Peroxidation in Subretinal Fluid: Some Light on the Prognosis Factors" Biomolecules 11, no. 4: 514. https://doi.org/10.3390/biom11040514
APA StyleBosch-Morell, F., García-Gen, E., Mérida, S., Penadés, M., Desco, C., & Navea, A. (2021). Lipid Peroxidation in Subretinal Fluid: Some Light on the Prognosis Factors. Biomolecules, 11(4), 514. https://doi.org/10.3390/biom11040514







