A Biorefinery Approach to the Biomass of the Seaweed Undaria pinnatifida (Harvey Suringar, 1873): Obtaining Phlorotannins-Enriched Extracts for Wound Healing
Abstract
:1. Introduction
2. Materials and Methods
2.1. Biomass Harvesting and Processing
2.2. Preliminary Sequential Extraction Screening
2.3. Optimized Sequential Extraction
2.4. Antioxidant Activity
2.4.1. Diphenyl-1-picrylhydrazyl (DPPH) Assay
2.4.2. Ferric Reducing Antioxidant Power (FRAP) Assay
2.5. Antibacterial Activity
2.5.1. Disc Diffusion Method
2.5.2. Microdilution Protocol
2.6. Cellular Activity
2.6.1. Cell Viability
2.6.2. Anti-Inflammatory Activity of Extracts (Nitric Oxide Production Inhibition)
2.7. Chemical Characterization of Extracts
2.7.1. Total Phenolic Content (TPC) by Folin–Ciocalteu (FC) Assay
2.7.2. Nuclear Magnetic Resonance (NMR) Analysis
2.7.3. Fourier Transform Infrared (FTIR) Analysis
2.8. Statistical Analysis
3. Results
3.1. Extraction Yield
3.2. Antioxidant Activity
3.3. Antimicrobial Activity
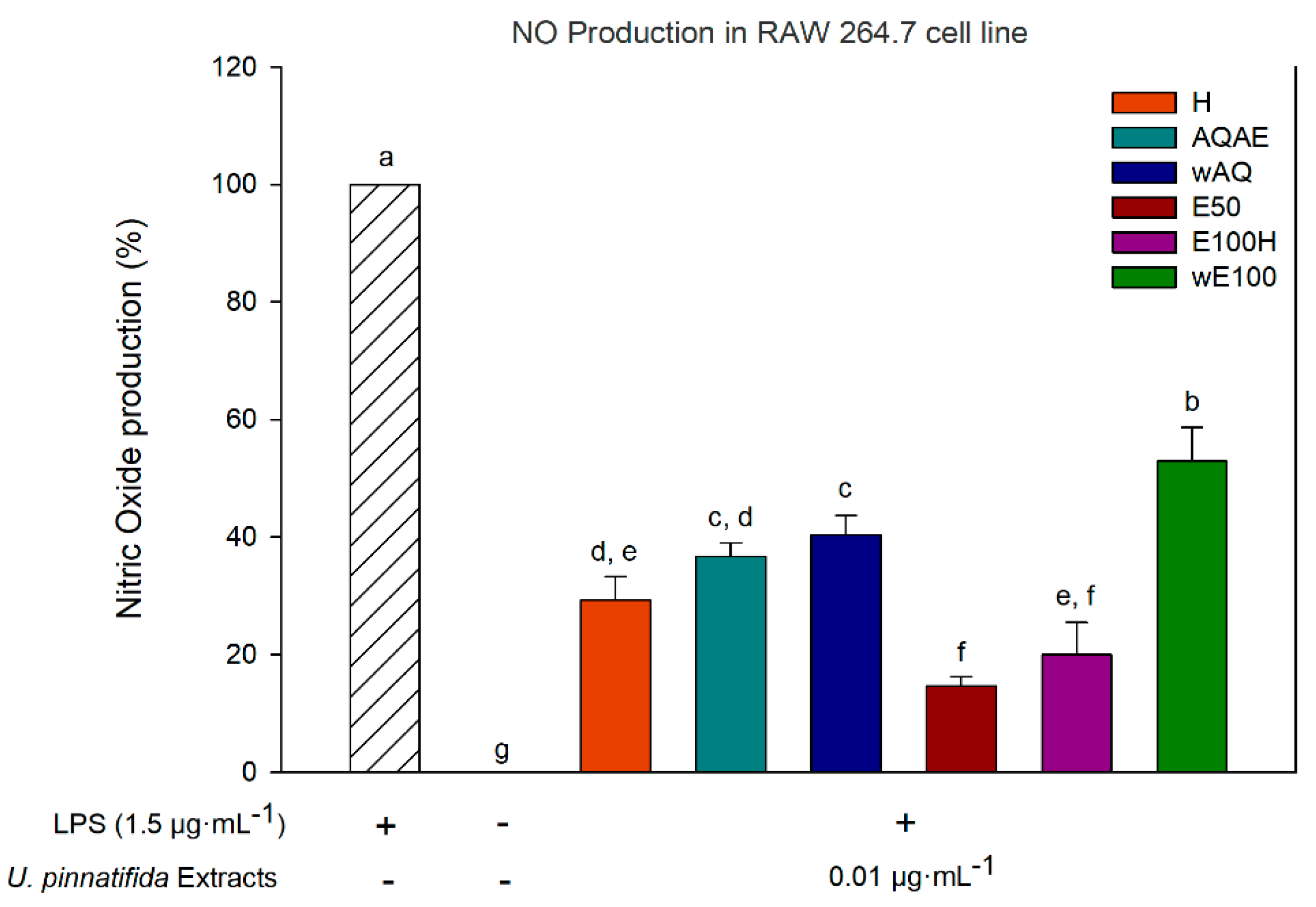
3.4. Anti-Inflammatory Activity (Inhibition of Nitric Oxide Production)
3.5. AQAE and wE100 Chemical Characterization
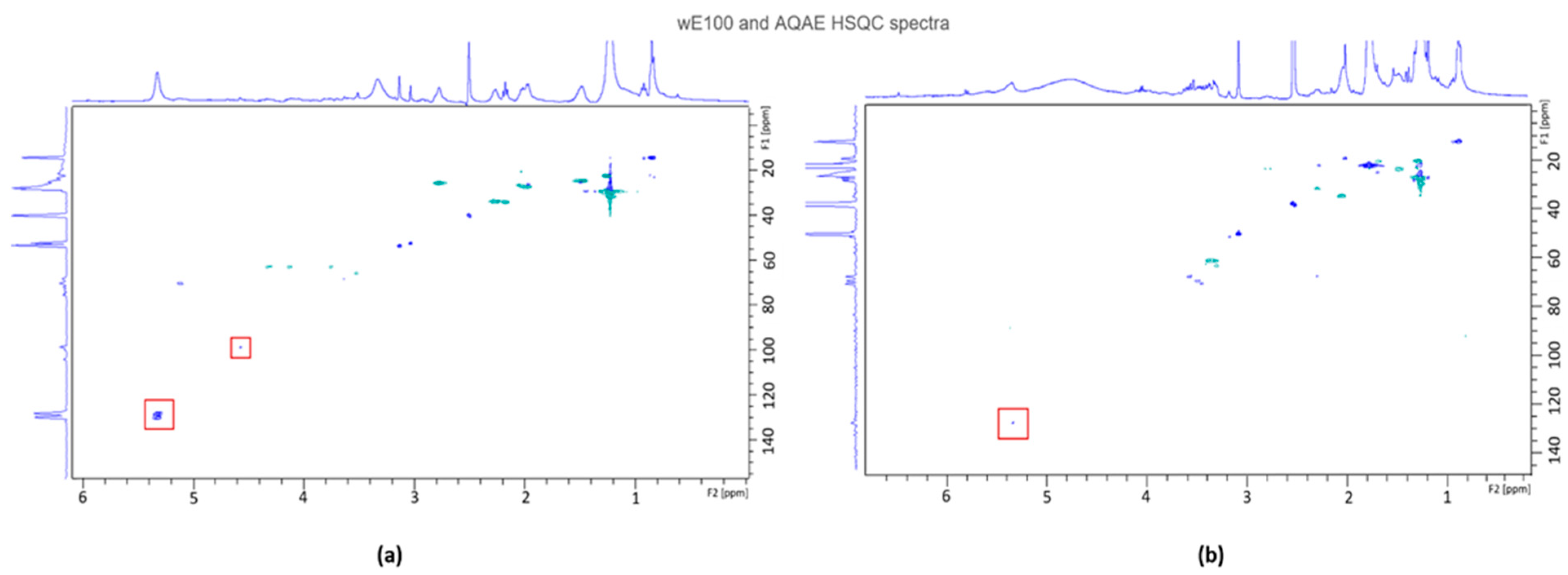
3.5.1. Nuclear Magnetic Resonance (NMR)
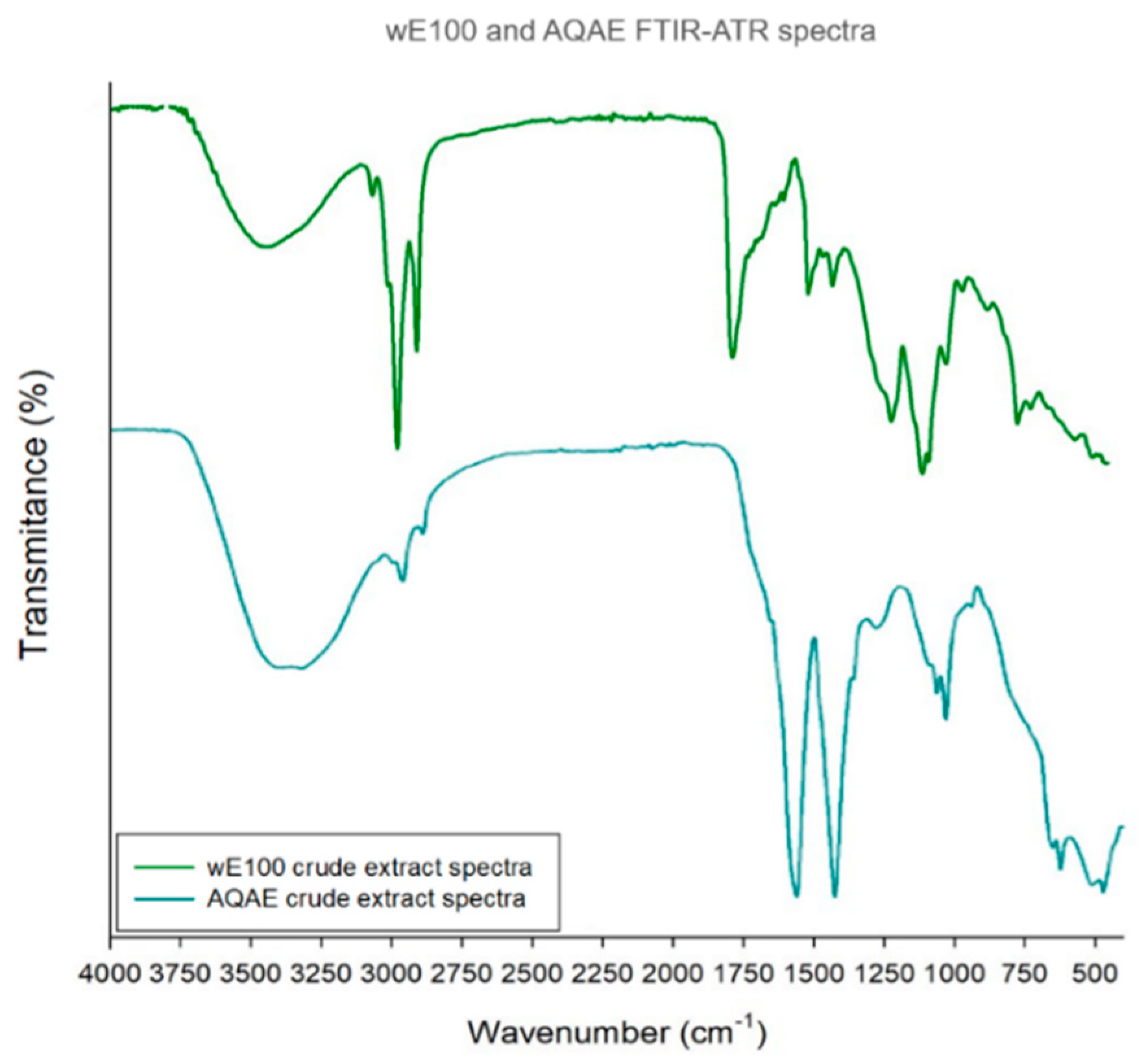
3.5.2. FTIR-ATR
3.5.3. Total Phenolic Content (TPC) Quantification
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Barbosa, M.; Lopes, G.; Andrade, P.B.; Valentão, P. Bioprospecting of brown seaweeds for biotechnological applications: Phlorotannin actions in inflammation and allergy network. Trends Food Sci. Technol. 2019, 86, 153–171. [Google Scholar] [CrossRef]
- Hu, J.; Yang, B.; Lin, X.; Zhou, X.F.; Yang, X.W.; Liu, Y. Bioactive Metabolites from Seaweeds. In Handbook of Marine Macroalgae: Biotechnology and Applied Phycology, Se-Kwon, K., 1st ed.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2011; pp. 262–284. ISBN 9780470979181. [Google Scholar]
- Puscaselu, R.G.; Lobiuc, A.; Dimian, M.; Covasa, M. Alginate: From food industry to biomedical applications and management of metabolic disorders. Polymers 2020, 12, 2417. [Google Scholar] [CrossRef] [PubMed]
- Stojadinovic, A.; Carlson, J.W.; Schultz, G.S.; Davis, T.A.; Elster, E.A. Topical advances in wound care. Gynecol. Oncol. 2008, 111, S70–S80. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.; Abu-Ghannam, N. Bioactive potential and possible health effects of edible brown seaweeds. Trends Food Sci. Technol. 2011, 22, 315–326. [Google Scholar] [CrossRef] [Green Version]
- Pádua, D.; Rocha, E.; Gargiulo, D.; Ramos, A.A. Bioactive compounds from brown seaweeds: Phloroglucinol, fucoxanthin and fucoidan as promising therapeutic agents against breast cancer. Phytochem. Lett. 2015, 14, 91–98. [Google Scholar] [CrossRef]
- Li, Y.; Fu, X.; Duan, D.; Liu, X.; Xu, J.; Gao, X. Extraction and identification of phlorotannins from the brown alga, Sargassum fusiforme (Harvey) Setchell. Mar. Drugs 2017, 15, 49. [Google Scholar] [CrossRef] [Green Version]
- Wang, H.M.D.; Li, X.C.; Lee, D.J.; Chang, J.S. Potential biomedical applications of marine algae. Bioresour. Technol. 2017, 244, 1407–1415. [Google Scholar] [CrossRef]
- O’Callaghan, S.; Galvin, P.; O’Mahony, C.; Moore, Z.; Derwin, R. ‘Smart’ wound dressings for advanced wound care: A review. J. Wound Care 2020, 29, 394–406. [Google Scholar] [CrossRef]
- Casas, G.; Scrosati, R.; Piriz, M.L. The invasive kelp Undaria pinnatifida (Phaeophyceae, Laminariales) reduces native seaweed diversity in Nuevo Gulf (Patagonia, Argentina). Biol. Invasions 2003, 6, 411–416. [Google Scholar] [CrossRef]
- Irigoyen, A.J.; Trobbiani, G.; Sgarlatta, M.P.; Raffo, M.P. Effects of the alien algae Undaria pinnatifida (Phaeophyceae, Laminariales) on the diversity and abundance of benthic macrofauna in Golfo Nuevo (Patagonia, Argentina): Potential implications for local food webs. Biol. Invasions 2011, 13, 1521–1532. [Google Scholar] [CrossRef]
- Veiga, P.; Torres, A.C.; Rubal, M.; Troncoso, J.; Sousa-Pinto, I. The invasive kelp Undaria pinnatifida (Laminariales, Ochrophyta) along the north coast of Portugal: Distribution model versus field observations. Mar. Pollut. Bull. 2014, 84, 363–365. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Park, Y.J.; Jeon, Y.J.; Ryu, B.M. Bioactivities of the edible brown seaweed, Undaria pinnatifida: A review. Aquaculture 2018, 495, 873–880. [Google Scholar] [CrossRef]
- Dias, J.R.; Granja, P.L.; Bártolo, P.J. Advances in electrospun skin substitutes. Prog. Mater. Sci. 2016, 84, 314–334. [Google Scholar] [CrossRef]
- Pinteus, S.; Lemos, M.F.L.; Alves, C.; Neugebauer, A.; Silva, J.; Thomas, O.P.; Botana, L.M.; Gaspar, H.; Pedrosa, R. Marine invasive macroalgae: Turning a real threat into a major opportunity—The biotechnological potential of Sargassum muticum and Asparagopsis armata. Algal Res. 2018, 34, 217–234. [Google Scholar] [CrossRef]
- Pasko, S.; Goldberg, J. Review of harvest incentives to control invasive species. Manag. Biol. Invasions 2014, 5, 263–277. [Google Scholar] [CrossRef] [Green Version]
- Baliano, A.P.; Pimentel, E.F.; Buzin, A.R.; Vieira, T.Z.; Romão, W.; Tose, L.V.; Lenz, D.; de Andrade, T.U.; Fronza, M.; Kondratyuk, T.P.; et al. Brown seaweed Padina gymnospora is a prominent natural wound-care product. Braz. J. Pharmacogn. 2016, 26, 714–719. [Google Scholar] [CrossRef] [Green Version]
- Félix, R.; Valentão, P.; Andrade, P.B.; Félix, C.; Novais, S.C.; Lemos, M.F.L. Evaluating the in vitro potential of natural extracts to protect lipids from oxidative damage. Antioxidants 2020, 9, 231. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kurahashi, T.; Fujii, J. Roles of antioxidative enzymes in wound healing. J. Dev. Biol. 2015, 3, 57–70. [Google Scholar] [CrossRef] [Green Version]
- Ryu, B.; Li, Y.; Qian, Z.-J.; Kim, M.-M.; Kim, S.-K. Differentiation of human osteosarcoma cells by isolated phlorotannins is subtly linked to COX-2, iNOS, MMPs, and MAPK signaling: Implication for chronic articular disease. Chem. Biol. Interact. 2009, 179, 192–201. [Google Scholar] [CrossRef] [PubMed]
- Santos, S.A.O.; Félix, R.; Pais, A.C.S.; Rocha, S.M.; Silvestre, A.J.D. The quest for phenolic compounds from macroalgae: A review of extraction and identification methodologies. Biomolecules 2019, 9, 847. [Google Scholar] [CrossRef] [Green Version]
- Cuong, D.X.; Hoan, N.X.; Dong, D.H.; Thuy, L.T.M.; van Thanh, N.; Ha, H.T.; Tuyen, D.T.T.T.; Chinh, D.X. Tannins: Extraction from Plants. In Tannins—Structural Properties, Biological Properties and Current Knowledge, 1st ed.; Aires, A., IntechOpen: London, UK, 2019. [Google Scholar]
- Wang, T.; Jónsdóttir, R.; Liu, H.; Gu, L.; Kristinsson, H.G.; Raghavan, S.; Ólafsdóttir, G. Antioxidant Capacities of Phlorotannins Extracted from the Brown Algae Fucus vesiculosus. J. Agric. Food Chem. 2012, 60, 5874–5883. [Google Scholar] [CrossRef]
- Schroeder, P.; Anggraeni, K.; Weber, U. The relevance of circular economy practices to the sustainable development goals. J. Ind. Ecol. 2019, 23, 77–95. [Google Scholar] [CrossRef] [Green Version]
- Carus, M.; Dammer, L. The Circular Bioeconomy—Concepts, opportunities, and limitations. Ind. Biotechnol. 2018, 14, 83–91. [Google Scholar] [CrossRef]
- Félix, R.; Félix, C.; Januário, A.P.; Carmona, A.M.; Baptista, T.; Gonçalves, R.A.; Sendão, J.; Novais, S.C.; Lemos, M.F.L. Tailoring shrimp aquafeed to tackle Acute Hepatopancreatic Necrosis Disease by inclusion of industry-friendly seaweed extracts. Aquaculture 2020, 529, 735661. [Google Scholar] [CrossRef]
- Dudonné, S.; Vitrac, X.; Coutiére, P.; Woillez, M.; Mérillon, J.M. Comparative study of antioxidant properties and total phenolic content of 30 plant extracts of industrial interest using DPPH, ABTS, FRAP, SOD, and ORAC assays. J. Agric. Food Chem. 2009, 57, 1768–1774. [Google Scholar] [CrossRef]
- Clinical and Laboratory Standards Institute (CLSI). Performance Standards for Antimicrobial Susceptibility Testing, Supplement M100S, 26th ed.; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2014. [Google Scholar]
- Clinical and Laboratory Standards Institute (CLSI). Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria That Grow Aerobically: Approved Standard, CLSI document M07-A10, 10th ed.; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2015. [Google Scholar]
- Benevides Bahiense, J.; Marques, F.M.; Figueira, M.M.; Vargas, T.S.; Kondratyuk, T.P.; Endringer, D.C.; Scherer, R.; Fronza, M. Potential anti-inflammatory, antioxidant and antimicrobial activities of Sambucus australis. Pharm. Biol. 2017, 55, 991–997. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Q.; Zhang, J.; Shen, J.; Silva, A.; Dennis, D.A.; Barrow, C.J. A simple 96-well microplate method for estimation of total polyphenol content in seaweeds. J. Appl. Phycol. 2006, 18, 445–450. [Google Scholar] [CrossRef] [Green Version]
- Coates, J. Interpretation of Infrared Spectra, A Practical Approach. In Encyclopedia of Analytical Chemistry: Applications, Theory and Instrumentation; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2000; pp. 10815–10837. [Google Scholar]
- Tenorio-Rodríguez, P.A.; Esquivel-Solis, H.; Murillo-Álvarez, J.I.; Ascencio, F.; Campa-Córdova, Á.I.; Angulo, C. Biosprospecting potential of kelp (Laminariales, Phaeophyceae) from Baja California Peninsula: Phenolic content, antioxidant properties, anti-inflammatory, and cell viability. J. Appl. Phycol. 2019. [Google Scholar] [CrossRef]
- Pereira, L.; Sousa, A.; Coelho, H.; Amado, A.M.; Ribeiro-Claro, P.J.A. Use of FTIR, FT-Raman and 13 C-NMR spectroscopy for identification of some seaweed phycocolloids. Biomol. Eng. 2003, 20, 223–228. [Google Scholar] [CrossRef] [Green Version]
- Sudha, G.; Balasundaram, A. Analysis of bioactive compounds in Padina pavonica using HPLC, UV-VIS and FTIR techniques. J. Pharmacogn. Phytochem. 2018, 7, 3192–3195. [Google Scholar]
- Abdelhamid, A.; Lajili, S.; Elkaibi, M.A.; Ben Salem, Y.; Abdelhamid, A.; Muller, C.D.; Majdoub, H.; Kraiem, J.; Bouraoui, A. optimized extraction, preliminary characterization and evaluation of the in vitro anticancer activity of phlorotannin-rich fraction from the brown seaweed, Cystoseira sedoides. J. Aquat. Food Prod. Technol. 2019, 28, 892–909. [Google Scholar] [CrossRef]
- Xuan Cuong, D. Antioxidant nano phlorotannin powder from brown algae Sargassum serratum: Spray Drying, antioxidant activities, physic-chemical characterization. J. Pharm. Res. Int. 2020, 32, 71–85. [Google Scholar] [CrossRef]
- Miyashita, K.; Mikami, N.; Hosokawa, M. Chemical and nutritional characteristics of brown seaweed lipids: A review. J. Funct. Foods 2013, 5, 1507–1517. [Google Scholar] [CrossRef]
- Takagi, T.; Sasaki, Y.; Motone, K.; Shibata, T.; Tanaka, R.; Miyake, H.; Mori, T.; Kuroda, K.; Ueda, M. Construction of bioengineered yeast platform for direct bioethanol production from alginate and mannitol. Appl. Microbiol. Biotechnol. 2017, 101, 6627–6636. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Yuen, A.K.L.; de Nys, R.; Masters, A.F.; Maschmeyer, T. Step by step extraction of bio-actives from the brown seaweeds, Carpophyllum flexuosum, Carpophyllum plumosum, Ecklonia radiata and Undaria pinnatifida. Algal Res. 2020, 52. [Google Scholar] [CrossRef]
- Abdelhamid, A.; Jouini, M.; Bel Haj Amor, H.; Mzoughi, Z.; Dridi, M.; Ben Said, R.; Bouraoui, A. Phytochemical analysis and evaluation of the antioxidant, anti-Inflammatory, and antinociceptive potential of phlorotannin-rich Fractions from three mediterranean brown seaweeds. Mar. Biotechnol. 2018, 20, 60–74. [Google Scholar] [CrossRef]
- Jiménez-Escrig, A.; Jiménez-Jiménez, I.; Pulido, R.; Saura-Calixto, F. Antioxidant activity of fresh and processed edible seaweeds. J. Sci. Food Agric. 2001, 81, 530–534. [Google Scholar] [CrossRef]
- Fung, A.; Hamid, N.; Lu, J. Fucoxanthin content and antioxidant properties of Undaria pinnatifida. Food Chem. 2013, 136, 1055–1062. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Jónsdóttir, R.; Ólafsdóttir, G. Total phenolic compounds, radical scavenging and metal chelation of extracts from Icelandic seaweeds. Food Chem. 2009, 116, 240–248. [Google Scholar] [CrossRef]
- Patra, J.K.; Lee, S.-W.; Park, J.G.; Baek, K.-H. Antioxidant and antibacterial properties of essential oil extracted from an edible seaweed Undaria Pinnatifida. J. Food Biochem. 2017, 41, e12278. [Google Scholar] [CrossRef]
- Gonçalves-Fernández, C.; Sineiro, J.; Moreira, R.; Gualillo, O. Extraction and characterization of phlorotannin-enriched fractions from the Atlantic seaweed Bifurcaria bifurcata and evaluation of their cytotoxic activity in murine cell line. J. Appl. Phycol. 2019, 31, 2573–2583. [Google Scholar] [CrossRef]
- Prabhasankar, P.; Ganesan, P.; Bhaskar, N.; Hirose, A.; Stephen, N.; Gowda, L.R.; Hosokawa, M.; Miyashita, K. Edible Japanese seaweed, wakame (Undaria pinnatifida) as an ingredient in pasta: Chemical, functional and structural evaluation. Food Chem. 2009, 115, 501–508. [Google Scholar] [CrossRef]
- Lee, J.-H.; Eom, S.-H.; Lee, E.-H.; Jung, Y.-J.; Kim, H.-J.; Jo, M.-R.; Son, K.-T.; Lee, H.-J.; Kim, J.H.; Lee, M.-S.; et al. In vitro antibacterial and synergistic effect of phlorotannins isolated from edible brown seaweed Eisenia bicyclis against acne-related bacteria. Algae 2014, 29, 47–55. [Google Scholar] [CrossRef]
- Cox, S.; Abu-ghannam, N.; Gupta, S. An Assessment of the Antioxidant and Antimicrobial Activity of Six Species of Edible Irish Seaweeds. Int. Food Res. J. 2010, 17, 205–220. [Google Scholar] [CrossRef]
- Jaiswal, A.K.; Rajauria, G.; Abu-Ghannam, N.; Gupta, S. Effect of different solvents on polyphenolic content, antioxidant capacity and antibacterial activity of irish york cabbage. J. Food Biochem. 2012, 36, 344–358. [Google Scholar] [CrossRef]
- Karpiński, T.M.; Adamczak, A. Fucoxanthin—An Antibacterial Carotenoid. Antioxidants 2019, 8, 239. [Google Scholar] [CrossRef] [Green Version]
- Navon-Venezia, S.; Kondratyeva, K.; Carattoli, A. Klebsiella pneumoniae: A major worldwide source and shuttle for antibiotic resistance. FEMS Microbiol. Rev. 2017, 41, 252–275. [Google Scholar] [CrossRef]
- Paczosa, M.K.; Mecsas, J. Klebsiella pneumoniae: Going on the offense with a strong defense. Microbiol. Mol. Biol. Rev. 2016, 80, 629–661. [Google Scholar] [CrossRef] [Green Version]
- Khan, M.N.A.; Cho, J.Y.; Lee, M.C.; Kang, J.Y.; Nam, G.P.; Fujii, H.; Hong, Y.K. Isolation of two anti-inflammatory and one pro-inflammatory polyunsaturated fatty acids from the brown seaweed Undaria pinnatifida. J. Agric. Food Chem. 2007, 55, 6984–6988. [Google Scholar] [CrossRef]
- Kim, S.; Kim, J.-I.; Choi, J.-W.; Kim, M.; Yoon, N.Y.; Choi, C.-G.; Choi, J.-S.; Kim, H.-R. Anti-inflammatory effect of hexane fraction from Myagropsis myagroides ethanolic extract in lipopolysaccharide-stimulated BV-2 microglial cells. J. Pharm. Pharmacol. 2013, 65, 895–906. [Google Scholar] [CrossRef]
- Kim, S.; Choi, S.I.; Kim, G.H.; Imm, J.Y. Anti-inflammatory effect of Ecklonia cava extract on porphyromonas gingivalis lipopolysaccharide-stimulated macrophages and a periodontitis rat model. Nutrients 2019, 11, 1143. [Google Scholar] [CrossRef] [Green Version]
- Catarino, M.D.; Silva, A.; Cruz, M.T.; Mateus, N.; Silva, A.M.S.; Cardoso, S.M. Phlorotannins from Fucus vesiculosus: Modulation of Inflammatory Response by Blocking NF-κB Signaling Pathway. Int. J. Mol. Sci. Artic. 2020, 21, 6897. [Google Scholar] [CrossRef] [PubMed]
- Dong, X.; Bai, Y.; Xu, Z.; Shi, Y.; Sun, Y.; Janaswamy, S.; Yu, C.; Qi, H. Phlorotannins from Undaria pinnatifida sporophyll: Extraction, antioxidant, and anti-Inflammatory activities. Mar. Drugs 2019, 17, 434. [Google Scholar] [CrossRef] [Green Version]
- Jung, H.A.; Jin, S.E.; Ahn, B.R.; Lee, C.M.; Choi, J.S. Anti-inflammatory activity of edible brown alga Eisenia bicyclis and its constituents fucosterol and phlorotannins in LPS-stimulated RAW264.7 macrophages. Food Chem. Toxicol. 2013, 59, 199–206. [Google Scholar] [CrossRef] [PubMed]
- Song, K.M.; Ha, S.J.; Lee, J.E.; Kim, S.H.; Kim, Y.H.; Kim, Y.; Hong, S.P.; Jung, S.K.; Lee, N.H. High yield ultrasonication extraction method for Undaria pinnatifida sporophyll and its anti-inflammatory properties associated with AP-1 pathway suppression. LWT Food Sci. Technol. 2015, 64, 1315–1322. [Google Scholar] [CrossRef]
- Eom, S.J.; Kim, Y.E.; Kim, J.E.; Park, J.; Kim, Y.H.; Song, K.M.; Lee, N.H. Production of Undaria pinnatifida sporophyll extract using pilot-scale ultrasound-assisted extraction: Extract characteristics and antioxidant and anti-inflammatory activities. Algal Res. 2020, 51, 102039. [Google Scholar] [CrossRef]
- Kim, S.M.; Shang, Y.F.; Um, B.-H. A preparative method for isolation of fucoxanthin from Eisenia bicyclis by centrifugal partition chromatography. Phytochem. Anal. 2011, 22, 322–329. [Google Scholar] [CrossRef]
- Komba, S.; Kotake-Nara, E.; Tsuzuki, W. Degradation of fucoxanthin to elucidate the relationship between the fucoxanthin molecular structure and its antiproliferative effect on Caco-2 Cells. Mar. Drugs 2018, 16, 275. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, P.; Wong, C.C.; Li, Y.; He, Y.; Mao, X.; Wu, T.; Ren, Y.; Chen, F. A novel strategy for isolation and purification of fucoxanthinol and fucoxanthin from the diatom Nitzschia laevis. Food Chem. 2019, 277, 566–572. [Google Scholar] [CrossRef]
- Koh, H.S.A.; Lu, J.; Zhou, W. Structure characterization and antioxidant activity of fucoidan isolated from Undaria pinnatifida grown in New Zealand. Carbohydr. Polym. 2019, 212, 178–185. [Google Scholar] [CrossRef]
- Synytsya, A.; Kim, W.J.; Kim, S.M.; Pohl, R.; Synytsya, A.; Kvasnička, F.; Čopíková, J.; Park, Y., II. Structure and antitumour activity of fucoidan isolated from sporophyll of Korean brown seaweed Undaria pinnatifida. Carbohydr. Polym. 2010, 81, 41–48. [Google Scholar] [CrossRef]
- Vishchuk, O.S.; Ermakova, S.P.; Zvyagintseva, T.N. Sulfated polysaccharides from brown seaweeds Saccharina japonica and Undaria pinnatifida: Isolation, structural characteristics, and antitumor activity. Carbohydr. Res. 2011, 346, 2769–2776. [Google Scholar] [CrossRef]
- Kadam, S.U.; Tiwari, B.K.; O’Donnell, C.P. Extraction, structure and biofunctional activities of laminarin from brown algae. Int. J. Food Sci. Technol. 2015, 50, 24–31. [Google Scholar] [CrossRef]
- Date, Y.; Sakata, K.; Kikuchi, J. Chemical profiling of complex biochemical mixtures from various seaweeds. Polym. J. 2012, 44, 888–894. [Google Scholar] [CrossRef] [Green Version]
- Zhang, R.; Yuen, A.K.L.; Magnusson, M.; Wright, J.T.; de Nys, R.; Masters, A.F.; Maschmeyer, T. A comparative assessment of the activity and structure of phlorotannins from the brown seaweed Carpophyllum flexuosum. Algal Res. 2018, 29, 130–141. [Google Scholar] [CrossRef]
- Jégou, C.; Kervarec, N.; Cérantola, S.; Bihannic, I.; Stiger-Pouvreau, V. NMR use to quantify phlorotannins: The case of Cystoseira tamariscifolia, a phloroglucinol-producing brown macroalga in Brittany (France). Talanta 2015, 135, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Karthik, R.; Manigandan, V.; Sheeba, R.; Saravanan, R.; Rajesh, P.R. Structural characterization and comparative biomedical properties of phloroglucinol from Indian brown seaweeds. J. Appl. Phycol. 2016, 28, 3561–3573. [Google Scholar] [CrossRef]
- Ford, L.; Theodoridou, K.; Sheldrake, G.N.; Walsh, P.J. A critical review of analytical methods used for the chemical characterisation and quantification of phlorotannin compounds in brown seaweeds. Phytochem. Anal. 2019, 1–13. [Google Scholar] [CrossRef]
- Cérantola, S.; Breton, F.; Gall, E.A.; Deslandes, E. Co-occurrence and antioxidant activities of fucol and fucophlorethol classes of polymeric phenols in Fucus spiralis. Bot. Mar. 2006, 49, 347–351. [Google Scholar] [CrossRef]
- Leyton, A.; Pezoa-Conte, R.; Barriga, A.; Buschmann, A.H.; Mäki-Arvela, P.; Mikkola, J.-P.; Lienqueo, M.E. Identification and efficient extraction method of phlorotannins from the brown seaweed Macrocystis pyrifera using an orthogonal experimental design. Algal Res. 2016, 16, 201–208. [Google Scholar] [CrossRef]
- Generalić Mekinić, I.; Skroza, D.; Šimat, V.; Hamed, I.; Čagalj, M.; Popović Perković, Z. Phenolic content of brown algae (Pheophyceae) species: Extraction, identification, and quantification. Biomolecules 2019, 9, 244. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Catarino, M.D.; Silva, A.M.S.; Mateus, N.; Cardoso, S.M. Optimization of phlorotannins extraction from Fucus vesiculosus and evaluation of their potential to prevent metabolic disorders. Mar. Drugs 2019, 17, 162. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ahn, D.-H.; Cho, S.; Hong, Y.-K.; Kim, A.; Cho, J.; Park, S.; Kang, S. The Antioxidant Properties of brown seaweed (Sargassum siliquastrum) Extracts. J. Med. Food 2007, 10, 479–485. [Google Scholar] [CrossRef]
- Montero, L.; Sánchez-Camargo, A.P.; García-Cañas, V.; Tanniou, A.; Stiger-Pouvreau, V.; Russo, M.; Rastrelli, L.; Cifuentes, A.; Herrero, M.; Ibáñez, E. Anti-proliferative activity and chemical characterization by comprehensive two-dimensional liquid chromatography coupled to mass spectrometry of phlorotannins from the brown macroalga Sargassum muticum collected on North-Atlantic coasts. J. Chromatogr. A 2016, 1428, 115–125. [Google Scholar] [CrossRef]
- Machu, L.; Misurcova, L.; Vavra Ambrozova, J.; Orsavova, J.; Mlcek, J.; Sochor, J.; Jurikova, T. Phenolic content and antioxidant capacity in algal food products. Molecules 2015, 20, 1118–1133. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aminina, N.M.; Karaulova, E.P.; Vishnevskaya, T.I.; Yakush, E.V.; Kim, Y.K.; Nam, K.H.; Son, K.T. Characteristics of polyphenolic content in brown algae of the Pacific Coast of Russia. Molecules 2020, 25, 3909. [Google Scholar] [CrossRef]
- López, A.; Rico, M.; Rivero, A.; Suárez De Tangil, M. The effects of solvents on the phenolic contents and antioxidant activity of Stypocaulon scoparium algae extracts. Food Chem. 2011, 125, 1104–1109. [Google Scholar] [CrossRef]
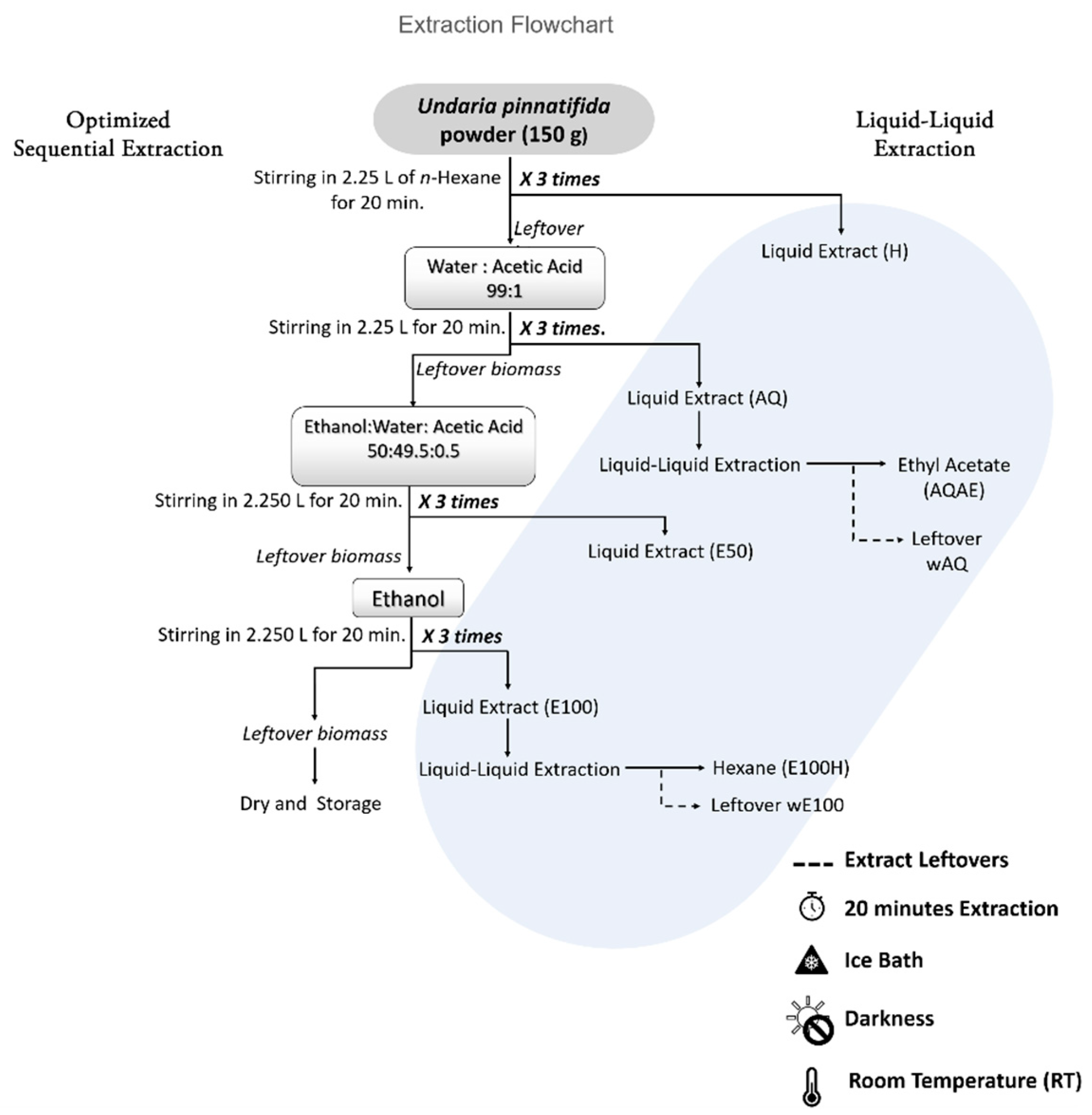

| Optimized Extraction Outputs | |||
|---|---|---|---|
| Extraction Solvent | Liquid–Liquid Solvent | Extract Name | Yield (%) |
| n-hexane | H | 0.5 | |
| Water:acetic acid (99:1) | Ethyl acetate | AQAE | 0.3 |
| Water:acetic acid (99:1) | wAQ | 21.9 | |
| Ethanol:water:acetic acid (50:49.5:0.5) | E50 | 6.1 | |
| Ethanol | n-hexane | E100H | 0.9 |
| Ethanol | wE100 | 1.1 | |
| Strains | Growth Inhibition (%) for Extracts at 1.5 mg·mL−1 | ||||||
|---|---|---|---|---|---|---|---|
| CP | H | AQAE | wAQ | E50 | E100H | wE100 | |
| S. aureus | 100.34 ± 3.16 a, b | 76.11 ± 12.64 a, b, c, d | 102.07 ± 1,53 a | 0.0 ± 26.15 f | 15.05 ± 9.99 e, f | 78.06 ± 9.06 a, b, c | 43.29 ± 2.85 d, e |
| P. aeruginosa | 101.17 ± 0.74 a | 38.99 ± 23.73 b | 0.0 ± 6.81 d | 1.75 ± 4.23 c | 0.0 ± 5.77 d | 0.0 ± 0.0 d | 44.79 ± 8.12 b |
| K. pneumoniae | 100.85 ± 3.75 a | 19.46 ± 5.03 c, d | 1.46 ± 1.08 e | 11.26 ± 0.53 c, d, e | 0.0 ± 1.57 e | 34.25 ± 7.81 b | 19.49 ± 5.42 c |
| E. coli | 99.93 ± 0,097 a | 40.98 ± 3.73 c | 16.24 ± 3.73 d | 25.59 ± 1.41d | 0.0 ± 2.56 e | 54.9 1± 4.80 b | 37.62 ± 4.44 c |
| P. mirabilis | 100.97 ± 0,95 a | 23.88 ± 3.24 c, d | 14.15 ± 3.83 d, e | 8.18 ± 1.71 e, f | 0.0 ± 3.45 f | 68.79 ± 6.95 b | 31.56 ± 1.04 c |
| WE100 and AQAE FTIR-ATR Spectra | ||||
|---|---|---|---|---|
| Extract | Main Bands | References | ||
| Wavenumber (cm−1) | Bond/Vibration | Functional Group | ||
| wE100 | 3366 | O-H stretch | Hydroxyl | [32,33,34,35,36,37] |
| 2922 | C-H stretch | Alkane | ||
| 2852 | ||||
| 1733 | C=O stretch | Aldehyde and esther | ||
| 1463 | C-H bend/C-C stretch | Alkane/Aromatic ring | ||
| 1168 | S=O stretch | Sulfonic acid hydrate | ||
| 1058 | C-O-C stretch | Unknown ether | ||
| 724 | C-H bond | Aromatic ring | ||
| AQAE | 3273 | O-H stretch | Hydroxyl | |
| 2933 | C-H stretch | Alkane | ||
| 2852 | ||||
| 1542 | N-H bend/C-N stretch | Protein amides | ||
| 1408 | S=O stretch | Sulfate | ||
| 1020 | C-O-C stretch | Unknown ether | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ferreira, C.A.M.; Félix, R.; Félix, C.; Januário, A.P.; Alves, N.; Novais, S.C.; Dias, J.R.; Lemos, M.F.L. A Biorefinery Approach to the Biomass of the Seaweed Undaria pinnatifida (Harvey Suringar, 1873): Obtaining Phlorotannins-Enriched Extracts for Wound Healing. Biomolecules 2021, 11, 461. https://doi.org/10.3390/biom11030461
Ferreira CAM, Félix R, Félix C, Januário AP, Alves N, Novais SC, Dias JR, Lemos MFL. A Biorefinery Approach to the Biomass of the Seaweed Undaria pinnatifida (Harvey Suringar, 1873): Obtaining Phlorotannins-Enriched Extracts for Wound Healing. Biomolecules. 2021; 11(3):461. https://doi.org/10.3390/biom11030461
Chicago/Turabian StyleFerreira, Carolina A. M., Rafael Félix, Carina Félix, Adriana P. Januário, Nuno Alves, Sara C. Novais, Juliana R. Dias, and Marco F. L. Lemos. 2021. "A Biorefinery Approach to the Biomass of the Seaweed Undaria pinnatifida (Harvey Suringar, 1873): Obtaining Phlorotannins-Enriched Extracts for Wound Healing" Biomolecules 11, no. 3: 461. https://doi.org/10.3390/biom11030461
APA StyleFerreira, C. A. M., Félix, R., Félix, C., Januário, A. P., Alves, N., Novais, S. C., Dias, J. R., & Lemos, M. F. L. (2021). A Biorefinery Approach to the Biomass of the Seaweed Undaria pinnatifida (Harvey Suringar, 1873): Obtaining Phlorotannins-Enriched Extracts for Wound Healing. Biomolecules, 11(3), 461. https://doi.org/10.3390/biom11030461













