Subnormothermic Perfusion with H2S Donor AP39 Improves DCD Porcine Renal Graft Outcomes in an Ex Vivo Model of Kidney Preservation and Reperfusion
Abstract
1. Introduction
2. Materials and Methods
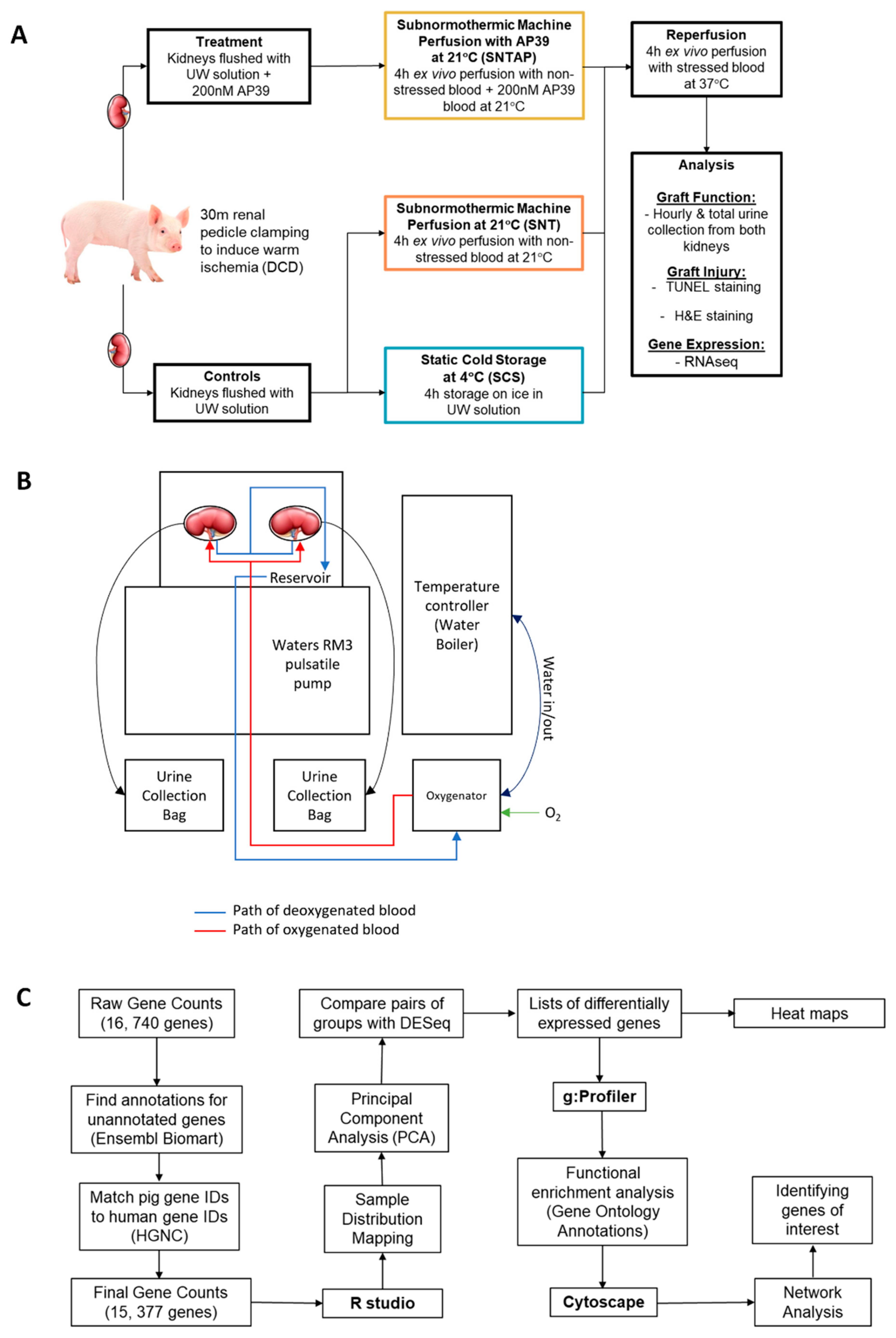
2.1. Animal Care and Surgery
2.2. Treatments and Ex Vivo Perfusion Setup
2.3. AP39
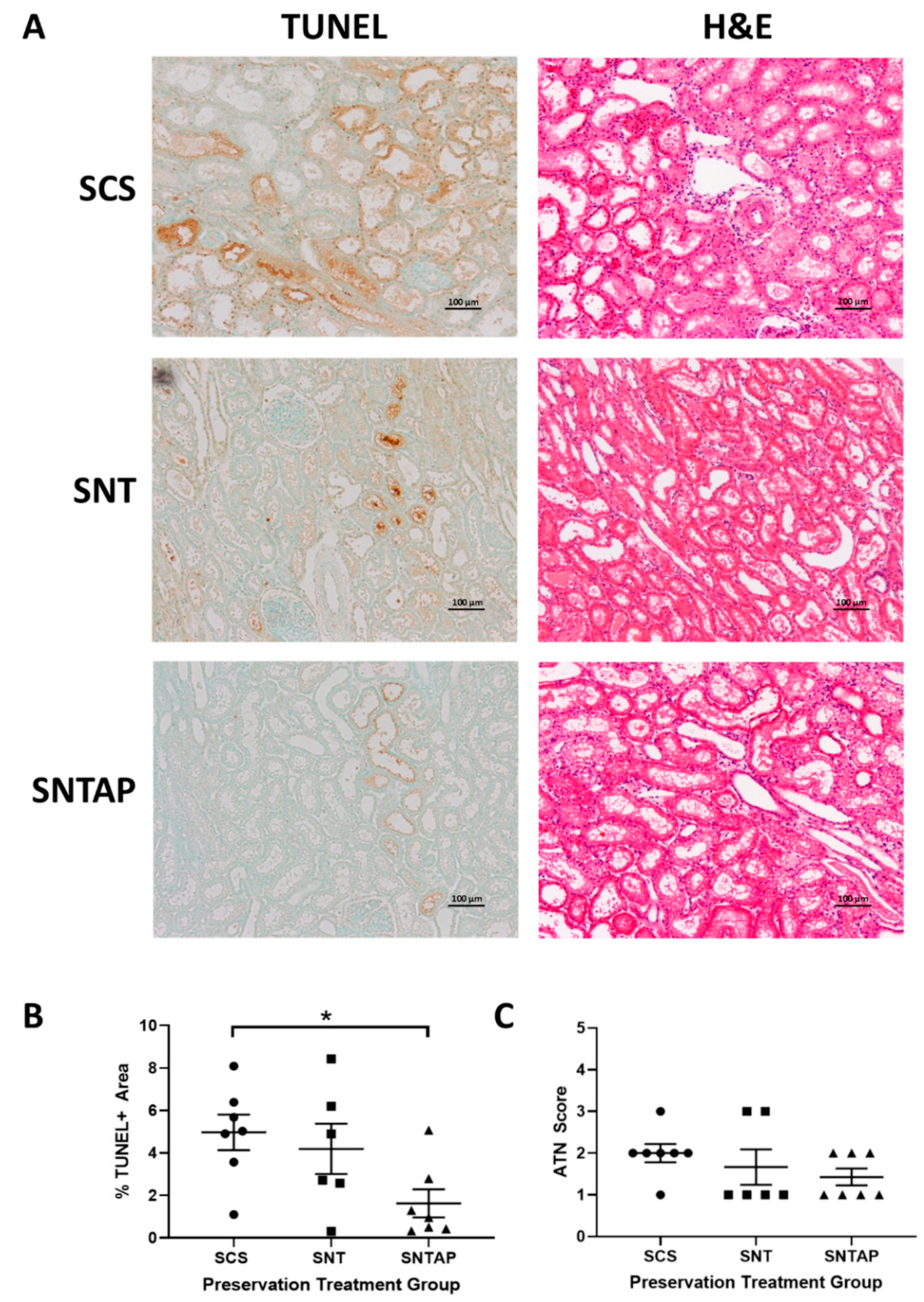
2.4. Histopathology Imaging and Quantification
2.5. Statistical Analysis
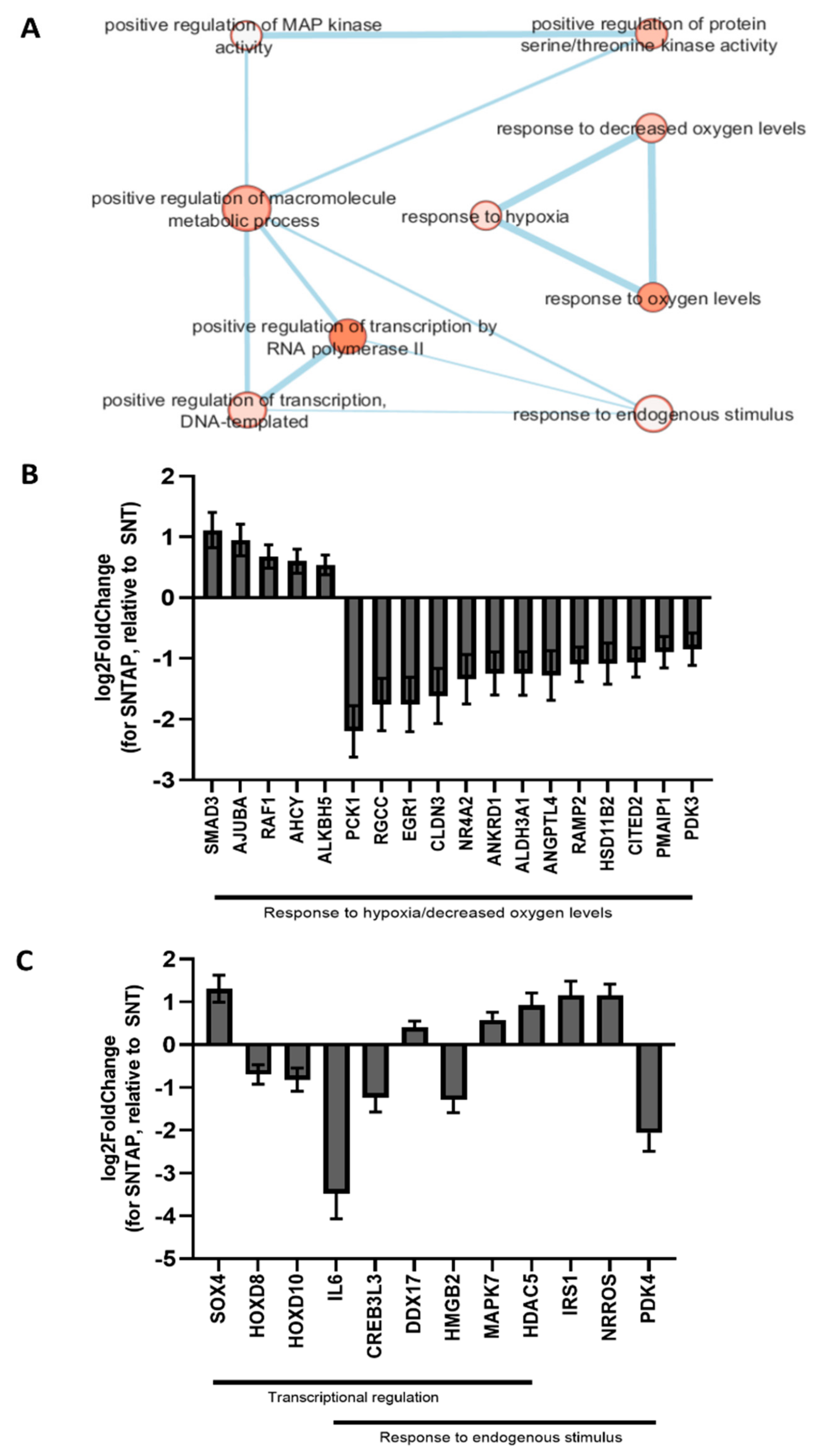
2.6. RNASeq
3. Results
3.1. Subnormothermic Perfusion with AP39-Supplemented Blood Improves Urine Output and Reduces Tissue Injury Compared to Static Cold Storage and Subnormothermic Perfusion without AP39
3.2. Adding AP39 to Blood During Subnormothermic Perfusion Leads to Differential Pro--Survival Gene Expression Patterns Compared to Static Cold Storage and Subnormothermic Perfusion without AP39
4. Discussion
5. Patent
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AJUBA | Lim-domain containing protein AJUBA |
| BAG3 | Bcl2-associated anthanogene (BAG) family chaperone regulator 3 |
| BCL10 | B-cell lymphoma/leukemia 10 |
| DDIT3 | DNA damage-inducible transcript 3 |
| EGR1 | Early growth response protein 1 |
| HOXD8/10 | Homeobox protein Hox-D8/10 |
| HSPA1A | Heat shock 70kDa protein 1A |
| HSPD1 | Heat shock protein 60kDa, mitochondrial |
| IL6 | Interleukin 6 |
| MAPK7 | Mitogen-activated protein kinase 7 |
| NRROS | Negative regulator of reactive oxygen species |
| PCK1 | Phosphoenolpyruvate carboxykinase, cytosolic |
| PDK3 | Pyruvate dehydrogenase kinase, mitochondrial |
| RGCC | Regulator of the cell cycle |
| SMAD3 | Mothers against decapentaplegic homolog 3 |
| TGF-β | Transforming growth factor β |
Appendix A
References
- Abe, K.; Kimura, H. The possible role of hydrogen sulfide as an endogenous neuromodulator. J. Neurosci. 1996, 16, 1066–1071. [Google Scholar] [CrossRef] [PubMed]
- Kimura, H. Hydrogen sulfide as a neuromodulator. Mol. Neurobiol. 2002, 26, 13–19. [Google Scholar] [CrossRef]
- Szabó, C. Hydrogen sulphide and its therapeutic potential. Nat. Rev. Drug Discov. 2007, 6, 917–935. [Google Scholar] [CrossRef] [PubMed]
- Wolfe, R.A.; Ashby, V.B.; Milford, E.L.; Ojo, A.O.; Ettenger, R.E.; Agodoa, L.Y.C.; Held, P.J.; Port, F.K. Comparison of Mortality in All Patients on Dialysis, Patients on Dialysis Awaiting Transplantation, and Recipients of a First Cadaveric Transplant. N. Engl. J. Med. 1999, 341, 1725–1730. [Google Scholar] [CrossRef] [PubMed]
- Hart, A.; Smith, J.M.; Skeans, M.A.; Gustafson, S.K.; Wilk, A.R.; Castro, S.; Robinson, A.; Wainright, J.L.; Snyder, J.J.; Kasiske, B.L.; et al. OPTN/SRTR 2017 Annual Data Report: Kidney. Am. J. Transplant. 2019, 19, 19–123. [Google Scholar] [CrossRef]
- Nemati, E.; Einollahi, B.; Pezeshki, M.L.; Porfarziani, V.; Fattahi, M.R. Does Kidney Transplantation With Deceased or Living Donor Affect Graft Survival? Nephrourol. Mon. 2014, 6. [Google Scholar] [CrossRef]
- Singh, R.P.; Farney, A.C.; Rogers, J.; Zuckerman, J.; Reeves-Daniel, A.; Hartmann, E.; Iskandar, S.; Adams, P.; Stratta, R.J. Kidney transplantation from donation after cardiac death donors: Lack of impact of delayed graft function on post-transplant outcomes. Clin. Transplant. 2011, 25, 255–264. [Google Scholar] [CrossRef]
- Akoh, J.A. Kidney donation after cardiac death. World J. Nephrol. 2012, 1, 79. [Google Scholar] [CrossRef]
- Tingle, S.J.; Figueiredo, R.S.; Moir, J.A.G.; Goodfellow, M.; Thompson, E.R.; Ibrahim, I.K.; Bates, L.; Talbot, D.; Wilson, C.H. Hypothermic machine perfusion is superior to static cold storage in deceased donor kidney transplantation: A meta-analysis. Clin. Transplant. 2020, 34, e13814. [Google Scholar] [CrossRef] [PubMed]
- Dion, M.S.; McGregor, T.B.; McAlister, V.C.; Luke, P.P.; Sener, A. Hypothermic machine perfusion improves Doppler ultrasonography resistive indices and long-term allograft function after renal transplantation: A single-centre analysis. BJU Int. 2015, 116, 932–937. [Google Scholar] [CrossRef]
- Lee, C.Y.; Mangino, M.J. Preservation methods for kidney and liver. Organogenesis 2009, 5, 105–112. [Google Scholar] [CrossRef] [PubMed]
- Guibert, E.E.; Petrenko, A.Y.; Balaban, C.L.; Somov, A.Y.; Rodriguez, J.V.; Fuller, B.J. Organ Preservation: Current Concepts and New Strategies for the Next Decade. Transfus. Med. Hemother. 2011, 38, 125–142. [Google Scholar] [CrossRef]
- Kayler, L.; Yu, X.; Cortes, C.; Lubetzky, M.; Friedmann, P. Impact of Cold Ischemia Time in Kidney Transplants From Donation After Circulatory Death Donors. Transplant. Direct. 2017, 3, e177. [Google Scholar] [CrossRef]
- Dugbartey, G.J.; Bouma, H.R.; Saha, M.N.; Lobb, I.; Henning, R.H.; Sener, A. A Hibernation-Like State for Transplantable Organs: Is Hydrogen Sulfide Therapy the Future of Organ Preservation? Antioxid. Redox Signal. 2018, 28, 1503–1515. [Google Scholar] [CrossRef] [PubMed]
- Lobb, I.; Jiang, J.; Lian, D.; Liu, W.; Haig, A.; Saha, M.N.; Torregrossa, R.; Wood, M.E.; Whiteman, M.; Sener, A. Hydrogen Sulfide Protects Renal Grafts Against Prolonged Cold Ischemia-Reperfusion Injury via Specific Mitochondrial Actions. Am. J. Transplant. 2017, 17, 341–352. [Google Scholar] [CrossRef]
- Lobb, I.; Davison, M.; Carter, D.; Liu, W.; Haig, A.; Gunaratnam, L.; Sener, A. Hydrogen Sulfide Treatment Mitigates Renal Allograft Ischemia-Reperfusion Injury during Cold Storage and Improves Early Transplant Kidney Function and Survival Following Allogeneic Renal Transplantation. J. Urol. 2015, 194, 1806–1815. [Google Scholar] [CrossRef]
- Juriasingani, S.; Akbari, M.; Luke, P.; Sener, A. Novel therapeutic strategies for renal graft preservation and their potential impact on the future of clinical transplantation. Curr. Opin. Organ Transplant. 2019, 24, 385–390. [Google Scholar] [CrossRef]
- Kaths, J.M.; Echeverri, J.; Chun, Y.M.; Cen, J.Y.; Goldaracena, N.; Linares, I.; Dingwell, L.S.; Yip, P.M.; John, R.; Bagli, D.; et al. Continuous Normothermic Ex Vivo Kidney Perfusion Improves Graft Function in Donation After Circulatory Death Pig Kidney Transplantation. Transplantation 2017, 101, 754–763. [Google Scholar] [CrossRef] [PubMed]
- Kaths, J.M.; Echeverri, J.; Linares, I.; Cen, J.Y.; Ganesh, S.; Hamar, M.; Urbanellis, P.; Yip, P.; John, R.; Bagli, D.; et al. Normothermic Ex Vivo Kidney Perfusion Following Static Cold Storage-Brief, Intermediate, or Prolonged Perfusion for Optimal Renal Graft Reconditioning? Am. J. Transplant. 2017, 17, 2580–2590. [Google Scholar] [CrossRef]
- Hosgood, S.A.; Nicholson, M.L. First in Man Renal Transplantation After Ex Vivo Normothermic Perfusion. Transplantation 2011, 92, 735–738. [Google Scholar] [CrossRef] [PubMed]
- Hoyer, D.P.; Gallinat, A.; Swoboda, S.; Wohlschläger, J.; Rauen, U.; Paul, A.; Minor, T. Subnormothermic machine perfusion for preservation of porcine kidneys in a donation after circulatory death model. Transpl. Int. 2014, 27, 1097–1106. [Google Scholar] [CrossRef]
- Juriasingani, S.; Akbari, M.; Chan, J.Y.; Whiteman, M.; Sener, A. H2S supplementation: A novel method for successful organ preservation at subnormothermic temperatures. Nitric Oxide 2018, 81, 57–66. [Google Scholar] [CrossRef]
- Bhattacharjee, R.N.; Ruthirakanthan, A.; Sun, Q.; Richard-Mohamed, M.; Luke, S.; Jiang, L.; Aquil, S.; Sharma, H.; Tun-Abraham, M.E.; Alharbi, B.; et al. Subnormothermic Oxygenated Perfusion Optimally Preserves Donor Kidneys Ex Vivo. Kidney Int. Rep. 2019, 4, 1323–1333. [Google Scholar] [CrossRef]
- Le Trionnaire, S.; Perry, A.; Szczesny, B.; Szabo, C.; Winyard, P.G.; Whatmore, J.L.; Wood, M.E.; Whiteman, M. The synthesis and functional evaluation of a mitochondria-targeted hydrogen sulfide donor, (10-oxo-10-(4-(3-thioxo-3H-1,2-dithiol-5-yl)phenoxy)decyl)triphenylphosphonium bromide (AP39). Med. Chem. Commun. 2014, 5, 728–736. [Google Scholar] [CrossRef]
- Reimand, J.; Isserlin, R.; Voisin, V.; Kucera, M.; Tannus-Lopes, C.; Rostamianfar, A.; Wadi, L.; Meyer, M.; Wong, J.; Xu, C.; et al. Pathway enrichment analysis and visualization of omics data using g:Profiler, GSEA, Cytoscape and EnrichmentMap. Nat. Protoc. 2019, 14, 482–517. [Google Scholar] [CrossRef] [PubMed]
- Maathuis, M.H.J.; de Groot, M.; Ploeg, R.J.; Leuvenink, H.G.D. Deterioration of Endothelial and Smooth Muscle Cell Function in DCD Kidneys After Static Cold Storage in IGL-1 or UW. J. Surg. Res. 2009, 152, 231–237. [Google Scholar] [CrossRef]
- Xia, M.; Chen, L.; Muh, R.W.; Li, P.-L.; Li, N. Production and Actions of Hydrogen Sulfide, a Novel Gaseous Bioactive Substance, in the Kidneys. J. Pharmacol. Exp. Ther. 2009, 329, 1056–1062. [Google Scholar] [CrossRef] [PubMed]
- Hosgood, S.A.; Nicholson, M.L. Hydrogen sulphide ameliorates ischaemia-reperfusion injury in an experimental model of non-heart-beating donor kidney transplantation. Br. J. Surg. 2010, 97, 202–209. [Google Scholar] [CrossRef]
- Zagli, G.; Patacchini, R.; Trevisani, M.; Abbate, R.; Cinotti, S.; Gensini, G.F.; Masotti, G.; Geppetti, P. Hydrogen sulfide inhibits human platelet aggregation. Eur. J. Pharmacol. 2007, 559, 65–68. [Google Scholar] [CrossRef] [PubMed]
- Grambow, E.; Mueller-Graf, F.; Delyagina, E.; Frank, M.; Kuhla, A.; Vollmar, B. Effect of the hydrogen sulfide donor GYY4137 on platelet activation and microvascular thrombus formation in mice. Platelets 2014, 25, 166–174. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, A.; Olah, G.; Szczesny, B.; Wood, M.E.; Whiteman, M.; Szabo, C. AP39, A Mitochondrially Targeted Hydrogen Sulfide Donor, Exerts Protective Effects in Renal Epithelial Cells Subjected to Oxidative Stress in Vitro and in Acute Renal Injury in Vivo. Shock 2016, 45, 88–97. [Google Scholar] [CrossRef]
- Zhu, C.; Su, Y.; Juriasingani, S.; Zheng, H.; Veramkovich, V.; Jiang, J.; Sener, A.; Whiteman, M.; Lacefield, J.; Nagpal, D.; et al. Supplementing preservation solution with mitochondria-targeted H2S donor AP39 protects cardiac grafts from prolonged cold ischemia–reperfusion injury in heart transplantation. Am. J. Transplant. 2019, 19, 3139–3148. [Google Scholar] [CrossRef]
- Chiarini, A.; Liu, D.; Armato, U.; Dal Prà, I. Bcl10 crucially nucleates the pro-apoptotic complexes comprising PDK1, PKCζ and caspase-3 at the nuclear envelope of etoposide-treated human cervical carcinoma C4-I cells. Int. J. Mol. Med. 2015, 36, 845–856. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Simms, L.A.; Young, J.; Wicking, C.; Meltzer, S.J.; Jass, J.R.; Leggett, B.A. The apoptotic regulatory gene, BCL10, is mutated in sporadic mismatch repair deficient colorectal cancers. Cell Death Differ. 2000, 7, 236–237. [Google Scholar] [CrossRef]
- Li, T.; Su, L.; Lei, Y.; Liu, X.; Zhang, Y.; Liu, X. DDIT3 and KAT2A proteins regulate TNFRSF10A and TNFRSF10B expression in endoplasmic reticulum stress-mediated apoptosis in human lung cancer cells. J. Biol. Chem. 2015, 290, 11108–11118. [Google Scholar] [CrossRef] [PubMed]
- Franceschelli, S.; Rosati, A.; Lerose, R.; De Nicola, S.; Turco, M.C.; Pascale, M. Bag3 gene expression is regulated by heat shock factor 1. J. Cell. Physiol. 2008, 215, 575–577. [Google Scholar] [CrossRef]
- Zhang, Z.; Fan, J.; Becker, K.G.; Graff, R.D.; Lee, G.M.; Francomano, C.A. Comparison of gene expression profile between human chondrons and chondrocytes: A cDNA microarray study. Osteoarthr. Cartil. 2006, 14, 449–459. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Dokladny, K.; Moseley, P.L.; Ma, T.Y. Physiologically relevant increase in temperature causes an increase in intestinal epithelial tight junction permeability. Am. J. Physiol. Gastrointest. Liver Physiol. 2006, 290. [Google Scholar] [CrossRef] [PubMed]
- Tesser-Gamba, F.; Lopes, L.J.d.S.; Petrilli, A.S.; Toledo, S.R.C. MAPK7 gene controls proliferation, migration and cell invasion in osteosarcoma. Mol. Carcinog. 2016, 55, 1700–1713. [Google Scholar] [CrossRef] [PubMed]
- Shu, S.; Wang, Y.; Zheng, M.; Liu, Z.; Cai, J.; Tang, C.; Dong, Z. Hypoxia and Hypoxia-Inducible Factors in Kidney Injury and Repair. Cells 2019, 8, 207. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.H.; Xia, D.; Kim, S.W.; Holla, V.; Menter, D.G.; DuBois, R.N. Human enhancer of filamentation 1 is a mediator of hypoxia-inducible factor-1α-mediated migration in colorectal carcinoma cells. Cancer Res. 2010, 70, 4054–4063. [Google Scholar] [CrossRef] [PubMed]
- Prigione, A.; Rohwer, N.; Hoffmann, S.; Mlody, B.; Drews, K.; Bukowiecki, R.; Blümlein, K.; Wanker, E.E.; Ralser, M.; Cramer, T.; et al. HIF1α modulates cell fate reprogramming through early glycolytic shift and upregulation of PDK1-3 and PKM2. Stem Cells 2014, 32, 364–376. [Google Scholar] [CrossRef] [PubMed]
- Ai, H.; Yang, B.; Li, J.; Xie, X.; Chen, H.; Ren, J. Population history and genomic signatures for high-altitude adaptation in Tibetan pigs. BMC Genom. 2014, 15, 834. [Google Scholar] [CrossRef]
- Su, T.; Liu, P.; Ti, X.; Wu, S.; Xue, X.; Wang, Z.; Dioum, E.; Zhang, Q. HIF1α, EGR1 and SP1 co-regulate the erythropoietin receptor expression under hypoxia: An essential role in the growth of non-small cell lung cancer cells. Cell Commun. Signal. 2019, 17, 152. [Google Scholar] [CrossRef]
- Foxler, D.E.; Bridge, K.S.; James, V.; Webb, T.M.; Mee, M.; Wong, S.C.K.; Feng, Y.; Constantin-Teodosiu, D.; Petursdottir, T.E.; Bjornsson, J.; et al. The LIMD1 protein bridges an association between the prolyl hydroxylases and VHL to repress HIF-1 activity. Nat. Cell Biol. 2012, 14, 201–208. [Google Scholar] [CrossRef]
- Yang, H.; Zhou, J.; Mi, J.; Ma, K.; Fan, Y.; Ning, J.; Wang, C.; Wei, X.; Zhao, H.; Li, E. HOXD10 acts as a tumor-suppressive factor via inhibition of the RHOC/AKT/MAPK pathway in human cholangiocellular carcinoma. Oncol. Rep. 2015, 34, 1681–1691. [Google Scholar] [CrossRef] [PubMed]
- Mansour, M.A.; Senga, T. HOXD8 exerts a tumor-suppressing role in colorectal cancer as an apoptotic inducer. Int. J. Biochem. Cell Biol. 2017, 88, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Noubade, R.; Wong, K.; Ota, N.; Rutz, S.; Eidenschenk, C.; Valdez, P.A.; Ding, J.; Peng, I.; Sebrell, A.; Caplazi, P.; et al. NRROS negatively regulates reactive oxygen species during host defence and autoimmunity. Nature 2014, 509, 235–239. [Google Scholar] [CrossRef] [PubMed]
- Qin, Y.; Garrison, B.S.; Ma, W.; Rossi, D.J.; Lu, C.; Correspondence, T.A.S. A Milieu Molecule for TGF-β Required for Microglia Function in the Nervous System. Cell 2018, 174, 156–171.e16. [Google Scholar] [CrossRef] [PubMed]
- Sureshbabu, A.; Muhsin, S.A.; Choi, M.E. TGF-β signaling in the kidney: Profibrotic and protective effects. Am. J. Physiol. Ren. Physiol. 2016, 310, F596–F606. [Google Scholar] [CrossRef] [PubMed]
- Su, H.; Lei, C.T.; Zhang, C. Interleukin-6 signaling pathway and its role in kidney disease: An update. Front. Immunol. 2017, 8, 405. [Google Scholar] [CrossRef] [PubMed]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Juriasingani, S.; Ruthirakanthan, A.; Richard-Mohamed, M.; Akbari, M.; Aquil, S.; Patel, S.; Al-Ogaili, R.; Whiteman, M.; Luke, P.; Sener, A. Subnormothermic Perfusion with H2S Donor AP39 Improves DCD Porcine Renal Graft Outcomes in an Ex Vivo Model of Kidney Preservation and Reperfusion. Biomolecules 2021, 11, 446. https://doi.org/10.3390/biom11030446
Juriasingani S, Ruthirakanthan A, Richard-Mohamed M, Akbari M, Aquil S, Patel S, Al-Ogaili R, Whiteman M, Luke P, Sener A. Subnormothermic Perfusion with H2S Donor AP39 Improves DCD Porcine Renal Graft Outcomes in an Ex Vivo Model of Kidney Preservation and Reperfusion. Biomolecules. 2021; 11(3):446. https://doi.org/10.3390/biom11030446
Chicago/Turabian StyleJuriasingani, Smriti, Aushanth Ruthirakanthan, Mahms Richard-Mohamed, Masoud Akbari, Shahid Aquil, Sanjay Patel, Rafid Al-Ogaili, Matthew Whiteman, Patrick Luke, and Alp Sener. 2021. "Subnormothermic Perfusion with H2S Donor AP39 Improves DCD Porcine Renal Graft Outcomes in an Ex Vivo Model of Kidney Preservation and Reperfusion" Biomolecules 11, no. 3: 446. https://doi.org/10.3390/biom11030446
APA StyleJuriasingani, S., Ruthirakanthan, A., Richard-Mohamed, M., Akbari, M., Aquil, S., Patel, S., Al-Ogaili, R., Whiteman, M., Luke, P., & Sener, A. (2021). Subnormothermic Perfusion with H2S Donor AP39 Improves DCD Porcine Renal Graft Outcomes in an Ex Vivo Model of Kidney Preservation and Reperfusion. Biomolecules, 11(3), 446. https://doi.org/10.3390/biom11030446





