On the Search of a Silver Bullet for the Preparation of Bioinspired Molecular Electrets with Propensity to Transfer Holes at High Potentials
Abstract
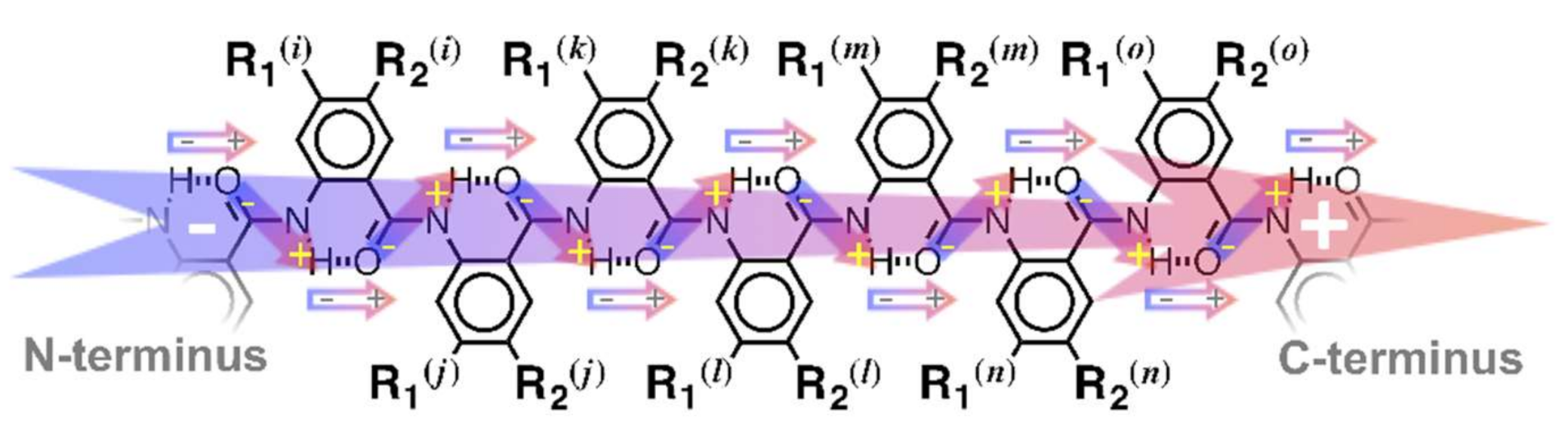
1. Introduction
2. Results and Discussion
2.1. Choice of Substrates
2.2. Synthesis Design
2.3. Testing Beyond Ag2O
2.4. Microwave vs. Conventional Heating
2.5. Box-Containing Oligomers
3. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Derr, J.B.; Tamayo, J.; Clark, J.A.; Morales, M.; Mayther, M.F.; Espinoza, E.M.; Rybicka-Jasinska, K.; Vullev, V.I. Multifaceted aspects of charge transfer. Phys. Chem. Chem. Phys. 2020, 22, 21583–21629. [Google Scholar] [CrossRef]
- Vullev, V.I. From Biomimesis to Bioinspiration: What’s the Benefit for Solar Energy Conversion Applications? J. Phys. Chem. Lett. 2011, 2, 503–508. [Google Scholar] [CrossRef]
- Marcus, R.A.; Sutin, N. Electron Transfers in Chemistry and Biology. Biochim. Biophys. Acta 1985, 811, 265–322. [Google Scholar] [CrossRef]
- Beratan, D.N.; Betts, J.N.; Onuchic, J.N. Protein Electron-Transfer Rates Set by the Bridging Secondary and Tertiary Structure. Science 1991, 252, 1285–1288. [Google Scholar] [CrossRef] [PubMed]
- Beratan, D.N.; Onuchic, J.N.; Winkler, J.R.; Gray, H.B. Electron-Tunneling Pathways in Proteins. Science 1992, 258, 1740–1741. [Google Scholar] [CrossRef] [PubMed]
- Gray, H.B.; Winkler, J.R. Electron tunneling through proteins. Q. Rev. Biophys. 2003, 36, 341–372. [Google Scholar] [CrossRef] [PubMed]
- Skourtis, S.S.; Balabin, I.A.; Kawatsu, T.; Beratan, D.N. Protein dynamics and electron transfer: Electronic decoherence and non-Condon effects. Proc. Natl. Acad. Sci. USA 2005, 102, 3552–3557. [Google Scholar] [CrossRef]
- Beratan, D.N.; Liu, C.R.; Migliore, A.; Polizzi, N.F.; Skourtis, S.S.; Zhang, P.; Zhang, Y.Q. Charge Transfer in Dynamical Biosystems, or The Treachery of (Static) Images. Acc. Chem. Res. 2015, 48, 474–481. [Google Scholar] [CrossRef] [PubMed]
- Ing, N.L.; El-Naggar, M.Y.; Hochbaum, A.I. Going the Distance: Long-Range Conductivity in Protein and Peptide Bioelectronic Materials. J. Phys. Chem. B 2018, 122, 10403–10423. [Google Scholar] [CrossRef]
- Derr, J.B.; Tamayo, J.; Espinoza, E.M.; Clark, J.A.; Vullev, V.I. Dipole-induced effects on charge transfer and charge transport. Why do molecular electrets matter? Can. J. Chem. 2018, 96, 843–858. [Google Scholar] [CrossRef]
- Marcus, R.A. Exchange reactions and electron transfer reactions including isotopic exchange. Theory of oxidation-reduction reactions involving electron transfer. Part 4—A statistical-mechanical basis for treating contributions from solvent, ligands, and inert salt. Discuss. Faraday Soc. 1960, 21–31. [Google Scholar] [CrossRef]
- Yomosa, S. Charge-Transfer Molecular Compounds in Biological Systems. Sup. Prog. Theor. Phys. 1967, 40, 249–263. [Google Scholar] [CrossRef]
- Steffen, M.A.; Lao, K.Q.; Boxer, S.G. Dielectric Asymmetry in the Photosynthetic Reaction-Center. Science 1994, 264, 810–816. [Google Scholar] [CrossRef] [PubMed]
- Galoppini, E.; Fox, M.A. Effect of the electric field generated by the helix dipole on photoinduced intramolecular electron transfer in dichromophoric alpha-helical peptides. J. Am. Chem. Soc. 1996, 118, 2299–2300. [Google Scholar] [CrossRef]
- Fox, M.A.; Galoppini, E. Electric field effects on electron transfer rates in dichromophoric peptides: The effect of helix unfolding. J. Am. Chem. Soc. 1997, 119, 5277–5285. [Google Scholar] [CrossRef]
- Knorr, A.; Galoppini, E.; Fox, M.A. Photoinduced intramolecular electron transfer in dichromophore-appended alpha-helical peptides: Spectroscopic properties and preferred conformations. J. Phys. Org. Chem. 1997, 10, 484–498. [Google Scholar] [CrossRef]
- Xia, B.; Bao, D.; Upadhyayula, S.; Jones, G.; Vullev, V.I. Anthranilamides as Bioinspired Molecular Electrets: Experimental Evidence for a Permanent Ground-State Electric Dipole Moment. J. Org. Chem. 2013, 78, 1994–2004. [Google Scholar] [CrossRef]
- Bao, D.; Upadhyayula, S.; Larsen, J.M.; Xia, B.; Georgieva, B.; Nunez, V.; Espinoza, E.M.; Hartman, J.D.; Wurch, M.; Chang, A.; et al. Dipole-Mediated Rectification of Intramolecular Photoinduced Charge Separation and Charge Recombination. J. Am. Chem. Soc. 2014, 136, 12966–12973. [Google Scholar] [CrossRef]
- Ashraf, M.K.; Pandey, R.R.; Lake, R.K.; Millare, B.; Gerasimenko, A.A.; Bao, D.; Vullev, V.I. Theoretical design of bioinspired macromolecular electrets based on anthranilamide derivatives. Biotechnol. Prog. 2009, 25, 915–922. [Google Scholar] [CrossRef] [PubMed]
- Larsen, J.M.; Espinoza, E.M.; Hartman, J.D.; Lin, C.-K.; Wurch, M.; Maheshwari, P.; Kaushal, R.K.; Marsella, M.J.; Beran, G.J.O.; Vullev, V.I. Building blocks for bioinspired electrets: Molecular-level approach to materials for energy and electronics. Pure Appl. Chem. 2015, 87, 779–792. [Google Scholar] [CrossRef]
- Larsen, J.M.; Espinoza, E.M.; Vullev, V.I. Bioinspired molecular electrets: Bottom-up approach to energy materials and applications. J. Photon. Energy 2015, 5, 055598. [Google Scholar] [CrossRef]
- Espinoza, E.M.; Larsen, J.M.; Vullev, V.I. What Makes Oxidized N-Acylanthranilamides Stable? J. Phys. Chem. Lett. 2016, 7, 758–764. [Google Scholar] [CrossRef]
- Espinoza, E.M.; Larsen-Clinton, J.M.; Krzeszewski, M.; Darabedian, N.; Gryko, D.T.; Vullev, V.I. Bioinspired Approach Toward Molecular Electrets: Synthetic Proteome for Materials. Pure Appl. Chem. 2017, 89, 1777–1797. [Google Scholar] [CrossRef]
- Larsen-Clinton, J.M.; Espinoza, E.M.; Mayther, M.F.; Clark, J.; Tao, C.; Bao, D.; Larino, C.M.; Wurch, M.; Lara, S.; Vullev, V.I. Fluorinated aminoanthranilamides: Non-native amino acids for bringing proteomic approaches to charge-transfer systems. Phys. Chem. Chem. Phys. 2017, 19, 7871–7876. [Google Scholar] [CrossRef] [PubMed]
- Krzeszewski, M.; Espinoza, E.M.; Cervinka, C.; Derr, J.B.; Clark, J.A.; Borchardt, D.; Beran, G.J.O.; Gryko, D.T.; Vullev, V.I. Dipole Effects on Electron Transfer are Enormous. Angew. Chem. Int. Ed. 2018, 57, 12365–12369. [Google Scholar] [CrossRef] [PubMed]
- Espinoza, E.M.; Bao, D.; Krzeszewski, M.; Gryko, D.T.; Vullev, V.I. Is it common for charge recombination to be faster than charge separation? Int. J. Chem. Kinet. 2019, 51, 657–668. [Google Scholar] [CrossRef]
- Derr, J.B.; Clark, J.A.; Morales, M.; Espinoza, E.M.; Vadhin, S.; Vullev, V.I. Solvent-induced selectivity of Williamson etherification in the pursuit of amides resistant against oxidative degradation. RSC Adv. 2020, 10, 24419–24424. [Google Scholar] [CrossRef]
- Rybicka-Jasinska, K.; Vullev, V.I. Molecular electrets—Why do dipoles matter for charge transfer and excited-state dynamics? J. Photochem. Photobiol. A 2020, 401, 112779. [Google Scholar] [CrossRef]
- Skonieczny, K.; Espinoza, E.M.; Derr, J.B.; Morales, M.; Clinton, J.M.; Xia, B.; Vullev, V.I. Biomimetic and bioinspired molecular electrets. How to make them and why does the established peptide chemistry not always work? Pure Appl. Chem. 2020, 92, 275–299. [Google Scholar] [CrossRef]
- Shin, Y.-G.K.; Newton, M.D.; Isied, S.S. Distance Dependence of Electron Transfer Across Peptides with Different Secondary Structures: The Role of Peptide Energetics and Electronic Coupling. J. Am. Chem. Soc. 2003, 125, 3722–3732. [Google Scholar] [CrossRef]
- Upadhyayula, S.; Bao, D.; Millare, B.; Sylvia, S.S.; Habib, K.M.M.; Ashraf, K.; Ferreira, A.; Bishop, S.; Bonderer, R.; Baqai, S.; et al. Permanent Electric Dipole Moments of Carboxyamides in Condensed Media: What Are the Limitations of Theory and Experiment? J. Phys. Chem. B 2011, 115, 9473–9490. [Google Scholar] [CrossRef]
- Yasutomi, S.; Morita, T.; Imanishi, Y.; Kimura, S. A Molecular Photodiode System That Can Switch Photocurrent Direction. Science 2004, 304, 1944–1947. [Google Scholar] [CrossRef]
- Cordes, M.; Kottgen, A.; Jasper, C.; Jacques, O.; Boudebous, H.; Giese, B. Influence of amino acid side chains on long-distance electron transfer in peptides: Electron hopping via "Stepping Stones". Angew. Chem. Int. Edit. 2008, 47, 3461–3463. [Google Scholar] [CrossRef] [PubMed]
- Shlizerman, C.; Atanassov, A.; Berkovich, I.; Ashkenasy, G.; Ashkenasy, N. De Novo Designed Coiled-Coil Proteins with Variable Conformations as Components of Molecular Electronic Devices. J. Am. Chem. Soc. 2010, 132, 5070–5076. [Google Scholar] [CrossRef]
- Becucci, L.; Guryanov, I.; Maran, F.; Guidelli, R. Effect of a Strong Interfacial Electric Field on the Orientation of the Dipole Moment of Thiolated Aib-Oligopeptides Tethered to Mercury on Either the N- or C-Terminus. J. Am. Chem. Soc. 2010, 132, 6194–6204. [Google Scholar] [CrossRef]
- Chen, Y.; Viereck, J.; Harmer, R.; Rangan, S.; Bartynski, R.A.; Galoppini, E. Helical peptides design for molecular dipoles functionalization of wide band gap oxides. J. Am. Chem. Soc. 2020, 142, 3489–3498. [Google Scholar] [CrossRef] [PubMed]
- Gray, H.B.; Winkler, J.R. Long-range electron transfer. Proc. Natl. Acad. Sci. USA 2005, 102, 3534–3539. [Google Scholar] [CrossRef]
- Vullev, V.I.; Jones, G., II. Photoinduced charge transfer in helical polypeptides. Res. Chem. Intermed. 2002, 28, 795–815. [Google Scholar] [CrossRef]
- Jones, G., II; Lu, L.N.; Vullev, V.; Gosztola, D.; Greenfield, S.; Wasielewski, M. Photoactive peptides. 6. Photoinduced electron transfer for pyrenesulfonamide conjugates of tryptophan-containing peptides. Mitigation of fluoroprobe behavior in N-terminal labeling experiments. Bioorg. Med. Chem. Lett. 1995, 5, 2385–2390. [Google Scholar] [CrossRef]
- O’Donnell, J.F.; Mann, C.K. Controlled-Potential Oxidation of Aliphatic Amides. J. Electroanal. Chem. Interf. Electrochem. 1967, 13, 157–162. [Google Scholar] [CrossRef]
- Espinoza, E.M.; Clark, J.A.; Soliman, J.; Derr, J.B.; Morales, M.; Vullev, V.I. Practical Aspects of Cyclic Voltammetry: How to Estimate Reduction Potentials When Irreversibility Prevails. J. Electrochem. Soc. 2019, 166, H3175–H3187. [Google Scholar] [CrossRef]
- Venkatramani, R.; Keinan, S.; Balaeff, A.; Beratan, D.N. Nucleic acid charge transfer: Black, white and gray. Coord. Chem. Rev. 2011, 255, 635–648. [Google Scholar] [CrossRef] [PubMed]
- Guo, S.; Bao, D.; Upadhyayula, S.; Wang, W.; Guvenc, A.B.; Kyle, J.R.; Hosseinibay, H.; Bozhilov, K.N.; Vullev, V.I.; Ozkan, C.S.; et al. Photoinduced Electron Transfer Between Pyridine Coated Cadmium Selenide Quantum Dots and Single Sheet Graphene. Adv. Funct. Mater. 2013, 23, 5199–5211. [Google Scholar] [CrossRef]
- Lu, H.; Bao, D.; Penchev, M.; Ghazinejad, M.; Vullev, V.I.; Ozkan, C.S.; Ozkan, M. Pyridine-coated lead sulfide quantum dots for polymer hybrid photovoltaic devices. Adv. Sci. Lett. 2010, 3, 101–109. [Google Scholar] [CrossRef]
- Purdie, T.; Irvine, J.C. The alkylation of sugars. J. Chem. Soc. 1903, 83, 1021–1037. [Google Scholar] [CrossRef]
- Wurtz, A. Note sur un nouveau mode de formation de l’éther ordinaire et de ses homologues. Ann. Chim. Phys. 1856, 46, 222–226. [Google Scholar]
- Lander, G.D. LXIV—Alkylation by means of dry silver oxide and alkyl halides. J. Chem. Soc. Trans. 1900, 77, 729–753. [Google Scholar] [CrossRef][Green Version]
- Thiem, J.; Wessel, H.-P. Reaktionen von Benzyliden-D-tetrosen. Liebigs Ann. Chem. 1982, 595–606. [Google Scholar] [CrossRef]
- Sauthier, M.; Mortreux, A.; Suisse, I. From conventional to greener catalytic approaches for carbohydrates etherification. Carbohydr. Chem. R. Soc. Chem. 2014, 40, 73–98. [Google Scholar]
- Hudson, C.S. Relations between rotatory power and structure in the sugar groups. XIV. The determination of ring structures in the glucose, mannose and rhamnose series. J. Am. Chem. Soc. 1926, 48, 1434–1443. [Google Scholar] [CrossRef]
- Sugihara, J.M.; Wolfrom, M.L. Maltotriose and its crystalline β-D-hendecaacetate. J. Am. Chem. Soc. 1949, 71, 3357–3359. [Google Scholar] [CrossRef]
- Whistler, R.L.; Kazeniac, S.J. Preparation of crystalline methyl 4-O-methyl-α-D-glucopyranoside and its triacetate. J. Am. Chem. Soc. 1954, 76, 3044–3045. [Google Scholar] [CrossRef]
- Purdie, T.; Irvine, J.C. The stereoisomeric tetramethyl methylglucosides and tetramethyl glucose. J. Chem. Soc. 1904, 85, 1049–1070. [Google Scholar] [CrossRef]
- Mahmud, I.; Garrett, T.J. Mass Spectrometry Techniques in Emerging Pathogens Studies: COVID-19 Perspectives. J. Am. Soc. Mass Spectrom. 2020, 31, 2013–2024. [Google Scholar] [CrossRef]
- Cipollo, J.F.; Parsons, L.M. Glycomics and glycoproteomics of viruses: Mass spectrometry applications and insights toward structure-function relationships. Mass Spectrom. Rev. 2020, 39, 371–409. [Google Scholar] [CrossRef] [PubMed]
- Smith, D.F.; Cummings, R.D.; Song, X. History and future of shotgun glycomics. Biochem. Soc. Trans. 2019, 47, 1–11. [Google Scholar] [CrossRef]
- Nurcombe, V.; Ling, L.; Hondermarck, H.; Cool, S.M.; Smith, R.A.A. Bringing Heparan Sulfate Glycomics Together with Proteomics for the Design of Novel Therapeutics: A Historical Perspective. Proteomics 2019, 19, 1800466. [Google Scholar] [CrossRef] [PubMed]
- Sureshan, K.M.; Das, T.; Shashidhar, M.S.; Gonnade, R.G.; Bhadbhade, M.M. Sulfonate protecting groups: Synthesis of D- and L-myo-inositol-1,3,4,5-tetrakisphosphate precursors by a novel silver(I) oxide-mediated O-alkylation of 2,4(6)-di-O-acyl-6(4)-O-sulfonyl-myo-inositol 1,3,5-orthoformate derivatives through intramolecular assistance of the sulfonyl group. Eur. J. Org. Chem. 2003, 1035–1041. [Google Scholar]
- Ren, B.; Lv, J.; Zhang, Y.; Tian, J.; Dong, H. Highly Efficient Selective Benzylation of Carbohydrates Catalyzed by Iron(III) with Silver Oxide and Bromide Anion as Co-catalysts. ChemCatChem 2017, 9, 950–953. [Google Scholar] [CrossRef]
- Das, T.; Shashidhar, M.S. Silver(I) oxide assisted O-alkylation of 2,3-di-O-benzyl-myo-inositol-1,3,5-orthoformate and its 6-O-substituted derivatives: Transannular participation of oxygen. Carbohydr. Res. 1997, 297, 243–249. [Google Scholar] [CrossRef]
- Harbury, P.B.; Zhang, T.; Kim, P.S.; Alber, T. A Switch between 2-Stranded, 3-Stranded and 4-Stranded Coiled Coils in Gcn4 Leucine-Zipper Mutants. Science 1993, 262, 1401–1407. [Google Scholar] [CrossRef]
- Robertson, D.E.; Farid, R.S.; Moser, C.C.; Urbauer, J.L.; Mulholland, S.E.; Pidikiti, R.; Lear, J.D.; Wand, A.J.; DeGrado, W.F.; Dutton, P.L. Design and synthesis of multi-heme proteins. Nature 1994, 368, 425–432. [Google Scholar] [CrossRef]
- Shoemaker, K.R.; Kim, P.S.; York, E.J.; Stewart, J.M.; Baldwin, R.L. Tests of the helix dipole model for stabilization of α-helices. Nature 1987, 326, 563–567. [Google Scholar] [CrossRef]
- Kerppola, T.; Curran, T. Zen and the art of Fos and Jun. Nature 1995, 373, 199–200. [Google Scholar] [CrossRef][Green Version]
- Rabanal, F.; DeGrado, W.F.; Dutton, P.L. Toward the synthesis of a photosynthetic reaction center maquette: A cofacial porphyrin pair assembled between two subunits of a synthetic four-helix bundle multiheme protein. J. Am. Chem. Soc. 1996, 118, 473–474. [Google Scholar] [CrossRef]
- Jones, G., II; Vullev, V.; Braswell, E.H.; Zhu, D. Multistep Photoinduced Electron Transfer in a de Novo Helix Bundle: Multimer Self-Assembly of Peptide Chains Including a Chromophore Special Pair. J. Am. Chem. Soc. 2000, 122, 388–389. [Google Scholar] [CrossRef]
- Kornilova, A.Y.; Wishart, J.F.; Xiao, W.; Lasey, R.C.; Fedorova, A.; Shin, Y.-K.; Ogawa, M.Y. Design and Characterization of A Synthetic Electron-Transfer Protein. J. Am. Chem. Soc. 2000, 122, 7999–8006. [Google Scholar] [CrossRef][Green Version]
- Jones, G., II; Vullev, V.I. Contribution of a Pyrene Fluorescence Probe to the Aggregation Propensity of Polypeptides. Org. Lett. 2001, 3, 2457–2460. [Google Scholar] [CrossRef] [PubMed]
- Jones, G., II; Vullev, V.I. Photoinduced Electron Transfer between Non-Native Donor-Acceptor Moieties Incorporated in Synthetic Polypeptide Aggregates. Org. Lett. 2002, 4, 4001–4004. [Google Scholar] [CrossRef] [PubMed]
- Vullev, V.I.; Jones, G. Photoinduced electron transfer in alkanoylpyrene aggregates in conjugated polypeptides. Tetrahedron Lett. 2002, 43, 8611–8615. [Google Scholar] [CrossRef]
- Fedorova, A.; Chaudhari, A.; Ogawa, M.Y. Photoinduced electron-transfer along α-helical and coiled-coil metallopeptides. J. Am. Chem. Soc. 2003, 125, 357–362. [Google Scholar] [CrossRef]
- Jones, G., II; Zhou, X.; Vullev, V.I. Photoinduced electron transfer in alpha -helical polypeptides: Dependence on conformation and electron donor-acceptor distance. Photochem. Photobiol. Sci. 2003, 2, 1080–1087. [Google Scholar] [CrossRef]
- Ghirlanda, G.; Osyczka, A.; Liu, W.; Antolovich, M.; Smith, K.M.; Dutton, P.L.; Wand, A.J.; DeGrado, W.F. De Novo Design of a D2-Symmetrical Protein that Reproduces the Diheme Four-Helix Bundle in Cytochrome bc1. J. Am. Chem. Soc. 2004, 126, 8141–8147. [Google Scholar] [CrossRef]
- Di Santo, J.P. A defining factor for natural killer cell development. Nat. Immunol. 2009, 10, 1051–1052. [Google Scholar] [CrossRef]
- Murphy, T.L.; Tussiwand, R.; Murphy, K.M. Specificity through cooperation: BATF-IRF interactions control immune-regulatory networks. Nat. Rev. Immunol. 2013, 13, 499–509. [Google Scholar] [CrossRef]
- Jang, Y.; Champion, J.A. Self-Assembled Materials Made from Functional Recombinant Proteins. Acc. Chem. Res. 2016, 49, 2188–2198. [Google Scholar] [CrossRef] [PubMed]
- Jay, A. The methylation reaction in carbohydrate analysis. J. Carbohydr. Chem. 1996, 15, 897–923. [Google Scholar] [CrossRef]
- Hansch, C.; Leo, A.; Taft, R.W. A Survey of Hammett Substituent Constants and Resonance and Field Parameters. Chem. Rev. 1991, 91, 165–195. [Google Scholar] [CrossRef]
- Jain, A.; Ong, S.P.; Hautier, G.; Chen, W.; Richards, W.D.; Dacek, S.; Cholia, S.; Gunter, D.; Skinner, D.; Ceder, G.; et al. Commentary: The Materials Project: A materials genome approach to accelerating materials innovation. APL Mater. 2013, 1, 011002. [Google Scholar] [CrossRef]
- Hansen, L.D.; Izatt, R.M.; Christensen, J.J. Thermodynamics of metal-halide coordination in aqueous solution. I. ’Equilibrium constants for several mercury(I)- and mercury(II)-halide systems as a function of temperature. Inorg. Chem. 1963, 2, 1243–1245. [Google Scholar] [CrossRef]
- Clever, H.L.; Johnston, F.J. The solubility of some sparingly soluble lead salts: An evaluation of the solubility in water and aqueous electrolyte solution. J. Phys. Chem. Ref. Data 1980, 9, 751–784. [Google Scholar] [CrossRef]
- Chao, E.E.; Cheng, K.L. Stepwise titration of some anion mixtures and determination of Ksp of silver precipitates with silver ion selective electrode. Anal. Chem. 1976, 48, 267–271. [Google Scholar] [CrossRef]
- Alexander, R.; Ko, E.C.F.; Mac, Y.C.; Parker, A.J. Solvation of ions. XI. Solubility products and instability constants in water methanol, formamide, dimethylformamide, dimethylacetamide, dimethyl sulfoxide, acetonitrile, and hexamethylphosphorotriamide. J. Am. Chem. Soc. 1967, 89, 3703–3712. [Google Scholar] [CrossRef]
- Mussini, P.R.; Cipolli, A.; Mussini, T.; Rondinini, S. Standard aqueous potentials for the {thallium amalgam|thallium(I) iodide} electrode, and thermodynamic solubility product of thallium(I) iodide at temperatures from 298.15 K to 328.15 K. J. Chem. Thermodyn. 1993, 25, 1055–1059. [Google Scholar] [CrossRef]
- Davies, C.W.; Robinson, R.A. The solubility product of thallous iodide at 25°. Trans. Faraday Soc. 1937, 33, 633–635. [Google Scholar] [CrossRef]
- Subalakshmi, A.; Kavitha, B.; Karthika, A.; Nikhil, S.; Srinivasan, N.; Rajarajan, M.; Suganthi, A. Design of Mn and Zr incorporated Ag2O nanoparticles and their enhanced photocatalytic activity driven by visible light irradiation for degradation of rose bengal dye. New J. Chem. 2021, 45, 1876–1886. [Google Scholar] [CrossRef]
- Ono, S.; Tomizawa, R.; Nagai, T. Ion conduction mechanisms of silver halides under high pressures. Phase Transit. 2020, 93, 856–864. [Google Scholar] [CrossRef]
- Waghorne, W.E. Solubilities of the silver halides in aqueous mixtures of methanol, acetonitrile, and dimethylsulfoxide. Monatsh. Chem. 2003, 134, 655–667. [Google Scholar] [CrossRef]
- Becquerel, A.-E. Mémoire sur les effets électriques produits sous l’influence des rayons solaires. Comptes Rendus 1839, 9, 561–567. [Google Scholar]
- Abeyweera, S.C.; Rasamani, K.D.; Sun, Y. Ternary Silver Halide Nanocrystals. Acc. Chem. Res. 2017, 50, 1754–1761. [Google Scholar] [CrossRef]
- Van Renterghem, W.; Goessens, C.; Schryver, D.; Van Landuyt, J.; Bollen, D.; De Keyzer, R.; Van Roost, C. Influence of twinning on the morphology of AgBr and AgCl microcrystals. J. Imaging Sci. Technol. 2001, 45, 349–356. [Google Scholar]
- Lee, H.-Y.; An, M. Selective reduction of the nitro-group using Co2(CO)8-H2O. Bull. Korean Chem. Soc. 2004, 25, 1717–1719. [Google Scholar] [CrossRef]
- Purc, A.; Espinoza, E.M.; Nazir, R.; Romero, J.J.; Skonieczny, K.; Jeżewski, A.; Larsen, J.M.; Gryko, D.T.; Vullev, V.I. Gating That Suppresses Charge Recombination–The Role of Mono-N-Arylated Diketopyrrolopyrrole. J. Am. Chem. Soc. 2016, 138, 12826–12832. [Google Scholar] [CrossRef] [PubMed]

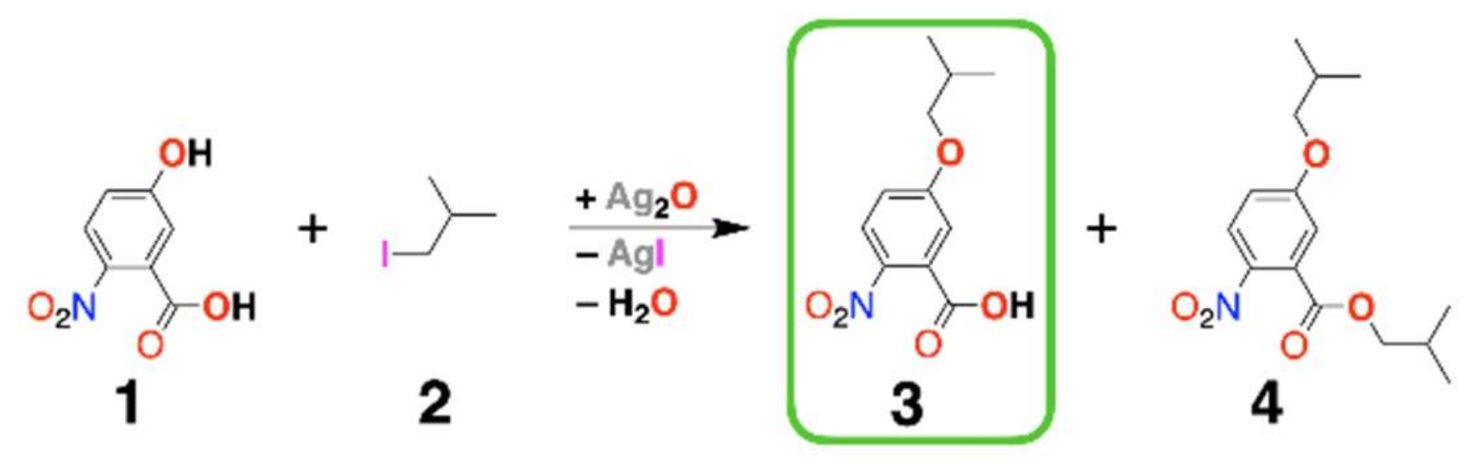

| n(Ag2O)/n(1) a,b,c | Yield of 3 d | Yield of 4 d |
| 1 | 9% | 18% |
| 1.5 | 10% | 23% |
| 2 | 20% | 20% |
| Previous studies: e n(Cs2CO3)/n(1) | ||
| 3 | 40% | 0% |
| Oxide or Iodide | Crystal Lattice a | ΔGf(0)/eV per Atom b | ρ/g cm−3 c | Yield/% d |
|---|---|---|---|---|
| Ag2O | cubic (mm, Pnm [224]), stable | −0.328 | 6.78 | 40 (90) e |
| triclinic (1, P1 [1]) | −0.219 | 6.87 | ||
| trigonal (mm, Pm1 [164]) | −0.208 | 8.61 | ||
| AgI | cubic (3m, F3m [216]), stable | −0.281 | 5.32 | — |
| cubic (mm, Fmm [225]) | −0.188 | 6.64 | ||
| cubic (mm, Pmm [221]) | −0.109 | 6.80 | ||
| hexagonal (6mm, P63mc [186]) | −0.280 | 5.33 | ||
| hexagonal (6mm, P63mc [186]) | −0.234 | 5.29 | ||
| tetragonal (2m, Im2 [119]) | −0.279 | 5.32 | ||
| tetragonal (4/mmm, P4/nmm [129]) | −0.256 | 5.55 | ||
| monoclinic (2/m, P21/m [11]) | −0.177 | 6.69 | ||
| Tl2O | trigonal (m, Rm [166]) stable | −0.826 | 9.41 | 0 |
| TlI | cubic (mm, Fmm [225]) stable | −0.682 | 6.05 | — |
| cubic (mm, Pmm [221]) | −0.629 | 6.98 | ||
| orthorhombic (mmm, Cmcm [63]) | −0.658 | 6.55 | ||
| PbO | orthorhombic (mmm, Pbcm [57]) stable | −1.477 | 8.31 | 0 |
| orthorhombic (mmm, Pbcm [57]) | −1.458 | 8.74 | ||
| orthorhombic (mm2, Pca21 [29]) | −1.456 | 7.89 | ||
| tetragonal (4/mmm, P4/nmm [129]) | −1.476 | 8.47 | ||
| tetragonal (4/mmm, P42/mmc [131]) | −1.064 | 8.37 | ||
| PbI2 | hexagonal (6mm, P63mc [186]) stable | −0.668 | 5.12 | — |
| hexagonal (6mm, P63mc [186]) | −0.668 | 5.13 | ||
| trigonal (3m, P3m1 [156]) | −0.668 | 5.34 | ||
| trigonal (m, Pm1 [164]) | −0.667 | 5.36 | ||
| trigonal (m, Rm1 [166]) | −0.668 | 5.11 | ||
| trigonal (m, Rm1 [166]) | −0.666 | 5.34 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Derr, J.B.; Rybicka-Jasińska, K.; Espinoza, E.M.; Morales, M.; Billones, M.K.; Clark, J.A.; Vullev, V.I. On the Search of a Silver Bullet for the Preparation of Bioinspired Molecular Electrets with Propensity to Transfer Holes at High Potentials. Biomolecules 2021, 11, 429. https://doi.org/10.3390/biom11030429
Derr JB, Rybicka-Jasińska K, Espinoza EM, Morales M, Billones MK, Clark JA, Vullev VI. On the Search of a Silver Bullet for the Preparation of Bioinspired Molecular Electrets with Propensity to Transfer Holes at High Potentials. Biomolecules. 2021; 11(3):429. https://doi.org/10.3390/biom11030429
Chicago/Turabian StyleDerr, James Bennett, Katarzyna Rybicka-Jasińska, Eli Misael Espinoza, Maryann Morales, Mimi Karen Billones, John Anthony Clark, and Valentine Ivanov Vullev. 2021. "On the Search of a Silver Bullet for the Preparation of Bioinspired Molecular Electrets with Propensity to Transfer Holes at High Potentials" Biomolecules 11, no. 3: 429. https://doi.org/10.3390/biom11030429
APA StyleDerr, J. B., Rybicka-Jasińska, K., Espinoza, E. M., Morales, M., Billones, M. K., Clark, J. A., & Vullev, V. I. (2021). On the Search of a Silver Bullet for the Preparation of Bioinspired Molecular Electrets with Propensity to Transfer Holes at High Potentials. Biomolecules, 11(3), 429. https://doi.org/10.3390/biom11030429







