Evaluation of In Vitro Capsaicin Release and Antimicrobial Properties of Topical Pharmaceutical Formulation
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Plant Material and Extraction Procedure
2.3. HPLC Analyses
2.4. Formulation with Capsaicin Extract
2.5. Evaluation of Rheological Properties
2.6. In Vitro Release of Capsaicin from Carbopol-Based Formulation
2.7. Antimicrobial Susceptibility Testing
2.8. Antibacterial Activity Assay
2.9. Antifungal Activity Assay
2.10. Statistical Analysis
3. Results and Discussion
3.1. Content of Capsaicin in Ethanolic Extracts and Carbopol Gel Formulation
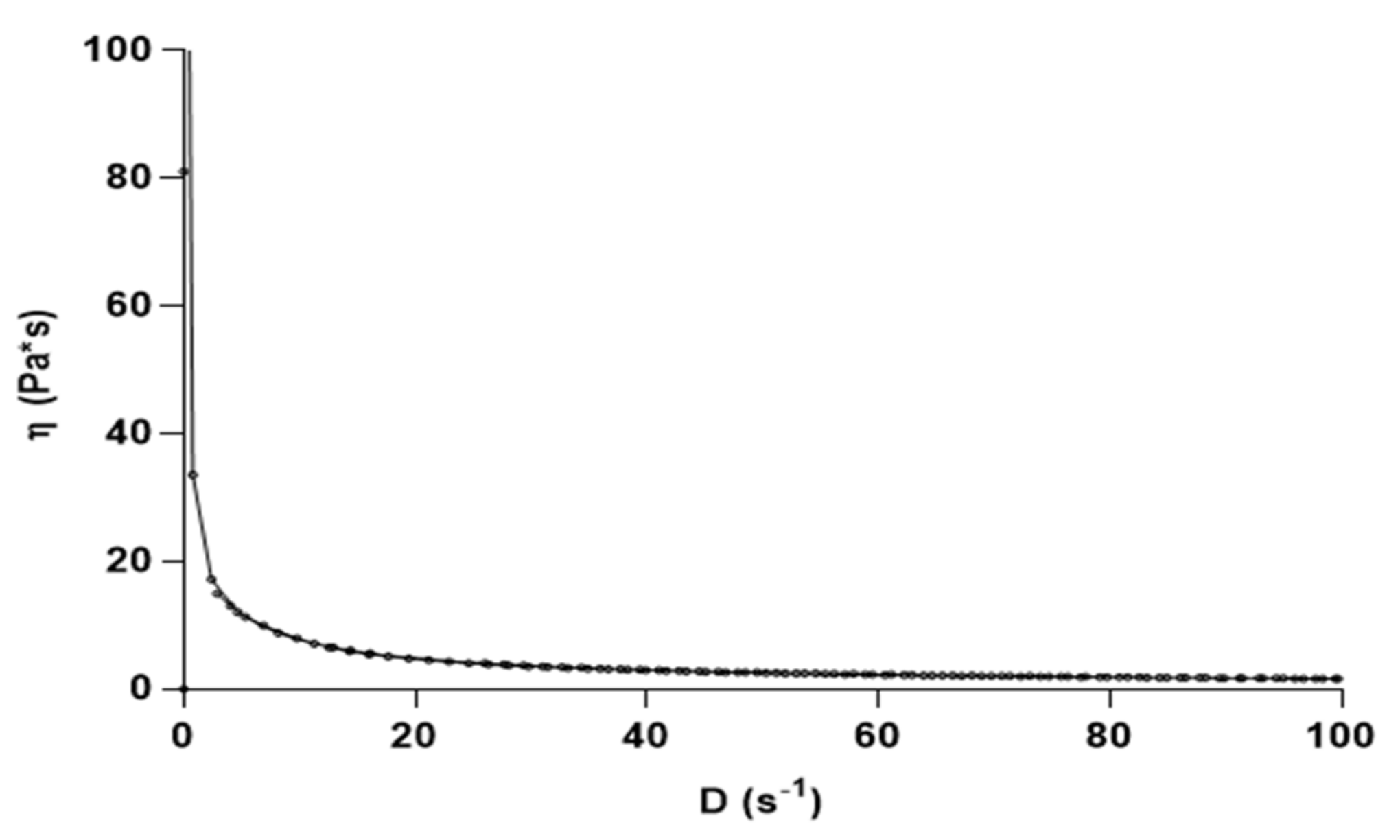
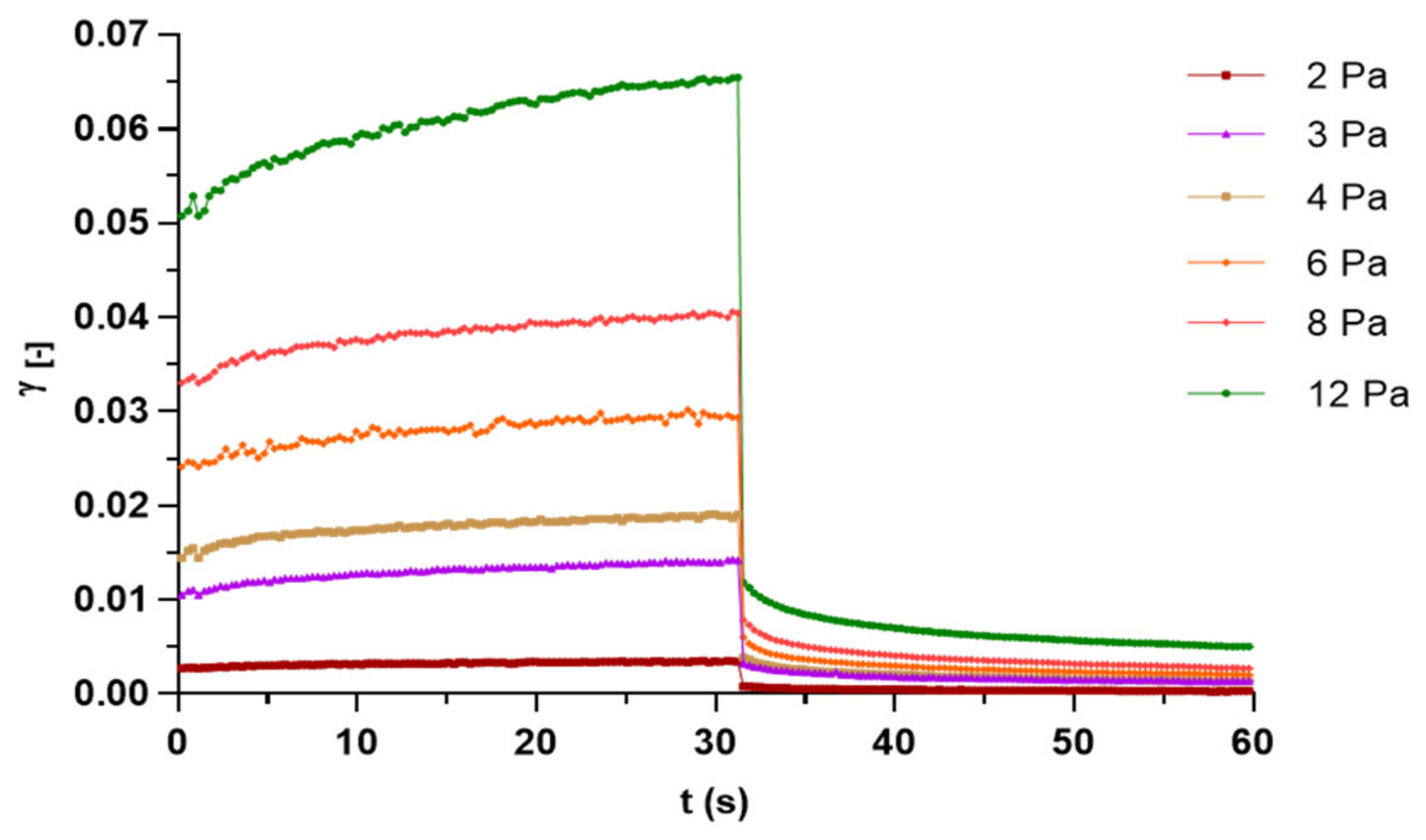
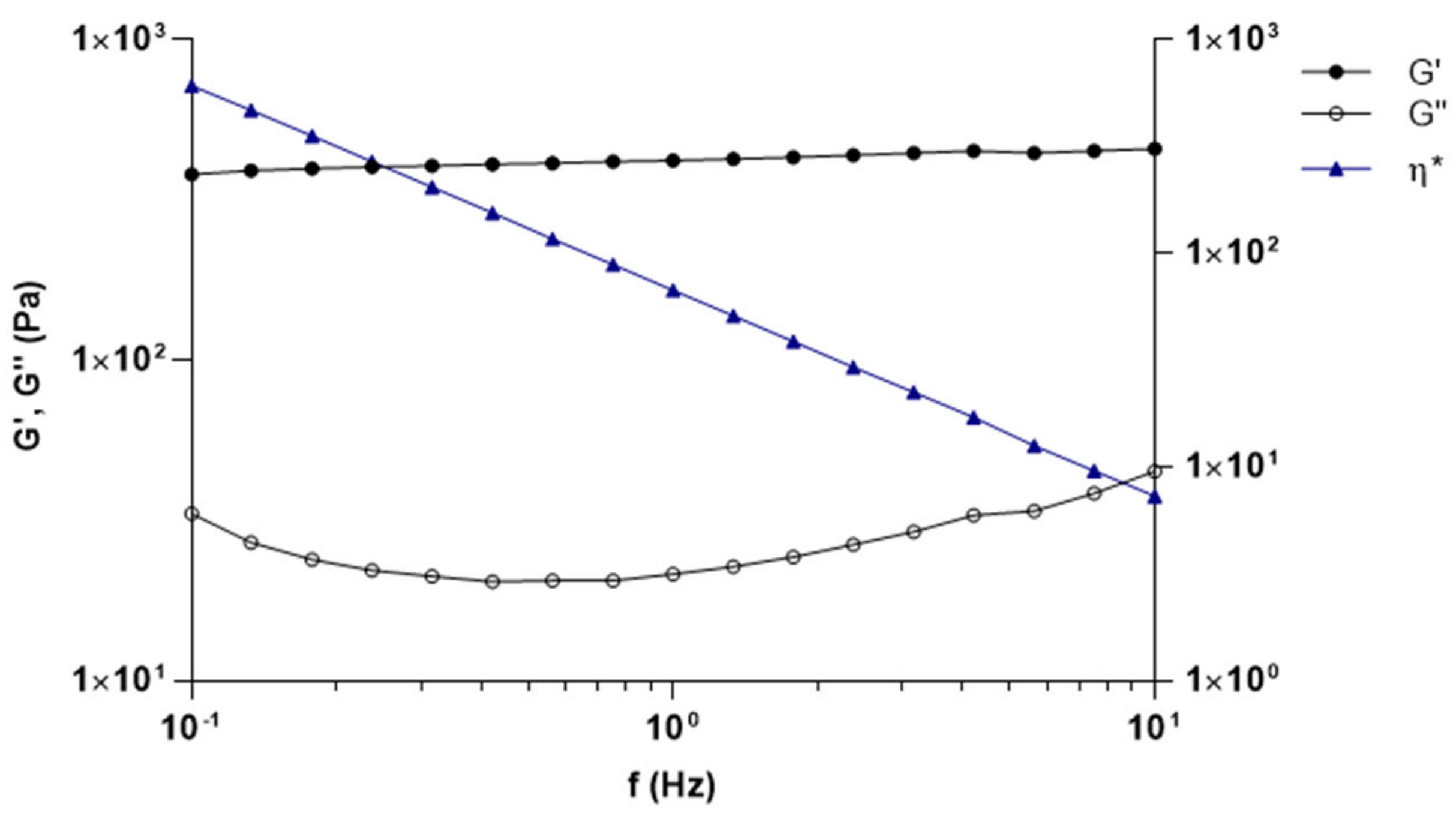
3.2. Characterization of Capsaicin Formulations
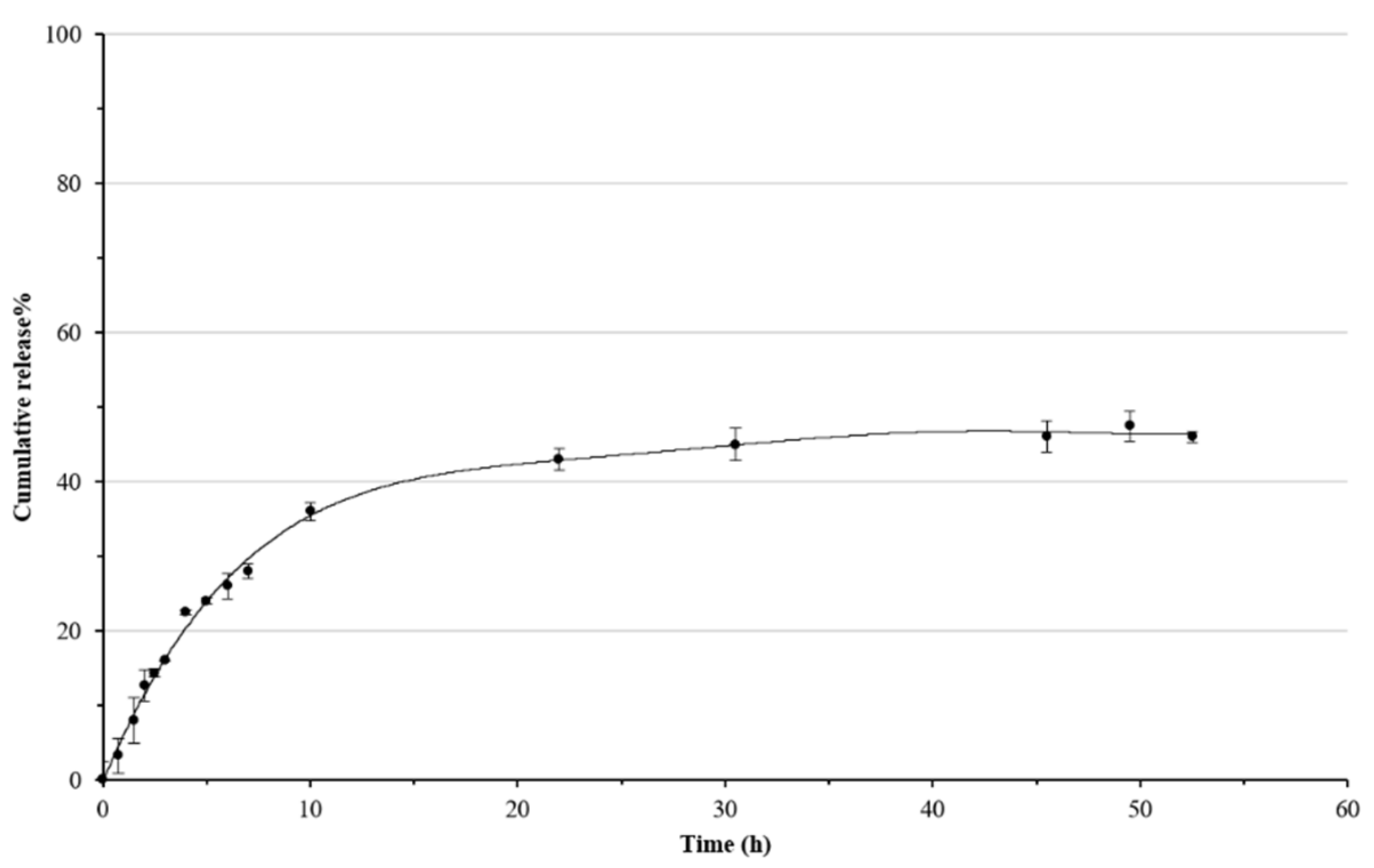
3.3. In Vitro Release Assay
3.4. Antimicrobial Properties
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Babbar, S.; Marier, J.-F.; Mouksassi, M.-S.; Beliveau, M.; Vanhove, G.F.; Chanda, S.; Bley, K. Pharmacokinetic Analysis of Capsaicin After Topical Administration of a High-Concentration Capsaicin Patch to Patients With Peripheral Neuropathic Pain. Ther. Drug Monit. 2009, 31, 502–510. [Google Scholar] [CrossRef] [PubMed]
- Peng, X.; Wen, X.; Pan, X.; Wang, R.; Chen, B.; Wu, C. Design and In Vitro Evaluation of Capsaicin Transdermal Controlled Release Cubic Phase Gels. Aaps Pharmscitech 2010, 11, 1405–1410. [Google Scholar] [CrossRef]
- Hayman, M.; Kam, P.C. Capsaicin: A review of its pharmacology and clinical applications. Curr. Anaesth. Crit. Care 2008, 19, 338–343. [Google Scholar] [CrossRef]
- Kosuge, S.; Furuta, M. Studies on the pungent principle of Capsicum. Part XIV: Chemical constitution of the pungent princi-ple. Agric. Biol. Chem. 1970, 34, 248–256. [Google Scholar]
- Schumacher, M.; Pasvankas, G. Topical capsaicin formulations in the management of neuropathic pain. Prog. Drug Res. 2014, 68, 105–128. [Google Scholar]
- Derry, S.; Lloyd, R.; Moore, R.A.; McQuay, H.J. Topical capsaicin for chronic neuropathic pain in adults. Cochrane Database Syst. Rev. 2009, 4, CD007393. [Google Scholar] [CrossRef]
- Arora, R.; Gill, N.S.; Chauhan, G.; Rana, A.C. An Overview about Versatile Molecule Capsaicin. Int. J. Pharm. Sci. Drug Res. 2011, 3, 280–286. [Google Scholar]
- Luo, X.-J.; Peng, J.; Li, Y.-J. Recent advances in the study on capsaicinoids and capsinoids. Eur. J. Pharmacol. 2011, 650, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Cowan, M.M. Plant Products as Antimicrobial Agents. Clin. Microbiol. Rev. 1999, 12, 564–582. [Google Scholar] [CrossRef]
- Dorantes, L.; Colmenero, R.; Hernandez, H.; Mota, L.; Jaramillo, M.E.; Fernandez, E.; Solano, C. Inhibition of growth of some foodborne pathogenic bacteria by Capsicum annuum extracts. Int. J. Food Microbiol. 1999, 57, 125–128. [Google Scholar] [CrossRef]
- Xing, F.; Cheng, G.; Yi, K. Study on the antimicrobial activities of the capsaicin microcapsules. J. Appl. Polym. Sci. 2006, 102, 1318–1321. [Google Scholar] [CrossRef]
- Omolo, M.A.; Wong, Z.Z.; Mergen, K.; Hastings, J.C.; Le, N.C.; Reil, H.A.; Case, K.A.; Baumler, D.J. Antimicrobial Properties of Chili Peppers. J. Infect. Dis. Ther. 2014, 2, 1–8. [Google Scholar] [CrossRef]
- Tayseer, I.; Aburjai, T.; Abu-Qatouseh, L.; Al-Karabieh, N.; Ahmed, W.; Al-Samydai, A. In vitro Anti-Helicobacter pylori Activity of Capsaicin. J. Pure Appl. Microbiol. 2020, 14, 279–286. [Google Scholar] [CrossRef]
- Chatterjee, S.; Asakura, M.; Chowdhury, N.; Neogy, S.B.; Sugimoto, N.; Haldar, S.; Awasthi, S.P.; Hinenoya, A.; Aoki, S.; Yamasaki, S. Capsaicin, a potential inhibitor of chol-era toxin production in Vibrio cholerae. FEMS Microbiol. Lett. 2010, 306, 54–60. [Google Scholar] [CrossRef] [PubMed]
- Kalia, N.P.; Mahajan, P.; Mehra, R.; Nargotra, A.; Sharma, J.P.; Koul, S.; Khan, I.A. Capsaicin, a novel inhibitor of the NorA efflux pump, reduces the intracellular invasion of Staphylococcus aureus. J. Antimicrob. Chemother. 2012, 67, 2401–2408. [Google Scholar] [CrossRef] [PubMed]
- Qiu, J.; Niu, X.; Wang, J.; Xing, Y.; Leng, B.; Dong, J.; Li, H.; Luo, M.; Zhang, Y.; Dai, X.; et al. Capsaicin protects mice from community-associated methicillin-resistant Staphylococcus aureus pneumonis. PLoS ONE 2012, 7, e33032. [Google Scholar]
- Zhou, Y.; Guan, X.; Zhu, W.; Liu, Z.; Wang, X.; Yu, H.; Wang, H. Capsaicin inhibits Porphyromonas gingivalis growth, biofilm formation, gingivomucosal inflammatory cytokine secretion, and in vitro osteoclastogenesis. Eur. J. Clin. Microbiol. Infect. Dis. 2013, 33, 211–219. [Google Scholar] [CrossRef]
- Nascimento, P.L.A.; Nascimento, T.C.E.S.; Ramos, N.S.M.; Silva, G.R.; Gomes, J.E.G.; Falcão, R.E.A.; Moreira, K.A.; Porto, A.L.F.; Silva, T.M.S. Quantification, Antioxidant and Antimicrobial Activity of Phenolics Isolated from Different Extracts of Capsicum frutescens (Pimenta Malagueta). Molecules 2014, 19, 5434–5447. [Google Scholar] [CrossRef]
- Marini, E.; Magi, G.; Mingoia, M.; Pugnaloni, A.; Facinelli, B. Antimicrobial and Anti-Virulence Activity of Capsaicin Against Erythromycin-Resistant, Cell-Invasive Group A Streptococci. Front. Microbiol. 2015, 6, 1281. [Google Scholar] [CrossRef] [PubMed]
- Fieira, C.; Oliveira, F.; Calegari, R.P.; Machado, A.; Coelho, A.R. In vitro and in vivo antifungal activity of natural inhibitors against Penicillium expansum. Food Sci. Technol. 2013, 33, 40–46. [Google Scholar] [CrossRef]
- Ndashe, K.; Nghake, S.; Pola, E.; Sakala, E.M.; Kabwali, E.; Moonga, L.; Kefi, A.S.; Hangombe, B.M. The Potential of Capsicum an-num Extracts to Prevent Lactococcosis in Tilapia. bioRxiv 2020. [Google Scholar] [CrossRef]
- Farmacopea Ufficiale della Repubblica Italiana—Droghe Vegetali e Preparazioni; Ministero della Sanita, Ist. Poligrafico Dello Stato: Roma, Italy, 1991; pp. 100–105. ISBN 9788824001809.
- Wagnerit, H.; Bladt, S. Plant Drug Analysis, A Thin Layer Chromatography Atlas, 2nd ed.; Springer: Berlin/Heidelberg, Germany; New York, NY, USA, 2001; pp. 289–292. [Google Scholar]
- Goci, E.; Haloçi, E.; Vide, K.; Malaj, L. Application and comparison of three different extraction methods of capsaicin from cap-sicum fruits. Albanian J. Pharm. Sci. 2014, 1, 16–19. [Google Scholar]
- Abdullah Al Othman, Z.; Ahmed, Y.; Habila, M.; Ghafar, A. Determination of Capsaicin and Dihydrocapsaicin in Capsi-cum Fruit Samples using High Performance Liquid Chromatography. Molecules 2011, 16, 8919–8929. [Google Scholar] [CrossRef]
- Soetarno, S.; Sukrasn, O.; Yulinah, E. Antimicrobial activities of the ethanol extracts of capsicum fruits with different levels of pungency. J. Mat. Dan Sains 1997, 2, 57–63. [Google Scholar]
- Di Stefano, A.; D’Aurizio, E.; Trubiani, O.; Grande, R.; Di Campli, E.; Di Giulio, M.; Di Bartolomeo, S.; Sozio, P.; Iannitelli, A.; Nostro, A.; et al. Viscoelastic properties of Staphylococcus aureus and Staphylococcus epidermidis mono-microbial bio-films. Microb. Biotechnol. 2009, 2, 634–641. [Google Scholar] [CrossRef] [PubMed]
- Iannitelli, A.; Grande, R.; Di Stefano, A.; Di Giulio, M.; Sozio, P.; Bessa, L.J.; Laserra, S.; Paolini, C.; Protasi, F.; Cellini, L. Potential antibacterial activity of carvacrol-loaded poly(DL-lactide-co-glycolide) (PLGA) nanoparticles against microbial biofilm. Int. J. Mol. Sci. 2011, 12, 5039–5051. [Google Scholar] [CrossRef]
- Clinical and Laboratory Standards Institute. Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria That Grow Aerobically. Approved Standard, 10th ed.; CLSI Document M07-A10; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2015. [Google Scholar]
- Angelini, P.; Venanzoni, R.; Flores, G.A.; Tirillini, B.; Orlando, G.; Recinella, L.; Chiavaroli, A.; Brunetti, L.; Leone, S.; Di Simone, S.C.; et al. Evaluation of Antioxidant, Antimicrobial and Tyrosinase Inhibitory Activities of Extracts from Tricholosporum goniospermum, an Edible Wild Mushroom. Antibiotics 2020, 9, 513. [Google Scholar] [CrossRef]
- Clinical and Laboratory Standards Institute. Reference Method for Broth Dilution Antifungal Susceptibility Testing of Yeast, 4th ed.; CLSI Standard M27; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2017. [Google Scholar]
- Clinical and Laboratory Standards Institute. Performance Standards for Antifungal Susceptibility Testing of Yeasts, 1st ed.; CLSI Supplement M60; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2017. [Google Scholar]
- Pagiotti, R.; Angelini, P.; Rubini, A.; Tirillini, B.; Granetti, B.; Venanzoni, R. Identification and characterisation of human pathogenic filamentous fungi and susceptibility to Thymus schimperi essential oil. Mycoses 2010, 54, e364–e376. [Google Scholar] [CrossRef]
- Goodwin, D.C.; Hertwig, K.M. Peroxidase-catalyzed oxidation of capsaicinoids: Steady-state and transient state kinetic studies. Arch. Biochem. Biophys. 2003, 417, 18–26. [Google Scholar] [CrossRef]
- Szolcsányi, J. Forty years in capsaicin research for sensory pharmacology and physiology. Neuropeptides 2004, 38, 377–384. [Google Scholar] [CrossRef]
- Thomas, B.V.; Schreiber, A.A.; Weisskopf, C.P. Simple Method for Quantitation of Capsaicinoids in Peppers Using Capillary Gas Chromatography. J. Agric. Food Chem. 1998, 46, 2655–2663. [Google Scholar] [CrossRef]
- Lopez-Hernandez, J.; Oruna-Concha, M.J.; Simal-Lozano, J.; Gonzales-Castro, M.J.; Vazquez-Blanco, M.E. Determination of capsaicin and dihydrocapsaicin in cayenne pepper and padron peppers by HPLC. Dtsch. Lebensm. Rundsch. 1996, 92, 393–395. [Google Scholar]
- Cheema, S.K.; Pant, M.R. Estimation of capsaicin in seven cultivated varieties of Capsicum annuum L. Res. J. Pharm. Biol. Chem. Sci. 2011, 2, 701. [Google Scholar]
- Naseem, Z.; Imran, K.; Sajila, H.; Ali, J.; Inam, S.M.; Malik, S.M.; Farah, A. Estimation of capsaicin in different chili varieties using different Extraction Techniques and HPLC method: A Review. Pak. J. Food Sci. 2016, 26, 54–60. [Google Scholar]
- Chytil, M.; Pekař, M. Effect of new hydrophobic modification of hyaluronan on its solution properties: Evaluation of self-aggregation. Carbohydr. Polym. 2009, 76, 443–448. [Google Scholar] [CrossRef]
- Vinogradov, A.M.; Winston, M.; Rupp, C.J.; Stoodley, P. Rheology of biofilms formed from the dental plaque pathogen Strepto-coccus mutans. Biofilms 2004, 1, 49–56. [Google Scholar] [CrossRef]
- Iannitti, T.; Bingöl, A.Ö.; Rottigni, V.; Palmieri, B. A new highly viscoelastic hyaluronic acid gel: Rheological properties, bio-compatibility and clinical investigation in esthetic and restorative surgery. Int. J. Pharm. 2013, 456, 583–592. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Mooney, D.J. Designing hydrogels for controlled drug delivery. Nat. Rev. Mater. 2016, 1, 16071. [Google Scholar] [CrossRef]
- Scalzo, M.; Orlandi, C.; Simonetti, N.; Cerreto, F. Study of Interaction Effects of Polyacrylic Acid Polymers (Carbopol 940) on Antimicrobial Activity of Methyl Parahydroxybenzoate Against Some Gram-negative, Gram-positive Bacteria and Yeast. J. Pharm. Pharmacol. 1996, 48, 1201–1205. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.-Y.; Hong, C.-T.; Chiu, W.-T.; Fang, J.-Y. In vitro and in vivo evaluations of topically applied capsaicin and nonivamide from hydrogels. Int. J. Pharm. 2001, 224, 89–104. [Google Scholar] [CrossRef]
| Pepper Type | Capsaicin (mg/g) 1 | Yield 2 |
|---|---|---|
| Caspicum annuum | 2.109 ± 0.028 | 0.21% |
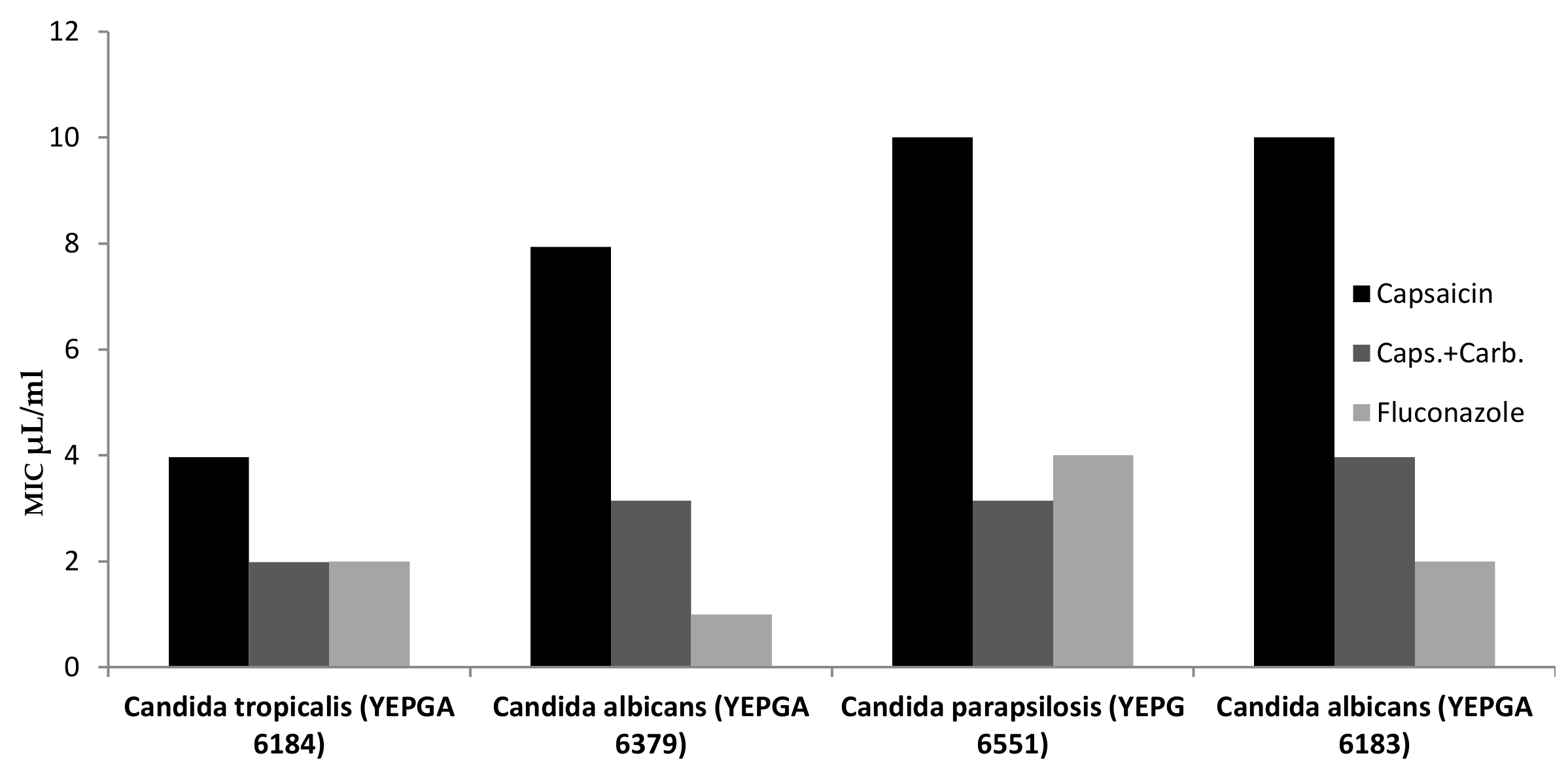
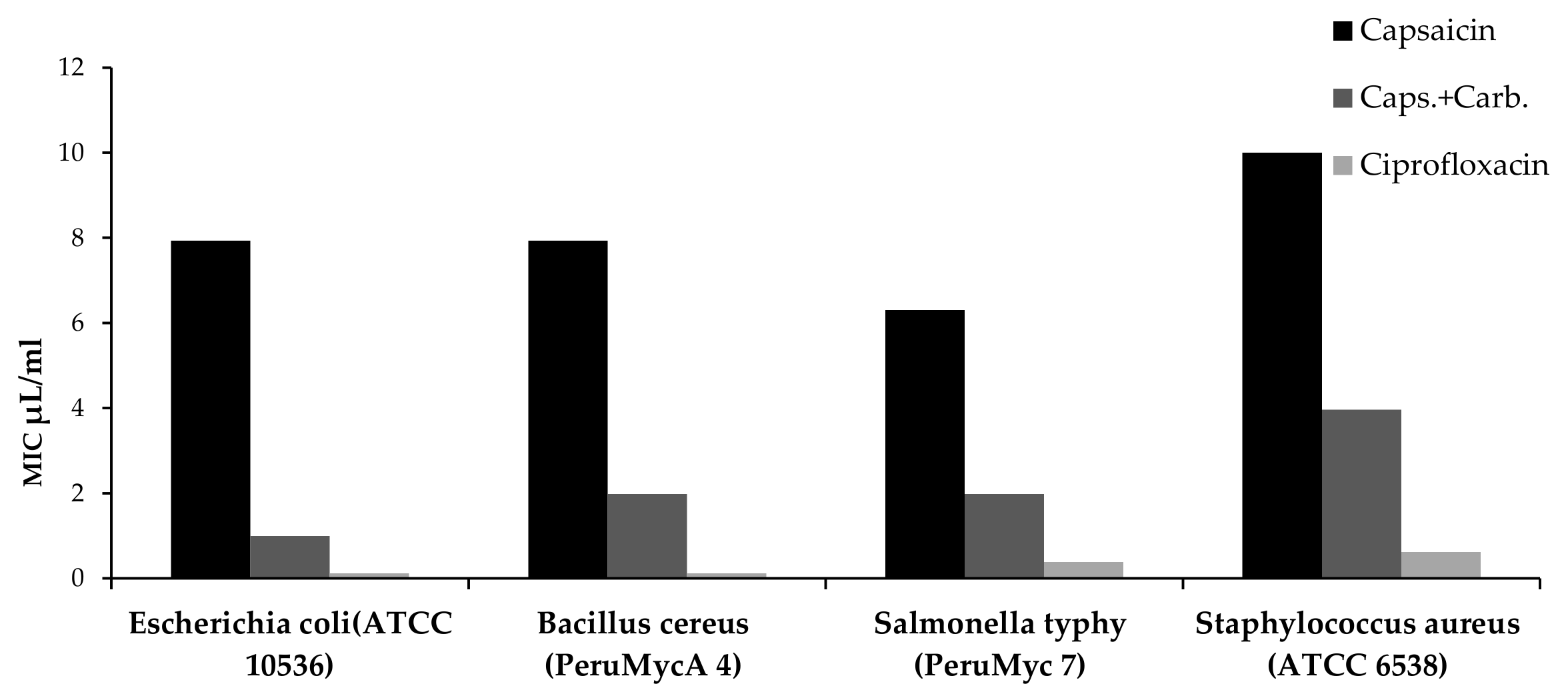
| Microrganisms (ID) | Minimum Inhibitory Concentration (MIC) µL/mL | ||
|---|---|---|---|
| Yeasts | Capsaicina | Caps.+Carb. | Fluconazole |
| Candida tropicalis (YEPGA 6184) | 3.968 (2.5–5) | 1.984 (1.25–2.5) | 2 |
| Candida albicans (YEPGA 6379) | 7.937 (5–10) | 3.149 (2.5–5) | 1 |
| Candida parapsilosis (YEPG 6551) | >10 | 3.149 (2.5–5) | 4 |
| Candida albicans (YEPGA 6183) | >10 | 3.968 (2.5–5) | 2 |
| Bacteria | Ciprofloxacin | ||
| Escherichia coli (ATCC 10536) | 7.937 (5–10) | 0.992 (0.625–1.25) | <0.12 |
| Bacillus cereus (Peru MycA 4) | 7.937 (5–10) | 1.984 (1.25–2.5) | <0.12 |
| Salmonella typhy (Peru Myc 7) | 6.299 (5–10) | 1.984 (1.25–2.5) | 0.38 (0.49–0.24) |
| Staphylococcus aureus (ATCC 6538) | >10 | 3.968 (2.5–5) | 0.62 (0.98–0.49) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Goci, E.; Haloci, E.; Di Stefano, A.; Chiavaroli, A.; Angelini, P.; Miha, A.; Cacciatore, I.; Marinelli, L. Evaluation of In Vitro Capsaicin Release and Antimicrobial Properties of Topical Pharmaceutical Formulation. Biomolecules 2021, 11, 432. https://doi.org/10.3390/biom11030432
Goci E, Haloci E, Di Stefano A, Chiavaroli A, Angelini P, Miha A, Cacciatore I, Marinelli L. Evaluation of In Vitro Capsaicin Release and Antimicrobial Properties of Topical Pharmaceutical Formulation. Biomolecules. 2021; 11(3):432. https://doi.org/10.3390/biom11030432
Chicago/Turabian StyleGoci, Enkelejda, Entela Haloci, Antonio Di Stefano, Annalisa Chiavaroli, Paola Angelini, Ajkuna Miha, Ivana Cacciatore, and Lisa Marinelli. 2021. "Evaluation of In Vitro Capsaicin Release and Antimicrobial Properties of Topical Pharmaceutical Formulation" Biomolecules 11, no. 3: 432. https://doi.org/10.3390/biom11030432
APA StyleGoci, E., Haloci, E., Di Stefano, A., Chiavaroli, A., Angelini, P., Miha, A., Cacciatore, I., & Marinelli, L. (2021). Evaluation of In Vitro Capsaicin Release and Antimicrobial Properties of Topical Pharmaceutical Formulation. Biomolecules, 11(3), 432. https://doi.org/10.3390/biom11030432












