Testosterone in Female Depression: A Meta-Analysis and Mendelian Randomization Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Meta-Analysis
2.1.1. Search Strategy
2.1.2. Inclusion Criteria
2.1.3. Exclusion Criteria
2.1.4. Data Extraction
2.1.5. Statistical Analysis
2.2. Mendelian Randomization
3. Results
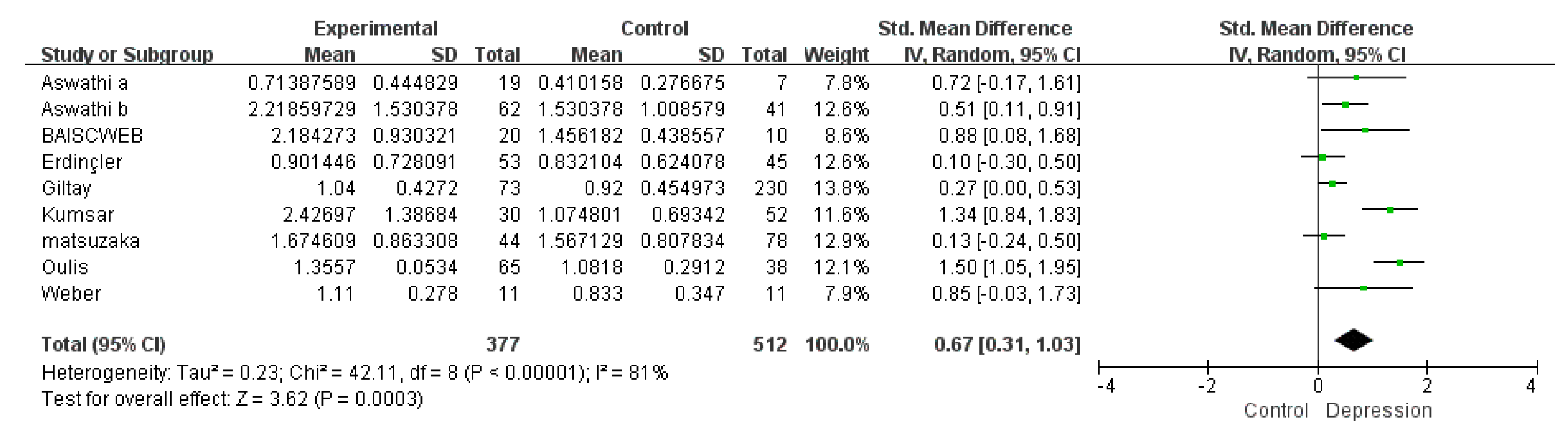
3.1. Meta-Analysis
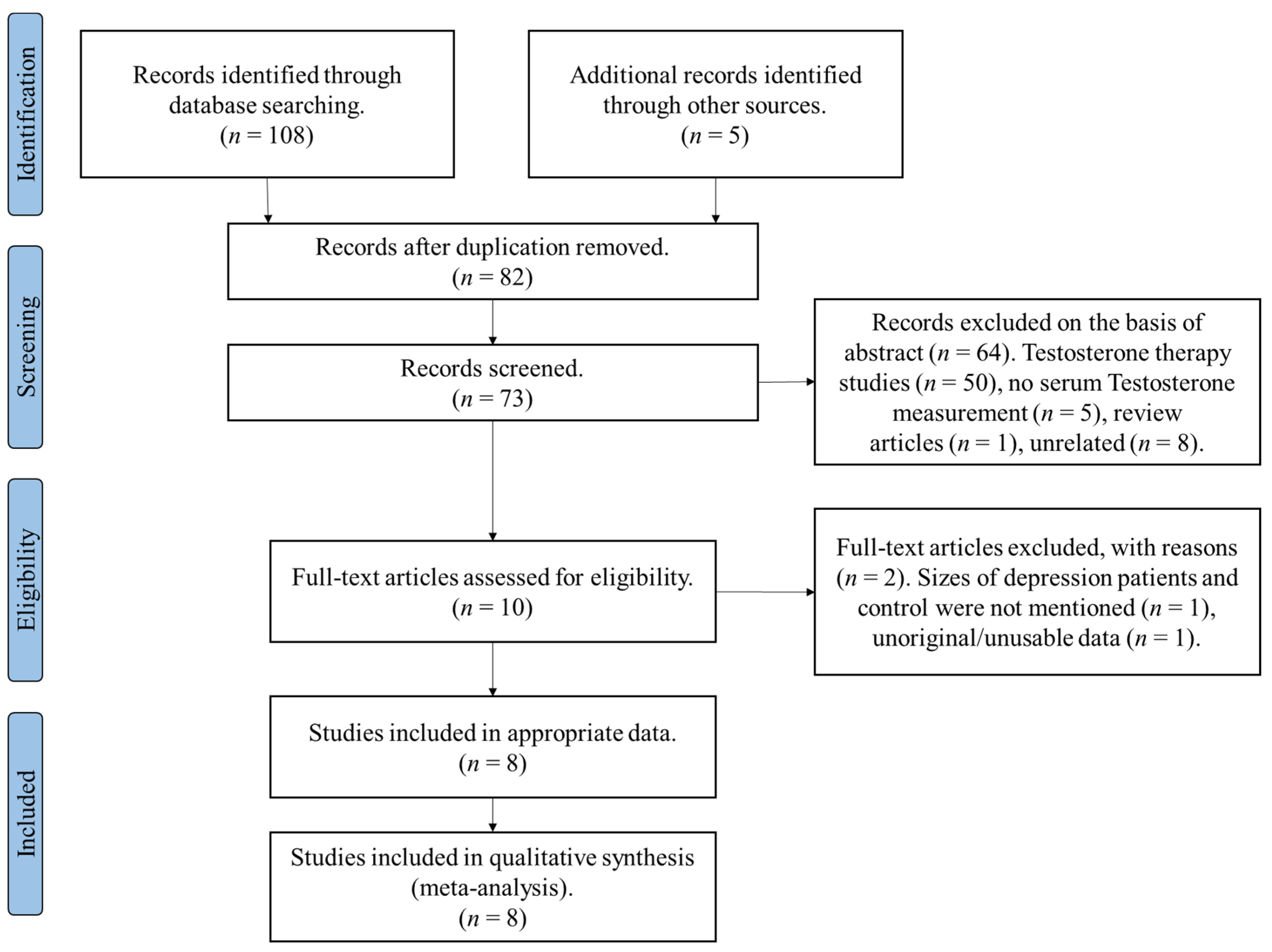
3.1.1. Included Studies and Participant Details
| Study | Year | Ethnicity | Menopause Status | Case/Con | Age (Se) |
|---|---|---|---|---|---|
| Baischer [36] | 1994 | European | Premenopausal | 20/10 | 32.5 (11) |
| Weber [37] | 2000 | European | 11/11 | 48.1 (18) | |
| Erdinçler [44] | 2004 | Turkish | Postmenopausal | 34/53 | 70 (7.7) |
| Matsuzaka [45] | 2004 | Japanese | 44/78 | 53.1 (23) | |
| Kumsar [32] | 2013 | Turkish | Premenopausal | 52/30 | 31.9 (7.3) |
| Aswathi [35] | 2015 | Indian | Premenopausal | 81/41 | 23.2 (2.7) |
| Giltay [46] | 2017 | European | Postmenopausal | 230/73 | 70.9 (7.3) |
| Oulis [47] | 2014 | European | 38/65 | 54.7 (13.4) |
3.1.2. A Meta-Analysis of Overall Testosterone Levels
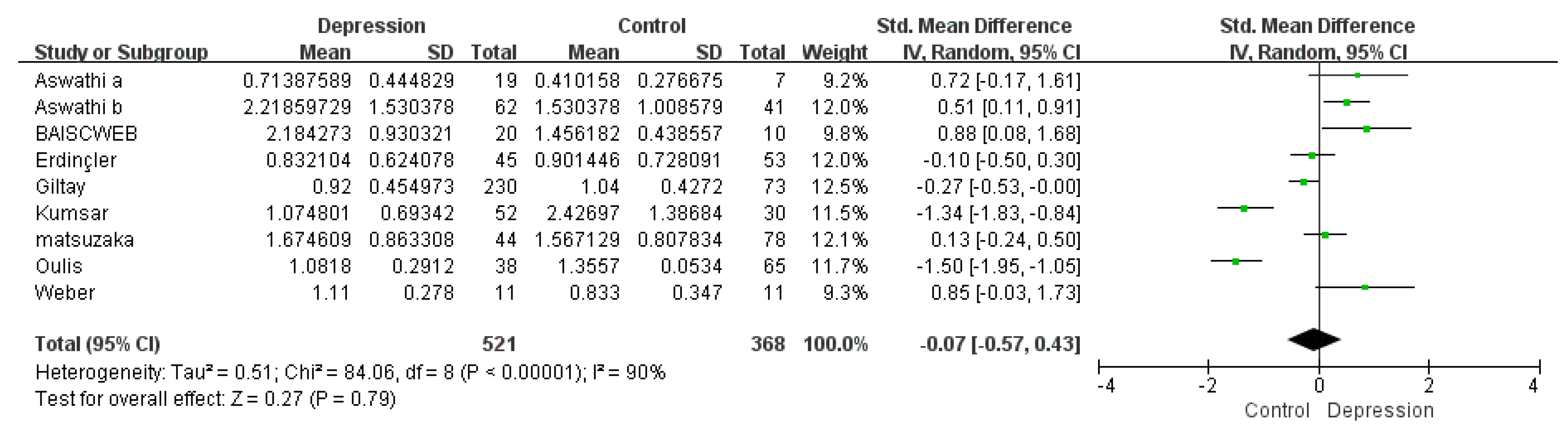
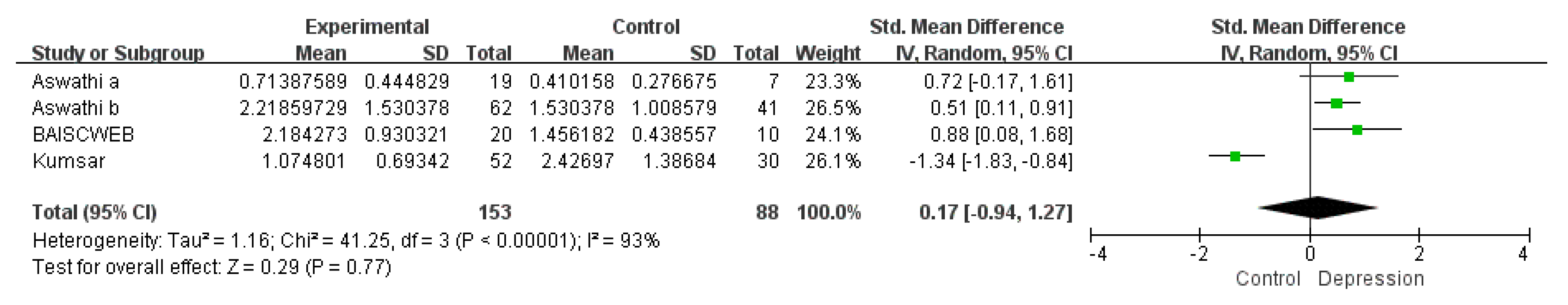
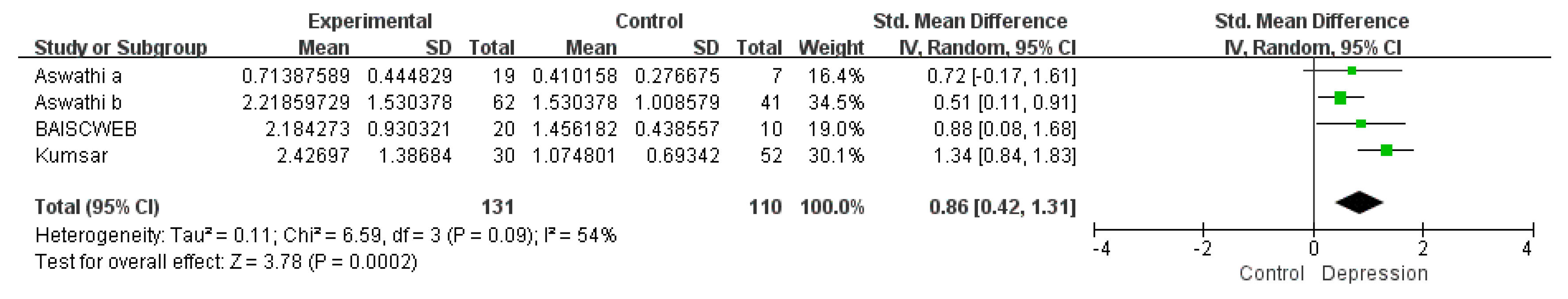
3.1.3. Subgroup Meta-Analysis
3.1.4. Absolute SMD Meta-Analysis
3.2. Mendelian Randomization
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kessler, R.C.; Bromet, E.J. The Epidemiology of Depression across Cultures. Annu. Rev. Public Health 2013, 34, 119–138. [Google Scholar] [CrossRef]
- Bromet, E.; Andrade, L.H.; Hwang, I.; Sampson, N.A.; Alonso, J.; De Girolamo, G.; De Graaf, R.; Demyttenaere, K.; Hu, C.; Iwata, N.; et al. Cross-national epidemiology of DSM-IV major depressive episode. BMC Med. 2011, 9, 90. [Google Scholar] [CrossRef]
- Vos, T.; Flaxman, A.D.; Naghavi, M.; Lozano, R.; Michaud, C.; Ezzati, M.; Shibuya, K.; Salomon, J.A.; Abdalla, S.; Aboyans, V.; et al. Years lived with disability (YLDs) for 1160 se-quelae of 289 diseases and injuries 1990–2010: A systematic analysis for the Global Burden of Disease Study 2010. Lancet 2012, 380, 2163–2196. [Google Scholar] [CrossRef]
- Beck, A.; Crain, A.L.; Solberg, L.I.; Unützer, J.; Glasgow, R.E.; Maciosek, M.V.; Whitebird, R. Severity of Depression and Magnitude of Productivity Loss. Ann. Fam. Med. 2011, 9, 305–311. [Google Scholar] [CrossRef] [PubMed]
- Angst, J.; Gamma, A.; Gastpar, M.; Mendlewicz, J.; Tylee, A. Gender differences in depression. Eur. Arch. Psychiatry Clin. Neurosci. 2002, 252, 201–209. [Google Scholar] [CrossRef] [PubMed]
- Weissman, M.M.; Bland, R.C.; Canino, G.J.; Faravelli, C.; Greenwald, S.; Hwu, H.G.; Joyce, P.R.; Karam, E.G.; Lee, C.K.; Lellouch, J.; et al. Cross-national epidemiology of major depression and bipolar disorder. JAMA 1996, 276, 293–299. [Google Scholar] [CrossRef] [PubMed]
- Jantaratnotai, N.; Mosikanon, K.; Lee, Y.; McIntyre, R.S. The interface of depression and obesity. Obes. Res. Clin. Pract. 2017, 11, 1–10. [Google Scholar] [CrossRef]
- Durdiakova, J.; Ostatnikova, D.; Celec, P. Testosterone and its metabolites--modulators of brain functions. Acta Neurobiol. Exp. 2011, 71, 434–454. [Google Scholar]
- Spivak, B.; Maayan, R.; Mester, R.; Weizman, A. Plasma testosterone levels in patients with combat-related posttraumatic stress disorder. Neuropsychobiology 2003, 47, 57–60. [Google Scholar] [CrossRef]
- Zarrouf, F.A.; Artz, S.; Griffith, J.; Sirbu, C.; Kommor, M. Testosterone and depression: Systematic review and meta-analysis. J. Psychiatr. Pract. 2009, 15, 289–305. [Google Scholar] [CrossRef] [PubMed]
- Seidman, S.N.; Walsh, B.T. Testosterone and depression in aging men. Am. J. Geriatr. Psychiatry 1999, 7, 18–33. [Google Scholar] [CrossRef]
- Juraska, J.M.; Sisk, C.L.; DonCarlos, L.L. Sexual differentiation of the adolescent rodent brain: Hormonal influences and de-velopmental mechanisms. Horm. Behav. 2013, 64, 203–210. [Google Scholar] [CrossRef] [PubMed]
- McHenry, J.; Carrier, N.; Hull, E.; Kabbaj, M. Sex differences in anxiety and depression: Role of testosterone. Front. Neuroendocr. 2014, 35, 42–57. [Google Scholar] [CrossRef] [PubMed]
- Walther, A.; Breidenstein, J.; Miller, R. Association of Testosterone Treatment with Alleviation of Depressive Symptoms in Men: A Systematic Review and Meta-analysis. JAMA Psychiatry 2019, 76, 31–40. [Google Scholar] [CrossRef] [PubMed]
- Altemus, M.; Sarvaiya, N.; Epperson, C.N. Sex differences in anxiety and depression clinical perspectives. Front. Neuroendocr. 2014, 35, 320–330. [Google Scholar] [CrossRef] [PubMed]
- Barrett-Connor, E.; Von Muhlen, D.G.; Kritz-Silverstein, D. Bioavailable testosterone and depressed mood in older men: The Rancho Bernardo Study. J. Clin. Endocrinol. Metab. 1999, 84, 573–5777. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Nieschlag, E.; Swerdloff, R.; Behre, H.M.; Hellstrom, W.J.; Gooren, L.J.; Kaufman, J.M.; Legros, J.-J.; Lunenfeld, B.; Morales, A.; et al. Investigation, treatment and monitoring of late-onset hypogonadism in males. Int. J. Androl. 2009, 32, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Seidman, S.N. Testosterone Deficiency and Mood in Aging Men: Pathogenic and Therapeutic Interactions. World J. Biol. Psychiatry 2003, 4, 14–20. [Google Scholar] [CrossRef]
- Margolese, H.C. The male menopause and mood: Testosterone decline and depression in the aging male—Is there a link? J. Geriatr. Psychiatry Neurol. 2000, 13, 93–101. [Google Scholar] [CrossRef]
- Bhasin, S.; Cunningham, G.R.; Hayes, F.J.; Matsumoto, A.M.; Snyder, P.J.; Swerdloff, R.S.; Montori, V.M. Testosterone Therapy in Men with Androgen Deficiency Syndromes: An Endocrine Society Clinical Practice Guideline. J. Clin. Endocrinol. Metab. 2010, 95, 2536–2559. [Google Scholar] [CrossRef]
- Amiaz, R.; Seidman, S.N. Testosterone and depression in men. Curr. Opin. Endocrinol. Diabetes Obes. 2008, 15, 278–283. [Google Scholar] [CrossRef]
- Diblasio, C.J.; Derweesh, I.H.; Malcolm, J.B.; Maddox, M.M.; Aleman, M.A.; Wake, R.W. Contemporary analysis of erectile, voiding, and oncologic outcomes following primary targeted cryoablation of the prostate for clinically localized prostate cancer. Int. Braz. J. Urol. 2008, 34, 443–450. [Google Scholar] [CrossRef][Green Version]
- Rabkin, J.G.; Wagner, G.J.; Rabkin, R. A double-blind, placebo-controlled trial of testosterone therapy for HIV-positive men with hypogonadal symptoms. Arch. Gen. Psychiatry 2000, 57, 141–147. [Google Scholar] [CrossRef]
- Wang, C.; Alexander, G.; Berman, N.; Salehian, B.; Davidson, T.; McDonald, V.; Steiner, B.; Hull, L.; Callegari, C.; Swerdloff, R.S.; et al. Testosterone replacement therapy im-proves mood in hypogonadal men--a clinical research center study. J. Clin. Endocrinol. Metab. 1996, 81, 3578–3583. [Google Scholar]
- Pope, H.G.; Cohane, G.H.; Kanayama, G.; Siegel, A.J.; Hudson, J.I. Testosterone Gel Supplementation for Men With Refractory Depression: A Randomized, Placebo-Controlled Trial. Am. J. Psychiatry 2003, 160, 105–111. [Google Scholar] [CrossRef] [PubMed]
- Kanayama, G.; Amiaz, R.; Seidman, S.; Pope, H.G. Testosterone supplementation for depressed men: Current research and suggested treatment guidelines. Exp. Clin. Psychopharmacol. 2007, 15, 529–538. [Google Scholar] [CrossRef] [PubMed]
- Ivandić, A.; Prpić-Krizevac, I.; Sucić, M.; Jurić, M. Hyperinsulinemia and sex hormones in healthy premenopausal women: Relative contribution of obesity, obesity type, and duration of obesity. Metabolism 1998, 47, 13–19. [Google Scholar] [CrossRef]
- Glassman, A.H.; Shapiro, P.A. Depression and the Course of Coronary Artery Disease. Am. J. Psychiatry 1998, 155, 4–11. [Google Scholar] [CrossRef]
- Albert, K.; Pruessner, J.; Newhouse, P. Estradiol levels modulate brain activity and negative responses to psychosocial stress across the menstrual cycle. Psychoneuroendocrinology 2015, 59, 14–24. [Google Scholar] [CrossRef] [PubMed]
- Hayaki, J.; Holzhauer, C.G.; Epstein, E.E.; Cook, S.; Gaba, A.; Lorenzo, A.C.; McCrady, B.S. Menstrual cycle phase, alcohol consumption, alcohol cravings, and mood among women in outpatient treatment for alcohol use disorder. Psychol. Addict. Behav. 2020, 34, 680–689. [Google Scholar] [CrossRef] [PubMed]
- Steiner, M.; Dunn, E.; Born, L. Hormones and mood: From menarche to menopause and beyond. J. Affect. Disord. 2003, 74, 67–83. [Google Scholar] [CrossRef]
- Kumsar, S.; Kumsar, N.A.; Saglam, H.S.; Kose, O.; Budak, S.; Adsan, O. Testosterone levels and sexual function disorders in de-pressive female patients: Effects of antidepressant treatment. J. Sex. Med. 2014, 11, 529–535. [Google Scholar] [CrossRef]
- van Noorden, M.S.; Giltay, E.J.; den Hollander-Gijsman, M.E.; van der Wee, N.J.; van Veen, T.; Zitman, F.G. Gender differences in clinical characteristics in a naturalistic sample of depressive outpatients: The Leiden Routine Outcome Monitoring Study. J. Affect. Disord. 2010, 125, 116–123. [Google Scholar] [CrossRef] [PubMed]
- Hui, L.Y.; Wang, Y.W.; Zhou, F.L.; Ma, X.C.; Yan, R.Z.; Zhang, L.; Wang, Q.; Yu, X. Association Between MKP-1, BDNF, and Gonadal Hor-mones with Depression on Perimenopausal Women. J. Womens Health 2016, 25, 71–77. [Google Scholar] [CrossRef]
- Aswathi, A.; Rajendiren, S.; Nimesh, A.; Philip, R.R.; Kattimani, S.; Jayalakshmi, D.; Ananthanarayanan, P.; Dhiman, P. High serum testosterone levels during postpartum period are associated with postpartum depression. Asian J. Psychiatry 2015, 17, 85–88. [Google Scholar] [CrossRef]
- Baischer, W.; Koinig, G.; Hartmann, B.; Huber, J.; Langer, G. Hypothalamic-pituitary-gonadal axis in depressed premenopau-sal women: Elevated blood testosterone concentrations compared to normal controls. Psychoneuroendocrinology 1995, 20, 553–559. [Google Scholar] [CrossRef]
- Weber, B.; Lewicka, S.; Deuschle, M.; Colla, M.; Heuser, I. Testosterone, androstenedione and dihydrotestosterone concentra-tions are elevated in female patients with major depression. Psychoneuroendocrinology 2000, 25, 765–771. [Google Scholar] [CrossRef]
- Stanikova, D.; Zsido, R.G.; Luck, T.; Pabst, A.; Enzenbach, C.; Bae, Y.J.; Thiery, J.; Ceglarek, U.; Engel, C.; Wirkner, K.; et al. Testosterone imbalance may link depression and in-creased body weight in premenopausal women. Transl. Psychiatry 2019, 9, 160. [Google Scholar] [CrossRef] [PubMed]
- Grover, S.; Del Greco, M.F.; Stein, C.M.; Ziegler, A. Mendelian Randomization. Methods Mol. Biol. 2017, 1666, 581–628. [Google Scholar] [PubMed]
- Syed, A.A.S.; He, L.; Shi, Y. The Potential Effect of Aberrant Testosterone Levels on Common Diseases: A Mendelian Ran-domization Study. Genes 2020, 11, 721. [Google Scholar] [CrossRef] [PubMed]
- Ruth, K.S.; The Endometrial Cancer Association Consortium; Day, F.R.; Tyrrell, J.; Thompson, D.J.; Wood, A.R.; Mahajan, A.; Beaumont, R.N.; Wittemans, L.; Martin, S.; et al. Using human genetics to understand the disease impacts of testosterone in men and women. Nat. Med. 2020, 26, 252–258. [Google Scholar] [CrossRef]
- Howard, D.M.; Adams, M.J.; Shirali, M.; Clarke, T.K.; Marioni, R.E.; Davies, G.; Coleman, J.R.I.; Alloza, C.; Shen, X.; Barbu, M.C.; et al. Genome-wide association study of depression phenotypes in UK Biobank identifies variants in excitatory synaptic pathways. Nat. Commun. 2018, 9, 1470. [Google Scholar] [CrossRef] [PubMed]
- Howard, D.M.; Adams, M.J.; Clarke, T.-K.; Hafferty, J.D.; Gibson, J.; Shirali, M.; Coleman, J.R.I.; Hagenaars, S.P.; Ward, J.; Wigmore, E.M.; et al. Genome-wide meta-analysis of depression identifies 102 independent variants and highlights the importance of the prefrontal brain regions. Nat. Neurosci. 2019, 22, 343–352. [Google Scholar] [CrossRef]
- Erdinçler, D.; Bugay, G.; Ertan, T.; Eker, E. Depression and sex hormones in elderly women. Arch. Gerontol. Geriatr. 2004, 39, 239–244. [Google Scholar] [CrossRef]
- Matsuzaka, H.; Maeshima, H.; Kida, S.; Kurita, H.; Shimano, T.; Nakano, Y.; Baba, H.; Suzuki, T.; Arai, H. Gender Differences in Serum Testosterone and Cortisol in Patients with Major Depressive Disorder Compared with Controls. Int. J. Psychiatry Med. 2013, 46, 203–221. [Google Scholar] [CrossRef] [PubMed]
- Giltay, E.J.; van der Mast, R.C.; Lauwen, E.; Heijboer, A.C.; de Waal, M.W.; Comijs, H.C. Plasma Testosterone and the Course of Major Depressive Disorder in Older Men and Women. Am. J. Geriatr. Psychiatry 2017, 25, 425–437. [Google Scholar] [CrossRef]
- Oulis, P.; Masdrakis, V.G.; Markianos, M. Testosterone and dehydroepiandrosterone sulfate in female anxious and non-anxious major depression. Int. J. Psychiatry Clin. Pract. 2014, 18, 21–24. [Google Scholar] [CrossRef]
- Lao, C.-K.; Chan, Y.-M.; Tong, H.H.-Y.; Chan, A. Underdiagnosis of depression in an economically deprived population in Macao, China. Asia-Pac. Psychiatry 2016, 8, 70–79. [Google Scholar] [CrossRef]
- Sigalas, P.D.; Barkla, X.; McArdle, P. Underdiagnosis of depression in young people. BMJ 2014, 348, g170. [Google Scholar] [CrossRef]
- Rohr, U.D. The impact of testosterone imbalance on depression and women’s health. Maturitas 2002, 41 (Suppl. 1), S25–S46. [Google Scholar] [CrossRef]
- Booth, A.J.D.; Grainger, D.A. Testosterone and men’s depression: The role of social behavior. J. Health Soc. Behav. 1999, 40, 130. [Google Scholar] [CrossRef]
| Outcome | SNPs | IVW (p-Val.) | WM (p-Val.) | MR-Egger Intercept (p-Val.) |
|---|---|---|---|---|
| Broad depression | 125 | 0.005 (0.20) | 0.011 (0.08) | 0.43 |
| MDD | 121 | 0.0216 (0.22) | 0.013 | 0.70 |
| Outcome | SNPs | IVW (p-Val.) | WM (p-Val.) | MR-Egger Intercept (p-Val.) |
|---|---|---|---|---|
| Broad depression | 92 | 0.0005 (0.94) | 0.0005 (0.95) | 0.80 |
| MDD | 91 | 0.0028 (0.92) | 0.006 (0.86) | 0.89 |
| Outcome | SNPs | IVW (p-Val.) | WM (p-Val.) | MR-Egger Intercept (p-Val.) |
|---|---|---|---|---|
| Broad depression | 170 | 0.009 (0.35) | 0.001 (0.93) | 0.18 |
| MDD | 173 | 0.043 (0.249) | 0.0003 (0.99) | 0.67 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maharjan, D.T.; Syed, A.A.S.; Lin, G.N.; Ying, W. Testosterone in Female Depression: A Meta-Analysis and Mendelian Randomization Study. Biomolecules 2021, 11, 409. https://doi.org/10.3390/biom11030409
Maharjan DT, Syed AAS, Lin GN, Ying W. Testosterone in Female Depression: A Meta-Analysis and Mendelian Randomization Study. Biomolecules. 2021; 11(3):409. https://doi.org/10.3390/biom11030409
Chicago/Turabian StyleMaharjan, Dhruba Tara, Ali Alamdar Shah Syed, Guan Ning Lin, and Weihai Ying. 2021. "Testosterone in Female Depression: A Meta-Analysis and Mendelian Randomization Study" Biomolecules 11, no. 3: 409. https://doi.org/10.3390/biom11030409
APA StyleMaharjan, D. T., Syed, A. A. S., Lin, G. N., & Ying, W. (2021). Testosterone in Female Depression: A Meta-Analysis and Mendelian Randomization Study. Biomolecules, 11(3), 409. https://doi.org/10.3390/biom11030409






