Phytochemical Analysis and Biological Activities of the Ethanolic Extract of Daphne sericea Vahl Flowering Aerial Parts Collected in Central Italy
Abstract
1. Introduction
2. Material and Methods
2.1. Plant Material
2.2. Chemicals
2.3. Extraction Procedure
2.4. Isolation and Identification of the Metabolites
2.5. NMR Analysis
2.6. MS Analysis
2.7. NMR and MS Data of the Isolated Compounds
2.8. Antioxidant Activity
2.9. Cytotoxic Activity
2.10. Statistical Analysis
3. Results and Discussion
3.1. Phytochemistry
3.2. Chemotaxonomy
3.3. Biological Activities
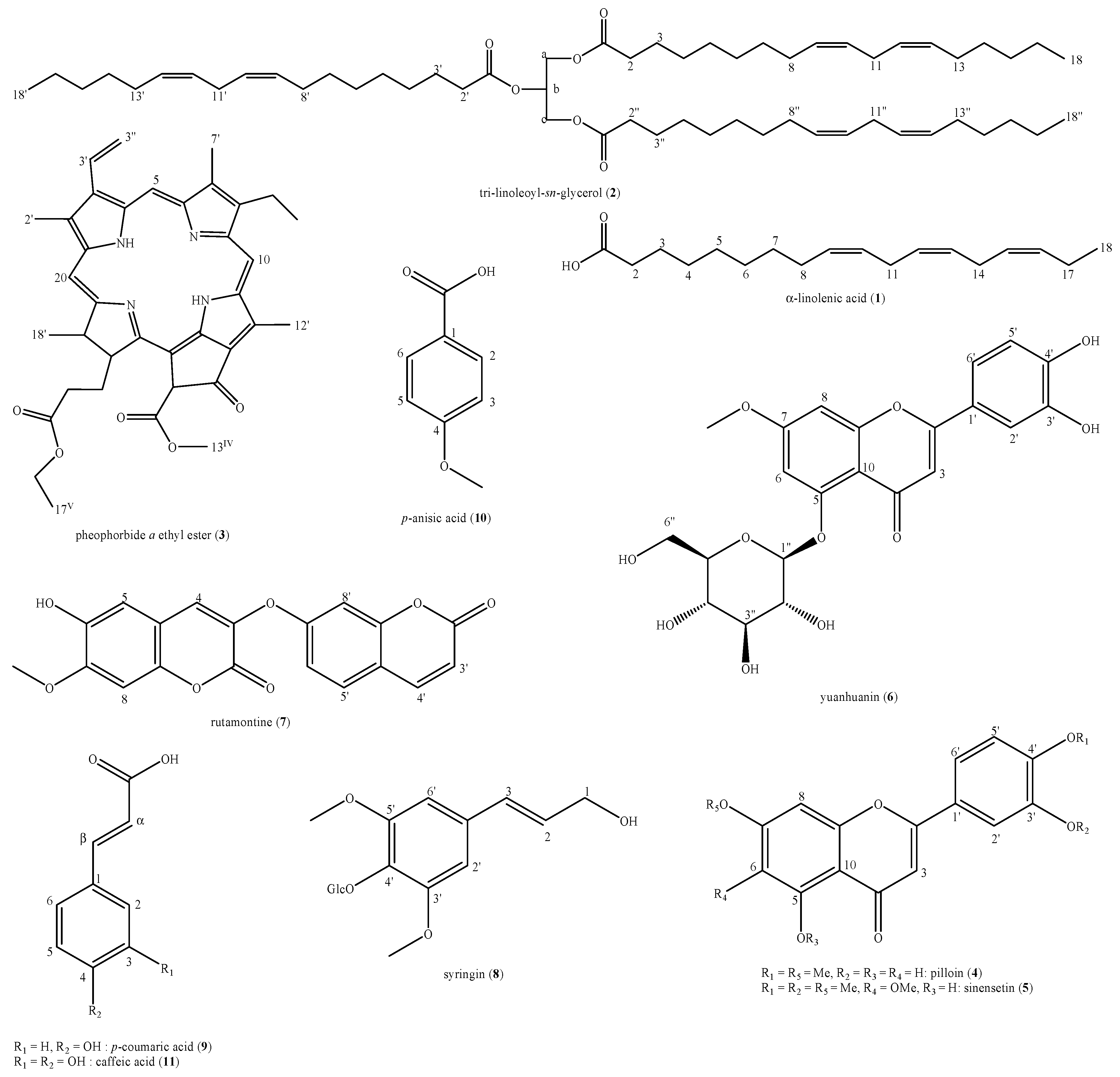
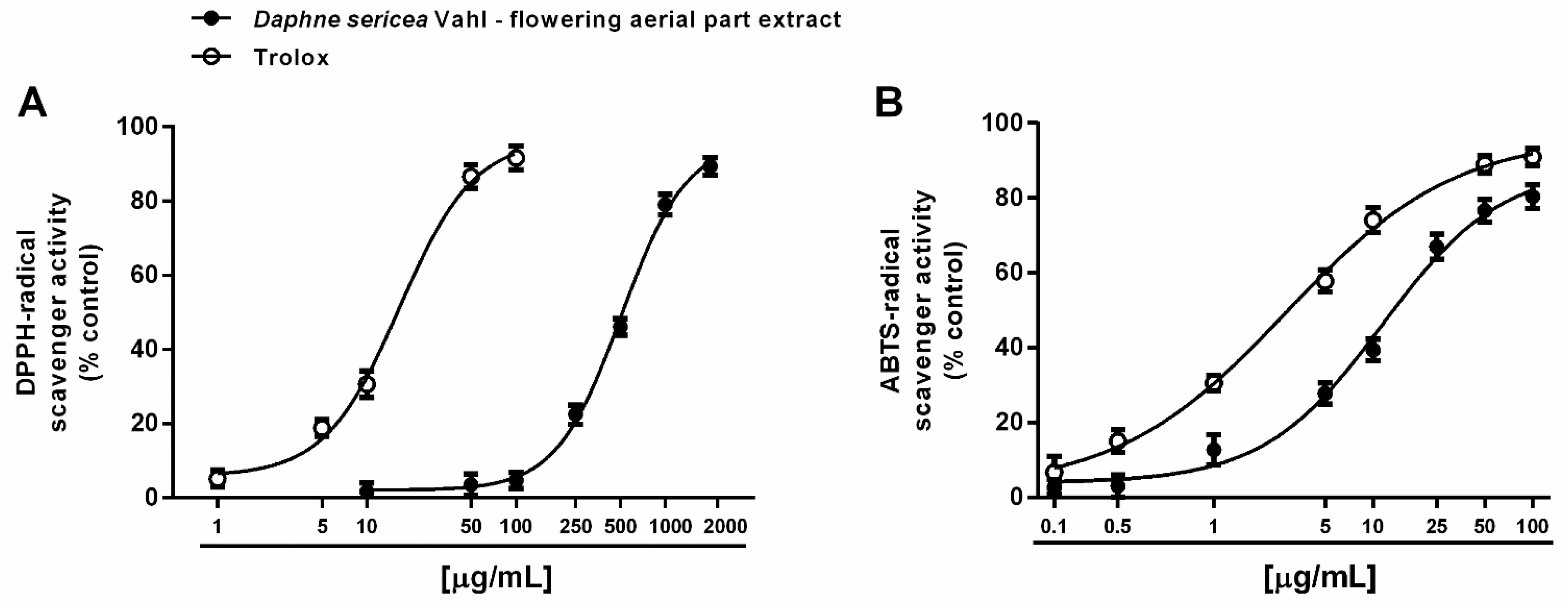
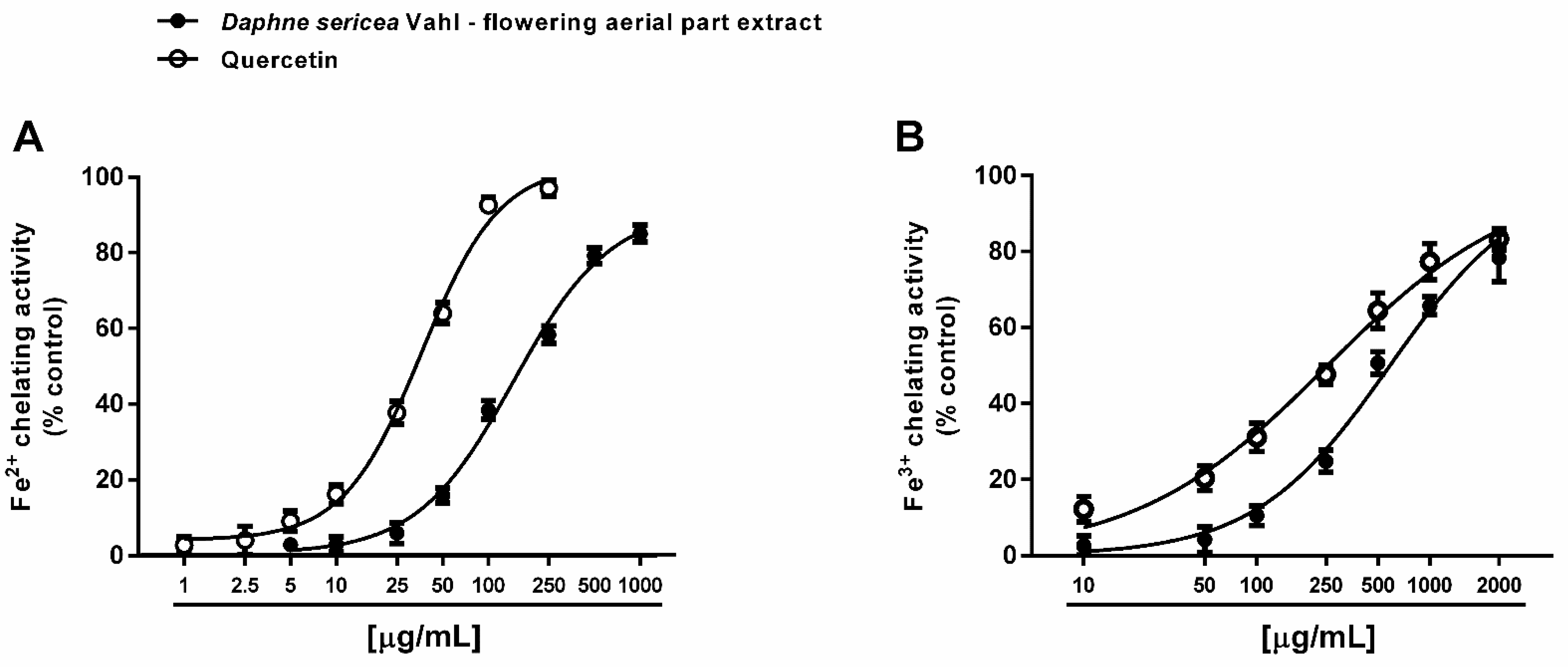
3.3.1. Antioxidant Activity
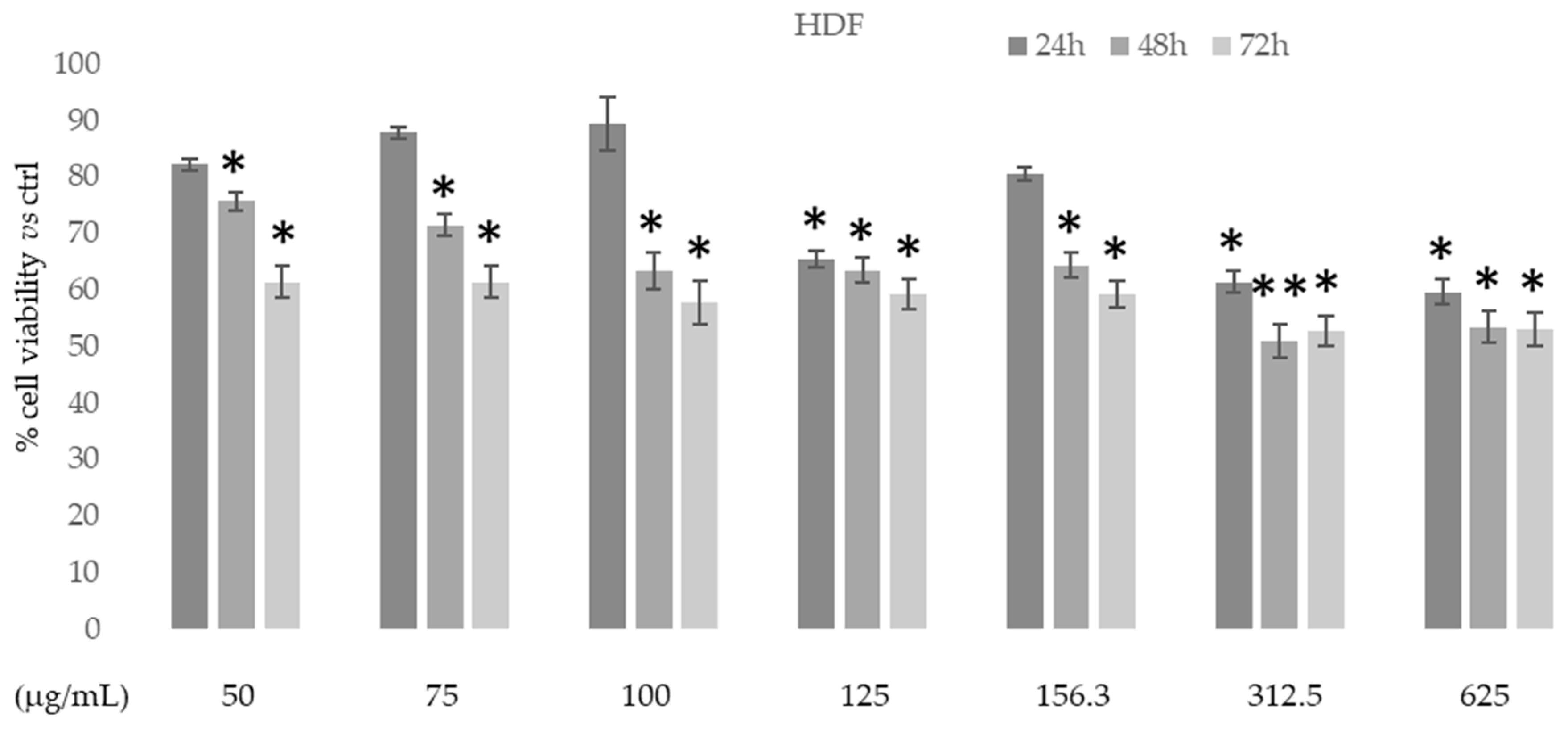
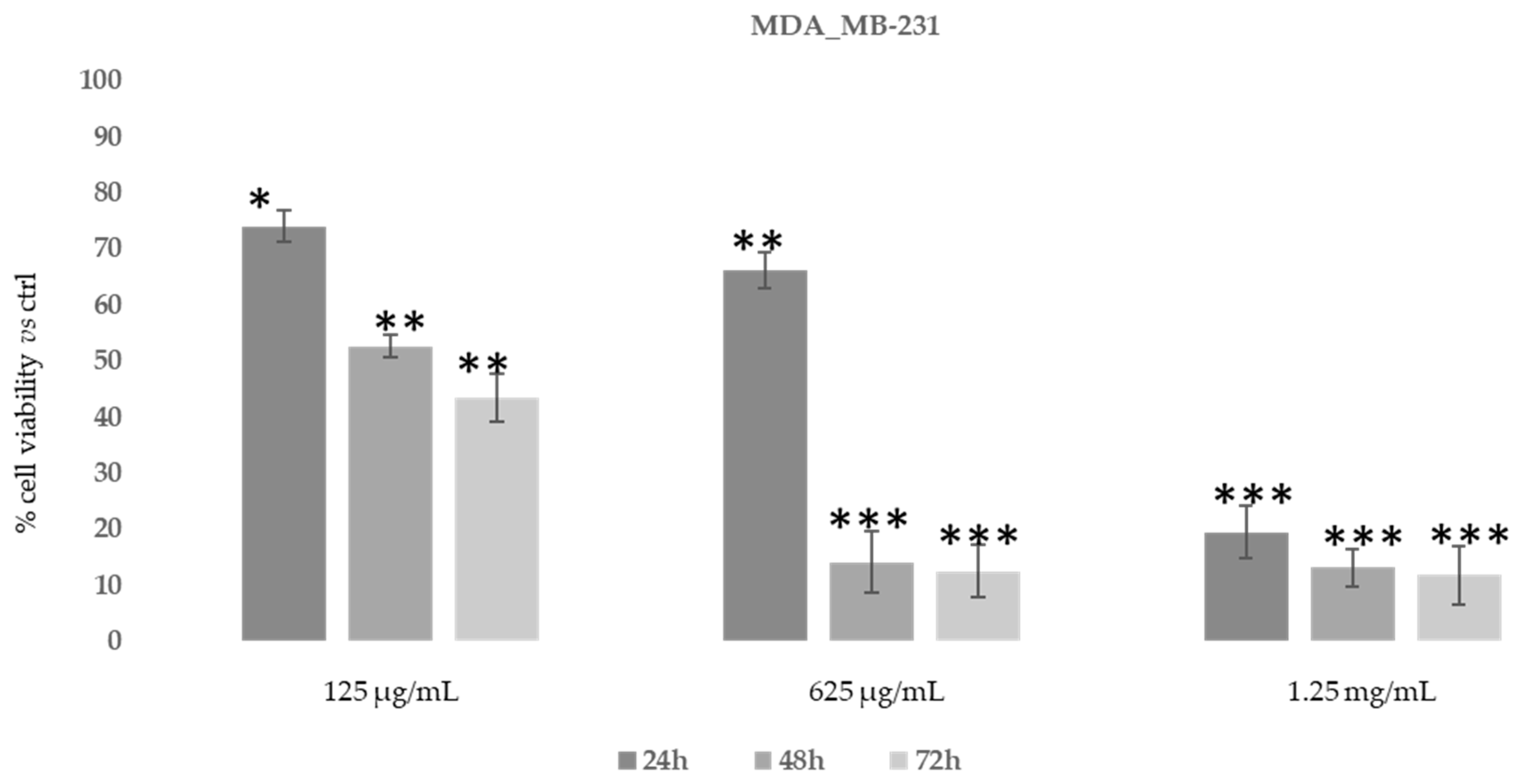
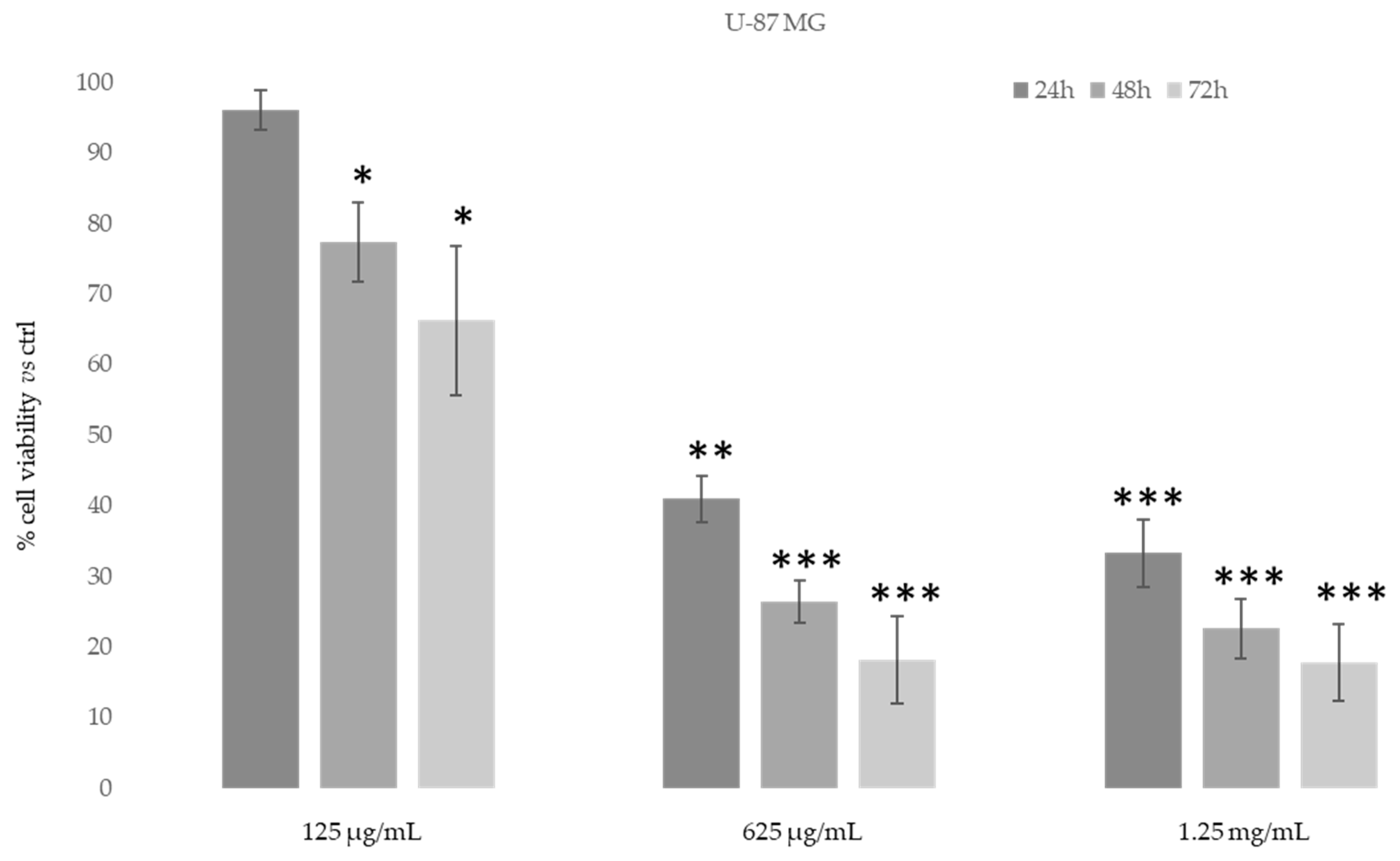
3.3.2. Cytotoxic Activity
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Vahl, M. Symbola Ebotanicae, Sive Plantarum, Tam Earum, Quas in Itinere, in Primis Orientali, Collegit Petrus Forskål, Quam Aliarum, Recen-tius Detectarum, Exactiores Descriptiones, nec non Observationes Circa Quasdam Plantas Dudum Cognitas; Pars prima; Nicolaus Möller & fil.: Copenhagen, Denmark, 1970; p. 28. [Google Scholar]
- Tutin, T.G.; Heywood, V.H.; Burges, N.A.; Moore, D.M.; Valentine, D.H.; Walters, S.M.; Webb, D.A. Flora Europaea; Cambridge University Press: Cambridge, UK, 1968; Volume 2. [Google Scholar]
- Pignatti, S.; Guarino, R.; La Rosa, M. Flora d’Italia, 2nd ed.; Edagricole—Edizioni Agricole di New Business Media: Milano, Italy, 2017; Volume 2. [Google Scholar]
- Pedrol, J. Thymelaeaceae. Euro+MedPlantbase—The Information Resource for Euro-Mediterranean Plant Diversity. Thymelaeaceae Juss. 2011. Available online: http://ww2.bgbm.org/euroPlusMed/PTaxonDetail.asp?UUID=0B0F7461-92DE-47A0-854C-5E9E595081C2 (accessed on 25 January 2021).
- Bartolucci, F.; Peruzzi, L.; Galasso, G.; Albano, A.; Alessandrini, A.; Ardenghi, N.M.G.; Astuti, G.; Bacchetta, G.; Ballelli, S.; Banfi, E.; et al. An updated checklist of the vascular flora native to Italy. Plant Biosyst. 2018, 152, 179–303. [Google Scholar] [CrossRef]
- Güzel, Y.; Güzelşemme, M.; Miski, M. Ethnobotany of medicinal plants used in Antakya: A multicultural district in Hatay province of Turkey. J. Ethnopharmacol. 2015, 174, 118–152. [Google Scholar] [CrossRef]
- Türkmen, N.; Kirici, S.; Özgüven, M.; İnan, M.; Kaya, D.A. An investigation of dye plants and their colourant substances in the eastern Mediterranean region of Turkey. Bot. J. Linn. Soc. 2004, 146, 71–77. [Google Scholar] [CrossRef]
- Moshiashvili, G.; Tabatadze, N.; Mshvildadze, V. The genus Daphne: A review of its traditional uses, phytochemistry and pharmacology. Fitoterapia 2020, 143, 1–39. [Google Scholar] [CrossRef] [PubMed]
- Tongur, T.; Erkan, N.; Ayranci, E. Investigation of the composition and antioxidant activityof acetone and methanol extracts of Daphne sericea L. and Daphne gnidioides L. J. Food Sci. Technol. 2018, 55, 1396–1406. [Google Scholar] [CrossRef] [PubMed]
- Ulubelen, A.; Bucher, R.; Mabry, T.J. Flavone 5-O-glucosides from Daphne sericea. Phytochemistry 1982, 21, 801–803. [Google Scholar] [CrossRef]
- Kupchan, S.M.; Baxter, R.L. Mezerein: Antileukemic principle isolated from Daphne mezereum L. Science 1975, 21, 652–653. [Google Scholar] [CrossRef]
- Bahadırlı, N.P.; Türkmen, M. Essential oil compositions of Daphne sericea vahl. flowers with hydrodistillation method. Am. J. Essent. Oils Nat. Prod. 2020, 8, 6–9. [Google Scholar]
- Venditti, A. What is and what should never be: Artifacts, improbable phytochemicals, contaminants and natural products. Nat. Prod. Res. 2020, 34, 1014–1031. [Google Scholar] [CrossRef]
- Sciubba, F.; Di Cocco, M.E.; Gianferri, R.; Impellizzeri, D.; Mannina, L.; De Salvador, F.R.; Venditti, A.; Delfini, M. Metabolic profile of different Italian cultivars of hazelnut (Corylus avellana) by nuclear magnetic resonance spectroscopy. Nat. Prod. Res. 2014, 28, 1075–1081. [Google Scholar] [CrossRef]
- Venditti, A.; Serrilli, A.M.; Di Cecco, M.; Ciaschetti, G.; Bianco, A. Coumarins and other components of Daphne oleoides Schreb. subsp. oleoides from Majella National Park. Biochem. Syst. Ecol. 2019, 83, 39–46. [Google Scholar] [CrossRef]
- Tsai, Y.S.; Chen, C.T.; Yeh, H.C.; Li, H.T.; Chen, C.Y. Chemical constituents of the leaves of Elaeagnus grandifolia. Chem. Nat. Compd. 2019, 55, 334–336. [Google Scholar] [CrossRef]
- Nagase, H.; Omae, N.; Omori, A.; Nakagawasai, O.; Tadano, T.; Yokosuka, A.; Sashida, Y.; Mimaki, Y.; Tamakuni, T.; Ohizumi, Y. Nobiletin and its related flavonoids with CRE-dependent transcription-stimulating and neuritegenic activities. Biochem. Biophys. Res. Commun. 2005, 337, 1330–1336. [Google Scholar] [CrossRef]
- Kabouche, Z.; Benkiki, N.; Seguin, E.; Bruneau, C. A new dicoumarinyl ether and two rare furocoumarins from Ruta montana. Fitoterapia 2003, 74, 194–196. [Google Scholar] [CrossRef]
- Kort, R.; Vonk, H.; Xu, X.; Hoff, W.D.; Crielaard, W.; Hellingwerf, K.J. Evidence for trans-cis isomerization of the p-coumaric acid chromophores as the photochemical basis of the photocycle of photoactive yellow protein. FEBS Lett. 1996, 382, 73–78. [Google Scholar] [CrossRef]
- Xu, W.; Jin, H.; Zhang, W.; Hu, X.; Zhang, W.; Fu, J.; Su, J.; Yan, S.; Shen, Y. Chemical constituents from Daphne pedunculata. Chem. Nat. Compd. 2009, 45, 417–419. [Google Scholar] [CrossRef]
- Saha, R.; Ghosh, A.; Saha, B. Kinetics of micellar catalysis on oxidation of p-anisaldehyde to p-anisic acid in aqueous medium at room temperature. Chem. Eng. Sci. 2013, 99, 23–27. [Google Scholar] [CrossRef]
- Frezza, C.; Venditti, A.; Rossi, G.; Serafini, I.; Pitorri, M.; Ciccòla, A.; Foddai, S.; Bianco, A.; Serafini, M. Phytochemical study on the leaves of Wollemia nobilis. Biochem. Syst. Ecol. 2017, 74, 63–66. [Google Scholar] [CrossRef]
- Venditti, A.; Frezza, C.; Maggi, F.; Lupidi, G.; Bramucci, M.; Quassinti, L.; Giuliani, C.; Cianfaglione, K.; Papa, F.; Serafini, M.; et al. Phytochemistry, micromorphology and bioactivities of Ajuga chamaepitys (L.) Schreb. (Lamiaceae, Ajugoideae): Two new harpagide derivatives and an unusual iridoid glycosides pattern. Fitoterapia 2016, 113, 35–43. [Google Scholar] [CrossRef]
- Venditti, A.; Maggi, F.; Quassinti, L.; Bramucci, M.; Lupidi, G.; Ornano, L.; Ballero, M.; Sanna, C.; Bruno, M.; Rosselli, S.; et al. Bioactive constituents of Juniperus turbinata Guss. from La Maddalena Archipelago. Chem. Biodivers. 2018, 15, 1–15. [Google Scholar] [CrossRef]
- Frezza, C.; Venditti, A.; Sciubba, F.; Tomai, P.; Antonetti, M.; Franceschin, M.; Di Cocco, M.E.; Gentili, A.; Delfini, M.; Serafini, M.; et al. Phytochemical profile of Euphorbia peplus L. collected in Central Italy and NMR semi-quantitative analysis of the diterpenoid fraction. J. Pharma Biomed. Anal. 2018, 160, 152–159. [Google Scholar] [CrossRef]
- Venditti, A.; Frezza, C.; Vincenti, F.; Brodella, A.; Sciubba, F.; Montesano, C.; Franceschin, M.; Sergi, M.; Foddai, S.; Di Cocco, M.E.; et al. A syn-ent-labdadiene derivative with a rare spiro-β-lactone function from the male cones of Wollemia nobilis. Phytochemistry 2019, 158, 91–95. [Google Scholar] [CrossRef] [PubMed]
- Venditti, A.; Frezza, C.; Rossi, G.; Serafini, I.; Ciccola, A.; Sciubba, F.; Foddai, S.; Tomassini, L.; Bianco, A.; Serafini, M. A new bicyclic monoterpene glucoside and a new biflavone from the male reproduction organs of Wollemia nobilis. Fitoterapia 2019, 133, 62–69. [Google Scholar] [CrossRef]
- Venditti, A.; Ukwueze, S.E. A possible glycosidic benzophenone with full substitution on B-ring from Psidium guajava leaves. Nat. Prod. Res. 2017, 31, 739–741. [Google Scholar] [CrossRef] [PubMed]
- Di Sotto, A.; Vecchiato, M.; Abete, L.; Toniolo, C.; Giusti, A.M.; Mannina, L.; Locatelli, M.; Nicoletti, M.; Di Giacomo, S. Capsicum annuum L. var. Cornetto di Pontecorvo PDO: Polyphenolic profile and in vitro biological activities. J. Funct. Foods 2018, 40, 679–691. [Google Scholar] [CrossRef]
- Di Sotto, A.; Checconi, P.; Celestino, I.; Locatelli, M.; Carissimi, S.; De Angelis, M.; Rossi, V.; Limongi, D.; Toniolo, C.; Martinoli, L.; et al. Antiviral and antioxidant activity of a hydroalcoholic extract from Humulus lupulus L. Oxid. Med. Cell. Longev. 2018, 2018, 1–14. [Google Scholar] [CrossRef]
- Akowuah, G.A.; Zhari, I.; Norhayati, I.; Sadikun, A.; Khamsah, S.M. Sinensetin, eupatorin, 3′-hydroxy-5,6,7,4′-tetramethoxyflavone and rosmarinic acid contents and antioxidative effect of Orthosiphon stamineus from Malaysia. Food Chem. 2004, 87, 559–566. [Google Scholar] [CrossRef]
- Yam, M.F.; Lim, V.; Salman, I.M.; Ameer, O.Z.; Ang, L.F.; Rosidah, N.; Abdulkarim, M.F.; Abdullah, G.Z.; Basir, R.; Sadikun, A.; et al. HPLC and anti-inflammatory studies of the flavonoid rich chloroform extract fraction of Orthosiphon stamineus leaves. Molecules 2010, 15, 4452–4466. [Google Scholar] [CrossRef] [PubMed]
- Laavola, M.; Nieminen, R.; Yam, M.F.; Sadikun, A.; Asmawi, M.Z.; Basir, R.; Welling, J.; Vapaatalo, H.; Korhonen, R.; Moilanen, E. Flavonoids eupatorin and sinensetin present in Orthosiphon stamineus leaves inhibit inflammatory gene expression and STAT1 activation. Planta Med. 2012, 78, 779–786. [Google Scholar] [CrossRef] [PubMed]
- Hossain, M.A.; Ismail, Z. Quantification and enrichment of sinensetin in the leaves of Orthosiphon stamineus. Arab. J. Chem. 2016, 9, 1338–1341. [Google Scholar] [CrossRef]
- Liang, S.; Tian, J.-M.; Feng, Y.; Liu, X.-H.; Xiong, Z.; Zhang, W.-D. Flavonoids from Daphne aurantiaca and their inhibitory activities against nitric oxide production. Chem. Pharm. Bull. 2011, 59, 653–656. [Google Scholar] [CrossRef]
- Xu, W.C.; Shen, J.G.; Jiang, J.Q. Phytochemical and biological studies of the plants from the genus Daphne. Chem. Biodivers. 2011, 8, 1215–1233. [Google Scholar] [CrossRef]
- Huang, S.Z.; Ma, Q.Y.; Liu, Y.Q.; Zhou, J.; Zhao, Y.X. Chemical constituents from Daphne acutiloba. China J. Chin. Mat. Med. 2013, 38, 64–69. [Google Scholar]
- Venditti, A.; Sanna, C.; Lorenzetti, L.M.; Ballero, M.; Bianco, A. New coumarinyl ethers in Daphne oleoides SCHREB. collected from Sardinia Island. Chem. Biodivers. 2017, 14, 1–8. [Google Scholar] [CrossRef]
- Niwa, M.; Sugino, H.; Takashima, S.; Sakai, T.; Wu, Y.-C.; Wu, T.-S.; Kuoh, C.-S. A new coumarin glucoside from Daphne arisanensis. Chem. Pharm. Bull. 1991, 39, 2422–2424. [Google Scholar] [CrossRef]
- Wei, Z.; Weidong, Z.; Tingzhao, L.; Runhui, L.; Peng, F.; Huiliang, L. Phenolic constituents from Daphne odora var. atrocaulis. Nat. Prod. Res. Dev. 2005, 17, 26–28. [Google Scholar]
- Hu, X.-J.; Jin, H.-Z.; Yan, L.; Zhang, W.-D. Chemical constituents of Daphne retusa. Nat. Prod. Res. Dev. 2011, 23, 20–24. [Google Scholar]
- Malafronte, N.; Vassallo, A.; Dal Piaz, F.; Bader, A.; Braca, A.; De Tommasi, N. Biflavonoids from Daphne linearifolia Hart. Phytochem. Lett. 2012, 5, 621–625. [Google Scholar] [CrossRef]
- Liang, S.; Shen, Y.-H.; Tian, J.-M.; Wu, Z.-J.; Jin, H.-Z.; Zhang, W.-D.; Yan, S.-K. Phenylpropanoids from Daphne feddei and their inhibitory activities against NO production. J. Nat. Prod. 2008, 71, 1902–1905. [Google Scholar] [CrossRef]
- Chen, Y.C.; Ma, Q.Y.; Huang, S.Z.; Guo, Z.K.; Zhao, Y.X.; Hua, Y. Analyses on chemical constituents of Daphne holosericea. J. Southwest For. Univ. 2014, 34, 97–101. [Google Scholar]
- Prior, R.L.; Wu, X.; Schaich, K. Standardized Methods for the Determination of antioxidant capacity and phenolics in foods and dietary supplements. J. Agric. Food Chem. 2005, 53, 4290–4302. [Google Scholar] [CrossRef]
- Müller, L.; Fröhlich, K.; Böhm, V. Comparative antioxidant activities of carotenoids measured by ferric reducing antioxidant power (FRAP), ABTS+ bleaching assay (αTEAC), DPPH assay and peroxyl radical scavenging assay. Food Chem. 2011, 129, 139–148. [Google Scholar] [CrossRef]
- Khodadadian, Z.; Hassanpour-Ezatti, M.; Mousavi, S.Z.; Asgarpanah, J. Analgesic and anti-inflammatory potential of aerial parts of the Daphne mucronate Royle extract in mice: Opioid-independent action. Asian Pac. J. Trop. Biomed. 2016, 6, 198–201. [Google Scholar] [CrossRef]
- Kupeli, E.; Tosun, A.; Yesilada, E. Assessment of anti-inflammatory and antinociceptive activities of Daphne pontica L. (Thymelaeaceae). J. Ethnopharmacol. 2007, 113, 332–337. [Google Scholar] [CrossRef] [PubMed]
- Süntar, I.; KüpeliAkkol, E.; Keles, H.; Yesilada, E.; Sarker, S.D.; Arroo, R. Efficacy of Daphne oleoides subsp. kurdica used for wound healing: Identification of active compounds through bioassay guided isolation technique. J. Ethnopharmacol. 2012, 141, 1058–1070. [Google Scholar] [CrossRef] [PubMed]
- Atay-Balkan, I.; Taşkın, T.; Doğan, H.T.; Deniz, I.; Akaydın, G. A comparative investigation on the in vitro anti-inflammatory, antioxidant and antimicrobial potentials of subextracts from the aerial parts of Daphne oleoides Schreb. subsp. oleoides. Ind. Crop Prod. 2017, 95, 695–703. [Google Scholar] [CrossRef]
- Toniolo, C.; Nicoletti, M.; Maggi, F.; Venditti, A. HPTLC determination of chemical composition variability in raw materials used in botanicals. Nat. Prod. Res. 2014, 28, 119–126. [Google Scholar] [CrossRef]
- Zengin, G.; Arkan, T.; Aktumsek, A.; Guler, G.O.; Cakmak, Y.S. A study on antioxidant capacities and fatty acid compositions of two Daphne species from Turkey: New sources of antioxidants and essential fatty acids. J. Food. Biochem. 2012, 37, 646–653. [Google Scholar] [CrossRef]
- Li, Z.; Chen, X.; Liu, Z.; Ye, W.; Li, L.; Qian, L.; Ding, H.; Li, P.; Aung, L.H.H. Recent advances: Molecular mechanism of RNA oxidation and its role in various diseases. Front. Mol. Biosci. 2020, 7, 1–7. [Google Scholar] [CrossRef]
- Quintanar, L.; Lim, M.H. Metal ions and degenerative diseases. J. Biol. Inorg. Chem. 2019, 24, 1137–1139. [Google Scholar] [CrossRef] [PubMed]
- Sharifi-Rad, M.; Anil Kumar, N.V.; Zucca, P.; Varoni, E.M.; Dini, L.; Panzarini, E.; Rajkovic, J.; Tsouh Fokou, P.V.; Azzini, E.; Peluso, I.; et al. Lifestyle, oxidative stress, and antioxidants: Back and forth in the pathophysiology of chronic diseases. Front. Physiol. 2020, 11, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Nagai, R.; Murray, D.B.; Metz, T.O.; Baynes, J.W. Chelation: A fundamental mechanism of action of AGE inhibitors, AGE breakers, and other inhibitors of diabetes complications. Diabetes 2012, 61, 549–559. [Google Scholar] [CrossRef] [PubMed]
- Di Sotto, A.; Locatelli, M.; Macone, A.; Toniolo, C.; Cesa, S.; Carradori, S.; Eufemi, M.; Mazzanti, G.; Di Giacomo, S. Hypoglycemic, antiglycation, and cytoprotective properties of a phenol-rich extract from waste peel of Punica granatum L. var. Dente di Cavallo DC2. Molecules 2019, 24, 3103. [Google Scholar] [CrossRef] [PubMed]
- Grael, C.F.F.; Kanashiro, A.; Kabeya, L.M.; Jordão, C.O.; Takeara, R.; Gobbo-Neto, L.; Polizello, A.C.M.; Lucisano-Valim, Y.M.; Lopes, N.P.; Lopes, J.L.C. In vitro study of antioxidant and scavenger properties of phenolic compounds from Lychnophora species. Química Nova 2010, 33, 867–870. [Google Scholar] [CrossRef]
- Kim, D.-S.; Lim, S.-B. Semi-continuous subcritical water extraction of flavonoids from Citrus unshiu peel: Their antioxidant and enzyme inhibitory activities. Antioxidants 2020, 9, 360. [Google Scholar] [CrossRef] [PubMed]
- Es-Safi, N.-E.; Kollmann, A.; Khlifi, S.; Ducrot, P.-H. Antioxidative effect of compounds isolated from Globularia alypum L. structure–activity relationship. LWT Food Sci. Technol. 2007, 40, 1246–1252. [Google Scholar] [CrossRef]
- Qiu, J.; Gao, F.; Shen, G.; Li, C.; Han, X.; Zhao, Q.; Zhao, D.; Hua, X.; Pang, Y. Metabolic engineering of the phenylpropanoid pathway enhances the antioxidant capacity of Saussurea involucrata. PLoS ONE 2013, 8, e70665. [Google Scholar] [CrossRef] [PubMed]
- Kiliç, I.; Yeşiloğlu, Y. Spectroscopic studies on the antioxidant activity of p-coumaric acid. Spectrochim. Acta Part A 2013, 115, 719–724. [Google Scholar]
- Maksymiak, M.M.; Zięba, M.; Orchel, A.; Musiał-Kulik, M.; Kowalczuk, M.; Adamus, G. Bioactive (co)oligoesters as potential delivery systems of p-anisic acid for cosmetic purposes. Materials 2020, 13, 4153. [Google Scholar] [CrossRef]
- Gülçin, I. Antioxidant activity of caffeic acid (3,4-dihydroxycinnamic acid). Toxicology 2006, 217, 213–220. [Google Scholar] [CrossRef]
- Ivanova, A.; Gerasimova, E.; Gazizullina, E. Study of antioxidant properties of agents from the perspective of their action mechanisms. Molecules 2020, 25, 4251. [Google Scholar] [CrossRef]
- Montecillo-Aguado, M.; Tirado-Rodriguez, B.; Tong, Z.; Vega, O.M.; Morales-Martínez, M.; Abkenari, S.; Pedraza-Chaverri, J.; Huerta-Yepez, S. Importance of the role of ω-3 and ω-6 polyunsaturated fatty acids in the progression of brain cancer. Brain Sci. 2020, 10, 381. [Google Scholar] [CrossRef] [PubMed]
- Michelis, F.; Tiligada, E.; Skaltsa, H.; Lazari, D.; Skaltsounis, A.L.; Delitheos, A. Effects of the flavonoid pilloin isolated from Marrubium cylleneum on mitogen-induced lymphocyte transformation. Pharm. Biol. 2002, 40, 245–248. [Google Scholar] [CrossRef]
- Choi, C.-H.; Sun, K.-H.; An, C.-S.; Yoo, J.-C.; Hahm, K.-S.; Leed, I.-H.; Sohng, J.-K.; Kim, Y.-C. Reversal of P-glycoprotein-mediated multidrug resistance by 5,6,7,3′,4′-pentamethoxyflavone (sinensetin). Biochem. Biophys. Res. Commun. 2002, 295, 832–840. [Google Scholar] [CrossRef]
- Qawoogh, S.S.; Shahiwal, A. Identification of potential anticancer phytochemicals against colorectal cancer by structure-based docking studies. J. Recept. Sign. Transduct. 2020, 40, 67–76. [Google Scholar] [CrossRef] [PubMed]
- Xia, N. Syringin exhibits anti-cancer effects in HeLa human cervical cancer cells by inducing apoptosis, cell cycle arrest and inhibition of cell migration. Bangladesh J. Pharmacol. 2016, 11, 838–843. [Google Scholar] [CrossRef]
- Lall, N.; Kishore, N.; Binneman, B.; Twilley, D.; van de Venter, M.; du Plessis-Stoman, D. Cytotoxicity of syringin and 4-methoxycinnamyl alcohol isolated from Foeniculum vulgare on selected human cell lines. Nat. Prod. Res. 2015, 29, 1752–1756. [Google Scholar] [CrossRef] [PubMed]
- Jaganathan, S.K.; Supriyanto, E.; Mandal, M. Events associated with apoptotic effect of p-coumaric acid in HCT-15 colon cancer cells. World J. Gastroenterol. 2013, 19, 7726–7734. [Google Scholar] [CrossRef]
- Pelinson, L.P.; Assmann, C.E.; Palma, T.V.; Mânica da Cruz, I.B.; Mainardi Pillat, M.; Mânica, M.; Stefanello, N.; Cezimbra Weis, G.C.; de Oliveira Alves, A.; Melazzo de Andrade, C.; et al. Antiproliferative and apoptotic effects of caffeic acid on SK-Mel-28 human melanoma cancer cells. Mol. Biol. Rep. 2019, 46, 2085–2092. [Google Scholar] [CrossRef]
- Rezakhani, N.; Goliaei, B.; Parivar, K.; Nikoofar, A.R. Effects of X-irradiation and sinensetin on apoptosis induction in MDA-MB-231 human breast cancer cells. Int. J. Rad. Res. 2020, 18, 75–82. [Google Scholar]
- Khan, S.S.; Iqbal, M.A.; Majid, A.M.S.A. Effect of crystallization of caffeic acid enhanced stability and dual biological efficacy. Cogent Biol. 2016, 2, 1–8. [Google Scholar] [CrossRef]

| Antioxidant Activity Assays | IC50 (Confidence Limits) μg/mL | |
|---|---|---|
| Daphne sericea Vahl Flowering Aerial Parts Extract | Positive Control | |
| DPPH· radical scavenger activity | 506.8 (404.6–634.9) | 16.5 (8.5–133.7) a |
| ABTS·+ radical scavenger activity | 11.4 (6.7–19.4) | 2.8 (1.7–4.7) a |
| Ferrous ion chelating activity | 143.8 (83.6–247.3) | 36.4 (28.1–47.2) b |
| Ferric ion chelating activity | 551.2 (270.6–969.1) | 273.0 (198.7–375.1) b |
| Pearson r (Confidence Limits; R Square) | ||||
|---|---|---|---|---|
| DPPH· scavenger activity | ABTS·+ scavenger activity | Ferrous ion chelating activity | Ferric ion chelating activity | |
| DPPH· radical scavenger activity | 1 | - | - | - |
| ABTS·+ radical scavenger activity | nsc | 1 | - | - |
| Ferrous ion chelating activity | 0.89 * (0.27–0.99; 0.79) | nsc | 1 | - |
| Ferric ion chelating activity | 0.99 *** (0.93–0.99; 0.98) | nsc | 0.94 ** (0.55–0.99; 0.89) | 1 |
| Compounds | µg/mg Extract |
|---|---|
| Polyphenols (TAE) | 34.1 ± 2.8 |
| Tannins (TAE) | 10.1 ± 2.9 |
| Flavonoids (QE) | 8.4 ± 0.9 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Frezza, C.; Venditti, A.; De Vita, D.; Sciubba, F.; Tomai, P.; Franceschin, M.; Di Cecco, M.; Ciaschetti, G.; Di Sotto, A.; Stringaro, A.; et al. Phytochemical Analysis and Biological Activities of the Ethanolic Extract of Daphne sericea Vahl Flowering Aerial Parts Collected in Central Italy. Biomolecules 2021, 11, 379. https://doi.org/10.3390/biom11030379
Frezza C, Venditti A, De Vita D, Sciubba F, Tomai P, Franceschin M, Di Cecco M, Ciaschetti G, Di Sotto A, Stringaro A, et al. Phytochemical Analysis and Biological Activities of the Ethanolic Extract of Daphne sericea Vahl Flowering Aerial Parts Collected in Central Italy. Biomolecules. 2021; 11(3):379. https://doi.org/10.3390/biom11030379
Chicago/Turabian StyleFrezza, Claudio, Alessandro Venditti, Daniela De Vita, Fabio Sciubba, Pierpaolo Tomai, Marco Franceschin, Mirella Di Cecco, Giampiero Ciaschetti, Antonella Di Sotto, Annarita Stringaro, and et al. 2021. "Phytochemical Analysis and Biological Activities of the Ethanolic Extract of Daphne sericea Vahl Flowering Aerial Parts Collected in Central Italy" Biomolecules 11, no. 3: 379. https://doi.org/10.3390/biom11030379
APA StyleFrezza, C., Venditti, A., De Vita, D., Sciubba, F., Tomai, P., Franceschin, M., Di Cecco, M., Ciaschetti, G., Di Sotto, A., Stringaro, A., Colone, M., Gentili, A., Serafini, M., & Bianco, A. (2021). Phytochemical Analysis and Biological Activities of the Ethanolic Extract of Daphne sericea Vahl Flowering Aerial Parts Collected in Central Italy. Biomolecules, 11(3), 379. https://doi.org/10.3390/biom11030379












